Development of Recombinant Dihydrolipoamide Dehydrogenase Subunit Vaccine against Vibrio Infection in Large Yellow Croaker
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Animals
2.2. Cloning of V. alginolyticus DLD Gene
2.3. Expression and Purification of Recombinant DLD Protein
2.4. Immunization of Large Yellow Croaker with Recombinant DLD Protein
2.5. Determination of Lysozyme Activity in Serum of Large Yellow Croaker
2.6. Determination of Antibody Titer in Serum of Large Yellow Croaker
2.7. Expression Analysis of Immune-Related Genes by Real-Time PCR
2.8. Challenge Experiment of Large Yellow Croaker
2.9. Statistical Analysis
3. Results
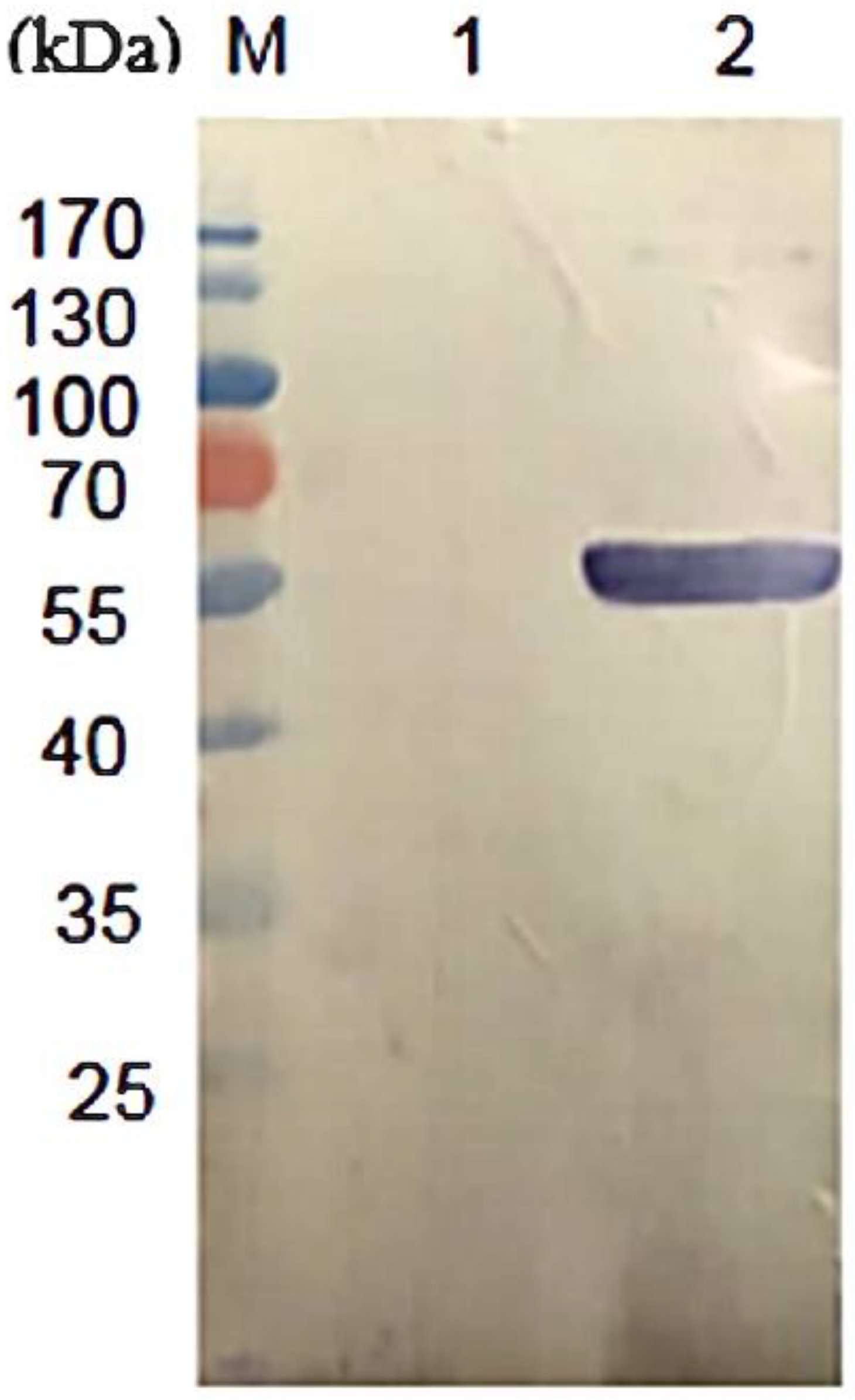
3.1. Expression and Purification of rDLD Protein
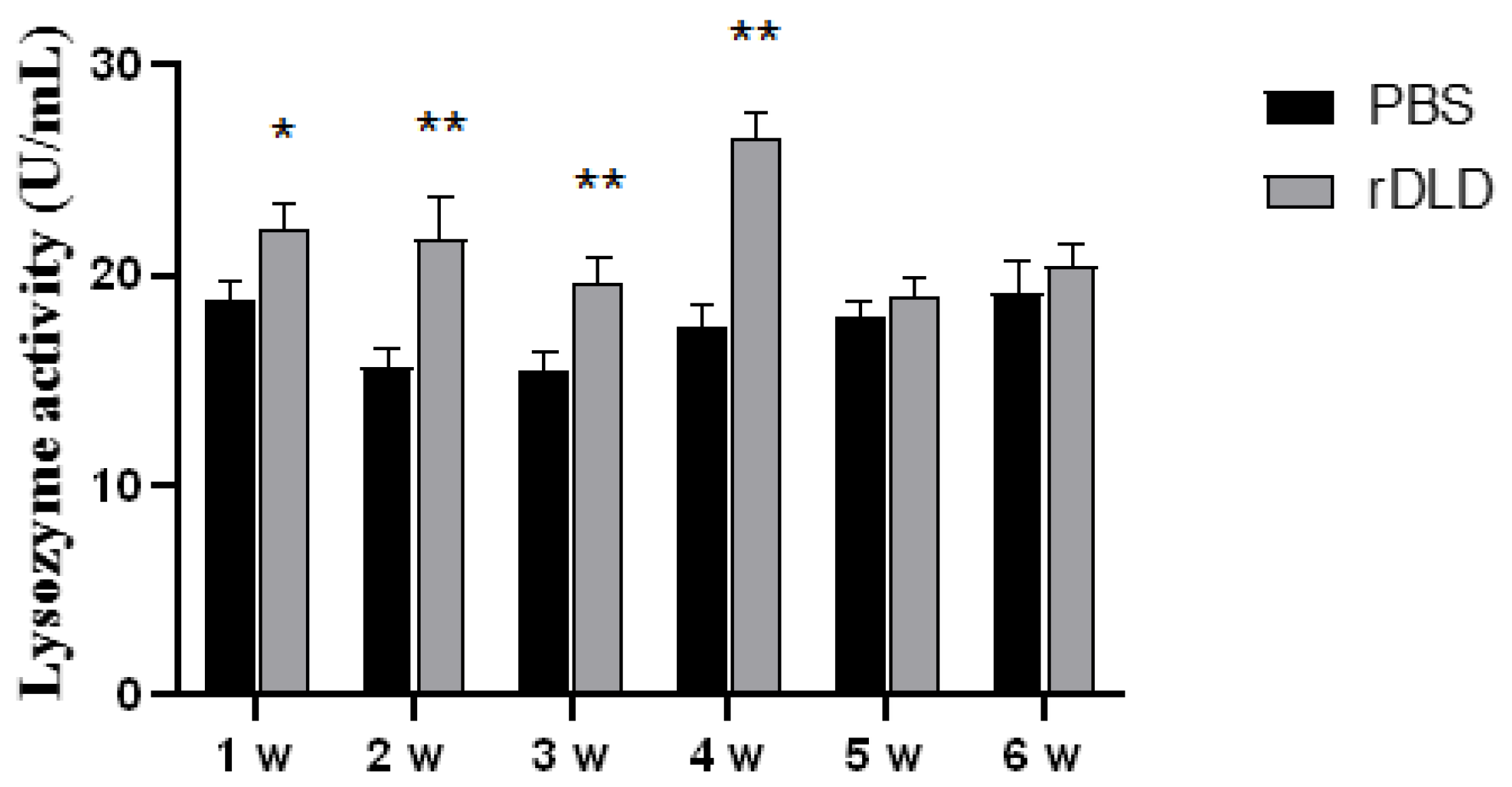
3.2. The rDLD Enhanced the Activity of Lysozyme
3.3. The rDLD-Induced Expression of Immune-Related Genes In Vivo
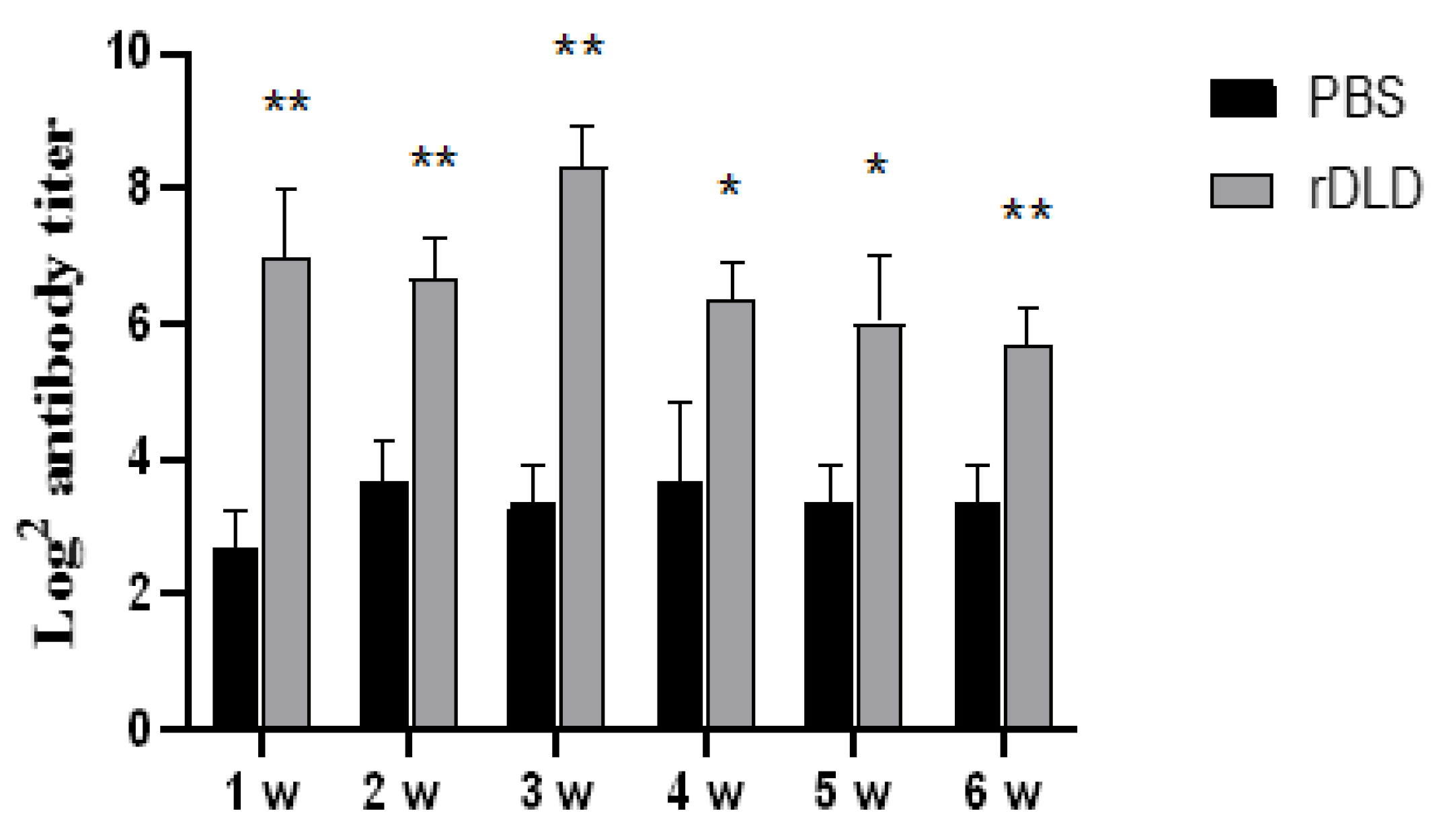
3.4. The rDLD-Increased Antibody Levels in Serum
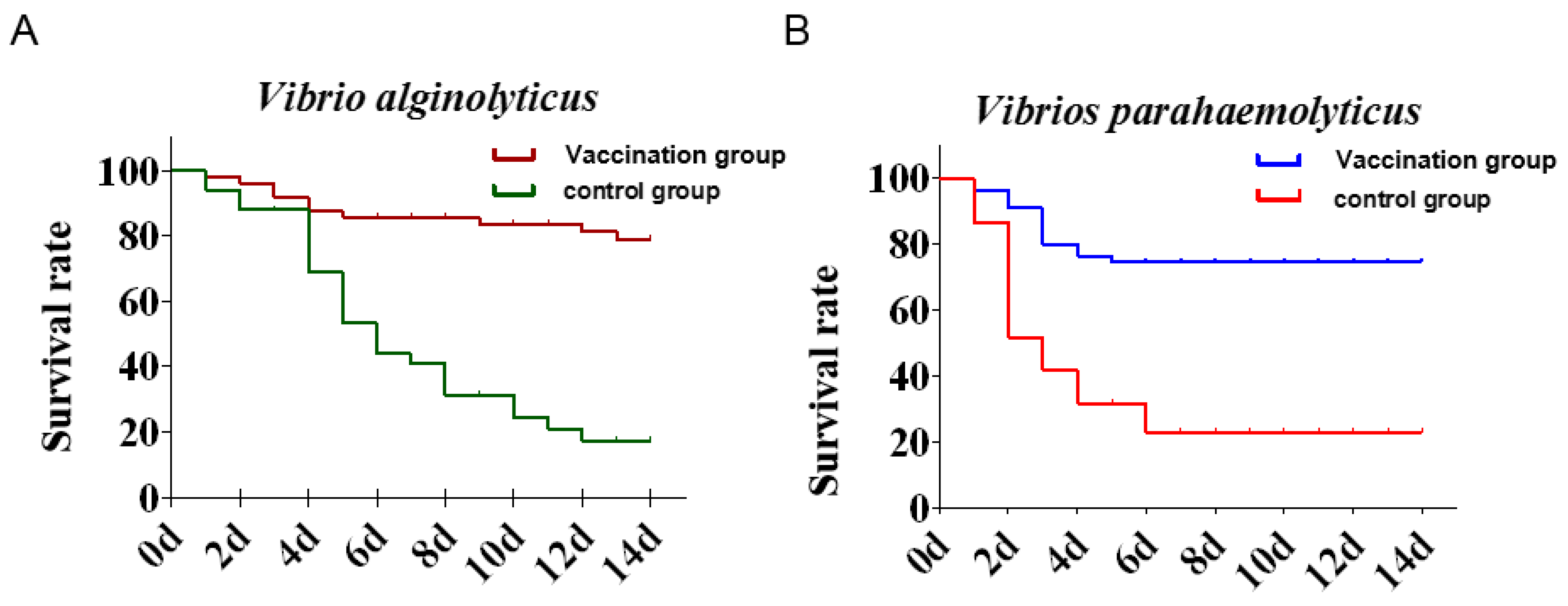
3.5. Vaccine Efficacy
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Chen, X.H.; Lin, K.B.; Wang, X.W. Outbreaks of an iridovirus disease in maricultured large yellow croaker, Larimichthys crocea (Richardson), in China. J. Fish Dis. 2003, 26, 615–619. [Google Scholar] [CrossRef]
- Chen, X.; Wu, Z.; Yu, S.; Wang, S.; Peng, X. Beta2-microglobulin is involved in the immune response of large yellow croaker to Aeromonas hydrophila: A proteomic based study. Fish Shellfish Immun. 2010, 28, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Zhijuan, M.; Dan, L.; Maocang, Y. Primary Study on Comprehensive Prevention Strategy against Vibriosis of Large Yellow Croaker, Pseudosciaena crocea. J. Huazhong Agric. 2004, 5, 16. [Google Scholar]
