The Effects of Silkworm-Derived Polysaccharide (Silkrose) on Ectoparasitic Infestations in Yellowtail (Seriola quinqueradiata) and White Trevally (Pseudocaranx dentex)
Abstract
1. Introduction
2. Materials and Methods
2.1. Silkrose
2.2. Experiment I (Laboratory-Based Experiment) Feeding Trial of Yellowtail with Silkrose in Tanks
2.2.1. Parasitic Challenge with Low Counts of Benedenia seriolae in Juvenile Yellowtail
2.2.2. Challenge with High Counts of Benedenia seriolae in Yellowtail
2.3. Experiment II (Field-Based Experiment) Feeding Trial of Yellowtail with Silkrose at Aquaculture Field Site
2.4. Experiment III (Field-Based Experiment) Feeding Trial of White Trevally (Pseudocaranx dentex) at Aquaculture Field Site
2.5. Measurement of Blood Cortisol Levels
2.6. Tissue Sampling and RNA Extraction for Differential Gene Expression Analysis of Yellowtail Epidermis
2.7. RNA-Seq Library Construction, Sequencing, and Bioinformatics Analysis
2.8. Confirmation of RNA-Seq Results Using Quantitative RT-PCR (qRT-PCR)
2.9. Statistical Analysis
3. Results
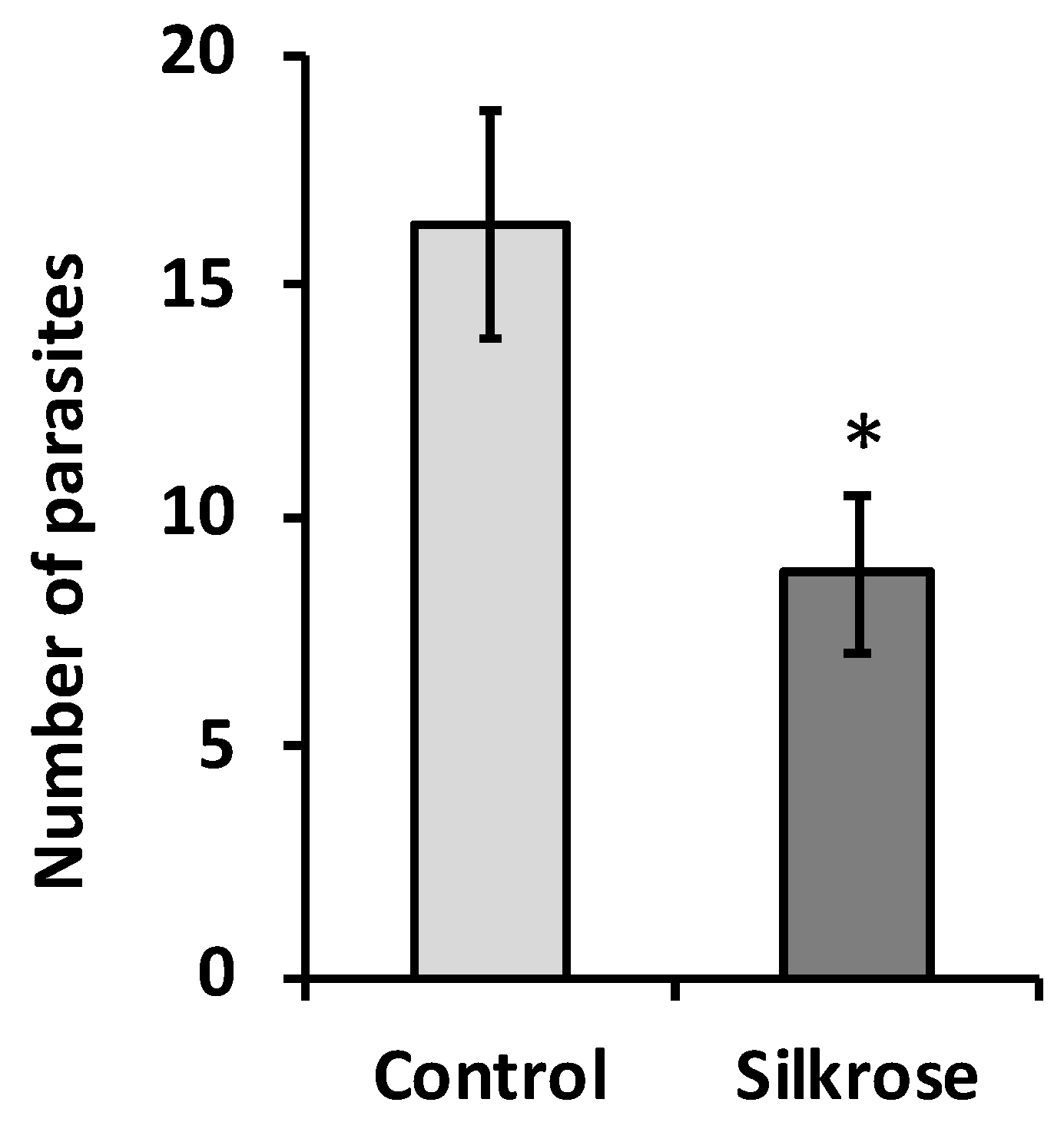
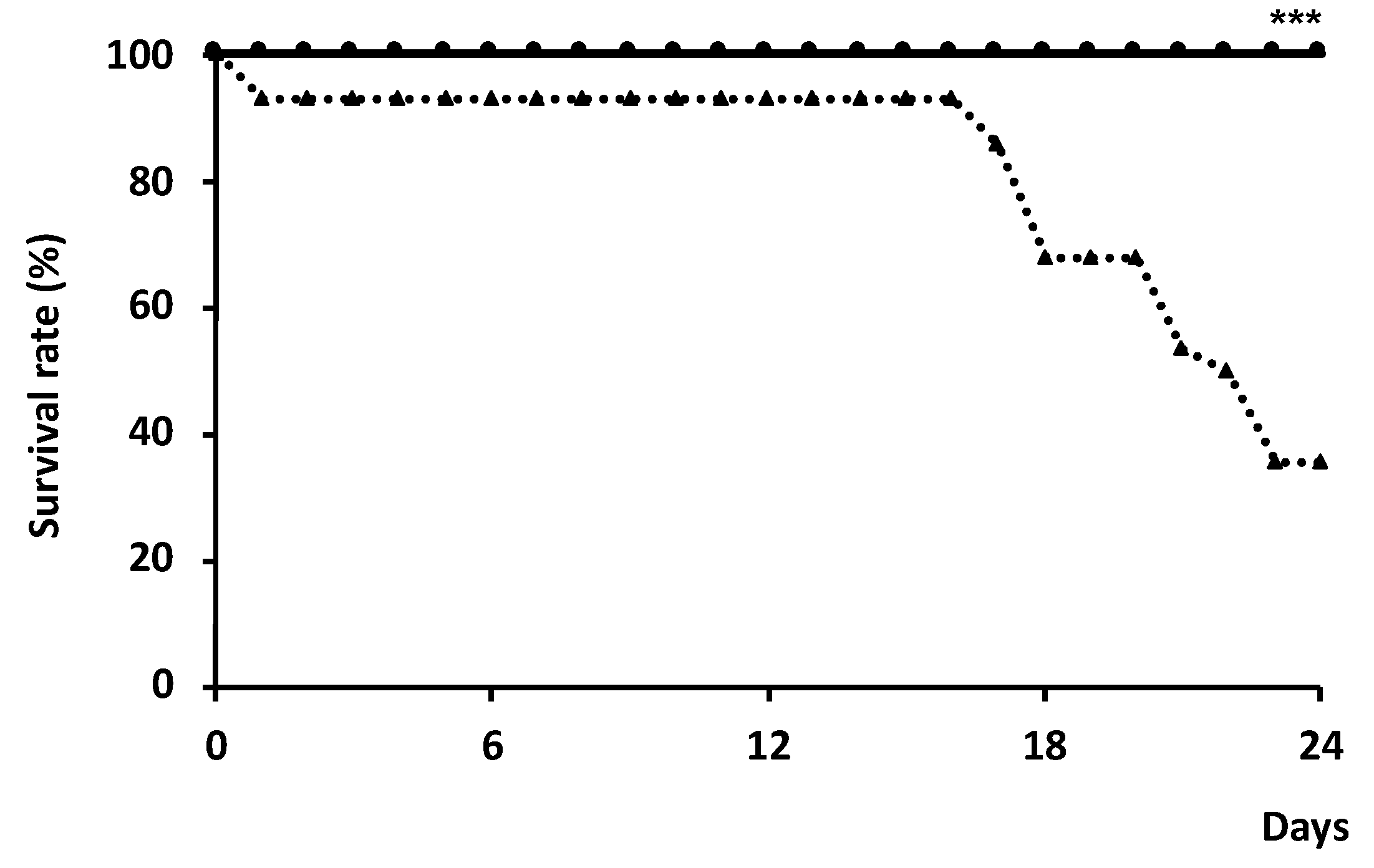
3.1. Effect of Silkrose on Ectoparasite Infection of Yellowtail Juveniles (Experimental I)
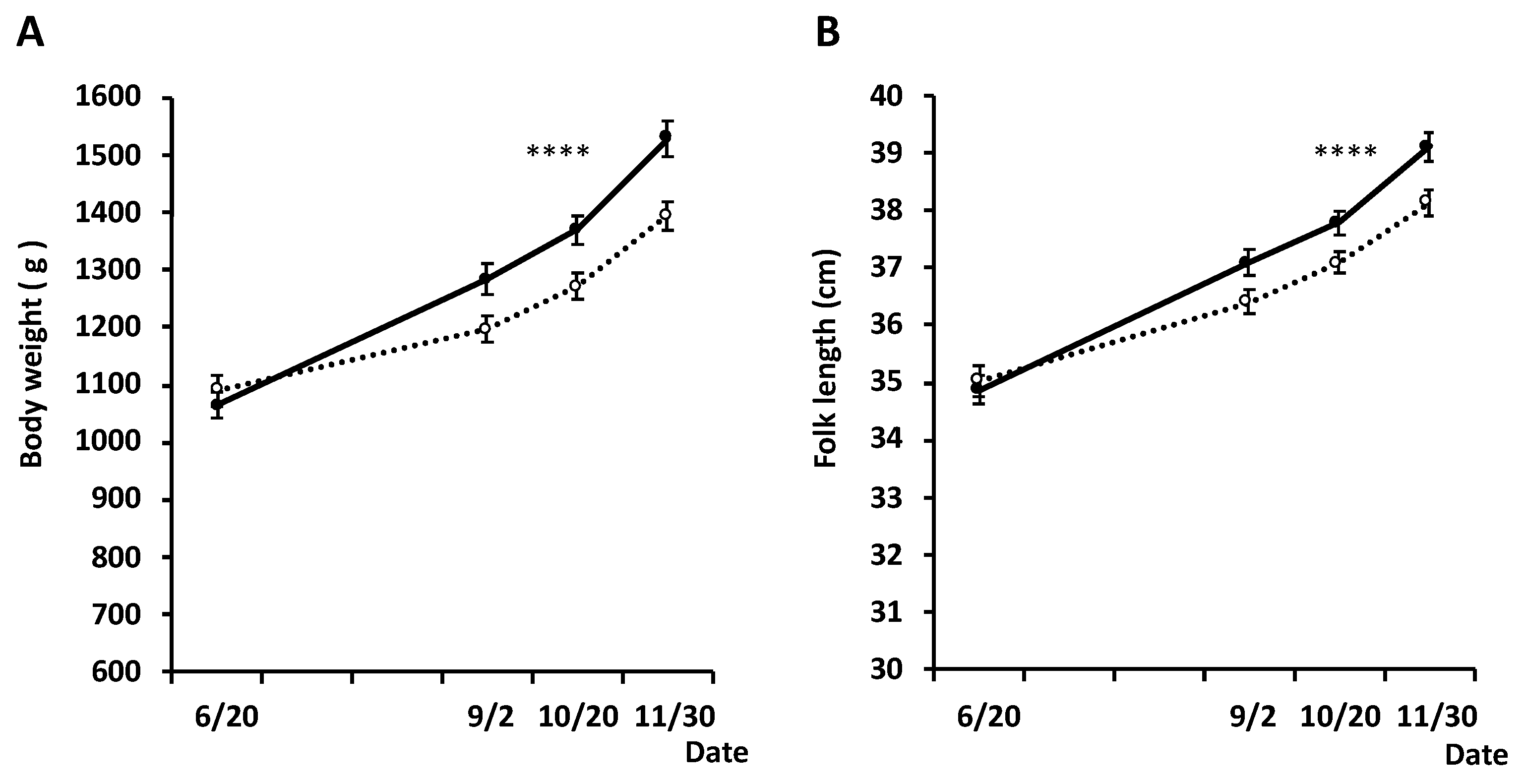
3.2. Effect of Silkrose on B. seriolae on Yellowtail at Aquaculture Field Site (Experimental II)
3.3. Effect of Silkrose on Parasites of White Trevally at Aquaculture Field Site (Experimental III)
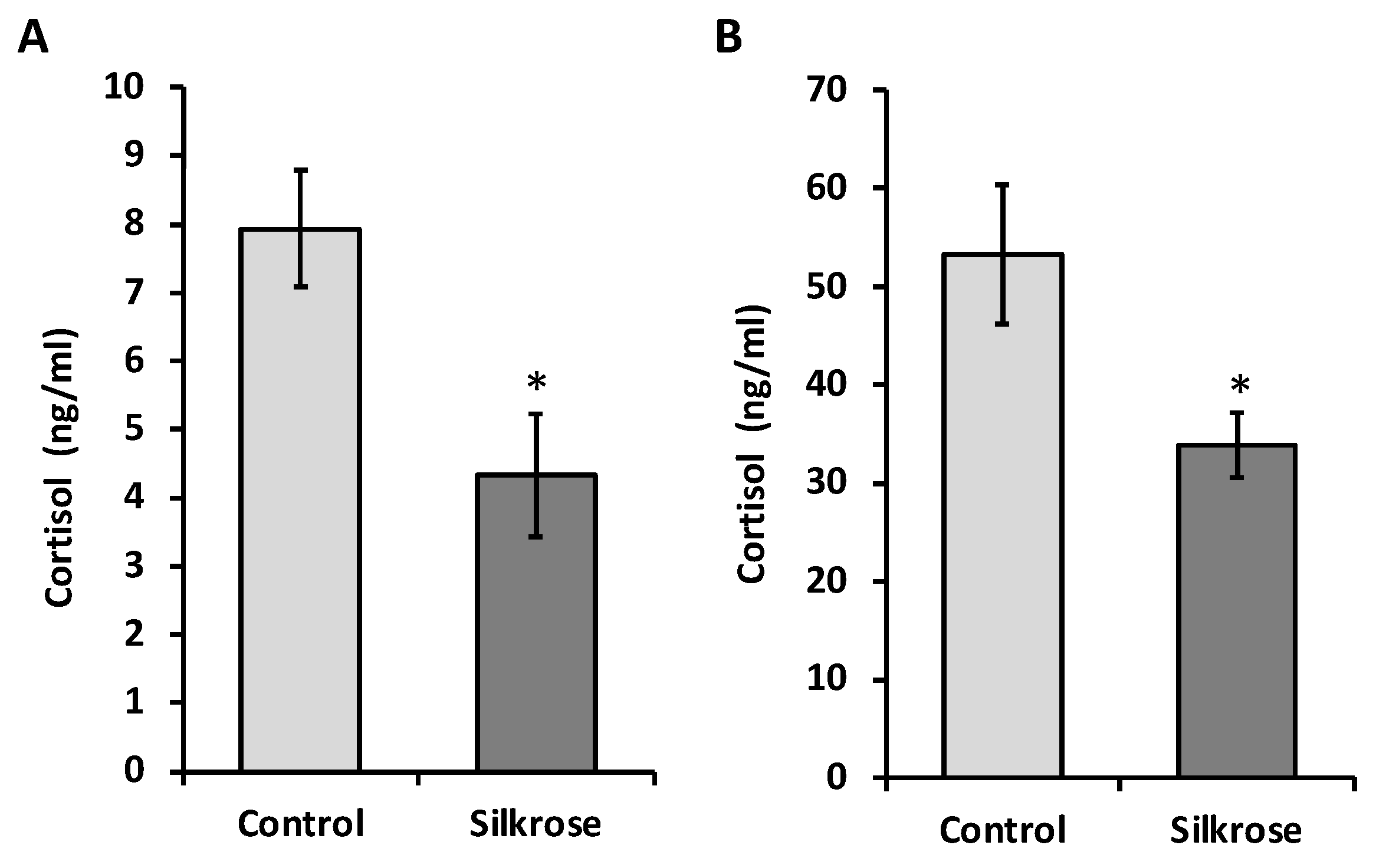
3.4. Effect of Silkrose Treatment on Blood Cortisol Levels in Yellowtail and White Trevally
3.5. Sequencing and Transcriptomic Analysis
3.6. Validation of RNA-Seq Results by Quantitative RT-PCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Costello, M.J. The global economic cost of sea lice to the salmonid farming industry. J. Fish Dis. 2009, 32, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Rodger, H.M. Fish disease causing economic impact in global aquaculture. In Fish Vaccines; Adams, A., Ed.; Springer Nature Switzerland AG: Cham, Switzerland, 2016; pp. 1–34. [Google Scholar]
- Nagasawa, K. Parasitic copepods of marine fish culture in Japan: A review. J. Nat. Hist. 2015, 49, 45–48. [Google Scholar] [CrossRef]
- Ogawa, K.; Yokoyama, H. Parasitic Diseases of cultured marine fish in Japan. Fish Pathol. 1998, 33, 303–309. [Google Scholar] [CrossRef]
- MacKinnon, B.M. Host response of Atlantic salmon (Salmo salar) to infection by sea lice (Caligus elongatus). Can. J. Fish. Aquat. Sci. 1993, 50, 789–792. [Google Scholar] [CrossRef]
- Bowers, J.M.; Mustafa, A.; Speare, D.J.; Conboy, G.A.; Brimacombe, M.; Sims, D.E.; Burka, J.F. The physiological response of Atlantic salmon, Salmo salar L., to a single experimental challenge with sea lice, Lepeophtheirus salmonis. J. Fish Dis. 2000, 23, 165–172. [Google Scholar] [CrossRef]
- Bruno, D.W.; Raynard, R.S. Studies on the use of hydrogen peroxide as a method for the control of sea lice on Atlantic salmon. Aquac. Int. 1994, 2, 10–18. [Google Scholar] [CrossRef]
