Could Modifying the Skin Microbiome, Diet, and Lifestyle Help with the Adverse Skin Effects after Stopping Long-Term Topical Steroid Use?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Gut Microbiome Intervention
2.2. Skin Microbiome Intervention
- Use the product on the skin at least 1× per day for a minimum of 4 months.
- To use the product, mix with a small amount of water to form a solution, and gently massage onto the body.
- Use no other cosmetics products and try to stick to this as rigidly as possible. Exemptions in extreme cases, or where it was unavoidable were allowed, e.g., wearing some makeup for an important business meeting.
- In the beginning, introduce the product slowly to the body by using it mixed with a small quantity of water once every couple of days, and slowly build up to using it 1× per day by the end of the first month.
2.3. Data Analysis
- y = rreq or p;
- x = degrees of freedom.
3. Results
3.1. Participant Information
3.2. Skin Condition
3.3. Correlation
4. Discussion
4.1. Does Addressing the Microbiome Show Promise for Helping People Suffering with TSW?
4.2. Are the Results Enough to Expand the Study in the Future?
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Gut Microbiome: Sample Collection, DNA Extraction and Analysis
References
- Wallen-Russell, C. The Role of Every-Day Cosmetics in Altering the Skin Microbiome: A Study Using Biodiversity. Cosmetics 2018, 6, 2. [Google Scholar] [CrossRef] [Green Version]
- Kathuria, P.; Silverberg, J.I. Association of pollution and climate with atopic eczema in US children. Pediatr. Allergy Immunol. 2016, 27, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Patruno, C.; Nisticò, S.P.; Fabbrocini, G.; Napolitano, M. COVID-19, quarantine, and atopic dermatitis. Med. Hypotheses 2020, 143, 109852. [Google Scholar] [CrossRef] [PubMed]
- Wallen-Russell, C.; Wallen-Russell, S. A new benchmark to determine what healthy western skin looks like in terms of biodiversity using standardised methodology. Cosmetics 2020, 7, 79. [Google Scholar] [CrossRef]
- Marrs, T.; Flohr, C. The role of skin and gut microbiota in the development of atopic eczema. Br. J. Dermatol. 2016, 175, 13–18. [Google Scholar] [CrossRef]
- Baviera, G.; Leoni, M.C.; Capra, L.; Cipriani, F.; Longo, G.; Maiello, N.; Ricci, G.; Galli, E. Microbiota in healthy skin and in atopic eczema. BioMed Res. Int. 2014, 2014, 436921. [Google Scholar] [CrossRef]
- Wallen-Russell, C.; Wallen-Russell, S. Meta Analysis of Skin Microbiome: New Link between Skin Microbiota Diversity and Skin Health with Proposal to Use This as a Future Mechanism to Determine Whether Cosmetic Products Damage the Skin. Cosmetics 2017, 4, 14. [Google Scholar] [CrossRef] [Green Version]
- Čelakovská, J.; Bukač, J. The severity of atopic dermatitis and analysis of the food hypersensitivity reactions. Food Agric. Immunol. 2015, 26, 896–908. [Google Scholar] [CrossRef]
- Belkaid, Y.; Hand, T.W. Role of the microbiota in immunity and inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef] [Green Version]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef] [PubMed]
- Prescott, S.L.; Larcombe, D.-L.; Logan, A.C.; West, C.; Burks, W.; Caraballo, L.; Levin, M.; Van Etten, E.; Horwitz, P.; Kozyrskyj, A.; et al. The skin microbiome: Impact of modern environments on skin ecology, barrier integrity, and systemic immune programming. World Allergy Organ. J. 2017, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Sanford, J.A.; Gallo, R.L. Functions of the skin microbiota in health and disease. Semin. Immunol. 2013, 25, 370–377. [Google Scholar] [CrossRef] [Green Version]