- Wu, Z.P. Experimental Study on Immune Protection of Trivalent Vaccine to Common Bacterial Diseases of Pseudosciaena crocea. J. Xiamen Univ. Nat. Sci. 2004, 43, 112–118. [Google Scholar]
- Zhao, Z.; Xiong, Y.; Zhang, C.; Jia, Y.J.; Qiu, D.K.; Wang, G.X.; Zhu, B. Optimization of the efficacy of a SWCNTs-based subunit vaccine against infectious spleen and kidney necrosis virus in mandarin fish. Fish Shellfish Immun. 2020, 106, 190–196. [Google Scholar] [CrossRef]
- Hastein, T.; Gudding, R.; Evensen, O. Bacterial vaccines for fish—An update of the current situation worldwide. Nat. Sci. 2005, 121, 55–74. [Google Scholar]
- Sommerset, I.; Krossøy, B.; Biering, E.; Frost, P. Vaccines for fish in aquaculture. Expert Rev. Vaccines 2005, 4, 89–101. [Google Scholar] [CrossRef]
- Thompson, F.L.; Klose, K.E. Vibrio 2005: The First International Conference on the Biology of Vibrios. J. Bacteriol. 2006, 188, 4592–4596. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Pei, C.; Sun, X.; Zhang, C.; Li, L.; Kong, X. Novel subunit vaccine based on grass carp reovirus VP35 protein provides protective immunity against grass carp hemorrhagic disease. Fish Shellfish Immun. 2018, 75, 91–98. [Google Scholar] [CrossRef]
- Fu, X.; Zhang, L.; Liu, L.; Lin, Q.; Liang, H.; Niu, Y.; Huang, Z.; Li, N. Identification of intron in ORF003 gene and its application for inactivation test of ISKNV. Microb. Pathog. 2020, 138, 103822. [Google Scholar] [CrossRef]
