Preferred and Optimal Swimming Speeds in Rainbow Trout (Oncorhynchus mykiss) at Three Temperatures
Abstract
1. Introduction
2. Materials and Methods
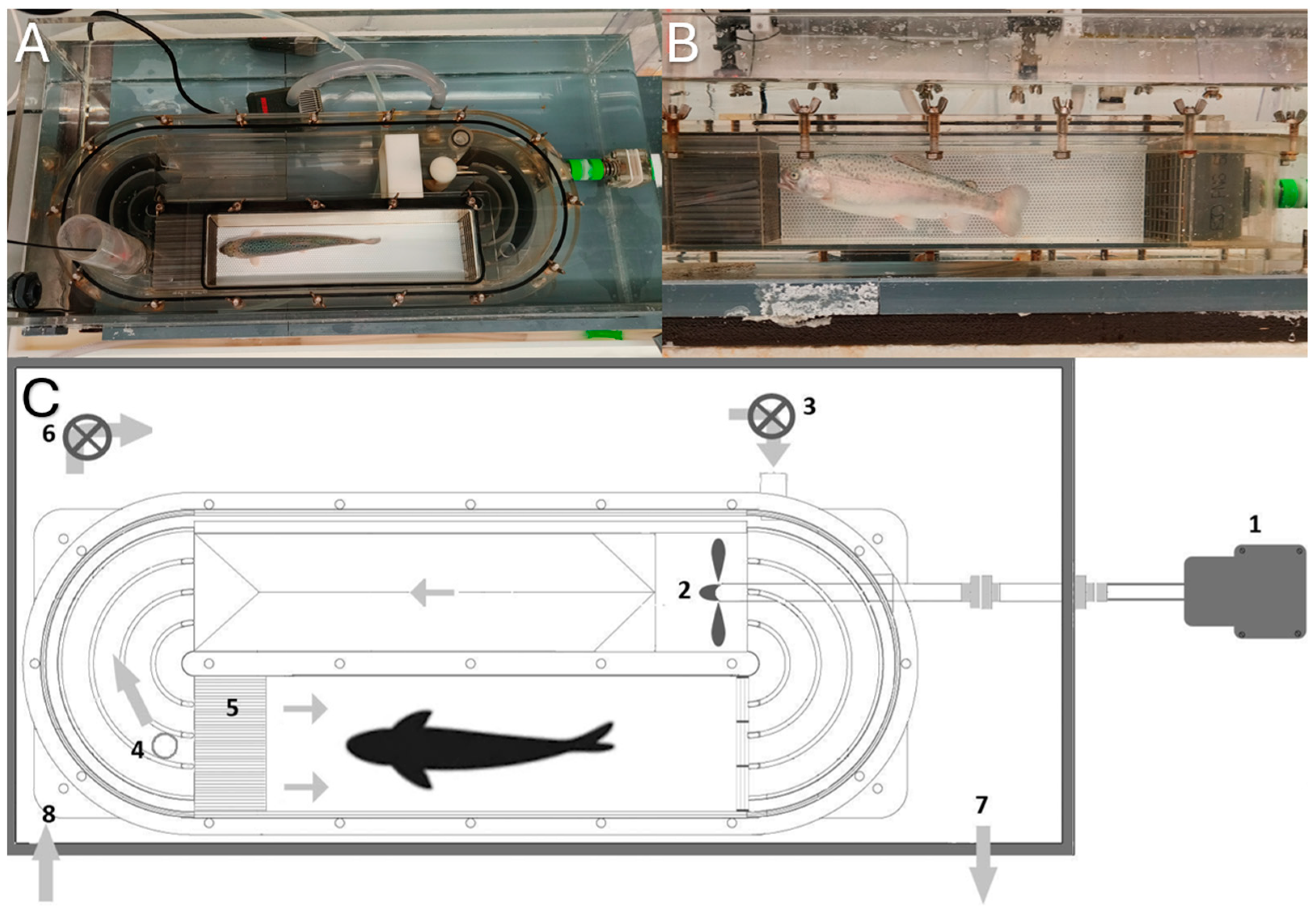
2.1. Experimental Design
2.2. Fish Husbandry
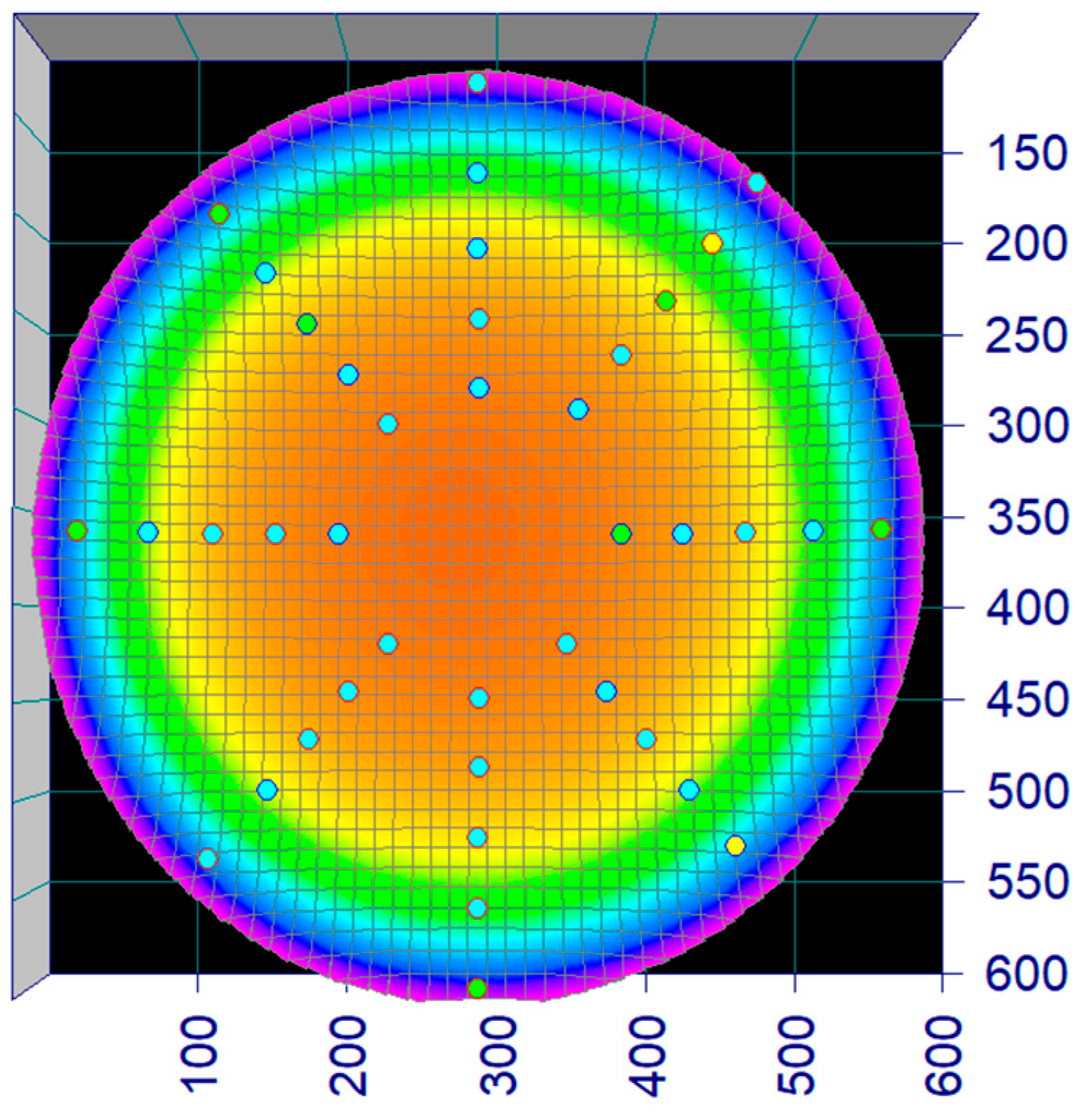
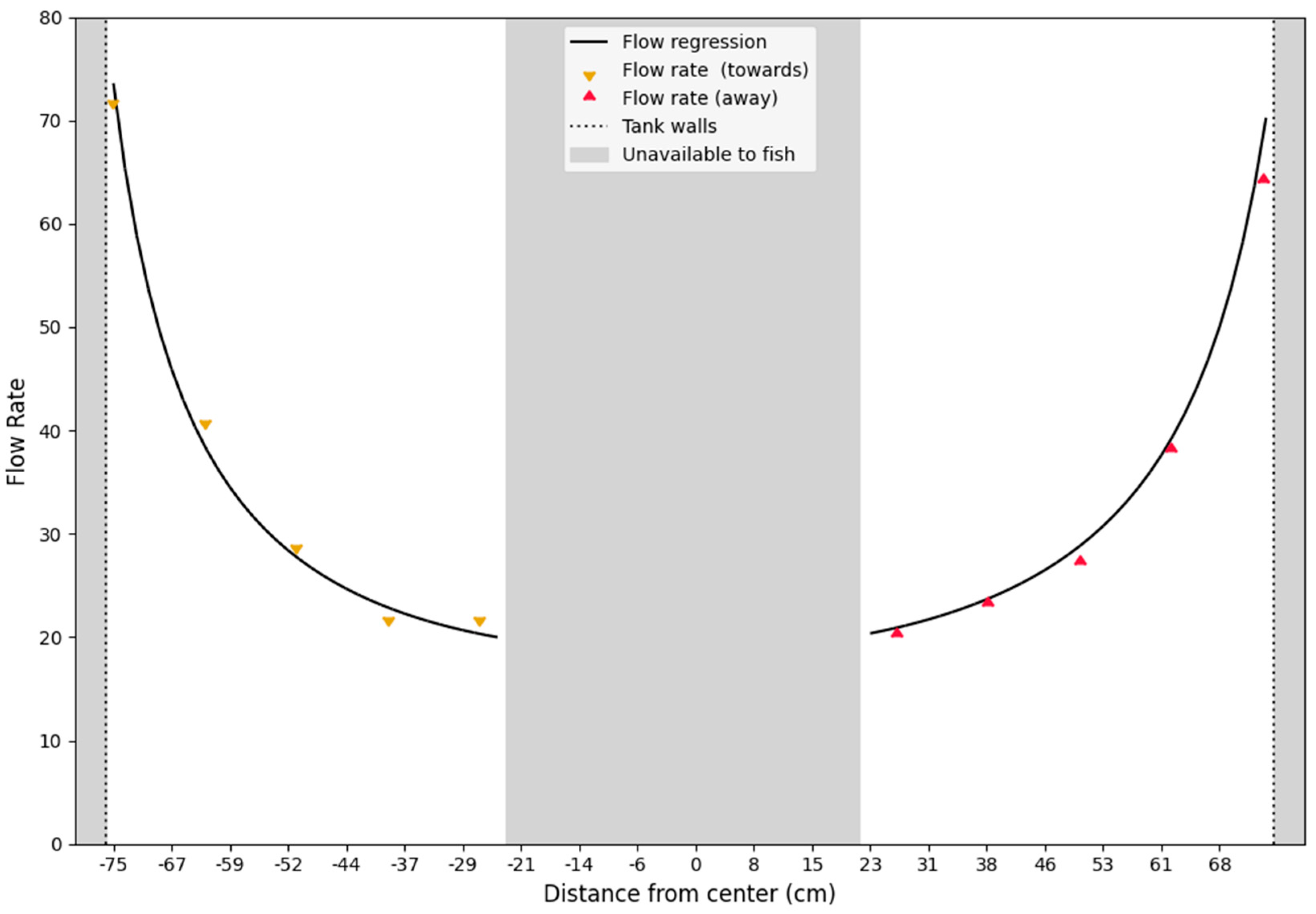
2.3. Flow Calibration and Validation
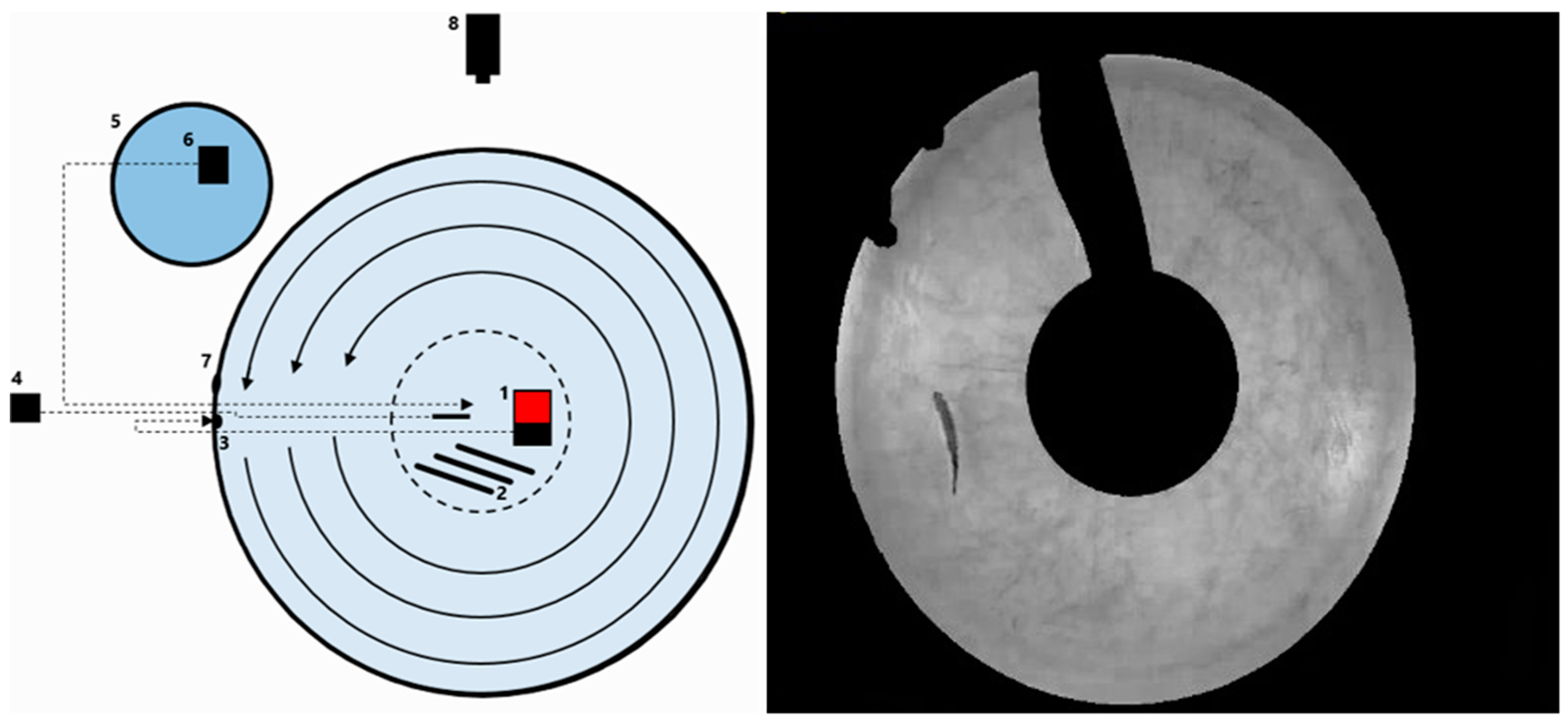
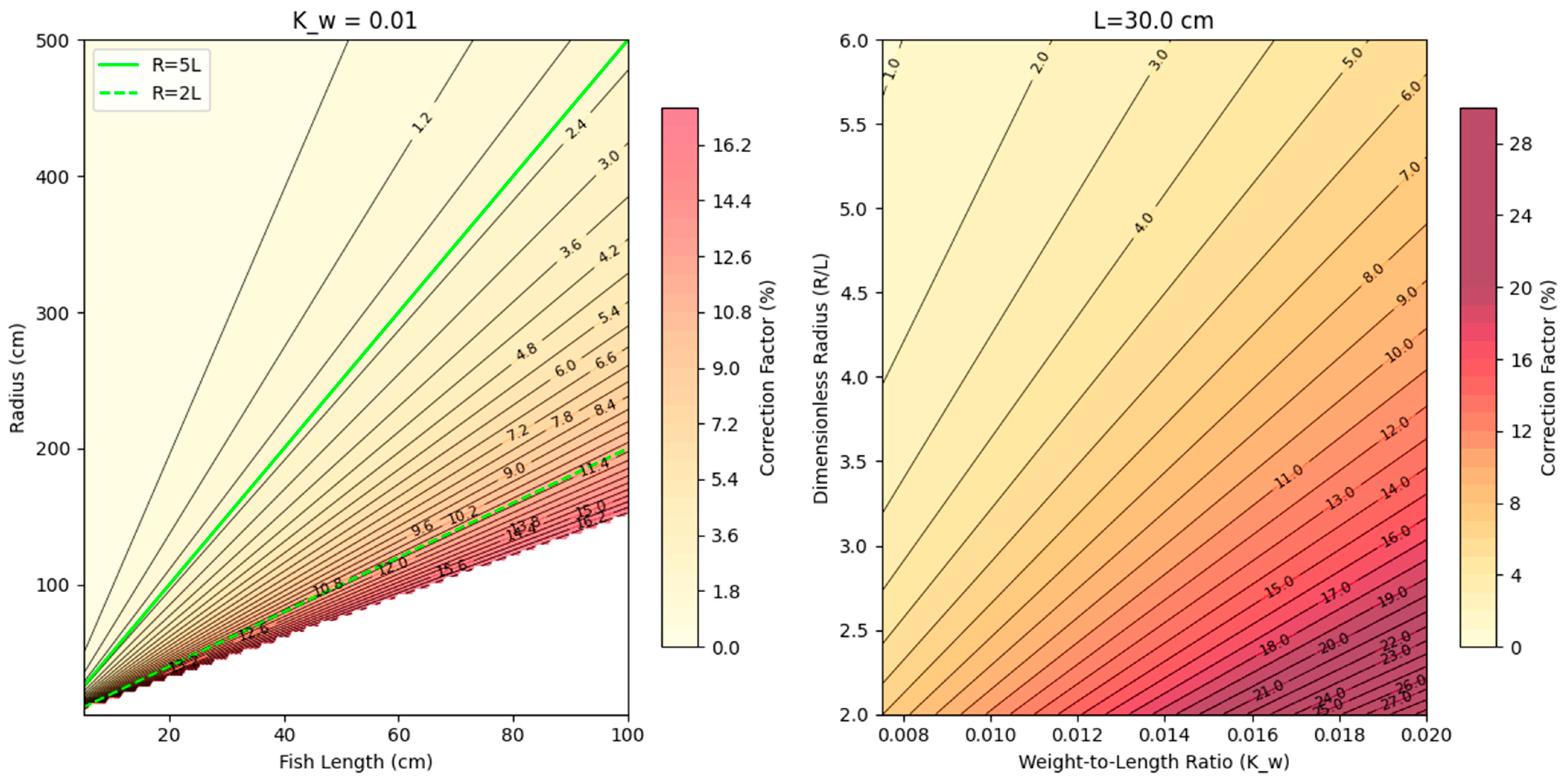
2.4. Preferred Swimming Speed (Upref)
2.5. Optimal Swimming Speed (Uopt)
2.6. Data Analysis
3. Results
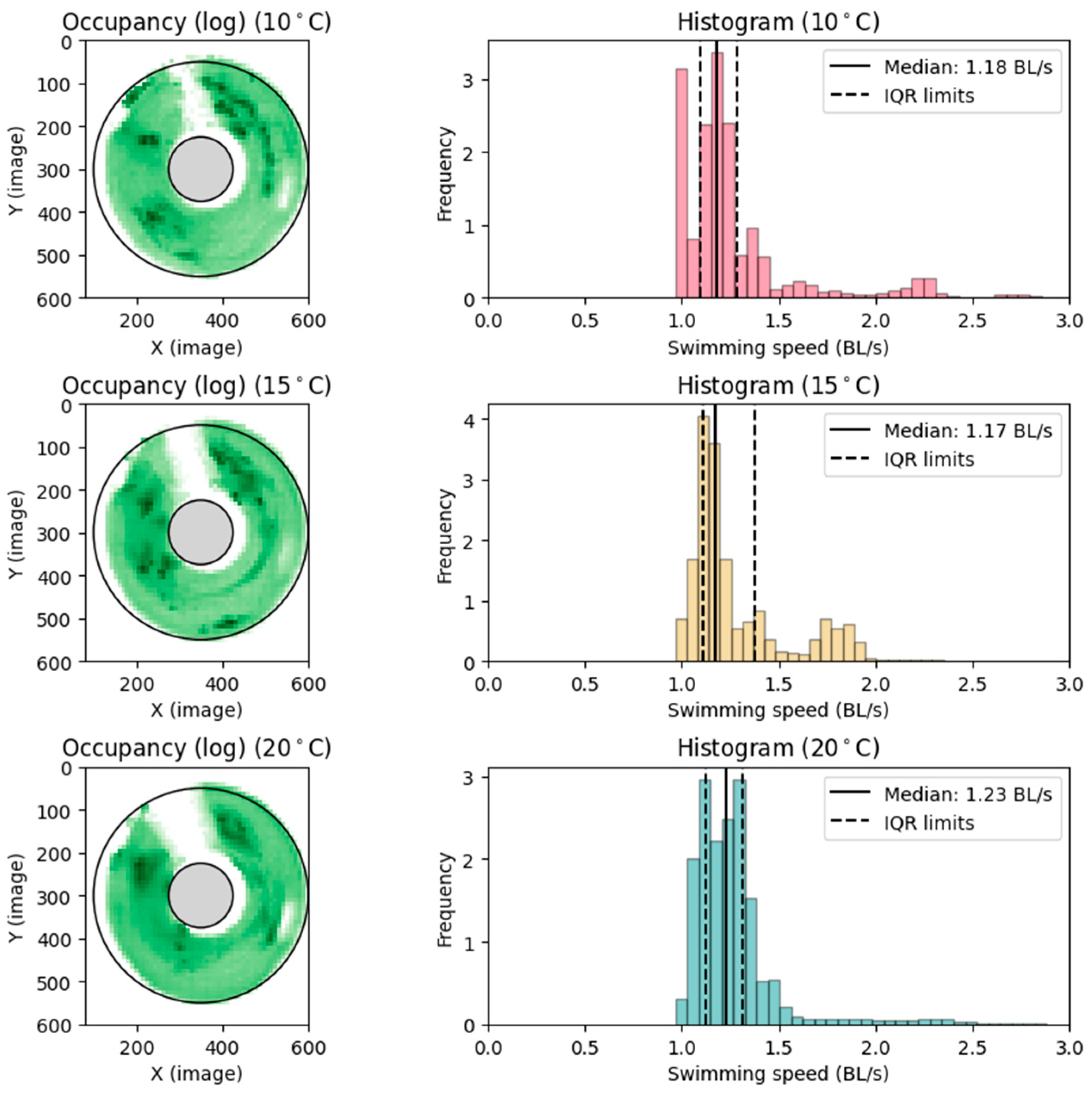
3.1. Preferred Swimming Speed
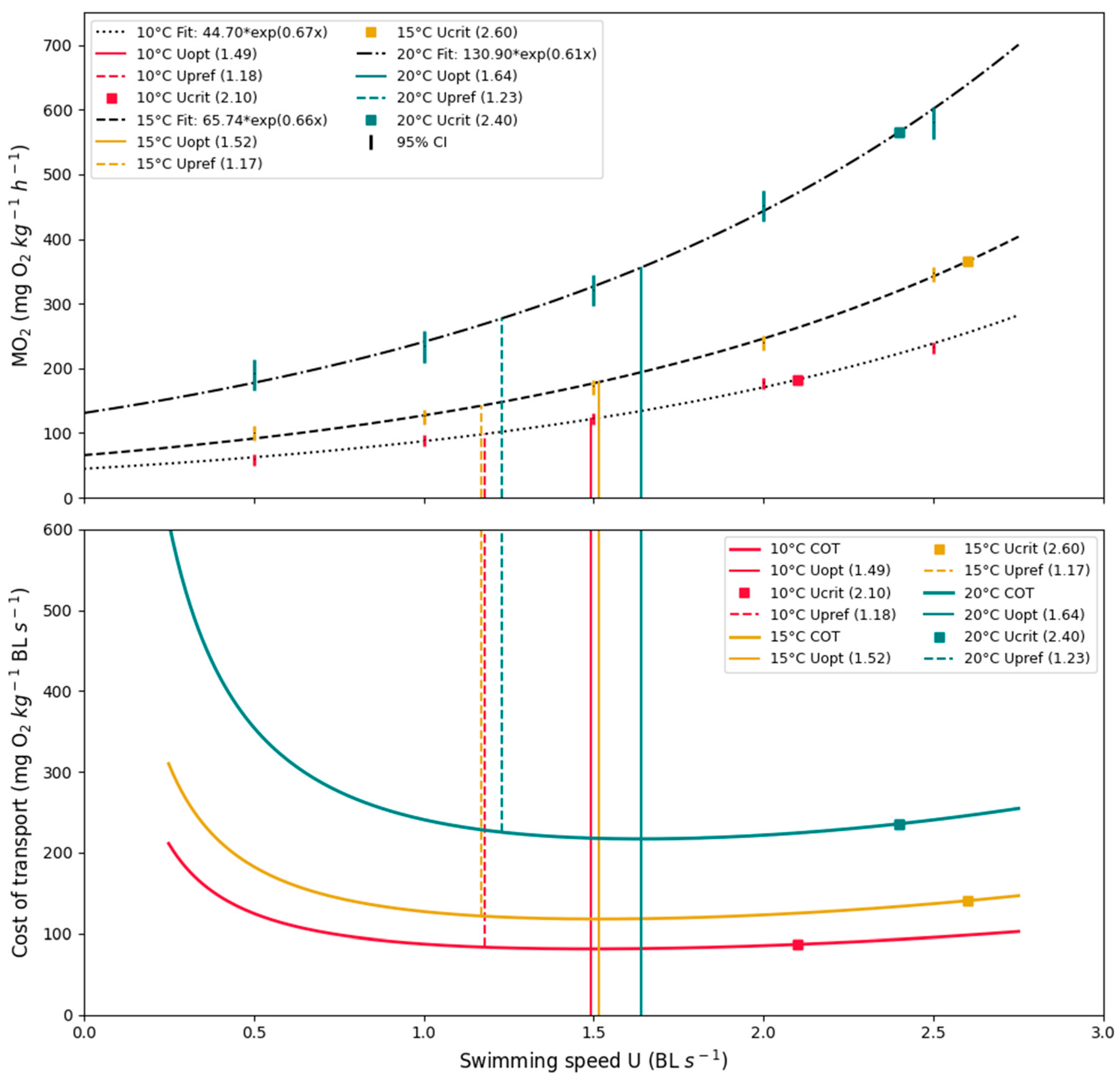
3.2. Energetics and Optimal Swimming Speed
3.3. Cost of Transport
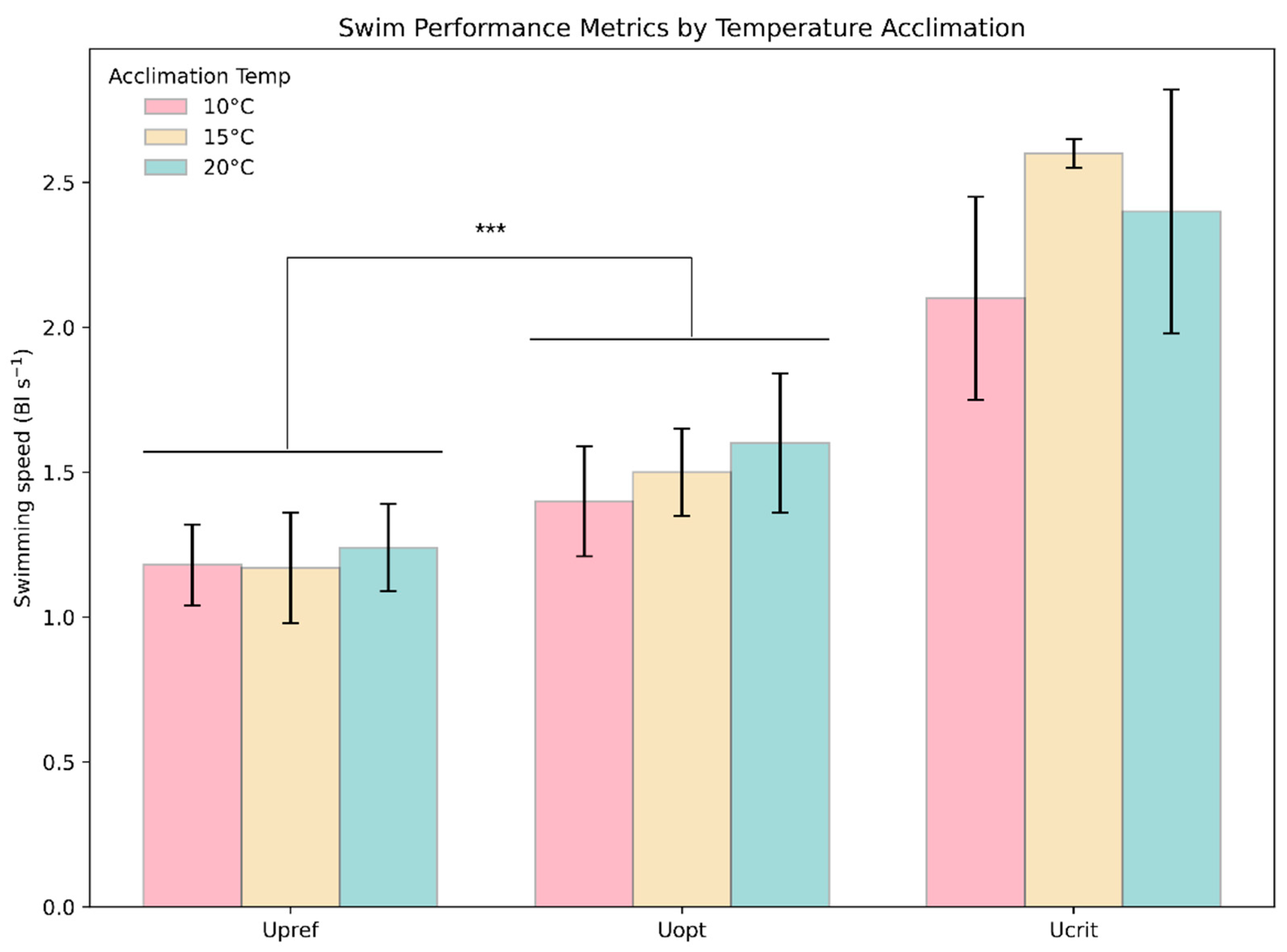
3.4. Comparison of Swimming Speeds
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brett, J.R. The swimming energetics of salmon. Sci. Am. 1965, 213, 80–87. [Google Scholar] [CrossRef]
- Videler, J.J. Fish Swimming; Springer Science & Business Media: Dordrecht, The Netherlands, 1993; p. 260. ISBN 978-0-412-40860-1. [Google Scholar]