- Hirazawa, N.; Ohtaka, T.; Hata, K. Challenge trials on the anthelmintic effect of drugs and natural agents against the monogenean Heterobothrium okamotoi in the tiger puffer Takifugu rubripes. Aquaculture 2000, 188, 1–13. [Google Scholar] [CrossRef]
- Hirazawa, N.; Mitsuboshi, T.; Hirata, T.; Shirasu, K. Susceptibility of spotted halibut Verasper variegates (Pleuronectidae) to infection by the monogenean Neobenedenia girellae (Capsalidae) and oral therapy trials using praziquantel. Aquaculture 2004, 238, 83–95. [Google Scholar] [CrossRef]
- Grave, K.; Horsberg, T.E.; Lunestad, B.T.; Litleskare, I. Consumption of drugs for sea lice infestations in Norwegian fish farms: Methods for assessment of treatment patterns and treatment rate. Dis. Aquat. Org. 2004, 60, 123–131. [Google Scholar] [CrossRef]
- Wood, A.T.; Taylor, R.S.; Quezada-Rodriguez, R.R.; Wynne, J.W. Hydrogen peroxide treatment of Atlantic salmon temporarily decreases oxygen consumption but has negligible effects on hypoxia tolerance and aerobic performance. Aquaculture 2021, 540, 736676. [Google Scholar] [CrossRef]
- Lieke, T.; Meinelt, T.; Hoseinifar, S.H.; Pan, B.; Straus, D.L.; Steinberg, C.E.W. Sustainable aquaculture requires environmental-friendly treatment strategies for fish diseases. Rev. Aquac. 2020, 12, 943–965. [Google Scholar] [CrossRef]
- Overton, K.; Dempster, T.; Oppedal, F.; Kristiansen, T.S.; Gsimervik, K.; Stien, L.H. Salmon lice treatments and salmon mortality in Norwegian aquaculture: A review. Rev. Aquac. 2019, 11, 1398–1417. [Google Scholar] [CrossRef]
- Ohashi, H.; Umeda, N.; Hirazawa, N.; Ozaki, Y.; Miura, C.; Miura, T. Antiparasitic effect of calcium and magnesium ion-free buffer treatments against a common monogenean Neobenedenia Girellae. Parasitology 2007, 134, 229–236. [Google Scholar] [CrossRef]
- Ohashi, H.; Umeda, N.; Hirazawa, N.; Ozaki, Y.; Miura, C.; Miura, T. Expression of vasa (vas)-related genes in germ cells and specific interference with gene functions by double-stranded RNA in the monogenean, Neobenedenia girellae. Int. J. Parasitol. 2007, 37, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, H.; Umeda, N.; Hirazawa, N.; Ozaki, Y.; Miura, C.; Miura, T. Purification and identification of a glycoprotein that induces the attachment of oncomiracidia of Neobenedenia girellae. Int. J. Parasitol. 2007, 37, 1483–1490. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Tran, G.; Heuzé, V.; Ankers, P. State-of-the-art on use of insects as animal feed. Anim. Feed Sci. Technol. 2014, 197, 1–33. [Google Scholar] [CrossRef]
- Van Huis, A. Prospects of insects as food and feed. Org. Agric. 2021, 11, 301–308. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; Khalil, R.H.; Metwally, A.A.; Shakweer, M.S.; Khallaf, M.A.; Hany, M.R.; Abdel-Latif, H.M.R. Effects of black soldier fly (Hermetia illucens L.) larvae meal on growth performance, organs-somatic indices, body composition, and hemato-biochemical variables of European sea bass, Dicentrarchus labrax. Aquaculture 2020, 522, 735136. [Google Scholar] [CrossRef]