- Zhu, B.; Zhang, C.; Yang, B.; Guo, Z.R.; Zheng, Y.Y.; Gong, Y.M.; Wang, G.X. Preliminary screening and immunogenicity analysis of antigenic epitopes of spring viremia of carp virus. Fish Shellfish Immun. 2019, 94, 833–841. [Google Scholar] [CrossRef]
- Li, N.; Yang, Z.; Bai, J.; Fu, X.; Liu, L.; Shi, C.; Wu, S. A shared antigen among Vibrio species: Outer membrane protein-OmpK as a versatile Vibriosis vaccine candidate in Orange-spotted grouper (Epinephelus coioides). Fish Shellfish Immun. 2010, 28, 952–956. [Google Scholar] [CrossRef]
- Tang, X.; Wang, H.; Liu, F.; Sheng, X.; Xing, J.; Zhan, W. Recombinant outer membrane protein T (OmpT) of Vibrio ichthyoenteri, a potential vaccine candidate for flounder (Paralichthys olivaceus). Microb. Pathog. 2019, 126, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Lun, J.; Xia, C.; Yuan, C.; Zhang, Y.; Zhong, M.; Huang, T.; Hu, Z. The outer membrane protein, LamB (maltoporin), is a versatile vaccine candidate among the Vibrio species. Vaccine 2014, 32, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.H.; Lu, Y.S.; Wu, Z.H.; Jian, J.C. Cloning, expression of Vibrio alginolyticus outer membrane protein-OmpU gene and its potential application as vaccine in crimson snapper, Lutjanus erythropterus Bloch. J. Fish Dis. 2013, 36, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Dong, C.; Weng, S.; He, J. Identification of outer membrane protein TolC as the major adhesin and potential vaccine candidate for Vibrio harveyi in hybrid grouper, Epinephelus fuscoguttatus (female symbol) × E. lanceolatus (male symbol). Fish Shellfish Immun. 