- Palstra, A.P.; Planas, J.V. Fish under exercise. Fish Physiol. Biochem. 2011, 37, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Anttila, K.; Järvilehto, M.; Mänttäri, S. The swimming performance of brown trout and whitefish: The effects of exercise on Ca2+ handling and oxidative capacity of swimming muscles. J. Comp. Physiol. B 2008, 178, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Castro, V.; Grisdale-Helland, B.; Jørgensen, S.M.; Helgerud, J.; Claireaux, G.; Farrell, A.P.; Krasnov, A.; Helland, S.J.; Takle, H. Disease resistance is related to inherent swimming performance in Atlantic salmon. BMC Physiol. 2013, 13, 1. [Google Scholar] [CrossRef]
- Nilsen, A.; Hagen, Ø.; Johnsen, C.A.; Prytz, H.; Zhou, B.; Nielsen, K.V.; Bjørnevik, M. The importance of exercise: Increased water velocity improves growth of Atlantic salmon in closed cages. Aquaculture 2019, 501, 537–546. [Google Scholar] [CrossRef]
- Ytrestøyl, T.; Takle, H.; Kolarevic, J.; Calabrese, S.; Timmerhaus, G.; Rosseland, B.O.; Teien, H.C.; Nilsen, T.O.; Handeland, S.O.; Stefansson, S.O.; et al. Performance and welfare of Atlantic salmon, Salmo salar L. post-smolts in recirculating aquaculture systems: Importance of salinity and water velocity. J. World Aquac. Soc. 2020, 51, 373–392. [Google Scholar] [CrossRef]
- Beamish, F.W. Swimming capacity. In Fish Physiology; Academic Press: Cambridge, MA, USA, 1978; Volume 7, pp. 101–187. [Google Scholar] [CrossRef]