- Chernysh, S.; Kim, S.I.; Bekker, G.; Pleskach, V.A.; Filatova, N.A.; Anikin, V.B.; Platonov, V.G.; Bulet, P. Antiviral and antitumor peptides from insects. Proc. Natl. Acad. Sci. USA 2002, 99, 12628–12632. [Google Scholar] [CrossRef]
- Gasco, L.; Finke, M.; van Huis, A. Can diets containing insects promote animal health? J. Insects Food Feed 2018, 4, 1–4. [Google Scholar] [CrossRef]
- Ratcliffe, N.; Azambuja, P.; Mello, C.B. Recent advances in developing insect natural products as potential modern day medicines. Evid. Based Complementary Altern. Med. 2014, 2014, 904958. [Google Scholar] [CrossRef]
- Chaklader, M.R.; Siddik, M.A.B.; Fotedar, R.; Howieson, J. Insect larvae, Hermetia illucens in poultry by-product meal for barramundi, Lates calcarifer modulates histomorphology, immunity and resistance to Vibrio harveyi. Sci. Rep. 2019, 9, 16703. [Google Scholar] [CrossRef]
- Henry, M.A.; Gai, F.; Enes, P.; Peréz-Jiménez, A.; Gasco, L. Effect of partial dietary replacement of fishmeal by yellow mealworm (Tenebrio molitor) larvae meal on the innate immune response and intestinal antioxidant enzymes of rainbow trout (Oncorhynchus mykiss). Fish Shellfish. Immunol. 2018, 83, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Ido, A.; Iwai, T.; Ito, K.; Ohta, T.; Mizushige, T.; Kishida, T.; Miura, C.; Miura, T. Dietary effects of housefly (Musca domestica) (Diptera: Muscidae) pupae on the growth performance and the resistance against bacterial pathogen in red sea bream (Pagrus major) (Perciformes: Sparidae). Appl. Entomol. Zool. 2015, 50, 213–221. [Google Scholar] [CrossRef]
- Ido, A.; Hashizume, A.; Ohta, T.; Takahashi, T.; Miura, C.; Miura, T. Replacement of Fish Meal by Defatted Yellow Mealworm (Tenebrio molitor) Larvae in Diet Improves Growth Performance and Disease Resistance in Red Seabream (Pargus major). Animals 2019, 9, 100. [Google Scholar] [CrossRef]
- Motte, C.; Rios, A.; Lefebvre, T.; Do, H.; Henry, M.; Jintasataporn, O. Replacing fish meal with defatted insect meal (Yellow mealworm Tenebrio molitor) improves the growth and immunity of pacific white shrimp (Litopenaeus vannamei). Animals 2019, 9, 258. [Google Scholar] [CrossRef]
- Xiao, X.; Jin, P.; Zheng, L.; Cai, M.; Yu, Z.; Yu, J.; Zhang, J. Effects of black soldier fly (Hermetia illucens) larvae meal protein as a fishmeal replacement on the growth and immune index of yellow catfish (Pelteobagrus fulvidraco). Aquac. Res. 2018, 49, 1569–1577. [Google Scholar] [CrossRef]
- Abdel-Latif, H.M.R.; Abdel-Tawwab, M.; Khalil, R.H.; Metwally, A.A.; Shakweer, M.S.; Ghetas, H.A.; Khallaf, M.A. Black soldier fly (Hermetia illucens) larvae meal in diets of European seabass: Effects on antioxidative capacity, non-specific immunity, transcriptomic responses, and resistance to the challenge with Vibrio Alginolyticus. Fish Shellfish Immunol. 2021, 114, 207–217. [Google Scholar] [CrossRef]
- Laiba Shafique, A.; Abdel-Latif, H.M.R.; Hassan, F.; Alagawany, M.; Naiel, M.A.E.; Dawood, M.A.O.; Sevdan Yilmaz, S.; Liu, Q. The Feasibility of Using Yellow Mealworms (Tenebrio molitor): Towards a Sustainable Aquafeed Industry. Animals 2021, 11, 811. [Google Scholar] [CrossRef]
