Abstract
Gly m 7, a novel soybean allergen, was recently reported. In this study, we attempted to detect Gly m 7 in various soybeans and processed soybean foods using raised anti-Gly m 7 antibodies and enzyme-linked streptavidin, specifically binding to the biotin moiety of Gly m 7. There was a large difference in Gly m 7 levels in various soybean-processed foods. When Gly m 7 levels were determined, all cultivars contained this allergen almost completely, but the biotin moiety detected by streptavidin varied, suggesting that biotinylated levels of Gly m 7 might differ among cultivars. The thermal stability of Gly m 7 was determined by heating soybean extracts. During detection using anti-peptide antibodies, detectable intact Gly m 7 was gradually reduced by heating. Gly m 7 was not detected by peptide or biotin detection in worm-wounded soybeans. Soybeans were immersed in distilled water as a pretreatment step for germination, and Gly m 7 levels were compared by immersion time (4–96 h). Intact Gly m 7 was rapidly degraded in detection by both peptide and biotin moieties. This suggested that Gly m 7 was degraded by some protease(s) during germination. These results would be useful for understanding the properties or risk assessment of Gly m 7, a newly discovered soybean allergen.
1. Introduction
Soybeans (Glycine max L.) are a common ingredient in many foods, including fermented foods, in both traditional Asian and Western cuisines. In addition, properties of soybean protein make it an excellent choice for food processing widely used as an additive in various processed foods. Soybeans also have various unique physiological effects, including lowering serum lipids [1,2,3]. Soybean protein has received a great deal of attention in recent years as a material for “veggie meat” [4,5].
However, consumption of soybeans can cause allergic reactions in some individuals, and various allergens have already been identified in soybeans [6]. The major allergens of soybeans are the Kunitz soybean trypsin inhibitor [7]; Gly m Bd 30 K [8,9]; Gly m Bd 28 K [10]; Gly m 5 [11,12], which has been identified as 7S globulin (β-conglycinin); and Gly m 6 [13], which has been identified as 11S globulin (glycinin). Gly m 8 (2S albumin) has been recently identified as a clinically important allergen in soybeans [14]. These allergens are thought to cause systemic reactions in patients with soybean food allergies. In contrast, oral allergy syndrome and severe allergic reactions, including anaphylaxis, caused by soybean protein-containing foods have recently been reported in patients with allergies to birch pollen (pollinosis). Starvation-associated message 22 (SAM 22: Gly m 4: PR-10), which has a molecular weight (MW) of 17 kDa, has been detailed to be the major causative allergen of this pollen-related food allergy [15,16]. In addition to Gly m 4, Gly m 3 has been identified as another pollen-related soybean allergen. This molecule is homologous to the Bet v 2 allergen, which has been identified as a profilin, a type of cytoskeletal cellular protein [17].
Recently, John J. et al. have reported Gly m 7, a seed biotinylated protein, as a new allergen in soybeans [18]. The amino acid sequence of Gly m 7 (Allergome 10,214, Gly m 7.0101) is shown in Figure 1. It is known that the MW of this molecule is about 70 kDa, the lysine residue at the 125 amino acid position may be biotinylated, and the function of this molecule is speculated to be a biotin source or carrier during plant germination [18,19]. The reactivity of this molecule with subjects allergic to soybeans and peanuts have been examined, and 18 out of 23 were positive [18]. In patients with a peanut allergy, it has been suggested that soybean Gly m 7 shows stronger basophil activation ability than Gly m 5. In addition, despite the low content of Gly m 7 in soybean, it has been reported that its reactivity in soy allergy patients is high [18].
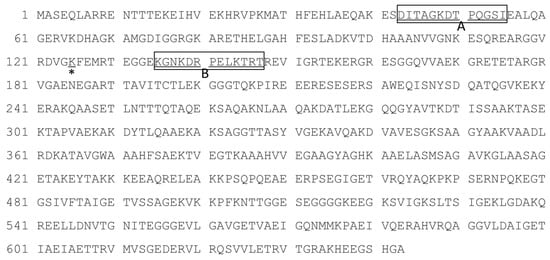
Figure 1.
Amino acid sequence of Gly m 7. Selected peptide antigen regions for antibody production are indicated as box A and box B. The putative biotin binding Lys (k) residue is indicated by * at 125 aa. The sequence data was referred from UniProtKB-C6K8D1 (C6K8D1_SOYBN) (Allergome 10214, Gly m 7.0101).
Gly m 7 is abundant during late embryogenesis in soybean seeds and is proposed to belong to the late-stage embryogenesis accumulating (LEA) protein family [18,19]. Generally, the LEA protein is structurally stable, and it is considered that Gly m 7 also shows thermal resistance to soybean cooking and processing, but the details are unknown.
Therefore, in this study, we produced antibodies against Gly m 7 to detect peptide moieties. In addition, we used the strong and specific binding between biotin and avidin to detect the biotin moiety of the biotinylated Gly m 7 protein in various soybeans and processed soybean foods under various conditions.
2. Materials and Methods
2.1. Materials
Horseradish peroxidase (HRP)-labeled anti-rabbit IgG and HRP-labeled streptavidin were obtained from Thermo Scientific (Waltham, MA, USA). ECLTM western blotting reagent and Hyperfilm-MP TM X-ray films were obtained from GE Healthcare (Piscataway, NJ, USA). The polyvinylidene fluoride (PVDF) membrane (Immobilon-P TM) was obtained from Millipore (Billerica, MA, USA).