- Castela, E.; Archier, E.; Devaux, S.; Gallini, A.; Aractingi, S.; Cribier, B.; Jullien, D.; Aubin, F.; Bachelez, H.; Joly, P.; et al. Topical corticosteroids in plaque psoriasis: A systematic review of efficacy and treatment modalities. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 36–46. [Google Scholar] [CrossRef]
- Uva, L.; Miguel, D.; Pinheiro, C.; Antunes, J.; Cruz, D.; Ferreira, J.; Filipe, P. Mechanisms of action of topical corticosteroids in psoriasis. Int. J. Endocrinol. 2012, 2012, 561018. [Google Scholar] [CrossRef] [Green Version]
- Hengge, U.R.; Ruzicka, T.; Schwartz, R.A.; Cork, M.J. Adverse effects of topical glucocorticosteroids. J. Am. Acad. Dermatol. 2006, 54, 1–15. [Google Scholar] [CrossRef]
- Wu, L.T.; Zhu, H.; Ghitza, U.E. Multicomorbidity of chronic diseases and substance use disorders and their association with hospitalization: Results from electronic health records data. Drug Alcohol Depend. 2018, 192, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. The antibiotic resistance crisis: Causes and threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Drake, L.A.; Dinehart, S.M.; Farmer, E.R.; Goltz, R.W.; Graham, G.F.; Hordinsky, M.K.; Lewis, C.W.; Pariser, D.M.; Webster, S.B.; Whitaker, D.C.; et al. Guidelines of care for the use of topical glucocorticosteroids. J. Am. Acad. Dermatol. 1996, 35, 615–619. [Google Scholar]
- Ference, J.D.; Last, A.R. Choosing Topical Corticosteroids. Am. Fam. Physician 2009, 79, 135–140. [Google Scholar]
- Juhasz, M.L.W.; Curley, R.A.; Rasmussen, A.; Malakouti, M.; Silverberg, N.; Jacob, S.E. Systematic Review of the Topical Steroid Addiction and Topical Steroid Withdrawal Phenomenon in Children Diagnosed With Atopic Dermatitis and Treated with Topical Corticosteroids. J. Dermatol. Nurses. Assoc. 2017, 9, 241–242. [Google Scholar] [CrossRef]
- Hajar, T.; Leshem, Y.A.; Hanifin, J.M.; Nedorost, S.T.; Lio, P.A.; Paller, A.S.; Block, J.; Simpson, E.L. A systematic review of topical corticosteroid withdrawal (“steroid addiction”) in patients with atopic dermatitis and other dermatoses. J. Am. Acad. Dermatol. 2015, 72, 541–549.e2. [Google Scholar] [CrossRef] [PubMed]
- Coondoo, A.; Phiske, M.; Verma, S.; Lahiri, K. Side-effects of topical steroids: A long overdue revisit. Indian Dermatol. Online J. 2014, 5, 416. [Google Scholar] [CrossRef]
- Sheary, B. Steroid Withdrawal Effects Following Long-term Topical Corticosteroid Use. Dermatitis 2018, 29, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Sengupta, S.; Coondoo, A.; Jana, A.K. Topical corticosteroid addiction and phobia. Indian J. Dermatol. 2014, 59, 465–468. [Google Scholar] [CrossRef]
- Rathi, S.; D’Souza, P. Rational and ethical use of topical corticosteroids based on safety and efficacy. Indian J. Dermatol. 2012, 57, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Sheary, B. Topical steroid withdrawal: A case series of 10 children. Acta Derm. Venereol. 2019, 99, 551–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukaya, M.; Sato, K.; Sato, M.; Kimata, H.; Fujisawa, S.; Dozono, H.; Yoshizawa, J.; Minaguchi, S. Topical steroid addiction in atopic dermatitis. Drug. Healthc. Patient Saf. 2014, 6, 131–138. [Google Scholar] [CrossRef] [Green Version]
- Fasano, A. Zonulin, regulation of tight junctions, and autoimmune diseases. Ann. N. Y. Acad. Sci. 2012, 1258, 25. [Google Scholar] [CrossRef] [Green Version]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. BMJ 2018, 361, 36–44. [Google Scholar] [CrossRef] [Green Version]
- Finlay, B.B.; Arrieta, M.-C. Let Them Eat Dirt. Saving Your Child from an Oversanitized World; Windmill Books: London, UK, 2016. [Google Scholar]