- Tudorache, C.; O’Keefe, R.A.; Benfey, T.J. Optimal swimming speeds reflect preferred swimming speeds of brook charr (Salvelinus fontinalis Mitchill, 1874). Fish Physiol. Biochem. 2011, 37, 307–315. [Google Scholar] [CrossRef]
- Houlihan, D.F.; Laurent, P. Effects of exercise training on the performance, growth, and protein turnover of rainbow trout (Salmo gairdneri). Can. J. Fish. Aquat. Sci. 1987, 44, 1614–1621. [Google Scholar] [CrossRef]
- Christiansen, J.S.; Ringø, E.; Jobling, M. Effects of sustained exercise on growth and body composition of first-feeding fry of Arctic charr, Salvelinus alpinus (L.). Aquaculture 1989, 79, 329–335. [Google Scholar] [CrossRef]
- Grünbaum, T.; Cloutier, R.; Le Francois, N.R. Positive effects of exposure to increased water velocity on growth of newly hatched Arctic charr, Salvelinus alpinus L. Aquac. Res. 2008, 39, 106–110. [Google Scholar] [CrossRef]
- Castro, V.; Grisdale-Helland, B.; Helland, S.J.; Kristensen, T.; Jørgensen, S.M.; Helgerud, J.; Claireaux, G.; Farrell, A.P.; Krasnov, A.; Takle, H. Aerobic training stimulates growth and promotes disease resistance in Atlantic salmon (Salmo salar). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2011, 160, 278–290. [Google Scholar] [CrossRef] [PubMed]