2.2. Preparation of Anti-Gly m 7 Antibodies
Rabbit polyclonal antibodies against Gly m 7 were obtained by immunizing rabbits with Gly m 7. Briefly, two synthesized peptides, “DITAGKDTPQGSI” (A) and “KGNKDRPELKTRT” (B), corresponding to the sequences of Gly m 7 (Figure 1), were conjugated to keyhole limpet hemocyanin and mix-immunized to rabbits. These sequences were determined as antigen peptides based on the hydrophobic/hydrophilic information of the protein and the sequence information presumed to be the surface of the molecule. After three immunizations, the final antigens were injected and boosted. Anti-serum was obtained from the rabbits. Antibody production was performed at Scrum Inc. (Tokyo, Japan).
2.3. Preparation of Food Samples
Various kinds of soymilk and processed soybean foods (kinu-tofu, momen-tofu, thick fried tofu, thin fried tofu, boiled soybeans, kome-miso (salty miso made from soybean and rice), shiro-miso (sweetened miso), and natto) were purchased at supermarkets near the university. The samples were diluted with distilled water, and the experiment was conducted at a constant dilution ratio. Solid processed soybean foods were weighed at a fixed amount (5 g), and distilled water (20 mL) was added and homogenized for 30 s with a food processor with blades and squeezed with quadrupled gauze to obtain an extract. By this operation, the food sample was finely crushed, mixed, and homogenized. Experiments were conducted under constant conditions of equal original sample weights. To compare relative levels of Gly m 7 in various soybean cultivars, 17 major soybean cultivars were obtained from a food company in Japan.
2.4. Preparation of Extracts from Dried Soybeans and Protein Analysis
Approximately 3 g of mature dry soybeans was immersed overnight in 30 mL of distilled water, and soaked soybeans were then homogenized for 30 s in 30 mL of distilled water using a commercial food mixer. The homogenate was filtered through four layers of gauze. The filtrate was used to determine protein concentrations, and subsequently analyzed by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) or enzyme-linked immunosorbent assay (ELISA) after dilution. The protein concentration of the extracted soybeans was determined using the Bradford method [20].
2.5. Antibodies to Soybean Allergens Other Than Gly m 7
A mouse monoclonal antibody against Gly m Bd 30 K [6] was kindly provided by Dr. Tadashi Ogawa (Professor Emeritus at Kyoto University). Rabbit polyclonal antibodies against Gly m 5 (7S globulin; β-conglycinin; α′, α, and β subunits) were obtained as previously described [21]. Mouse polyclonal antibodies against Gly m 6 (11S globulin; glycinin) were obtained by immunizing mice with purified 11S globulin in our laboratory. Rabbit polyclonal antibodies against Gly m 4 were obtained by immunizing rabbits with recombinant Gly m 4, as described previously [22]. Rabbit antibodies against soybean trypsin inhibitors were obtained from Rockland (Gilbertsville, PA, USA). The specific reactivity of these antibodies was confirmed in our previous studies.
2.6. Electrophoresis and Western Blotting
Extracted soybean proteins were subjected to SDS-PAGE [23]. Proteins on the gel were stained with Coomassie Brilliant Blue (CBB R-350, GE Healthcare) to visualize the total protein patterns. Western blotting analysis was conducted by transferring the SDS-PAGE gel onto an Immobilon-PTM PVDF membrane (Millipore) using a semi-dry blotting method [24]. The membrane was incubated in 10 mM phosphate-buffered saline (PBS) (pH 7.5) containing 0.1% Tween-20 (PBST) and 5% skim milk for blocking (blocking solution). The membrane was then incubated for 1 h at room temperature in a blocking buffer containing allergen-specific antibodies. After washing the membranes four times with PBST for 10 min, the bound primary antibodies were detected using HRP-conjugated goat anti-rabbit or anti-mouse IgG and an ECLTM western blotting kit (GE Healthcare). The resultant chemiluminescent signals were detected on an X-ray film (HyperfilmTM MP, GE Healthcare). Western blotting experiments were carried out three times, and band densities were determined using the Alpha EaseTM software (Alpha Innotech, San Leandro, CA, USA).
2.7. Detection of Biotin Moiety of Gly m 7
HRP-labeled streptavidin was used to detect the biotin moiety of Gly m 7. After SDS-PAGE, gels were blotted onto a PVDF membrane, and the membrane was blocked with blocking solution, as described above, and incubated with HRP-labeled streptavidin (1:1000 dilution) for 1 h. After incubation, the membrane was washed with PBST three times for 5 min with shaking. The ECL reagent was used for signal generation. The chemiluminescent signal was exposed to X-ray films (GE Healthcare).
2.8. ELISA for Determination of Antibody Titers
ELISA was carried out to evaluate titers of the antibodies produced. Briefly, soybean extracts were coated on an ELISA plate by sequential dilution. After sample coating and blocking, diluted antibodies (antisera) were added to wells. After reacting for 1 h at 37 °C and washing with PBST five times, HRP-labeled secondary antibodies were added to wells. The bound HRP-labeled secondary antibodies were detected by reacting with a tetramethylbenzidine peroxidase substrate (KPL, Gaithersburg, MD, USA) for 5–15 min. The reaction was stopped by adding 100 μL of 1 M phosphoric acid to amplify the signal. Absorption was measured at 450 nm using an ARVOsx-1 1420 multilabel counter (PerkinElmer Life Sciences, Boston, MA, USA). Measurements were performed three times, and the mean values were plotted.