- Ohta, T.; Ido, A.; Kusano, K.; Miura, C.; Miura, T. A Novel Polysaccharide in Insects Activates the Innate Immune System in Mouse. PLoS ONE 2014, 9, e114823. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.F.Z.; Ohta, T.; Ido, A.; Miura, C.; Miura, T. The dipterose of black soldier fly (Hermetia illucens) induces innate immune response through toll-like receptor pathway in mouse macrophage RAW264.7 cells. Biomolecules 2019, 9, 677. [Google Scholar] [CrossRef] [PubMed]
- Ohta, T.; Kusano, K.; Ido, A.; Miura, C.; Miura, T. Silkrose: A novel acidic polysaccharide from the silkmoth that can stimulate the innate immune response. Carbohydr. Polym. 2016, 136, 995–1001. [Google Scholar] [CrossRef]
- Ali, M.F.Z.; Yasin, I.A.; Ohta, T.; Hashizume, A.; Ido, A.; Takahashi, T.; Miura, C.; Miura, T. The silkrose of Bombyx mori effectively prevents vibriosis in penaeid prawns via the activation of innate immunity. Sci. Rep. 2018, 8, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.F.Z.; Kameda, K.; Kondo, F.; Iwai, T.; Kurniawan, R.A.; Ohta, T.; Ido, A.; Takahashi, T.; Miura, C.; Miura, T. Effects of dietary silkrose of Antheraea yamamai on gene expression profiling and disease resistance to Edwardsiella tarda in Japanese medaka (Oryzias latipes). Fish Shellfish Immunol. 2021, 114, 207–217. [Google Scholar] [CrossRef]
- Ali, M.F.Z.; Nakahara, S.; Otsu, Y.; Ido, A.; Miura, C.; Miura, T. Effects of functional polysaccharide from silkworm as an immunostimulant on transcriptional profiling and disease resistance in fish. J. Insects Food Feed 2021, 1–14. [Google Scholar] [CrossRef]
- Kinami, R.; Miyamoto, J.; Yoshinaga, T.; Ogawa, K.; Nagakura, Y. A practical method to distinguish between Neobenedenia girellae and Benedenia seriolae. Fish Pathol. 2005, 40, 63–66. [Google Scholar] [CrossRef]
- Ogawa, K. Caligus longipedis infection of cultured striped jack, Pseudocaranx dentex (Teleostei: Carangidae) in Japan. Fish Pathol. 1992, 27, 197–205. [Google Scholar] [CrossRef]
- Ogawa, K.; Shirakashi, S. Skin fluke infection of cultured marine fish. Fish Pathol. 2017, 52, 186–190. [Google Scholar] [CrossRef][Green Version]
- Neiffer, D.L.; Stamper, M.A. Fish sedation, anesthesia, analgesia, and euthanasia: Considerations, methods, and types of drugs. ILAR J. 2009, 50, 343–360. [Google Scholar] [CrossRef]
- Underwood, W.; Anthony, R. AVMA Guidelines for the Euthanasia of Animals: 2020 Edition; American Veterinary Medical Association: Schaumburg, IL, USA, 2020; Volume 30, pp. 42–90. [Google Scholar]
- Reilly, J.S. (Ed.) Euthanasia of Animals Used for Scientific Purposes; Australia and New Zealand Council for the Care of Animals in Research and Teaching, Department of Environmental Biology, Adelaide University: Adelaide, SA, Australia, 2001. [Google Scholar]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Joshi, N.A.; Fass, J.N. Sickle: A Sliding-Window, Adaptive, Quality-Based Trimming Tool for FastQ Files (Version 1.33). [Software]. 2011. Available online: https://github.com/najoshi/sickle (accessed on 2 April 2021).