- Ibarz, A.; Felip, O.; Fernández-Borràs, J.; Martín-Pérez, M.; Blasco, J.; Torrella, J.R. Sustained swimming improves muscle growth and cellularity in gilthead sea bream. J. Comp. Physiol. B 2011, 181, 209–217. [Google Scholar] [CrossRef]
- Palstra, A.P.; Mes, D.; Kusters, K.; Roques, J.A.; Flik, G.; Kloet, K.; Blonk, R.J. Forced sustained swimming exercise at optimal speed enhances growth of juvenile yellowtail kingfish (Seriola lalandi). Front. Physiol. 2015, 5, 506. [Google Scholar] [CrossRef]
- Tucker, V.A.; Catlett, R. Energetic cost of locomotion in animals. Read. Anim. Energetics 1973, 34, 109. [Google Scholar] [CrossRef] [PubMed]
- Steffensen, J.F.; Johansen, K.; Bushnell, P.G. An automated swimming respirometer. Comp. Biochem. Physiol. Part A Physiol. 1984, 79, 437–440. [Google Scholar] [CrossRef]
- Myrick, C.A.; Cech, J.J. Temperature influences on California rainbow trout physiological performance. Fish Physiol. Biochem. 2000, 22, 245–254. [Google Scholar] [CrossRef]
- Prescott, L.A.; Symonds, J.E.; Walker, S.P.; Miller, M.R.; Swift, L.; Herbert, N.A.; Semmens, J.M.; Carter, C.G. The mismatch between swimming speeds and flow regimes when optimizing exercise regimes to improve Chinook salmon, Oncorhynchus tshawytscha, performance. Aquaculture 2024, 585, 740705. [Google Scholar] [CrossRef]