2.9. Statistical Analysis
Results were expressed as the mean ± standard deviation (SD). Data were analyzed using the Tukey–Kramer or Dunnett’s method with the Stat View v. 5.0 (SAS Institute, Tokyo, Japan). For the comparison of two groups, Student’s t-test was used to determine significant differences. All experiments were performed at least three times. Statistical significance was defined as p < 0.05, indicated by different letters or asterisks.
3. Results
3.1. Preparation and Assessment of Anti-Gly m 7 Antibodies
Based on the amino acid sequence of Gly m 7, two highly antigenic peptide sequences, “DITAGKDTPQGSI” (A) and “KGNKDRPELKTRT” (B), were selected, and synthetic peptides were prepared (Figure 1). The synthesized peptide was conjugated to keyhole limpet hemocyanin as a carrier protein and subsequently administered to rabbits. The antisera obtained were used to assess the titer and reactivity. ELISA (Figure 2a) and western blotting (Figure 2b) were performed using soybean homogenates. As a result, a dose-dependent increase in the absorbance of the soybean extract was confirmed by ELISA. The increase in absorbance using preimmune antisera was obviously lower than that using the obtained antisera (Figure 2a). In addition, we detected immunoreactive protein bands at approximately 70 kDa, which is consistent with the MW of Gly m 7 (Figure 2b, lane 1). This band was not detected when preimmune antisera were used (data not shown). These results indicated that the obtained anti-Gly m 7 antibodies containing antisera were able to react with Gly m 7 in soybean. Depending on the type of sample to be analyzed, some reactive protein bands were detected in addition to the position considered to be Gly m 7 when using anti-Gly m 7 polyclonal antibody raised. Therefore, the specificity of this antibody used in this study might be not very high (data not shown).
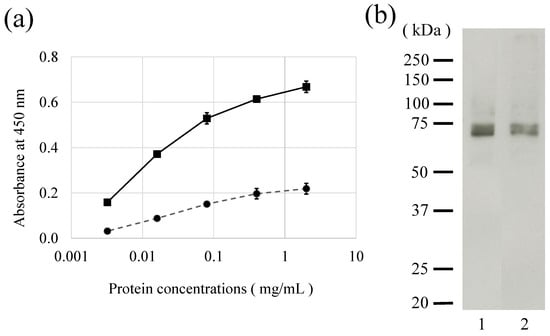
Figure 2.
Detection of Gly m 7 using raised anti-Gly m 7 antibodies and horseradish peroxidase-labeled streptavidin in soybean extracts. (a) Enzyme-linked immunosorbent assay (ELISA) results for detection of Gly m 7 using raised anti-Gly m 7 antibodies. Soybean extracts were immobilized on ELISA plates, and immunoreactions were performed using raised anti-Gly m 7 antibody (×1000 diluted) (solid line) and preimmune sera (×1000 diluted) (dashed line). (b) Detection of Gly m 7 using raised anti-Gly m 7 antibodies (lane 1) and horseradish peroxidase-labeled streptavidin (lane 2).
3.2. Detection of the Biotin Moiety of Gly m 7
HRP-labeled streptavidin was used to detect the biotin moiety in Gly m 7. When concentrations and blocking conditions of HRP-labeled streptavidin were optimized, it reacted specifically with an approximately 70 kDa protein band, which appeared to be in a position equal to the anti-Gly m 7 antibody-reactive band (Figure 2b lanes 1 and 2). This result indicated that the biotin moiety of Gly m 7 could be detected using HRP-labeled streptavidin under these conditions.
3.3. Detection of Gly m 7 in Various Processed Soybean Foods
The resulting antibodies were used to detect and compare the relative levels of Gly m 7 in various processed soybean foods (Figure 3a). The presence of a biotin moiety was also detected using HRP-labeled streptavidin (Figure 3b). Food samples were collected in aliquots, crushed, and subjected to western blotting in the same amount, and Gly m 7 was detected in kinu-tofu, momen-tofu, fried tofu (thick-type), fried tofu (thin-type), and boiled soybeans. The presence of Gly m 7 was particularly prevalent in tofu (lanes 2 and 3) and thick fried tofu (lane 4). In thin fried tofu and boiled soybeans, Gly m 7 levels were lower than in the previous three products, and it was not detected in fermented foods, such as kome-miso, shiro-miso, and natto. In thin fried tofu and boiled soybeans, Gly m 7 may have been degraded by factors, such as heating and pressurization. In miso and natto, both fermented foods, Gly m 7 might be degraded by proteolytic degradation during fermentation. Therefore, the allergen risk associated with this allergen might be low in these processed soybean foods. Detection of the biotin moiety using HRP-labeled streptavidin (Figure 3b) was also similar to the results obtained with anti-peptide antibodies for detecting the peptide moiety (Figure 3a).
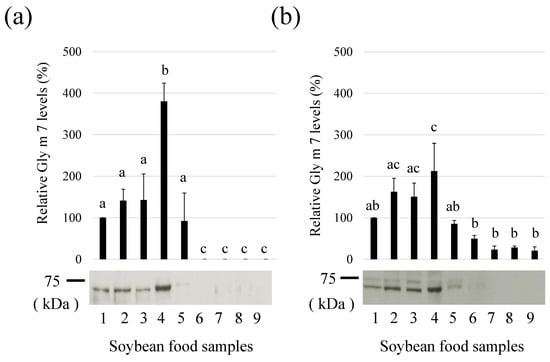
Figure 3.
