Larval Development in Tropical Gar (Atractosteus tropicus) Is Dependent on the Embryonic Thermal Regime: Ecological Implications under a Climate Change Context
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Animal Acquisition and Care
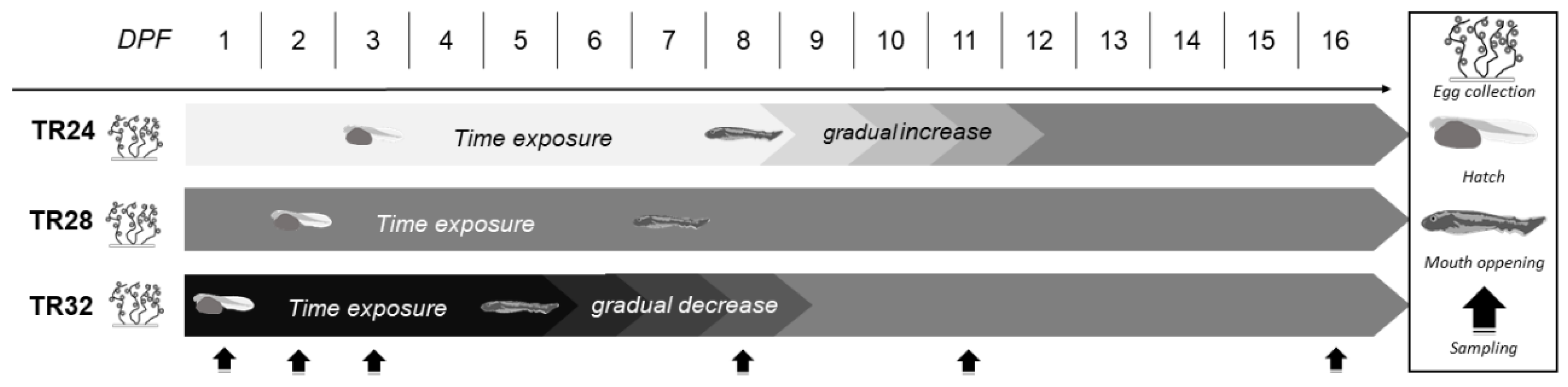
2.3. Experimental Design and Sampling
2.4. Survival and Growth
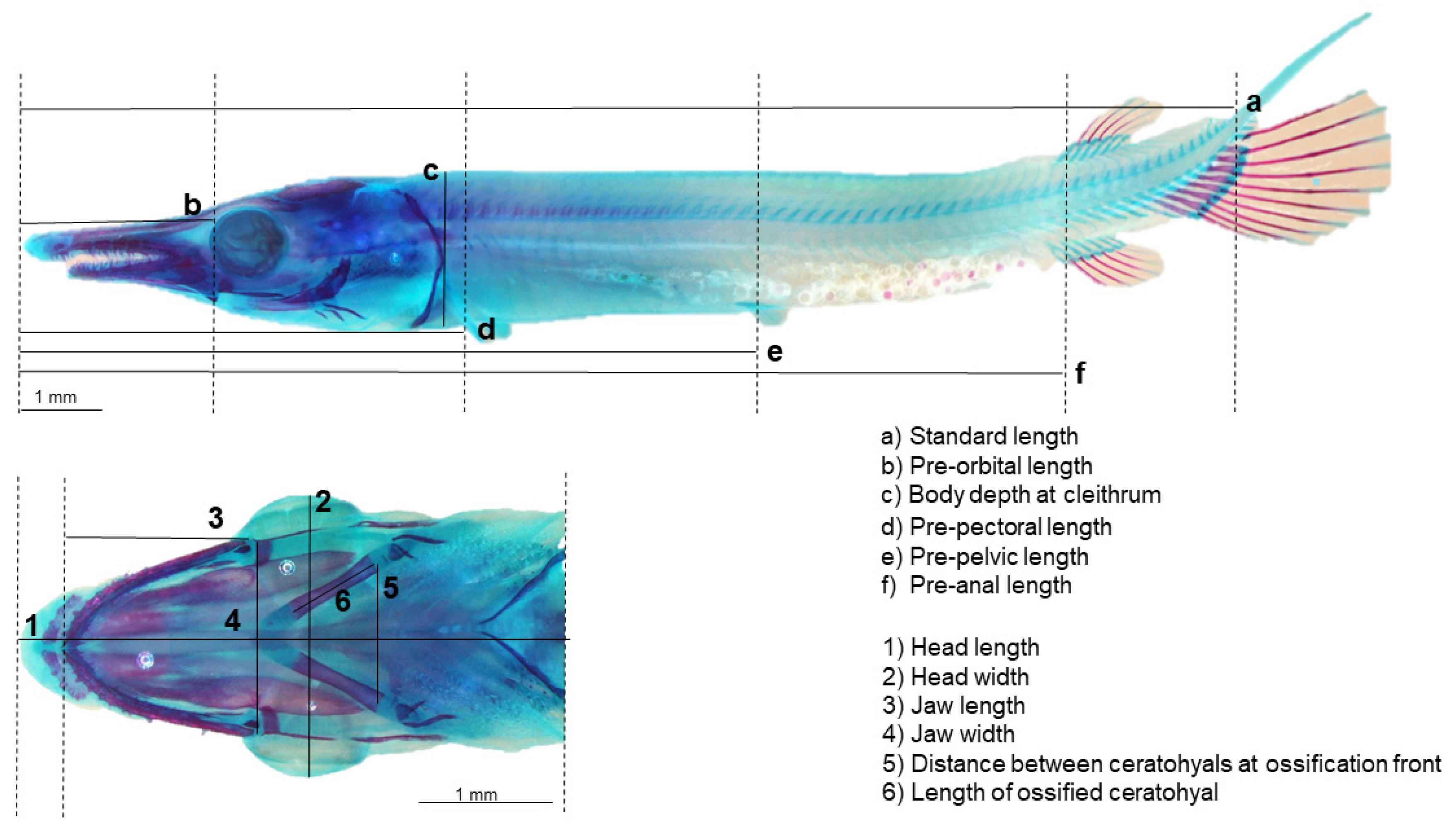
2.5. Skeletal Development Assessment and Body Morphology Biometrics
2.6. Statistical Analysis
3. Results
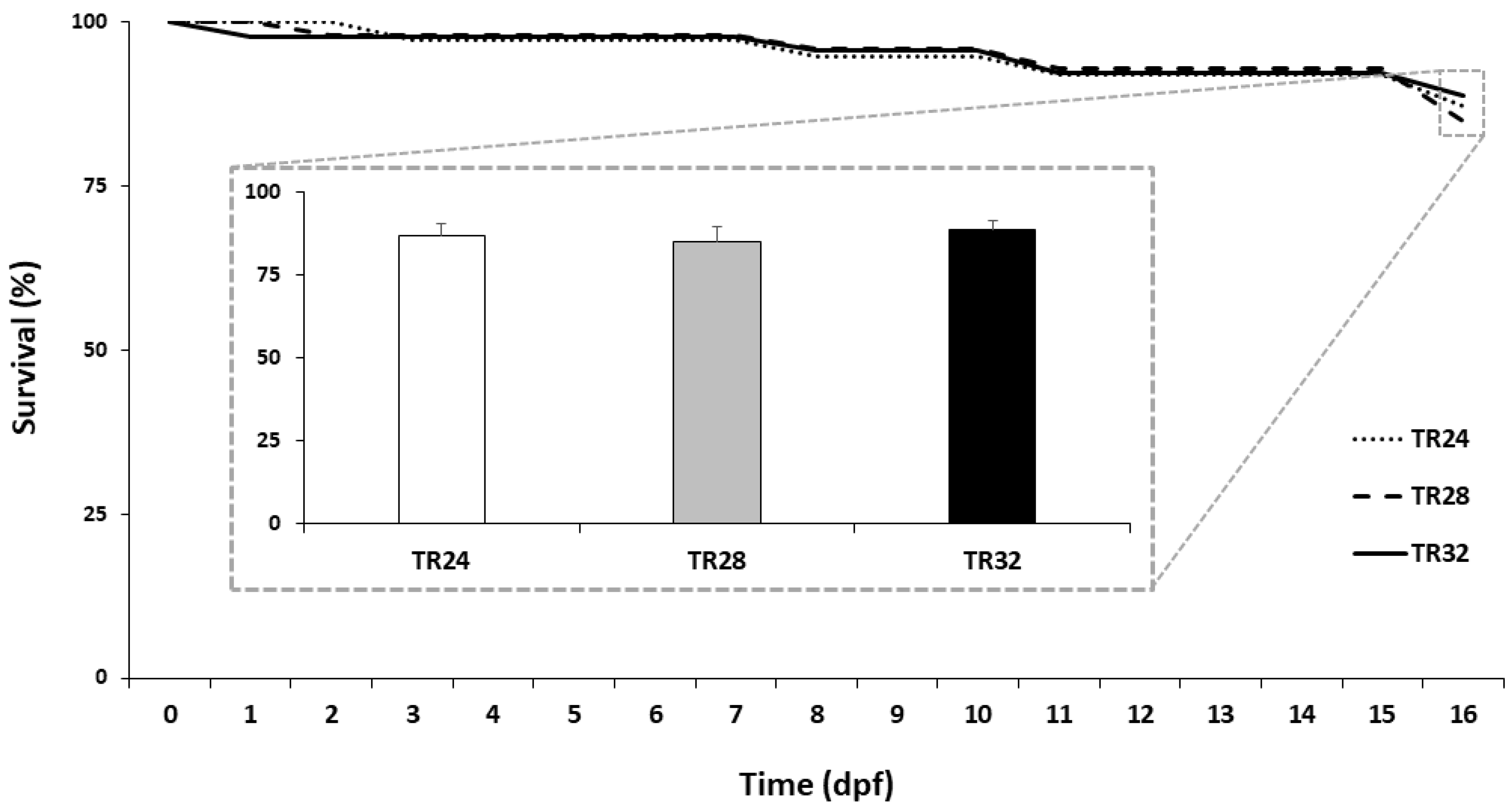
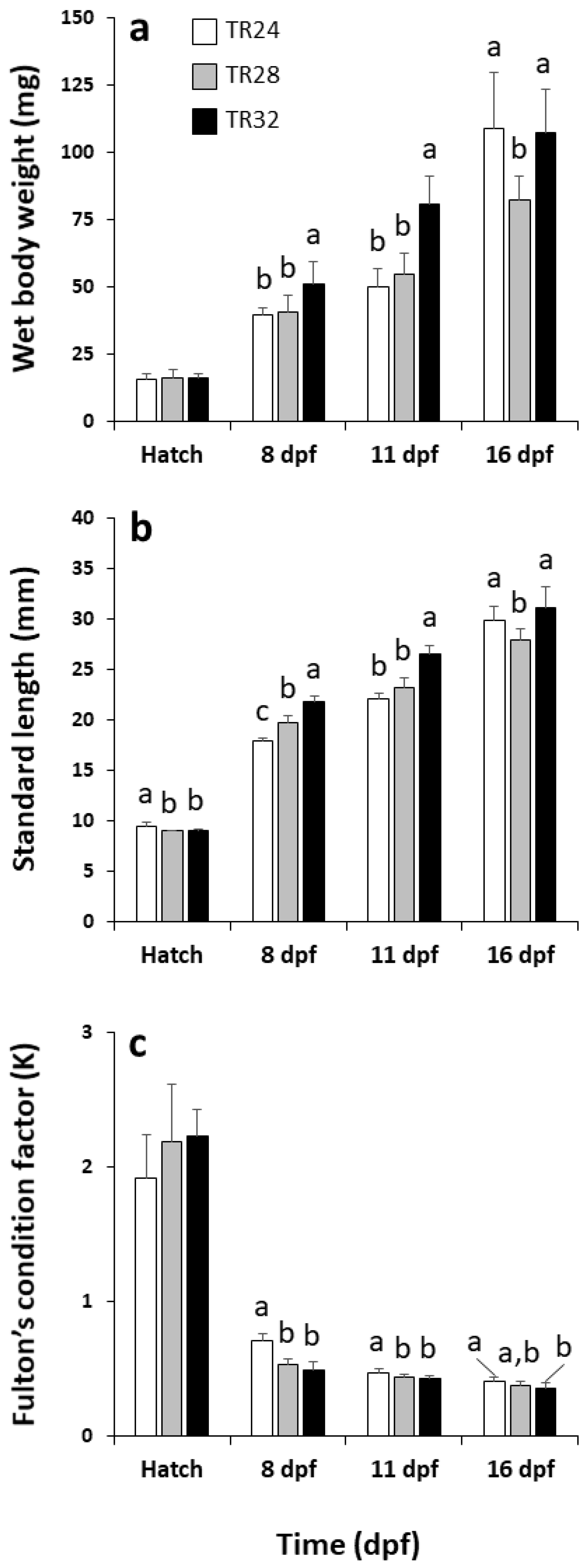
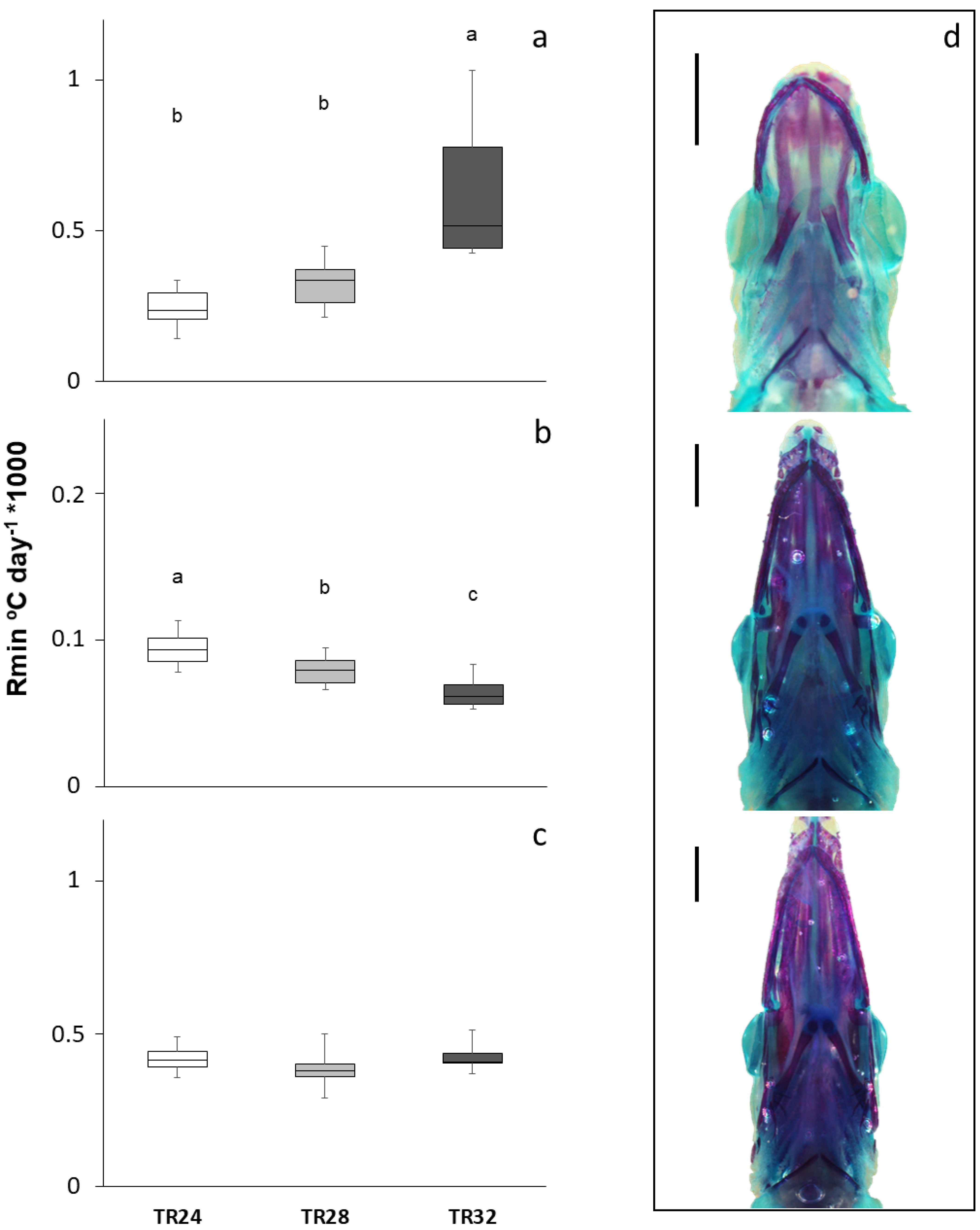
3.1. Hatching Rate, Survival and Growth Performance
3.2. Skeletal Development
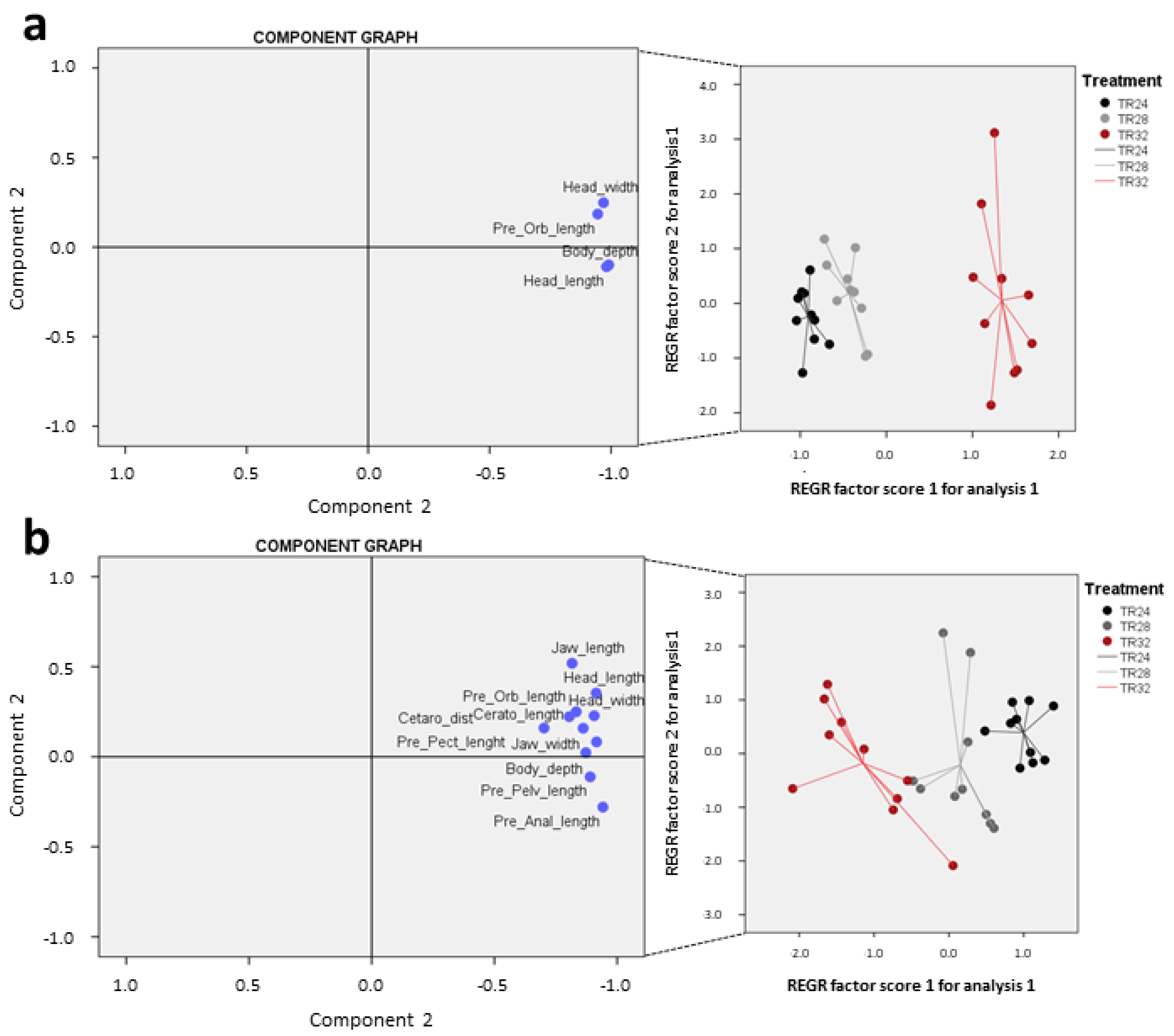
3.3. Body Morphology and Biometric Lengths
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arias, P.; Bellouin, N.; Coppolam, E.; Jones, R.; Krinner, G.; Marotzke, J.; Zickfeld, K. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Technical Summary; Cambridge University Press: Cambridge, UK, 2021; In Press. [Google Scholar]
- Enders, E.C.; Boisclair, D. Effects of environmental fluctuations on fish metabolism: Atlantic salmon Salmo salar a case study. J. Fish Biol. 2016, 88, 344–358. [Google Scholar] [CrossRef] [Green Version]
- Jonsson, B.; Jonsson, N. A review of the likely effects of climate change on anadromous Atlantic salmon Salmo salar and brown trout Salmo trutta, with particular reference to water temperature and flow. J. Fish Biol. 2009, 75, 2381–2447. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.Y.; Manzon, R.G.; Somers, C.M.; Boreham, D.R.; Wilson, J.Y. The effects of fluctuating temperature regimes on the embryonic development of lake whitefish (Coregonus clupeaformis). Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 2017, 214, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Marcos-López, M.; Gale, P.; Oidtmann, B.C.; Peeler, E.J. Assessing the impact of climate change on disease emergence in freshwater fish in the United Kingdom. Transbound. Emerg. Dis. 2010, 57, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Magel, J.M.T.; Dimoff, S.A.; Baum, J.K. Direct and indirect effects of climate change-amplified pulse heat stress events on coral reef fish communities. Ecol. Appl. 2020, 30, e02124. [Google Scholar] [CrossRef] [PubMed]
- Murdoch, A.; Mantyka-Pringle, C.; Sharma, S. The interactive effects of climate change and land use on boreal stream fish communities. Sci. Total Environ. 2020, 700, 134518. [Google Scholar] [CrossRef] [PubMed]
- Servili, A.; Canario, A.V.M.; Mouchel, O.; Muñoz-Cueto, J.A. Climate change impacts on fish reproduction are mediated at multiple levels of the brain-pituitary-gonad axis. Gen. Comp. Endocrinol. 2020, 291, 113439. [Google Scholar] [CrossRef]
- Abdo-de la Parra, M.I.; Martínez-Rodríguez, I.E.; González-Rodríguez, B.; Rodríguez-Ibarra, L.E.; Duncan, N.; Hernández, C. Efecto de la temperatura y salinidad del agua en la incubación de huevos de botete diana Sphoeroides annulatus. Rev. De Biol. Mar. Y Oceanogr. 2012, 47, 147–153. [Google Scholar] [CrossRef]
- Landsman, S.J.; Gingerich, A.J.; Philipp, D.P.; Suski, C.D. The effects of temperature change on the hatching success and larval survival of largemouth bass Micropterus salmoides and smallmouth bass Micropterus dolomieu. J. Fish Biol. 2011, 78, 1200–1212. [Google Scholar] [CrossRef]