- Kline, R.J.; Parkyn, D.C.; Murie, D.J. Empirical modelling of solid-blocking effect in a Blazka respirometer for gag, a large demersal reef fish. Adv. Zool. Bot. 2015, 3, 193–202. [Google Scholar] [CrossRef]
- He, P.; Wardle, C.S. Endurance at intermediate swimming speeds of Atlantic mackerel, Scomber scombrus L.; herring, Clupea harengus L.; and saithe, Pollachius virens L. J. Fish Biol. 1988, 33, 255–266. [Google Scholar] [CrossRef]
- Weihs, D. Effects of swimming path curvature on the energetics of fish motion. Fish. Bull. 1981, 79, 171, LCCN: 72625459. [Google Scholar]
- Steinhausen, M.F.; Steffensen, J.F.; Andersen, N.G. Tail beat frequency as a predictor of swimming speed and oxygen consumption of saithe (Pollachius virens) and whiting (Merlangius merlangus) during forced swimming. Mar. Biol. 2005, 148, 197–204. [Google Scholar] [CrossRef]
- Schurmann, H.; Steffensen, J.F. Effects of temperature, hypoxia and activity on the metabolism of juvenile Atlantic cod. J. Fish Biol. 1997, 50, 1166–1180. [Google Scholar] [CrossRef]
- Christensen, E.A.; Svendsen, M.B.; Steffensen, J.F. The combined effect of body size and temperature on oxygen consumption rates and the size-dependency of preferred temperature in European perch Perca fluviatilis. J. Fish Biol. 2020, 97, 794–803. [Google Scholar] [CrossRef] [PubMed]