Relative levels of Gly m 7 allergens in processed soybean food samples. (a) Gly m 7 levels were detected by anti-Gly m 7 antibodies (b) Relative levels of Gly m 7 in various processed soybean food samples using horseradish peroxidase-labeled streptavidins. Processed soybean food samples (10 µg (a) or 100 µg (b) protein/lane) were separated via sodium dodecyl sulphate–polyacrylamide gel electrophoresis, and the Gly m 7 levels were detected. There are significant differences (p < 0.05) between different lowercase letters. (lane 1, standard soymilk; lane 2, kinugoshi-tofu; lane 3, momen-tofu; lane 4, thick fried tofu; lane 5, thin fried tofu; lane 6, boiled soybeans; lane 7, kome-miso; lane 8, shiro-miso; lane 9, natto).
3.4. Detection of Gly m 7 in Various Soymilks
Relative levels of Gly m 7 were compared between commercially available soy milk beverages (Table 1). More Gly m 7 levels were detected in dense soymilk (samples No. 1, 2, 5, 6, 8, and 9; protein concentration > 30 mg/mL) than in other soymilks (samples No. 3, 4, and 7; protein concentration < 30 mg/mL) (Figure 4a(1)). Relative Gly m 7 levels and protein concentrations of these soymilk-related beverages were positively correlated, suggesting that concentrations of Gly m 7 were higher at higher protein concentrations (Figure 4a(2)). Therefore, it can be said that the risk of this allergen is also high in soymilk beverages with high protein concentrations. The detection of the biotin moiety using HRP-labeled streptavidin was also similar to results obtained with the anti-peptide antibodies (Figure 4b(1,2)). Therefore, biotin conjugation may show the presence of Gly m 7 in soymilk beverages.

Table 1.
Protein concentrations of tested soymilks.
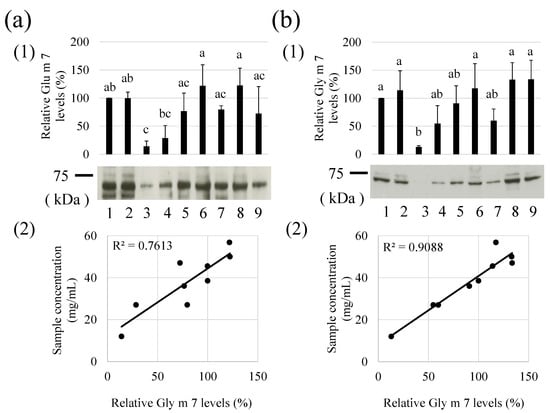
Figure 4.
Relative levels of Gly m 7 allergens in various soymilks. Soymilk samples were subjected to sodium dodecyl sulphate–polyacrylamide gel electrophoresis and western blot analysis using anti-Gly m 7 (a) or western blot-like analysis using horseradish peroxidase -labeled streptavidin (b). Experiments were performed three times, and the data are presented graphically, with bars representing means and error bars representing standard deviation (SD). There are significant differences (p < 0.05) between different lowercase letters. The representative band images are also shown in (1). The relations of sample concentrations and relative Gly m 7 levels are indicated in (2). The relative intensity of Sample 1 was calculated as 100. Sample numbers (1–9) correspond to the sample numbers in Table 1.
3.5. Detection of Gly m 7 in Various Soybean Cultivars
Relative levels of Gly m 7 in various major soybean cultivars were compared (Figure 5). In the detection of peptide moieties, total proteins or Gly m 7 were equally detected in any soybean cultivar (Figure 5a,b)), whereas differences were identified in the detection of Gly m 7 and biotin moieties (Figure 5c). Differences in the ratio of peptide to biotin moieties were also identified in each cultivar (data not shown). It was considered that the biotin bound to this molecule in each cultivar might not necessarily be the same amount.
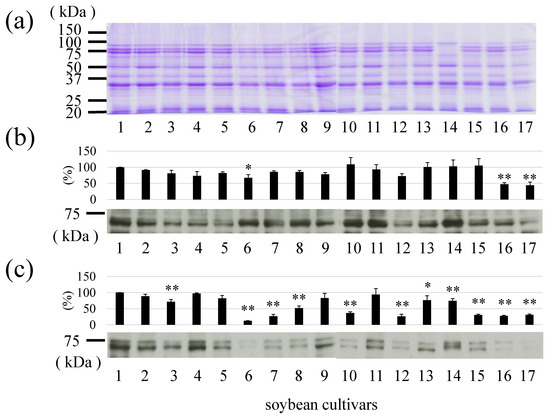
Figure 5.
Relative levels of Gly m 7 allergens in various soybean cultivars (1–17). (a) Coomassie Brilliant Blue staining of various soybeans. (b) Relative Gly m 7 levels detected by anti-Gly m 7 antibody. (c) Relative Gly m 7 levels detected by horseradish peroxidase-labeled streptavidin. *: p < 0.05, **: p < 0.01. (samples; 1, Tachinagaha; 2, Enrei; 3, Fukuyutaka; 4, Ryuuhou; 5, Ootsuru; 6, Yukihomare; 7, Toyokomachi; 8, Toyohomare; 9, Yukisizuka; 10, Oosuzu; 11, Nanbushirome; 12, Miyagishirome; 13, Tanrei; 14, Nagomimaru; 15, Tamahukura; 16, Toyoharuka; 17, Murayutaka).