- Mateus, A.P.; Costa, R.; Gisbert, E.; Pinto, P.; Andree, K.B.; Estévez, A.; Power, D.M. Thermal imprinting modifies bone homeostasis in cold-challenged sea bream (Sparus aurata). J. Exp. Biol. 2017, 220, 3442–3454. [Google Scholar] [CrossRef] [Green Version]
- Herbing, I.H.V. Effects of temperature on larval fish swimming performance: The importance of physics to physiology. J. Fish Biol. 2002, 61, 865–876. [Google Scholar] [CrossRef]
- Boglione, C.; Gisbert, E.; Gavaia, P.; Witten, P.E.; Moren, M.; Fontagné, S.; Koumoundouros, G. Skeletal anomalies in reared European fish larvae and juveniles. Part 2: Main typologies, occurrences and causative factors. Rev. Aquac. 2013, 5, S121–S167. [Google Scholar] [CrossRef] [Green Version]
- Georgakopoulou, E.; Katharios, P.; Divanach, P.; Koumoundouros, G. Effect of temperature on the development of skeletal deformities in Gilthead seabream (Sparus aurata Linnaeus, 1758). Aquac. Res. 2010, 308, 13–19. [Google Scholar] [CrossRef]
- Dionísio, G.; Campos, C.; Valente, L.M.P.; Conceição, L.E.C.; Cancela, M.L.; Gavaia, P.J. Effect of egg incubation temperature on the occurrence of skeletal deformities in Solea senegalensis. J. Appl. Ichthyol. 2012, 28, 471–476. [Google Scholar] [CrossRef]
- Yang, Q.; Ma, Z.; Zheng, P.; Jiang, S.; Qin, J.G.; Zhang, Q. Effect of temperature on growth, survival and occurrence of skeletal deformity in the golden pompano Trachinotus ovatus larvae. Indian J. Fish. 2016, 63, 74–82. [Google Scholar] [CrossRef] [Green Version]
- Pimentel, M.S.; Faleiro, F.; Marques, T.; Bispo, R.; Dionísio, G.; Faria, A.M.; Rosa, R. Foraging behaviour, swimming performance and malformations of early stages of commercially important fishes under ocean acidification and warming. Clim. Chang. 2016, 137, 495–509. [Google Scholar] [CrossRef] [Green Version]