- Schurmann, H.; Steffensen, J.F.; Lomholt, J.P. The influence of hypoxia on the preferred temperature of rainbow trout Oncorhynchus mykiss. J. Exp. Biol. 1991, 157, 75–86. [Google Scholar] [CrossRef]
- Chen, Z.; Snow, M.; Lawrence, C.S.; Church, A.R.; Narum, S.R.; Devlin, R.H.; Farrell, A.P. Selection for upper thermal tolerance in rainbow trout (Oncorhynchus mykiss Walbaum). J. Exp. Biol. 2015, 218, 803–812. [Google Scholar] [CrossRef]
- Thorarensen, H.; Gallaugher, P.; Farrell, A.P. Cardiac output in swimming rainbow trout, Oncorhynchus mykiss, acclimated to seawater. Physiol. Zool. 1996, 69, 139–153. [Google Scholar] [CrossRef]
- Brett, J.R. The respiratory metabolism and swimming performance of young sockeye salmon. J. Fish. Board Can. 1964, 21, 1183–1226. [Google Scholar] [CrossRef]
- Webb, P.W. The swimming energetics of trout: I. Thrust and power output at cruising speeds. J. Exp. Biol. 1971, 55, 489–520. [Google Scholar] [CrossRef]
- Davison, W.; Goldspink, G. The effect of prolonged exercise on the lateral musculature of the brown trout (Salmo trutta). J. Exp. Biol. 1977, 70, 1–12. [Google Scholar] [CrossRef]