3.6. Thermostability of Gly m 7
The thermal stability of Gly m 7 was determined by heating soybeans. The purpose of this experiment was to investigate the stability of this allergen when soybeans (extracts) as foods were heated. Soybean extract was prepared as described in Section 2.4. Obtained soybean extract (10 mg/mL in distilled water) was added in micro-tubes and heated at 100 °C using heat block. No obvious precipitates were observed, so the mixed samples were subjected onto SDS-PAGE and western blotting. In detection using anti-peptide antibodies, the detectable intact Gly m 7 around 70 kDa was gradually reduced by heating (100 °C) (Figure 6a,b)). The half-life of the apparent degradation was approximately 45 min. Similar results were obtained for the detection of biotin sites (Figure 6c). Therefore, this molecule showed heat stability during heating, and it was considered that biotin might not be separated by heating. Similar results have been reported in the previous study (18). They showed that Gly m 7 is a boiling-soluble protein since it did not precipitate when the extract was heated for 10 min. In addition, when compared with other major soybean allergens, Gly m 6 showed the highest thermal stability, followed by Gly m 4, Gly m 5, and Gly m 7, which showed similar thermal stability under the present conditions (Figure 6d).
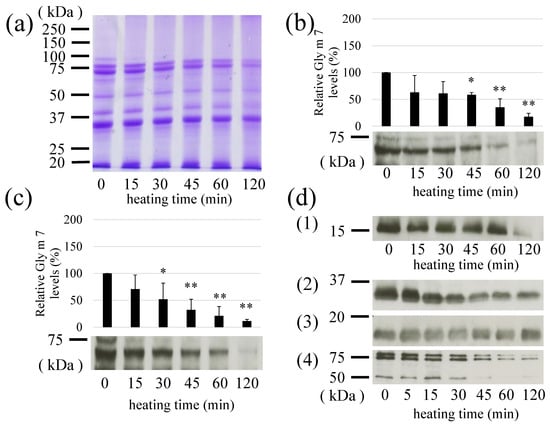
Figure 6.
Heat stability of Gly m 7 and other soybean allergens. Soybean extracts were heated for the indicated time and subsequently subjected to sodium dodecyl sulphate–polyacrylamide gel electrophoresis. (a) Coomassie Brilliant Blue staining of heated soybeans (b) Gly m 7 levels detected using anti-Gly m 7 antibodies (c) Gly m 7 levels detected using horseradish peroxidase-labeled streptavidin. The relative intensity at Time Point 0 was set at 100 %. Signal intensities were measured as described in the Materials and Methods section. Experiments were performed three times, and the data are presented graphically, with bars representing means and error bars representing the SD. *: p < 0.05, **: p < 0.01. (d) Time-dependent changes of other soybean allergens. (1) Gly m 4, (2) Gly m Bd 30K, (3) Gly m 6 (basic subunit), (4) Gly m 5.
3.7. Effect of Worm Wounding on Soybean Gly m 7 Levels
Gly m 7 levels of uninjured and worm-wounded soybeans were compared (Figure 7). In this experiment, the worm was identified to be Etiella zinckenella based on bite appearance (data not shown). The bite periods were not clear because the soybeans were wounded during storage time (half year). It was confirmed that Gly m 7 was not detected either by peptide detection (Figure 7c) or by biotin detection (Figure 7c) in worm-wounded soybeans. From this result, it was considered that the Gly m 7 peptide was degraded, rather than only the biotin being liberated from Gly m 7. In contrast, Gly m 4, a soybean allergen related to pollinosis, was clearly increased by worm wounding (Figure 7c). This result was consistent with that of our previous reports [23]. Total protein patterns of the two samples were quite similar, suggesting that the bulk soybean proteins did not change (Figure 7b).
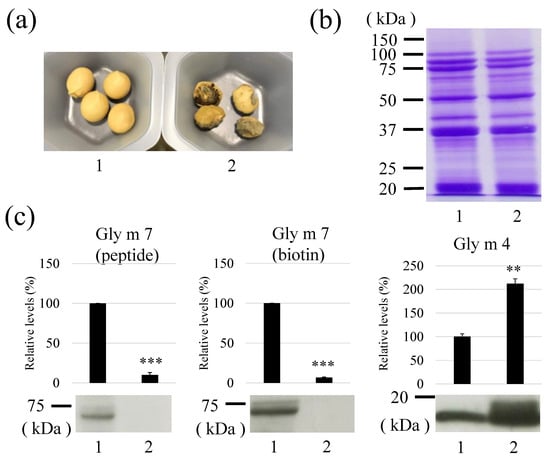
Figure 7.
Effect of worm wounding on the Gly m 7 and Gly m 4 allergen levels. Appearance (a) and protein profiled by Coomassie Brilliant Blue (CBB) staining (b), Gly m 7 and Gly m 4 levels detected by antibody or horseradish peroxidase (HRP)-labeled streptavidin of non-wounded (1) and worm-wounded mature soybeans (2) (c). Total proteins were extracted from mature soybeans (non-wounded and worm-wounded) and subjected to sodium dodecyl sulphate–polyacrylamide gel electrophoresis (60 μg/lane). Total protein was stained with CBB G-350, and Gly m 7 was detected by anti-Gly m 7 or HRP-labeled streptavidin as described in the Materials and Methods. Similarly, Gly m 4 levels were detected by anti-Gly m 4 antibody. Detected band intensities were expressed. Lane 1, non-wounded mature soybeans; Lane 2, worm-wounded mature soybeans. ***: p < 0.0001, **: p < 0.01, student’s t test.