- Crespel, A.; Zambonino-Infante, J.L.; Mazurais, D.; Koumoundouros, G.; Fragkoulis, S.; Quazuguel, P.; Claireaux, G. The development of contemporary European sea bass larvae (Dicentrarchus labrax) is not affected by projected ocean acidification scenarios. Mar. Biol. 2017, 164, 155. [Google Scholar] [CrossRef] [Green Version]
- Meehl, G.A.; Tebaldi, C. More intense, more frequent, and longer lasting heat waves in the 21st century. Science 2004, 305, 994–997. [Google Scholar] [CrossRef] [Green Version]
- Stillman, J.H. Heat waves, the new normal: Summertime temperature extremes will impact animals, ecosystems, and human communities. Physiology 2019, 34, 86–100. [Google Scholar] [CrossRef]
- Alfonso, S.; Gesto, M.; Sadoul, B. Temperature increase and its effects on fish stress physiology in the context of global warming. J. Fish Biol. 2021, 98, 1496–1508. [Google Scholar] [CrossRef]
- Braasch, I.; Gehrke, A.R.; Smith, J.J.; Kawasaki, K.; Manousaki, T.; Pasquier, J.; Amores, A.; Desvignes, T.; Batzel, P.; Postlethwait, J.H.; et al. The spotted gar genome illuminates vertebrate evolution and facilitates human-teleost comparisons. Nat. Genet. 2016, 48, 427–437. [Google Scholar] [CrossRef] [Green Version]
- Burggren, W.W.; Bautista, G.M.; Coop, S.C.; Couturier, G.M.; Delgadillo, S.P.; García, R.M.; González, C.A.A. Developmental cardiorespiratory physiology of the air-breathing tropical gar, Atractosteus tropicus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 311, R689–R701. [Google Scholar] [CrossRef] [Green Version]
- Martínez, G.; Peña, E.; Martínez, R.; Camarillo, S.; Burggren, W.; Álvarez, A. Survival, Growth, and Development in the Early Stages of the Tropical Gar Atractosteus tropicus: Developmental Critical Windows and the Influence of Temperature, Salinity, and Oxygen Availability. Fishes 2021, 6, 5. [Google Scholar] [CrossRef]