2019, 86, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, C.A.; Peterson, S.N.; Gill, S.R.; Cline, R.T.; White, O.; Fraser, C.M.; Smith, H.O.; Venter, J.C. Global transposon mutagenesis and a minimal Mycoplasma genome. Science 1999, 286, 2165–2169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hudson, P.; Gorton, T.S.; Papazisi, L.; Cecchini, K.; Frasca, S., Jr.; Geary, S.J. Identification of a virulence-associated determinant, dihydrolipoamide dehydrogenase (lpd), in Mycoplasma gallisepticum through in vivo screening of transposon mutants. Infect. Immun. 2006, 74, 931–939. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J. Adhesive Function of Putative Dihydrolipoamide Dehydrogenase of Streptococcus suis and Protective Vaccination with Recombinant Trivalent Protein Grpdh-MRP-HM6 in Swine; Shanghai Jiao Tong University: Shanghai, China, 2012. (In Chinese) [Google Scholar]
- de la Sierra, I.L.; Pernot, L.; Prangé, T.; Saludjian, P.; Schiltz, M.; Fourme, R.; Padrón, G. Molecular structure of the lipoamide dehydrogenase domain of a surface antigen from Neisseria meningitidis. J. Mol. Biol. 1997, 269, 129–141. [Google Scholar] [CrossRef]
- Pang, H.Y.; Li, Y.; Wu, Z.H.; Jian, J.C.; Lu, Y.S.; Cai, S.H. Immunoproteomic analysis and identification of novel immunogenic proteins from Vibrio harveyi. J. Appl. Microbiol. 2010, 109, 1800–1809. [Google Scholar] [CrossRef]
- Ao, J.; Mu, Y.; Xiang, L.X.; Fan, D.; Feng, M.; Zhang, S.; Shi, Q.; Zhu, L.Y.; Li, T.; Ding, Y.; et al. Genome sequencing of the perciform fish Larimichthys crocea provides insights into molecular and genetic mechanisms of stress adaptation. PLoS Genet. 2015, 11, e1005118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Yuan, X.; Mu, P.; Li, Q.; Ao, J.; Chen, X. Development of monoclonal antibody against IgM of large yellow croaker (Larimichthys crocea) and characterization of IgM(+) B cells. Fish Shellfish Immun. 2019, 91, 216–222. [Google Scholar] [CrossRef]