3.8. Effect of Immersion Times of Soybeans on Gly m 7 and Other Allergens
Soybeans were immersed in distilled water as a pretreatment step for germination, and Gly m 7 levels were compared by immersion time (4, 24, 48, 72, and 96 h). In this case, bulk proteins did not change (Figure 8a). It was confirmed that the longer the immersion time, the intact Gly m 7 was degraded more in detecting both peptide and biotin moieties (Figure 8b,c)). This suggested that Gly m 7 was degraded by some protease(s) during soybean germination. Interestingly, Gly m 7 was significantly reduced, but no significant reduction was observed in patterns of major proteins detected by CBB staining (Figure 8a) or in Gly m 4, Gly m 5, or Gly m 6 of other major allergens (Figure 8d). Therefore, this degradation may be a phenomenon specific to this allergen. Degradation intermediates were also detected at positions of approximately 50 kDa, and other MWs, such as that of intact Gly m 7, were degraded (Figure 8b,c).
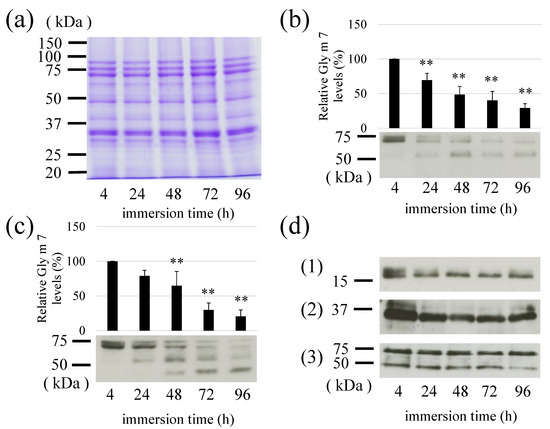
Figure 8.
Effect of soybean immersion time on Gly m 7 and other allergens. Dried mature soybeans were immersed in dH2O for the indicated time (4, 24, 48, 72, and 96 h), and the immersed soybeans were extracted to test proteins or allergen levels. (a) Total protein pattern detected by Coomassie Brilliant Blue staining; (b) Gly m 7 levels detected by raised anti-peptide antibody; (c) Gly m 7 biotin moiety detected by horseradish peroxidase-labeled streptavidin; (d) other allergen levels detected by the specific antibodies. (1) Gly m 4, (2) Gly m Bd30K, and (3) Gly m 5. **: p < 0.01.
4. Discussion
Gly m 7, recently reported as a new soybean allergen, undergoes modification (biotinylation) by biotin in molecules as a characteristic property. Taking advantage of this property, this molecule can be detected using HRP-labeled streptavidin. In this study, we generated anti-peptide antibodies against Gly m 7. The antibodies produced were able to detect putative intact Gly m 7 molecules (approximately 70 kDa) by western blotting. Using these multiple detection tools, we sought to characterize Gly m 7 in various soybeans and processed soybean foods. In some experiments, two close bands were detected at near 70 kDa by both methods. At this time, the substance and reasons for these two protein bands are unknown. However, since both of the two bands are also detected by biotin detection, we believe that they are both Gly m 7-related molecules. The reason of these putative molecular polymorphisms is unknown, but limited degradation and post-translational modifications are presumed. In the densitograph, the lower band of the major darker one is measured as Gly m 7.
When this molecule was detected in various processed soybean foods (Figure 3) and soy milk (Figure 4), food rich in proteins, such as tofu, showed higher levels of this molecule per constant weight. Similarly, the higher the protein concentration, the more abundant the presence of this molecule was for soymilk. The purpose of this experiment is to investigate whether this allergen level changes in various soymilks. Since there are various products with different concentrations of soymilk, in order to evaluate the risk of allergens when taking a sip as a “food”, it was necessary to evaluate with a constant dilution rate without adjusting the protein concentration. In both cases, the pattern of detection of the peptide portion of this molecule using anti-peptide antibodies and the pattern of biotin detection were similar, indicating that biotin might not be released in these processed foods and remained bound. This suggests that methods, such as adsorption of this allergen using avidin, may also be feasible in processed foods. For example, the biotin-bound Gly m 7 molecule may be specifically adsorbed and removed by a passing-through to an avidin column. In case of detection or capture of this allergen using ELISA or avidin column, the free biotin contaminated in food sample could interfere the processes. It is important to consider this possibility when using these procedures. In addition, Gly m 7 was not detected in fermented foods, such as natto and miso, where the protein content was lower than that of tofu, possibly because the protein was degraded by microbial proteases during fermentation. Other major soybean allergen levels have also been reported to significantly reduce in miso [25]. Therefore, it has been suggested that the risk of soy allergens is generally low in fermented foods, such as miso and natto.
When the relative levels of Gly m 7 in various soybean cultivars were compared, it was almost equally detected in any soybean cultivar in terms of peptide moieties, whereas biotin moieties identified differences in Gly m 7 detection (Figure 5). Differences in the ratio of peptide to biotin moieties were also identified for each cultivar. Therefore, it is suggested that the biotin bound to this molecule in each variety might be different. It suggests that different soybean cultivars may have different levels of biotin binding. In other words, it is possible that biotin is not bound to all Gly m 7 molecules, suggesting that the proportion may vary depending on the cultivars. It is unclear whether such differences result from differences in cultivars or from subtle differences in soybean seed maturity. The effect of biotin contained in this molecule on allergenicity is unknown, but if biotin modification affects allergen risk, it might vary between cultivars. In connection with this, we have recently reported that Gly m 7 levels in GM soybeans are comparable to those in non-GM soybeans [26].