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. 1000 Genome Project Data Processing Subgroup. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Sun, J.; Nishiyama, T.; Shimizu, K.; Kadota, K. TCC: An R package for comparing tag count data with robust normalization strategies. BMC Bioinform. 2013, 14, 219. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Barton, B.A.; Iwata, G.K. Physiological changes in fish from stress in aquaculture with emphasis on the response and effects of corticosteroids. Annu. Rev. Fish Dis. 1991, 1, 3–26. [Google Scholar] [CrossRef]
- Clark, T.D.; Donaldson, M.R.; Drenner, S.M.; Hinch, S.G.; Patterson, D.A.; Hills, J.; Ives, V.; Carter, J.J.; Cooke, S.J.; Farrell, A.P. The efficacy of field techniques for obtaining and storing blood samples from fishes. J. Fish Biol. 2011, 79, 1322–1333. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.M.; Liu, X.Y.; Xia, C.G.; Li, M.Y.; Niu, X.T.; Wang, G.Q.; Zhang, D.M. Effects of dietary Astragalus Propinquus Schischkin polysaccharides on growth performance, immunological parameters, antioxidants responses and inflammation-related gene expression in Channa argus. Comp. Biochem. Physiology. Part C Toxicol. Pharmacol. 2021, 249, 109121. [Google Scholar] [CrossRef]
- Safavi, S.V.; Kenari, A.A.; Tabarsa, M.; Esmaeili, M. Effect of sulfated polysaccharides extracted from marine macroalgae (Ulva intestinalis and Gracilariopsis persica) on growth performance, fatty acid profile, and immune response of rainbow trout (Oncorhynchus mykiss). J. Appl. Phycol. 2019, 31, 4021–4035. [Google Scholar] [CrossRef]
- Fuchs, V.I.; Schmidt, J.; Slater, M.J.; Buck, B.H.; Steinhagen, D. Influence of immunostimulant polysaccharides, nucleic acids, and Bacillus strains on the innate immune and acute stress response in turbots (Scophthalmus maximus) fed soy bean- and wheat-based diets. Fish Physiol. Biochem. 2017, 43, 1501–1515. [Google Scholar] [CrossRef]
- Franco Montoya, L.N.; Favero, G.C.; Zanuzzo, F.S.; Urbinati, E.C. Distinct β-glucan molecules modulates differently the circulating cortisol levels and innate immune responses in matrinxã (Brycon amazonicus). Fish Shellfish Immunol. 2018, 83, 314–320. [Google Scholar] [CrossRef]
- Mommsen, T.P.; Vijayan, M.M.; Moon, T.W. Cortisol in teleosts: Dynamics, mechanisms of action, and metabolic regulation. Rev. Fish Biol. Fish. 1999, 9, 211–268. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Passos, C.P.; Madureira, P.; Vilanova, M.; Coimbra, M.A. Structure—Function relationships of immunostimulatory polysaccharides: A review. Carbohydr. Polym. 2015, 132, 378–396. [Google Scholar] [CrossRef]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef]
- Yin, F.; Gao, Q.; Tang, B.; Sun, P.; Han, K.; Huang, W. Transcriptome and analysis on the complement and coagulation cascades pathway of large yellow croaker (Larimichthys crocea) to ciliate ectoparasite Cryptocaryon irritans infection. Fish Shellfish Immunol. 2016, 50, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ding, L.; Yu, Y.; Kong, W.; Yin, Y.; Huang, Z.; Zhang, X.; Xu, Z. The change of teleost skin commensal microbiota is associated with skin mucosal transcriptomic responses during parasitic infection by Ichthyophthirius multifillis. Front. Immunol. 2018, 9, 2972. [Google Scholar] [CrossRef]