- Fu, Q.; Wei, Z.; Chen, Y.; Xie, J.; Zhang, X.; He, T.; Chen, X. Development of monoclonal antibody against IgT of a perciform fish, large yellow croaker (Larimichthys crocea) and characterization of IgT(+) B cells. Dev. Comp. Immunol. 2021, 119, 104027. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Vasilescu, A.; Wang, Q.; Coffinier, Y.; Li, M.; Boukherroub, R.; Szunerits, S. Electrophoretic Approach for the Simultaneous Deposition and Functionalization of Reduced Graphene Oxide Nanosheets with Diazonium Compounds: Application for Lysozyme Sensing in Serum. ACS Appl. Mater. Inter. 2017, 9, 12823–12831. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Huang, J.; Li, J. Adjuvant Effect of Quillaja saponaria Saponin (QSS) on Protective Efficacy and IgM Generation in Turbot (Scophthalmus maximus) upon Immersion Vaccination. Int. J. Mol. Sci. 2016, 17, 325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liming, C. Expression and Purification of Dihydrolipoamide Dehydrogenase and D-Tyr-tRNATyr Deacylase of Vibrio alginolyticus and Cross-Protective Research of Vibrios; Guangdong Ocean University: Guangdong, China, 2014. [Google Scholar]
- Li, J.; Ma, S.; Woo, N.Y. Vaccination of Silver Sea Bream (Sparus sarba) against Vibrio alginolyticus: Protective Evaluation of Different Vaccinating Modalities. Int. J. Mol. Sci. 2015, 17, 40. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.W.; Sun, K.; Cheng, S.; Sun, L. Characterization of DegQVh, a serine protease and a protective immunogen from a pathogenic Vibrio harveyi strain. Appl. Environ. Microb. 2008, 74, 6254–6262. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Yu, L.; Qian, R. Cloning and expression of Vibrio harveyi OmpK and GAPDH genes and their potential application as vaccines in large yellow croakers Pseudosciaena crocea. J. Aquat. Anim. Health 2008, 20, 1–11. [Google Scholar] [CrossRef]
- Ina-Salwany, M.Y.; Al-saari, N.; Mohamad, A.; Mursidi, F.A.; Mohd-Aris, A.; Amal, M.N.A.; Kasai, H.; Mino, S.; Sawabe, T.; Zamri-Saad, M. Vibriosis in Fish: A Review on Disease Development and Prevention. J. Aquat. Anim. Health 2019, 31, 3–22. [Google Scholar] [CrossRef]
- Pang, H.; Chen, L.; Hoare, R.; Huang, Y.; Jian, J. Identification of DLD, by immunoproteomic analysis and evaluation as a potential vaccine antigen against three Vibrio species in Epinephelus coioides. Vaccine 2016, 34, 1225–1231. [Google Scholar] [CrossRef]
- Xu, Y.P.; Wang, T.T.; Sun, Y.X.; Liu, W.J.; You, J.S.; Jin, L.J. Current Research on Function and Application of Aquatic Animal Lysozyme. Fish. Sci. 2011, 30, 307–310. (In Chinese) [Google Scholar]
- Zheng, W.; Tian, C.; Chen, X. Molecular characterization of goose-type lysozyme homologue of large yellow croaker and its involvement in immune response induced by trivalent bacterial vaccine as an acute-phase protein. Immunol. Lett. 2007, 113, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Ao, J.; Chen, X. Molecular characterization and expression analysis of MHC class II alpha and beta genes in large yellow croaker (Pseudosciaena crocea). Mol. Biol. Rep. 2010, 37, 1295–1307. [Google Scholar] [CrossRef]