As shown in Figure 6, Gly m 7 was heat-stable, and it degraded gradually upon heating for up to approximately 2 h. Since biotin detection results were similar, biotin was not easily separated by heating in this case. Since biotin was bound to this molecule in many processed foods, heat processing does not seem to dislodge biotin. Although there were some differences, the thermal stability of this molecule was similar to that of other major soybean allergens. The aim of this study was to investigate the degree of degradation of various allergen molecules contained in soybean (extract) as a complex system, when heated, rather than to precisely test the thermal stability of isolated allergen proteins. Soybeans also contain high levels of stored protein, fiber, and oil, etc. These ingredients may exhibit matrix effects and affect the stability of allergen molecules. In addition, in terms of food safety assessment, we believe that it is beneficial to evaluate the stability of these allergens under unfractionated and crude conditions.
Previously, we have reported changes in various allergens in soybean and edamame (young soybeans) when they suffered worm wounding. Results have shown that two allergens related to pollinosis (Gly m 3 and Gly m 4) are significantly increased by worm damage (22). In particular, Gly m 4 belongs to the PR (pathogenesis-related)-10 family, and its increased expression in pests and worm wounding is reasonable. It is well-known that pathogen-related (PR) proteins are upregulated when plants are stressed by pathogens and worms. As a result of examining the effect of worm wounding on Gly m 7, it became clear that the protein disappeared (Figure 7). Under the same conditions, it was confirmed that it increased with respect to Gly m 4. Thus, variations in the content of different allergen types at the time of insect or worm damage are of interest in terms of the relationship between soybean cultivation management and allergenicity. However, the physiological significance of Gly m 7 disappearances due to worm damage is unknown.
When dried soybeans were immersed in water, it was hypothesized that Gly m 7 was specifically degraded by some proteases (Figure 8). In this process, degradation intermediates were detected at positions of approximately 50 kDa and other MWs. In this study, the dried soybeans were soaked in water for a long time. As a result, specific degradation of Gly m 7 was observed. Therefore, this process is slightly different than at the germination process. It is unclear whether this degradation is a physiologically meaningful phenomenon that occurs during germination or an artificial phenomenon. However, given that Gly m 7 is a biotin-binding protein, and its suggested role includes providing biotin during germination, it could be a physiological phenomenon. As the other soybean allergens examined in this study were not remarkably degraded by immersion alone, this simple method could potentially be applied as a Gly m 7-specific reduction method. This specific degradation of Gly m 7 during germination may be related to the physiological roles of this molecule in soybean.
In conclusion, in the present study, we conducted a variation analysis of levels of this allergen in foodstuffs and various treatments using a detection system that could detect the peptide and biotin parts of Gly m 7, a recently identified soybean allergen, and clarified some characteristic properties. This study allowed us to update the allergenicity of soybean and soybean foods. In the future, the clinical significance and risk evaluation of this allergen should be advanced. The development of an ELISA method for accurate quantification of Gly m 7 is also expected.
Author Contributions
Conceptualization, T.M.; methodology, T.M. and E.Y.; software, A.F.; validation, A.F. and E.Y.; formal analysis, T.M.; investigation, N.T., A.F. and A.M.; resources, T.M.; data curation, A.F. and N.T.; writing—original draft preparation, N.T.; writing—review and editing, A.F., N.Z. and T.M.; visualization, N.T. and A.F.; supervision, N.Z.; project administration, T.M.; funding acquisition, T.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by JSPS KAKENHI (Grant-in-Aid for Scientific Research (C)) Grant Numbers JP25450187 and JP16K07756 to T.M. This study was also supported in part by a grant from the Agricultural Technology and Innovation Research Institute (ATIRI), Kindai University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
The authors acknowledge Ryouhei Masaki for their experimental support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sirtori, C.R.; Agradi, E.; Conti, F.; Mantero, O.; Gatti, E. Soybean-protein diet in the treatment of type-II hyperlipoproteinaemia. Lancet 1977, 1, 275–277. [Google Scholar] [CrossRef]
- Sugano, M.; Koba, K. Dietary protein and lipid metabolism: A multifunctional effect. Ann. N. Y. Acad. Sci. 1993, 676, 215–222. [Google Scholar] [CrossRef]
- Torres, N.; Torre-Villalvazo, I.; Tovar, A.R. Regulation of lipid metabolism by soy protein and its implication in diseases mediated by lipid disorders. J. Nutr. Biochem. 2006, 17, 365–373. [Google Scholar] [CrossRef]
- Malav, O.P.; Talukder, S.; Gokulakrishnan, P.; Chand, S. Meat Analog: A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1241–1245. [Google Scholar] [CrossRef]