- Lefcheck, J.S.; Byrnes, J.E.K.; Isbell, F.; Gamfeldt, L.; Griffin, J.N.; Eisenhauer, N.; Hensel, M.J.S.; Hector, A.; Cardinale, B.J.; Duffy, J.E. Biodiversity enhances ecosystem multifunctionality across trophic levels and habitats. Nat. Commun. 2015, 6, 6936. [Google Scholar] [CrossRef]
- Balvanera, P.; Pfisterer, A.B.; Buchmann, N.; He, J.-S.; Nakashizuka, T.; Raffaelli, D.; Schmid, B. Quantifying the evidence for biodiversity effects on ecosystem functioning and services. Ecol. Lett. 2006, 9, 1146–1156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gamfeldt, L.; Hillebrand, H.; Jonsson, P.R. Multiple functions increase the importance of biodiversity for overall ecosystem functioning. Ecology 2008, 89, 1223–1231. [Google Scholar] [CrossRef]
- Thrupp, L.A. The importance of biodiversity in agroecosystems. J. Crop Improv. 2004, 12, 315–337. [Google Scholar] [CrossRef]
- Hooper, D.U.; Chapin, F.S.; Ewel, J.J.; Hector, A.; Inchausti, P.; Lavorel, S.; Lawton, J.H.; Lodge, D.M.; Loreau, M.; Naeem, S.; et al. Effects of biodiversity on ecosystem functioning: A consensus of current knowledge. Ecol. Monogr. 2005, 75, 3–35. [Google Scholar] [CrossRef]
- Wallen-Russell, C. Is There a Relationship between Transepidermal Water Loss and Microbial Biodiversity on the Skin? Cosmetics 2019, 6, 18. [Google Scholar] [CrossRef] [Green Version]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef] [Green Version]
- Wallen-Russell, C.; Wallen-Russell, S. Topical Probiotics Do Not Satisfy New Criteria for Effective Use Due to Insufficient Skin Microbiome Knowledge. Cosmetics 2021, 8, 90. [Google Scholar] [CrossRef]
- Mukaka, M.M. Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Med. J. 2012, 24, 69–71. [Google Scholar]
- Lee Rodgers, J.; Nicewander, W.A. Thirteen Ways to Look at the Correlation Coefficient. Am. Stat. 1988, 42, 59–66. [Google Scholar] [CrossRef]
- Sokal, R.R.; Rohlf, F.J. Biometry: The Principles and Practice of Statistics in Biological Research; W.H. Freeman and Co.: New York, NY, USA, 1995. [Google Scholar]
- Weatherhead, S.; Robson, S.C.; Reynolds, N.J. Eczema in pregnancy. Br. Med. J. 2007, 335, 152–154. [Google Scholar] [CrossRef] [PubMed]
- Sävervall, C.; Sand, F.L.; Thomsen, S.F. Dermatological diseases associated with pregnancy: Pemphigoid gestationis, polymorphic eruption of pregnancy, intrahepatic cholestasis of pregnancy, and atopic eruption of pregnancy. Dermatol. Res. Pract. 2015, 2015, 979635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, E.J.; Lee, J.A.; Park, J.J.; Kim, H.J.; Kim, N.S.; Byun, K.S.; Choi, G.S.; Moon, T.K. A study on seasonal variation of skin parameters in Korean males. Int. J. Cosmet. Sci. 2015, 37, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Long, X.; Ye, J.C.; Hou, J.; Senee, J.; Laurent, A.; Bazin, R.; Flament, F.; Adam, A.; Coutet, J.; et al. Influence of season on some skin properties: Winter vs. summer, as experienced by 354 Shanghaiese women of various ages. Int. J. Cosmet. Sci. 2011, 33, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Solomon, I.; Ilie, M.A.; Draghici, C.; Voiculescu, V.M.; Căruntu, C.; Boda, D.; Zurac, S. The impact of lifestyle factors on evolution of atopic dermatitis: An alternative approach (review). Exp. Ther. Med. 2019, 17, 1078–1084. [Google Scholar] [CrossRef] [Green Version]
- Fleischer, A.B. Atopic dermatitis: The relationship to temperature and seasonality in the United States. Int. J. Dermatol. 2019, 58, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Sheary, B.; Harris, M.F. Cessation of long-term topical steroids in adult atopic dermatitis: A prospective cohort study. Dermatitis 2020, 31, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Salem, I.; Ramser, A.; Isham, N.; Ghannoum, M.A. The gut microbiome as a major regulator of the gut-skin axis. Front. Microbiol. 2018, 9, 1459. [Google Scholar] [CrossRef] [Green Version]