- Bao, K.; Reinhardt, R.L. The differential expression of IL-4 and IL-13 and its impact on type-2 immunity. Cytokine 2015, 75, 25–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, K.D.; Zurawski, S.M.; Mosmann, T.R.; Zurawski, G. A family of small inducible proteins secreted by leukocytes are members of a new superfamily that includes leukocyte and fibroblast-derived inflammatory agents, growth factors, and indicators of various activation processes. J. Immunol. 1989, 142, 679–687. [Google Scholar]
- Mccormick, S.M.; Heller, N.M. Commentary: IL-4 and IL-13 receptors and signaling. Cytokine 2015, 75, 38–50. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J. T helper 2 (Th2) cell differentiation, type 2 innate lymphoid cell (ILC2) development and regulation of interleukin-4 (IL-4) and IL-13 production. Cytokine 2015, 75, 14–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Primers | Sequence (5′-3′) | Primers Information |
|---|---|---|
| DLD-F1 | CGCGGATCCATGAGCAAAGAAATTAAAGC | cloning |
| DLD-R1 | GGGAAGCTTTTACTTCTTCTTAACTGCTT | cloning |
| DLD-F2 | CGCGGATCCCGCGGATCCATGAGCAAAGAAATTA | cloning |
| DLD-R2 | CCGGAATTCGGGAAGCTTTTACTTCTTCTTAACTGC | cloning |
| IL-6-F | TGTTGTAAATAGTGGGTGTGTCG | qPCR |
| IL-6-R | GCTGTTCTCAAGTATGTGGCG | qPCR |
| IL-8-F | CTATCGTGGCACTCCTGGTT | qPCR |
| IL-8-R | GCAGGAATCACCTCCACTTGT | qPCR |
| IL-1β-F | CAGCTGTTCTCAAGTATGTGGC | qPCR |
| IL-1β-R | GTTGTAAATAGTGGGTGTGTCG | qPCR |
| IL-4/13A-F | TGGTACTGCTGGTCAATCCG | qPCR |
| IL-4/13A-R | TTTTGCCTTCAGCCAGATGT | qPCR |