- Cooke, S.J.; Chandroo, K.P.; Beddow, T.A.; Moccia, R.D.; McKinley, R.S. Swimming activity and energetic expenditure of captive rainbow trout (Oncorhynchus mykiss) estimated by electromyogram telemetry. Aquac. Res. 2000, 31, 495–505. [Google Scholar] [CrossRef]
- Thorstad, E.; Kland, F.; Finstad, B.; Sivertsgrd, R.; Bjorn, P.; McKinleyd, R. Migration speeds and orientation of Atlantic salmon and sea trout post-smolts in a Norwegian fjord system. Environ. Biol. Fishes 2004, 71, 305–311. [Google Scholar] [CrossRef]
| Temperature Acclimation Group | 10 °C | 15 °C | 20 °C |
|---|---|---|---|
| n | 10 | 10 | 11 |
| Body length (cm) | 24.2 ± 1.1 | 24.7 ± 0.5 | 24.7 ± 0.9 |
| Mass (g) | 187.5 ± 16.1 | 177.9 ± 12.7 | 162.7 ± 23.7 |
| Height (cm) | 6.0 ± 0.4 | 5.7 ± 0.5 | 5.1 ± 0.6 |
| Width (cm) | 2.7 ± 0.4 | 2.6 ± 0.2 | 2.6 ± 0.4 |
| Temperature Acclimation Group | 10 °C | 15 °C | 20 °C | |
|---|---|---|---|---|
| Upref (BL s−1) | 1.2 ± 0.14 | 1.2 ± 0.19 | 1.2 ± 0.15 | |
| Uopt (BL s−1) | 1.4 ± 0.19 | 1.5 ± 0.15 | 1.6 ± 0.24 | |
| Ucrit (BL s−1) | 2.1 ± 0.35 | 2.6 ± 0.05 | 2.4 ± 0.42 | |
| MO2 @ 0.5 BL s−1 (mg O2 kg−1 h−1) | 62.3 ± 4.8 | 99.2 ± 12.9 | 195.2 ± 30.9 | |
| AMR (mg O2 kg−1 h−1) | 188.9 ± 44.6 | 386.0 ± 72.6 | 550.6 ± 171.2 | |