- Skugor, S.; Glover, K.A.; Nilsen, F.; Krasnov, A. Local and systemic gene expression responses of Atlantic salmon (Salmo salar L.) to infection with the salmon louse (Lepeophtheirus salmonis). BMC Genom. 2008, 9, 115. [Google Scholar] [CrossRef]
- Tadiso, T.M.; Krasnov, A.; Skugor, S.; Afanasyev, S.; Hordvik, I.; Nilsen, F. Gene expression analyses of immune responses in Atlantic salmon during early stages of infection by salmon louse (Lepeophtheirus salmonis) revealed bi-phasic responses coinciding with the copepod-chalimus transition. BMC Genom. 2011, 12, 141. [Google Scholar] [CrossRef]
- Krasnov, A.; Skugor, S.; Todorcevic, M.; Glover, K.A.; Nilsen, F. Gene expression in Atlantic salmon skin in response to infection with the parasitic copepod Lepeophtheirus salmonis, cortisol implant, and their combination. BMC Genom. 2012, 13, 130. [Google Scholar] [CrossRef]
- Johnson, S.C.; Fast, M.D. (Eds.) Interactions between sea lice and their hosts. In Host-Parasite Interactions, 1st ed.; Taylor & Francis Group: London, UK, 2004; pp. 131–159. [Google Scholar]
- Grayfer, L.; Belosevic, M. Cytokine Regulation of Teleost Inflammatory Responses. In New Advances and Contributions to Fish Biology; Türker, H., Ed.; IntechOpen: London, UK, 2012; pp. 59–96. [Google Scholar] [CrossRef]
- Sigh, J.; Lindenstrøm, T.; Buchmann, K. Expression of pro-inflammatory cytokines in rainbow trout (Oncorhynchus mykiss) during an infection with Ichthyophthirius multifiliis. Fish Shellfish Immunol. 2004, 17, 75–86. [Google Scholar] [CrossRef]
- Cross, M.L. Localized cellular responses to Ichthyophthirius multifiliis: Protection or pathogenesis? Parasitol. Today 1994, 10, 364–368. [Google Scholar] [CrossRef]
- Johnson, S.C. A comparison of development and growth rates of Lepophtheirus salmonis (Copepoda: Caligidae) on naive Atlantic (Salmo salar) and Chinook (Oncorhynchus tshawytscha) salmon. In Pathogens of Wild and Farmed Fish: Sea Lice, 1st ed.; Boxshall, G.A., Defaye, D., Eds.; Ellis Horwood: Chichester, UK, 1993; pp. 68–80. [Google Scholar]
- Shields, R.J.; Goode, R.P. Host rejection of Lernaea cyprinacea L. (Copepoda). Crustaceana 1978, 35, 301–307. [Google Scholar] [CrossRef]
- Shariff, M. The histopathology of the eye of big head carp, Aristichthys noblis (Richardson), infested with Lernaea piscinae Harding, 1950. J. Fish Dis. 1981, 4, 161–168. [Google Scholar] [CrossRef]
- Woo, P.T.K.; Shariff, M. Lernea cyprinacea L. (Copepoda: Caligidea) in Helostoma temmincki Cuvier & Valenciennes: The dynamics of resistance in recovered and naive fish. J. Fish Dis. 1990, 13, 485–493. [Google Scholar] [CrossRef]
- Basu, N.; Nakano, T.; Grau, E.G.; Iwama, G.K. The effects of cortisol on heat shock protein 70 levels in two fish species. Gen. Comp. Endocrinol. 2001, 124, 97–105. [Google Scholar] [CrossRef]
- Sathiyaa, R.; Campbell, T.; Vijayan, M.M. Cortisol modulates HSP90 mRNA expression in primary cultures of trout hepatocytes. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2001, 129, 679–685. [Google Scholar] [CrossRef]