| IL-4/13B-F | AGTTCTTCTGTCGCGCTGAG | qPCR |
| IL-4/13B-R | GCTATGTATGTGCGGTTGCTG | qPCR |
| MHC IIα-F | TCAGCTGCACTCGTTCC | qPCR |
| MHC IIα-R | CCAGCATCAACGTTCCTC | qPCR |
| MHC IIβ-F | TCAGTTATTGGGAACCAGAATC | qPCR |
| MHC IIβ-R | CCACTCTCACCTGGAGTACAC | qPCR |
| CD8α-F | TGCTGCTCCGATTACGGTCA | qPCR |
| CD8α-R | TCACTCAATCTGGTGTTAGGCCA | qPCR |
| CD40-F | ATAGTGCATAGGCTGGAAAATGG | qPCR |
| CD40-R | GCTGTGGCTCAACACAGATTTAG | qPCR |
| β-action-F | GACCTGACAGACTACCTCATG | qPCR |
| β-action-R | AGTTGAAGGTGGTCTCGTGGA | qPCR |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Tan, Y.; Zhang, Z.; Huang, Y.; Mu, P.; Cui, Z.; Chen, X. Development of Recombinant Dihydrolipoamide Dehydrogenase Subunit Vaccine against Vibrio Infection in Large Yellow Croaker. Fishes 2022, 7, 17. https://doi.org/10.3390/fishes7010017
Li X, Tan Y, Zhang Z, Huang Y, Mu P, Cui Z, Chen X. Development of Recombinant Dihydrolipoamide Dehydrogenase Subunit Vaccine against Vibrio Infection in Large Yellow Croaker. Fishes. 2022; 7(1):17. https://doi.org/10.3390/fishes7010017
Chicago/Turabian StyleLi, Xiaomeng, Yuanzhen Tan, Zheng Zhang, Yupeng Huang, Pengfei Mu, Zhengwei Cui, and Xinhua Chen. 2022. "Development of Recombinant Dihydrolipoamide Dehydrogenase Subunit Vaccine against Vibrio Infection in Large Yellow Croaker" Fishes 7, no. 1: 17. https://doi.org/10.3390/fishes7010017
APA StyleLi, X., Tan, Y., Zhang, Z., Huang, Y., Mu, P., Cui, Z., & Chen, X. (2022). Development of Recombinant Dihydrolipoamide Dehydrogenase Subunit Vaccine against Vibrio Infection in Large Yellow Croaker. Fishes, 7(1), 17. https://doi.org/10.3390/fishes7010017