- Miller, R.R.; Minckley, W.L.; Norris, S.M. Freshwater Fishes of Mexico; University of Chicago Press: Chicago, IL, USA, 2005; p. 490. [Google Scholar]
- Resendez Medina, A. Estudio de los peces de la Laguna de Términos, Campeche, México. II. Última parte. Biótica 1981, 6, 345–430. [Google Scholar]
- Reséndez-Medina, A.; Salvadores, M.L. Contribución al conocimiento de la biología del pejelagarto Lepisosteus tropicus (Gill) y la tenguayaca Petenia splendida Günther, del estado de Tabasco. Biótica 1983, 8, 413–426. [Google Scholar]
- Mora, M.; Cabrera-Peña, J.; Galeano, G. Reproducción y alimentación del gaspar Atractosteus tropicus (Pisces: Lepisosteidae) en el refugio nacional de vida silvestre Caño Negro, Costa Rica. Rev. Biol. Trop. 1997, 45, 861–866. [Google Scholar]
- Barrientos-Villalobos, J.; Espinosa de los Monteros, A. Genetic variation and recent population history of the tropical gar Atractosteus tropicus Gill (Pisces: Lepisosteidae). J. Fish Biol. 2008, 73, 1919–1936. [Google Scholar] [CrossRef]
- Torres, J.R.; Barba, E.; Choix, F.J. Mangrove productivity and phenology in relation to hydroperiod and physical-chemistry properties of wáter and sediment in biosphere reserve, Centla wetland, Mexico. Trop. Cons. Sci. 2018, 11, 1–14. [Google Scholar] [CrossRef]
- Soares, D.; García, A. La Cuenca Del Río Usumacinta Desde la Perspectiva Del Cambio Climático; Instituto Mexicano de Tecnología del Agua: Jiutepec, Mexico, 2017; 422p, Available online: https://www.gob.mx/imta/documentos/la-cuenca-del-rio-usumacinta-desde-la-perspectiva-del-cambio-climatico (accessed on 13 December 2021).