- Jung, G.W.; Tse, J.E.; Guiha, I.; Rao, J. Prospective, randomized, open-label trial comparing the safety, efficacy, and tolerability of an acne treatment regimen with and without a probiotic supplement and minocycline in subjects with mild to moderate acne. J. Cutan. Med. Surg. 2013, 17, 114–122. [Google Scholar] [CrossRef]
- Fabbrocini, G.; Bertona, M.; Picazo, O.; Pareja-Galeano, H.; Monfrecola, G.; Emanuele, E. Supplementation with Lactobacillus rhamnosus SP1 normalises skin expression of genes implicated in insulin signalling and improves adult acne. Benef. Microbes 2016, 7, 625–630. [Google Scholar] [CrossRef] [Green Version]
- Nosrati, A.; Afifi, L.; Danesh, M.J.; Lee, K.; Yan, D.; Beroukhim, K.; Ahn, R.; Liao, W. Dietary modifications in atopic dermatitis: Patient-reported outcomes. J. Dermatolog. Treat. 2017, 28, 523–538. [Google Scholar] [CrossRef]
- Katta, R.; Schlichte, M. Diet and dermatitis: Food triggers. J. Clin. Aesthet. Dermatol. 2014, 7, 30–36. [Google Scholar] [PubMed]
- Manasson, J.; Reddy, S.M.; Neimann, A.L.; Segal, L.N.; Scher, J.U. Cutaneous Microbiota Features Distinguish Psoriasis from Psoriatic Arthritis. Arthritis Rheumatol. 2016, 68. Available online: https://acrabstracts.org/abstract/cutaneous-microbiota-features-distinguish-psoriasis-from-psoriatic-arthritis/ (accessed on 18 July 2018).
- Nakatsuji, T.; Chen, T.H.; Butcher, A.M.; Trzoss, L.L.; Nam, S.-J.; Shirakawa, K.T.; Zhou, W.; Oh, J.; Otto, M.; Fenical, W.; et al. A commensal strain of Staphylococcus epidermidis protects against skin neoplasia. Sci. Adv. 2018, 4, eaao4502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaidi, A.K.; Spaunhurst, K.; Sprockett, D.; Thomason, Y.; Mann, M.W.; Fu, P.; Ammons, C.; Gerstenblith, M.; Tuttle, M.S.; Popkin, D.L. Characterization of the facial microbiome in twins discordant for rosacea. Exp. Dermatol. 2018, 27, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Velegraki, A.; Cafarchia, C.; Gaitanis, G.; Iatta, R.; Boekhout, T. Malassezia Infections in Humans and Animals: Pathophysiology, Detection, and Treatment. PLoS Pathog. 2015, 11, e1004523. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.J.; Marsland, B.J.; Bunyavanich, S.; O’Mahony, L.; Leung, D.Y.M.; Muraro, A.; Fleisher, T.A. The microbiome in allergic disease: Current understanding and future opportunities-2017 PRACTALL document of the American Academy of Allergy, Asthma & Immunology and the European Academy of Allergy and Clinical Immunology. J. Allergy Clin. Immunol. 2017, 139, 1099–1110. [Google Scholar]
- Sherwani, M.A.; Tufail, S.; Muzaffar, A.F.; Yusuf, N. The skin microbiome and immune system: Potential target for chemoprevention? Photodermatol. Photoimmunol. Photomed. 2018, 34, 25–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Findley, K.; Oh, J.; Yang, J.; Conlan, S.; Deming, C.; Meyer, J.A.; Schoenfeld, D.; Nomicos, E.; Park, M.; Kong, H.H.; et al. Topographic diversity of fungal and bacterial communities in human skin. Nature 2013, 498, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Misic, A.M.; Gardner, S.E.; Grice, E.A. The Wound Microbiome: Modern Approaches to Examining the Role of Microorganisms in Impaired Chronic Wound Healing. Adv. Wound Care 2014, 3, 502–510. [Google Scholar] [CrossRef] [Green Version]
- Gardiner, M.; Vicaretti, M.; Sparks, J.; Bansal, S.; Bush, S.; Liu, M.; Darling, A.; Harry, E.; Burke, C.M. A longitudinal study of the diabetic skin and wound microbiome. PeerJ 2017, 5, e3543. [Google Scholar] [CrossRef]