- Galt, N.J.; McCormick, S.D.; Froehlich, J.M.; Biga, P.R. A comparative examination of cortisol effects on muscle myostatin and HSP90 gene expression in salmonids. Gen. Comp. Endocrinol. 2016, 237, 19–26. [Google Scholar] [CrossRef]
- Pedersen, M.E.; Vuong, T.T.; Rønning, S.B.; Kolset, S.O. Matrix metalloproteinases in fish biology and matrix turnover. Matrix Biol. 2015, 44, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Tom, M.; Chen, N.; Segev, M.; Herut, B.; Rinkevich, B. Quantifying fish metallothionein transcript by real time PCR for its utilization as an environmental biomarker. Mar. Pollut. Bull. 2004, 48, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Murashita, K.; Fukada, H.; Hosokawa, H.; Masumoto, T. Changes in cholecystokinin and peptide Y gene expression with feeding in yellowtail (Seriola quinqueradiata): Relation to pancreatic exocrine regulation. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2007, 146, 318–325. [Google Scholar] [CrossRef]



| Gene ID | Gene Name | Gene Symbol | Fold Change (log2) |
|---|---|---|---|
| Skin S-N/C-N | |||
| XM_023417751.1 | collagenase 3-like, transcript variant X2 | LOC111663530 | 5.668 |
| XM_023417750.1 | collagenase 3-like, transcript variant X1 | LOC111663530 | 5.666 |
| XM_023412500.1 | galactose-specific lectin nattectin-like | LOC111659519 | 5.160 |
| XM_023400417.1 | interleukin-1 receptor type 2-like | LOC111650527 | 4.283 |
| XM_023400815.1 | nuclear receptor subfamily 4 group A member 3, transcript variant X2 | nr4a3 | 3.844 |
| XM_023400817.1 | nuclear receptor subfamily 4 group A member 3, transcript variant X4 | nr4a3 | 3.721 |
| XM_023400816.1 | nuclear receptor subfamily 4 group A member 3, transcript variant X3 | nr4a3 | 3.654 |
| XM_023400814.1 | nuclear receptor subfamily 4 group A member 3, transcript variant X1 | nr4a3 | 3.647 |
| XM_023421640.1 | FOS like 1, AP-1 transcription factor subunit | fosl1 | 3.563 |
| XM_023417752.1 | collagenase 3-like | LOC111663531 | 3.395 |
| XM_023403589.1 | heat shock 70 kDa protein 1-like | LOC111653120 | 3.144 |
| XM_023405101.1 | tumor necrosis factor receptor superfamily member 9-like | LOC111654192 | 2.705 |
| XM_023395815.1 | heat shock 70 kDa protein 1 | LOC111646336 | 2.661 |
| XM_023427402.1 | matrix metallopeptidase 9 | mmp9 | 2.432 |
| XM_023431279.1 | suppressor of cytokine signaling 3-like | LOC111673508 | 2.395 |
| XM_023414302.1 | C-C motif chemokine 20-like | LOC111660877 | 2.366 |
| XM_023401030.1 | class I histocompatibility antigen, F10 alpha chain-like | LOC111651075 | 2.240 |
| XM_023419525.1 | heat shock protein family A (Hsp70) member 5 | hspa5 | 2.019 |
| XM_023415374.1 | complement C1q-like protein 4 | LOC111661717 | 4.688 |
| XM_023403629.1 | claudin-17-like | LOC111653151 | 3.745 |
| XM_023431223.1 | mucin-5AC-like | LOC111673465 | 3.745 |
| XM_023400530.1 | low affinity immunoglobulin gamma Fc region receptor II-like | LOC111650619 | 3.504 |
| XM_023412429.1 | lysozyme C-like | LOC111659460 | 3.089 |
| XM_023401049.1 | tumor necrosis factor receptor superfamily member 5-like | LOC111651097 | 2.919 |