- Galaviz Villa, I.; Sosa Villalobos, C.A. (Eds.) Fuentes Difusas y Puntuales de Contaminación. Calidad de las Aguas Superficiales y Subterráneas. Universidad Autónoma de Campeche. 2019. Available online: https://drive.google.com/file/d/1E1tcVEztzkG06ORUT6jAYgs_Lb1-A10F/view (accessed on 13 December 2021).
- Márquez-Couturier, G.; Vázquez-Navarrete, C.J.; Contreras-Sánchez, W.M.; Álvarez-González, C.A. Acuicultura Tropical Sus- Tentable: Una Estrategia Para la Producción y Conservación del Pejelagarto (Atractosteus tropicus) en Tabasco, México, 2nd ed.; Narciso Rovirosa, C.J., Ed.; Universidad Juárez Autónoma de Tabasco: Tabasco, México, 2015; Volume 3, p. 223. [Google Scholar]
- Ricker, W.E. Computation and interpretation of biological statistics of fish populations. Bull. Fish. Res. Board Can. 1975, 191, 1–382. [Google Scholar]
- Walker, M.B.; Kimmel, C.B. A two-color acid-free cartilage and bone stain for zebrafish larvae. Biotech. Histochem. 2007, 82, 23–28. [Google Scholar] [CrossRef]
- Tarasco, M.; Laizé, V.; Cardeira, J.; Cancela, M.L.; Gavaia, P.J. The zebrafish operculum: A powerful system to assess osteogenic bioactivities of molecules with pharmacological and toxicological relevance. Comp. Biochem. Physiol. Part C 2017, 197, 45–52. [Google Scholar] [CrossRef]
- Pimentel, M.S.; Faleiro, F.; Machado, J.; Pousão-Ferreira, P.; Rosa, R. Seabream larval physiology under ocean warming and acidification. Fishes 2020, 5, 1. [Google Scholar] [CrossRef] [Green Version]
- Huerta-Ortíz, M.; González, C.A.Á.; Couturier, G.M.; Sánchez, W.M.C.; Cerecedo, R.C.; Bores, E.G. Sustitución total de aceite de pescado con aceite vegetal en larvas de pejelagarto Atractosteus tropicus. Kuxulkab’ 2009, 15. [Google Scholar] [CrossRef]
- Frías-Quintana, C.A.; Álvarez-González, C.A.; Márquez-Couturier, G. Diseño de microdietas para el larvicultivo de pejelagarto Atractosteus tropicus, Gill 1863. Univ. Cienc. 2010, 26, 265–282. [Google Scholar]
- Guerrero-Zárate, R.; Álvarez-González, C.A.; Olvera-Novoa, M.A.; Perales-García, N.; Frías-Quintana, C.A.; Martínez-García, R.; Contreras-Sánchez, W.M. Partial characterization of digestive proteases in tropical gar Atractosteus tropicus juveniles. Fish Physiol. Biochem. 2014, 40, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Frías-Quintana, C.A.; Domínguez-Lorenzo, J.; Álvarez-González, C.A.; Tovar-Ramírez, D.; Martínez-García, R. Using cornstarch in microparticulate diets for larvicultured tropical gar (Atractosteus tropicus). Fish Physiol. Biochem. 2016, 42, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Cárdenas, L.; Hernández-Cortez, M.I.; Espinosa-Chaurand, D.; Castañeda-Chavez, M.R.; León-Fernández, A.E.; Valdez Hernández, E.F.; Álvarez-González, C.A. Effect of stocking density on growth, survival and condition factor in tropical gar (Atractosteus tropicus Gill, 1863) juveniles. Lat. Am. J. Aquat. Res. 2020, 48, 570–577. [Google Scholar] [CrossRef]
- Ferreira-Rodríguez, N.; Fernandez, I.; Cancela, M.L.; Pardo, I. Multibiomarker response shows how native and non-native freshwater bivalves differentially cope with heat-wave events. Aquatic. Conserv. Mar. Freshw. Ecosyst. 2018, 28, 934–943. [Google Scholar] [CrossRef]
- Politis, S.N.; Mazurais, D.; Servili, A.; Zambonino-Infante, J.L.; Miest, J.J.; Sorensen, S.R.; Butts, I.A. Temperature effects on gene expression and morphological development of European eel, Anguilla anguilla larvae. PLoS ONE 2017, 12, e0182726. [Google Scholar] [CrossRef]
- Mendoza Alfaro, R.; González, C.A.; Ferrara, A.M. Gar biology and culture: Status and prospects. Aquac. Res. 2008, 39, 748–763. [Google Scholar] [CrossRef]
- Aranda-Morales, S.A.; Peña-Marín, E.S.; Jiménez-Martínez, L.D.; Martínez-Burguete, T.; Martínez-Bautista, G.; Álvarez-Villagómez, C.S.; De la Rosa, S.; Camarillo-Coop, S.; Martínez-García, R.; Álvarez-González, C.A.; et al. Expression of ion transport proteins and routine metabolism in juveniles of tropical gar (Atractosteus tropicus) exposed to ammonia. Comp. Biochem. Physiol. Part C: Toxicol. Pharmacol. 2021, 250, 109166. [Google Scholar] [CrossRef] [PubMed]
- Córdova-de la Cruz, S.E.; Martínez-Bautista, G.; Peña-Marín, E.S.; Martínez-García, R.; Núñez-Nogueira, G.; Adams, R.H.; Burggren, W.W.; Alvarez-González, C.A. Morphological and cardiac alterations after crude oil exposure in the early-life stages of the tropical gar (Atractosteus tropicus). Environ. Sci. Pollut. Res. 2021, 1–12. [Google Scholar] [CrossRef]
- Navarro-Martin, L.; Vinas, J.; Ribas, L.; Diaz, N.; Gutierrez, A.; Di Croce, L.; Piferrer, F. DNA methylation of the gonadal aromatase (cyp 19a) promoter is involved in temperatura dependent sex ratio shifts in the European sea bass. PLoS Genet. 2011, 7, e1002447. [Google Scholar] [CrossRef] [Green Version]