| SMR (mg O2 kg−1 h−1) | 44.7 | 65.7 | 130.9 | |
| Aerobic scope @ 0.5 BL s−1 | 3.0 ± 0.6 | 3.8 ± 1.1 | 2.8 ± 0.7 | |
| Q10 | 2.5 | 3.8 | ||
| 3.1 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mittún, Ó.a.F.; Svendsen, M.B.S.; Andersen, L.E.J.; Bergsson, H.; Steffensen, J.F. Preferred and Optimal Swimming Speeds in Rainbow Trout (Oncorhynchus mykiss) at Three Temperatures. Fishes 2025, 10, 64. https://doi.org/10.3390/fishes10020064
Mittún ÓaF, Svendsen MBS, Andersen LEJ, Bergsson H, Steffensen JF. Preferred and Optimal Swimming Speeds in Rainbow Trout (Oncorhynchus mykiss) at Three Temperatures. Fishes. 2025; 10(2):64. https://doi.org/10.3390/fishes10020064
Chicago/Turabian StyleMittún, Ólavur av Fløtum, Morten Bo Søndergaard Svendsen, Lars Emil Juel Andersen, Heiðrikur Bergsson, and John Fleng Steffensen. 2025. "Preferred and Optimal Swimming Speeds in Rainbow Trout (Oncorhynchus mykiss) at Three Temperatures" Fishes 10, no. 2: 64. https://doi.org/10.3390/fishes10020064
APA StyleMittún, Ó. a. F., Svendsen, M. B. S., Andersen, L. E. J., Bergsson, H., & Steffensen, J. F. (2025). Preferred and Optimal Swimming Speeds in Rainbow Trout (Oncorhynchus mykiss) at Three Temperatures. Fishes, 10(2), 64. https://doi.org/10.3390/fishes10020064