- Singh, M.; Trivedi, N.; Enamala, M.K.; Kuppam, C.; Parikh, P.; Nikolova, M.P.; Chavali, M. Plant-based meat analogue (PBMA) as a sustainable food: A concise review. Eur. Food Res. Technol. 2021, 247, 2499–2526. [Google Scholar] [CrossRef]
- Ogawa, T.; Samoto, M.; Takahashi, K. Soybean allergens and hypoallergenic soybean products. J. Nutr. Sci. Vitaminol. 2000, 46, 271–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moroz, L.A.; Yang, W.H. Kunitz Soybean Trypsin Inhibitor—A Specific Allergen in Food Anaphylaxis. N. Engl. J. Med. 1980, 302, 1126–1128. [Google Scholar] [CrossRef]
- Ogawa, T.; Tsuji, H.; Bando, N.; Kitamura, K.; Zhu, Y.L.; Hirano, H.; Nishikawa, K. Identification of the soybean allergenic protein, Gly m Bd 30 K, with the soybean seed 34-kDa oil-body-associated protein. Biosci. Biotechnol. Biochem. 1993, 57, 1030–1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogawa, T.; Bando, N.; Tsuji, H.; Okajima, H.; Nishikawa, K.; Sasaoka, K. Investigation of the IgE-binding protein in soybeans by immunoblotting with the sera of the soybean-sensitive patient with atopic dermatitis. J. Nutr. Sci. Vitaminol. 1991, 37, 555–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuji, H.; Bando, N.; Hiemori, M.; Yamanishi, R.; Kimoto, M.; Nishikawa, K.; Ogawa, T. Purification and Characterization of Soybean Allergen Gly m Bd 28K. Biosci. Biotechnol. Biochem. 1997, 61, 942–947. [Google Scholar] [CrossRef]
- Ogawa, T.; Bando, N.; Tsuji, H.; Nishikawa, K.; Kitamura, K. α-Subunit of β-Conglycinin, an allergenic protein recognized by IgE Antibodies of soybean-sensitive patients with atopic dermatitis. Biosci. Biotechnol. Biochem. 1995, 59, 831–833. [Google Scholar] [CrossRef] [Green Version]
- Adachi, A.; Horikawa, T.; Shimizu, H.; Sarayama, Y.; Ogawa, T.; Sjolander, S.; Tanaka, A.; Moriyama, T. Soybean beta-conglycinin as the main allergen in a patient with food-dependent exercise-induced anaphylaxis by tofu: Food processing alters pepsin resistance. J. Clin. Exp. Allergy 2009, 39, 167–173. [Google Scholar] [CrossRef]
- Holzhauser, T.; Wackermann, O.; Ballmer-Weber, B.K.; Bindslev-Jensen, C.; Scibilia, J.; Perono-Garoffo, L.; Utsumi, S.; Poulsen, L.K.; Vieths, S. Soybean (Glycine max) allergy in Europe: Gly m 5 (β-conglycinin) and Gly m 6 (glycinin) are potential diagnostic markers for severe allergic reactions to soy. J. Allergy Clin. Immunol. 2009, 123, 452–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maruyama, N.; Sato, S.; Cabanos, C.; Tanaka, A.; Ito, K.; Ebisawa, M. Gly m 5/Gly m 8 fusion component as a potential novel candidate molecule for diagnosing soya bean allergy in Japanese children. Clin. Exp. Allergy 2018, 48, 1726–1734. [Google Scholar] [CrossRef]
- Kleine-Tabbe, J.; Vogel, L.; Crowell, D.N.; Haustein, U.F.; Vieths, S. Severe oral allergy syndrome and anaphylactic reactions caused by a Bet v 1- related PR-10 protein in soybean, SAM22. J. Allergy Clin. Immunol. 2002, 110, 797–804. [Google Scholar] [CrossRef]
- Mittag, D.; Vieths, S.; Vogel, L.; Becker, W.M.; Rihs, H.P.; Helbling, A.; Wüthrich, B.; Ballmer-Weber, B.K. Soybean allergy in patients allergic to birch pollen: Clinical investigation and molecular characterization of allergens. J. Allergy Clin. Immunol. 2004, 113, 148–154. [Google Scholar] [CrossRef]
- Rihs, H.P.; Chen, Z.; Ruëff, F.; Petersen, A.; Rozynek, P.; Heimann, H.; Baur, X. IgE binding of the recombinant allergen soybean profilin (rGly m 3) is mediated by conformational epitopes. J. Allergy Clin. Immunol. 1999, 104, 1293–1301. [Google Scholar] [CrossRef]
- Riascos, J.J.; Weissinger, S.M.; Weissinger, A.K.; Kulis, M.; Burks, A.W.; Pons, L. The Seed Biotinylated Protein of Soybean (Glycine max): A Boiling Resistant New Allergen (Gly m 7) with the Capacity to Induce IgE Mediated Allergic Responses. J. Agric. Food Chem. 2016, 64, 3890–3900. [Google Scholar] [CrossRef]
- Hsing, Y.; Tsou, C.; Hsu, T.; Chen, Z.; Hsieh, K.; Hsieh, J.; Chow, T. Tissue- and stage-specific expression of a soybean (Glycine max L.) seed-maturation, biotinylated protein. Plant Mol. Biol. 1998, 38, 481–490. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Moriyama, T.; Machidori, M.; Ozasa, S.; Maebuchi, M.; Urade, R.; Takahashi, K.; Ogawa, T.; Maruyama, N. A novel enzyme-linked immunosorbent assay for quantification of soybean beta-conglycinin, a major soybean storage protein, in soybean and soybean food products. J. Nutr. Sci. Vitaminol. 2005, 51, 34–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanafusa, K.; Murakami, H.; Ueda, T.; Yano, E.; Zaima, N.; Moriyama, T. Worm wounding increases levels of pollen-related food allergens in soybean (Glycine max). Biosci. Biotechnol. Biochem. 2018, 82, 1207–1215. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Kyhse-Andersen, J. Electroblotting of multiple gels: A simple apparatus without buffer tank for rapid transfer of proteins from polycrylamide to nitrocellulose. J. Biochem. Biophys. Methods 1984, 10, 203–209. [Google Scholar] [CrossRef]
- Moriyama, T.; Yano, E.; Suemori, Y.; Nakano, K.; Zaima, N.; Kawamura, Y. Hypoallergenicity of Various Miso Pastes Manu-factured in Japan. J. Nutr. Sci. Vitaminol. 2013, 59, 462–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuo, A.; Matsushita, K.; Fukuzumi, A.; Tokumasu, N.; Yano, E.; Zaima, N.; Moriyama, T. Comparison of Various Soybean Allergen Levels in Genetically and Non-Genetically Modified Soybeans. Foods 2020, 9, 522. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).