- Campos, C.; Valente, L.M.P.; Conceicao, L.E.C.; Engrola, S.; Fernandes, J.M.O. Temperature affects methylation of the myogenin putative promoter, its expression and muscle cellularity in Senegalese sole larvae. Epigenetics 2013, 8, 389–397. [Google Scholar] [CrossRef] [Green Version]
- Burgerhout, E.; Mommens, M.; Johnsen, H.; Aunsmo, A.; Santi, N.; Andersen, O. Genetic background and embryonic temperature affect DNA methylation and expression of myogenin and muscle development in Atlantic salmon (Salmo salar). PLoS ONE 2017, 12, e0179918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribas, L.; Liew, W.C.; Diaz, N.; Sreenivasan, R.; Orban, L.; Piferrer, F. Heat-induced masculinization in domesticated zebrafish is family-specific & yields a set of different gonadal transcriptomes. Proc. Natl. Acad. Sci. USA 2017, 114, E941–E950. [Google Scholar] [CrossRef] [Green Version]
- Di Santo, V. Ocean acidification and warming affect skeletal mineralization in a marine fish. Proc. R. Soc. B Biol. Sci. 2019, 286, 20182187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, C.Q.; Zhou, P.; Ren, Y.L.; Cao, L.H.; Wang, J.L. Physiological response and miRNA-mRNA interaction analysis in the head kidney of rainbow trout exposed to acute heat stress. J. Therm. Biol. 2019, 83, 134–141. [Google Scholar] [CrossRef]
- Valdivieso, A.; Ribas, L.; Monleon-Getino, A.; Orban, L.; Piferrer, F. Exposure of zebrafish to elevated temperature induces sex ratio shifts and alterations in the testicular epigenome of unexposed offspring. Environ. Res. 2020, 186, 109601. [Google Scholar] [CrossRef] [PubMed]
- Arratia, G.; Schultze, H.P. Palatoquadrate and its ossifications: Development and homology within osteichthyans. J. Morphol. 1991, 208, 1–81. [Google Scholar] [CrossRef]
- Long, W.L.; Ballard, W.W. Normal embryonic stages of the longnose gar, Lepisosteus osseus. BMC Dev. Biol. 2001, 1, 1–18. [Google Scholar] [CrossRef]
- Diogo, R.; Ziermann, J.M.; Molnar, J.; Siomava, N.; Abdala, V. Muscles of Chordates: Development, Homologies, and Evolution, 1st ed.; CRC Press: Boca Raton, FL, USA, 2018; ISBN 978-1-1385-7116-7. [Google Scholar]
- Grande, L. An Empirical Synthetic Pattern Study of Gars (Lepisosteiformes) and Closely Related Species, Based Montly on Skeletal Anatomy. The Resurrection of Holostei. Copeia. American Society of Icthyologists and Herpetolologists, 2010. ppt 6, 1187. Available online: https://www.jstor.org/stable/20787269 (accessed on 13 December 2021).
- Eames, B.F.; Amores, A.; Yan, Y.L.; Postlethwait, J.H. Evolution of the osteoblast: Skeletogenesis in gar and zebrafish. BMC Evol. Biol. 2012, 12, 27. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, M.; Bockmann, F.A.; de Carvalho, M.R. Homology of the fifth epibranchial and accessory elements of the ceratobranchials among gnathostomes: Insights from the development of ostariophysans. PLoS ONE 2013, 8, e62389. [Google Scholar] [CrossRef] [Green Version]
- Hilton, E.J.; Konstantinidis, P.; Schnell, N.K.; Dillman, C.B. Identity of a unique cartilage in the buccal cavity of gars (Neopterygii: Lepisosteiformes: Lepisosteidae). Copeia 2014, 1, 50–55. [Google Scholar] [CrossRef]
- Scherrer, R.; Hurtado, A.; Machado, E.G.; Debiais-Thibaud, M. MicroCT survey of larval skeletal mineralization in the Cuban gar Atractosteus tristoechus (Actinopterygii; Lepisosteiformes). MorphoMuseum 2017, 3. [Google Scholar] [CrossRef] [Green Version]
- Koumoundouros, G. Morpho-anatomical abnormalities in Mediterranean marine aquaculture. Recent Adv. Aquac. Res. 2010, 661, 125–148. [Google Scholar]
- Boglione, C.; Gavaia, P.; Koumoundouros, G.; Gisbert, E.; Moren, M.; Fontagné, S.; Witten, P.E. Skeletal anomalies in reared E uropean fish larvae and juveniles. Part 1: Normal and anomalous skeletogenic processes. Rev. Aquac. 2013, 5, S99–S120. [Google Scholar] [CrossRef] [Green Version]
- Fernández, I.; Granadeiro, L.; Darias, M.J.; Gavaia, P.J.; Andree, K.B.; Gisbert, E. Solea senegalensis skeletal ossification and gene expression patterns during metamorphosis: New clues on the onset of skeletal deformities during larval to juvenile transition. Aquaculture 2018, 496, 153–165. [Google Scholar] [CrossRef]
- Wang, L.H.; Tsai, C.L. Effects of temperature on the deformity and sex differentiation of tilapia, Oreochromis mossambicus. J. Exp. Zool. 2000, 286, 534–537. [Google Scholar] [CrossRef]
- Hansen, T.K.; Falk-Petersen, I.B. The influence of rearing temperature on early development and growth of spotted wolffish Anarhichas minor (Olafsen). Aquac. Res. 2001, 32, 369–378. [Google Scholar] [CrossRef]
- Sfakianakis, D.G.; Koumoundouros, G.; Divanach, P.; Kentouri, M. Osteological development of the vertebral column and of the fins in Pagellus erythrinus (L. 1758). Temperature effect on the developmental plasticity and morpho-anatomical abnormalities. Aquaculture 2004, 232, 407–424. [Google Scholar] [CrossRef]