| XM_023415322.1 | complement C1q tumor necrosis factor-related protein 3-like | LOC111661670 | 2.852 |
| XM_023422497.1 | mucin-2-like | LOC111666964 | 2.714 |
| XM_023416021.1 | claudin-4-like | LOC111662227 | 2.714 |
| XM_023418843.1 | C-C chemokine receptor type 7-like | LOC111664317 | 2.629 |
| XM_023429071.1 | galectin-2-like | LOC111671845 | 2.629 |
| XM_023404531.1 | cytokine inducible SH2 containing protein | cish | 2.609 |
| XM_023401015.1 | C-type mannose receptor 2-like | LOC111651055 | 2.547 |
| XM_023428424.1 | interleukin 4 induced 1 | il4i1 | 2.547 |
| XM_023394796.1 | interferon-inducible GTPase 5-like | LOC111645553 | 2.547 |
| XM_023395884.1 | interleukin 20 receptor subunit beta, transcript variant X1 | il20rb | 2.530 |
| XM_023398726.1 | RELT, TNF receptor | relt | 2.524 |
| XM_023399368.1 | immunoglobulin lambda-1 light chain-like | LOC111649636 | 2.366 |
| XM_023414416.1 | interleukin-1 beta-like | LOC111660964 | 2.366 |
| XM_023407830.1 | adherens junctions associated protein 1 | ajap1 | 2.366 |
| XM_023401390.1 | interferon regulatory factor 2 binding protein like | irf2bpl | 2.214 |
| XM_023419704.1 | NFKB inhibitor delta | nfkbid | 2.160 |
| XM_023430276.1 | heat shock protein HSP 90-alpha | LOC111672729 | 2.141 |
| XM_023399529.1 | claudin-23-like, transcript variant X2 | LOC111649834 | 2.115 |
| XM_023399530.1 | claudin-23-like, transcript variant X3 | LOC111649834 | 2.115 |
| XM_023399527.1 | claudin-23-like, transcript variant X1 | LOC111649834 | 2.115 |
| XM_023399111.1 | gap junction Cx32.2 protein-like | LOC111649336 | 2.095 |
| XM_023398423.1 | cathepsin L1-like | LOC111648648 | 2.044 |
| XM_023418047.1 | interleukin-22 receptor subunit alpha-2-like, transcript variant X1 | LOC111663746 | 2.004 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miura, T.; Nishikawa, M.; Otsu, Y.; Ali, M.F.Z.; Hashizume, A.; Miura, C. The Effects of Silkworm-Derived Polysaccharide (Silkrose) on Ectoparasitic Infestations in Yellowtail (Seriola quinqueradiata) and White Trevally (Pseudocaranx dentex). Fishes 2022, 7, 14. https://doi.org/10.3390/fishes7010014
Miura T, Nishikawa M, Otsu Y, Ali MFZ, Hashizume A, Miura C. The Effects of Silkworm-Derived Polysaccharide (Silkrose) on Ectoparasitic Infestations in Yellowtail (Seriola quinqueradiata) and White Trevally (Pseudocaranx dentex). Fishes. 2022; 7(1):14. https://doi.org/10.3390/fishes7010014
Chicago/Turabian StyleMiura, Takeshi, Munenori Nishikawa, Yuki Otsu, Muhammad Fariz Zahir Ali, Atsushi Hashizume, and Chiemi Miura. 2022. "The Effects of Silkworm-Derived Polysaccharide (Silkrose) on Ectoparasitic Infestations in Yellowtail (Seriola quinqueradiata) and White Trevally (Pseudocaranx dentex)" Fishes 7, no. 1: 14. https://doi.org/10.3390/fishes7010014
APA StyleMiura, T., Nishikawa, M., Otsu, Y., Ali, M. F. Z., Hashizume, A., & Miura, C. (2022). The Effects of Silkworm-Derived Polysaccharide (Silkrose) on Ectoparasitic Infestations in Yellowtail (Seriola quinqueradiata) and White Trevally (Pseudocaranx dentex). Fishes, 7(1), 14. https://doi.org/10.3390/fishes7010014