- Georgakopoulou, E.; Angelopoulou, A.; Kaspiris, P.; Divanach, P.; Koumoundouros, G. Temperature effects on cranial deformities in European sea bass, Dicentrarchus labrax (L.). J. Appl. Ichthyol. 2007, 23, 99–103. [Google Scholar] [CrossRef]
- Ytteborg, E.; Baeverfjord, G.; Torgersen, J.; Hjelde, K.; Takle, H. Molecular pathology of vertebral deformities in hyperthermic Atlantic salmon (Salmo salar). BMC Physiol. 2010, 10, 12. [Google Scholar] [CrossRef] [Green Version]
- Sfakianakis, D.G.; Georgakopoulou, E.; Papadakis, I.E.; Divanach, P.; Kentouri, M.; Koumoundouros, G. Environmental determinants of haemal lordosis in European sea bass, Dicentrarchus labrax (Linnaeus, 1758). Aquaculture 2006, 254, 54–64. [Google Scholar] [CrossRef]
- Martini, A.; Huysseune, A.; Witten, P.E.; Boglione, C. Plasticity of the skeleton and skeletal deformities in zebrafish (Danio rerio) linked to rearing density. J. Fish Biol. 2021, 98, 971–986. [Google Scholar] [CrossRef] [PubMed]
- Fernández, I.; Ortiz-Delgado, J.B.; Sarasquete, C.; Gisbert, E. Vitamin A effects on vertebral bone tissue homeostasis in gilthead sea bream (Sparus aurata) juveniles. J. Appl. Ichthyol. 2012, 28, 419–426. [Google Scholar] [CrossRef]
- Hall, B.K. Bones and Cartilage: Developmental and Evolutionary Skeletal Biology, 2nd ed.; Academic Press: Cambridge, MA, USA, 2015; ISBN 9780124166851. [Google Scholar]
- Fernández, I.; Darias, M.; Andree, K.B.; Mazurais, D.; Zambonino-Infante, J.L.; Gisbert, E. Coordinated gene expression during gilthead sea bream skeletogenesis and its disruption by nutritional hypervitaminosis A. BMC Dev. Biol. 2011, 11, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Fuiman, L.A.; Poling, K.R.; Higgs, D.M. Quantifying developmental progress for comparative studies of larval fishes. Copeia 1998, 602–611. [Google Scholar] [CrossRef]
- Seikai, T.; Tanangonan, J.B.; Tanaka, M. Temperature influence on larval growth and metamorphosis of the Japanese flounder Paralichthys olivaceus in the laboratory. Bull. Jpn. Soc. Sci. Fish. 1986, 52, 977–982. [Google Scholar] [CrossRef]
- Polo, A.; Yufera, M.; Pascual, E. Effects of temperature on egg and larval development of Sparus aurata L. Aquaculture 1991, 92, 367–375. [Google Scholar] [CrossRef]
- Koumoundouros, G.; Divanach, P.; Anezaki, L.; Kentouri, M. Temperature-induced ontogenetic plasticity in sea bass (Dicentrarchuslabrax). Mar. Biol. 2001, 139, 817–830. [Google Scholar] [CrossRef]
- Comabella, Y.; Hurtado, A.; Canabal, J.; Galano, T.G. Effect of temperature on hatching and growth of Cuban gar (Atractosteus tristoechus) larvae. Ecosistemas y Recursos Agropecuarios 2014, 1, 19–32. [Google Scholar]
- Combella, Y.; Azanza, J.; Hurtado, A.; Canabal, J.; García-Galano, T. Allometric growth in Cuban gar (Atractosteus tristoechus) larvae. Universidad Ciencia 2013, 29, 301–315. [Google Scholar]
- Kourkouta, C.; Printzi, A.; Geladakis, G.; Mitrizakis, N.; Papandroulakis, N.; Koumoundouros, G. Long lasting effects of early temperature exposure on the swimming performance and skeleton development of metamorphosing Gilthead seabream (Sparus aurata L.) larvae. Sci. Rep. 2021, 11, 8787. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cordova-de la Cruz, S.E.; Riesco, M.F.; Martínez-Bautista, G.; Calzada-Ruiz, D.; Martínez-Burguete, T.; Peña-Marín, E.S.; Álvarez-Gonzalez, C.A.; Fernández, I. Larval Development in Tropical Gar (Atractosteus tropicus) Is Dependent on the Embryonic Thermal Regime: Ecological Implications under a Climate Change Context. Fishes 2022, 7, 16. https://doi.org/10.3390/fishes7010016
Cordova-de la Cruz SE, Riesco MF, Martínez-Bautista G, Calzada-Ruiz D, Martínez-Burguete T, Peña-Marín ES, Álvarez-Gonzalez CA, Fernández I. Larval Development in Tropical Gar (Atractosteus tropicus) Is Dependent on the Embryonic Thermal Regime: Ecological Implications under a Climate Change Context. Fishes. 2022; 7(1):16. https://doi.org/10.3390/fishes7010016
Chicago/Turabian StyleCordova-de la Cruz, Simrith E., Marta F. Riesco, Gil Martínez-Bautista, Daniel Calzada-Ruiz, Talhia Martínez-Burguete, Emyr S. Peña-Marín, Carlos Alfonso Álvarez-Gonzalez, and Ignacio Fernández. 2022. "Larval Development in Tropical Gar (Atractosteus tropicus) Is Dependent on the Embryonic Thermal Regime: Ecological Implications under a Climate Change Context" Fishes 7, no. 1: 16. https://doi.org/10.3390/fishes7010016
APA StyleCordova-de la Cruz, S. E., Riesco, M. F., Martínez-Bautista, G., Calzada-Ruiz, D., Martínez-Burguete, T., Peña-Marín, E. S., Álvarez-Gonzalez, C. A., & Fernández, I. (2022). Larval Development in Tropical Gar (Atractosteus tropicus) Is Dependent on the Embryonic Thermal Regime: Ecological Implications under a Climate Change Context. Fishes, 7(1), 16. https://doi.org/10.3390/fishes7010016