- Salgado, V.R.; de Queiroz, A.T.L.; Sanabani, S.S.; de Oliveira, C.I.; Carvalho, E.M.; Costa, J.M.L.; Barral-Netto, M.; Barral, A. The microbiological signature of human cutaneous leishmaniasis lesions exhibits restricted bacterial diversity compared to healthy skin. Mem. Inst. Oswaldo Cruz 2016, 111, 241–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, L.J.; Liu, J. Human Microbiota and Ophthalmic Disease. Yale J. Biol. Med. 2016, 89, 325–330. [Google Scholar]
- Kong, H.H.; Oh, J.; Deming, C.; Conlan, S.; Grice, E.A.; Beatson, M.A.; Nomicos, E.; Polley, E.C.; Komarow, H.D.; NISC Comparative Sequence Program; et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res. 2012, 22, 850–859. [Google Scholar] [CrossRef] [Green Version]
- Rocha, L.A.; Ferreira de Almeida e Borges, L.; Gontijo Filho, P.P. Changes in hands microbiota associated with skin damage because of hand hygiene procedures on the health care workers. Am. J. Infect. Control 2009, 37, 155–159. [Google Scholar] [CrossRef]
- Blaser, M.J.; Falkow, S. What are the consequences of the disappearing human microbiota? Nat. Rev. Microbiol. 2009, 7, 887–894. [Google Scholar] [CrossRef]
- Holland, K.T.; Bojar, R.A. Cosmetics: What is their influence on the skin microflora? Am. J. Clin. Dermatol. 2002, 3, 445–449. [Google Scholar] [CrossRef]
- Costello, E.K.; Lauber, C.L.; Hamady, M.; Fierer, N.; Gordon, J.I.; Knight, R. Bacterial Community Variation in Human Body Habitats Across Space and Time. Science 2009, 326, 1694–1697. [Google Scholar] [CrossRef] [Green Version]
- Cogen, A.L.; Nizet, V.; Gallo, R.L. Skin microbiota: A source of disease or defence? Br. J. Dermatol. 2009, 158, 442–455. [Google Scholar] [CrossRef] [Green Version]
- Belkaid, Y.; Segre, J.A. Dialogue between skin microbiota and immunity. Science 2014, 346, 954–959. [Google Scholar] [CrossRef] [PubMed]
- Stingley, R.L.; Zou, W.; Heinze, T.M.; Chen, H.; Cerniglia, C.E. Metabolism of azo dyes by human skin microbiota. J. Med. Microbiol. 2010, 59, 108–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The Human Microbiome Project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef]
- Staudinger, T.; Pipal, A.; Redl, B. Molecular analysis of the prevalent microbiota of human male and female forehead skin compared to forearm skin and the influence of make-up. J. Appl. Microbiol. 2011, 110, 1381–1389. [Google Scholar] [CrossRef]
- Taylor, B.; Wadsworth, M.; Wadsworth, J.; Peckham, C. Changes in the reported prevalence of childhood eczema since the 1939–45 war. Lancet 1984, 324, 1255–1257. [Google Scholar] [CrossRef]
- Simpson, C.R.; Newton, J.; Hippisley-Cox, J.; Sheikh, A. Trends in the epidemiology and prescribing of medication for eczema in England. J. R. Soc. Med. 2009, 102, 108–117. [Google Scholar] [CrossRef] [Green Version]
- Burd, R.M. Psoriasis: A general overview. Br. J. Hosp. Med. 2006, 67, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Prescott, S.L.; Tang, M.L.K. The Australasian Society of Clinical Immunology and Allergy position statement: Summary of allergy prevention in children. Med. J. Aust. 2005, 182, 464–467. [Google Scholar] [CrossRef]
- Srinivas, G.; Möller, S.; Wang, J.; Künzel, S.; Zillikens, D.; Baines, J.F.; Ibrahim, S.M. Genome-wide mapping of gene–microbiota interactions in susceptibility to autoimmune skin blistering. Nat. Commun. 2013, 4, 2462. [Google Scholar] [CrossRef] [Green Version]
- Asher, M.I.; Montefort, S.; Björkstén, B.; Lai, C.K.; Strachan, D.P.; Weiland, S.K.; Williams, H.; ISAAC Phase Three Study Group. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet 2006, 368, 733–743. [Google Scholar] [CrossRef]
- Shaw, T.E.; Currie, G.P.; Koudelka, C.W.; Simpson, E.L. Eczema Prevalence in the United States: Data from the 2003 National Survey of Children’s Health. J. Investig. Dermatol. 2011, 131, 67–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goossens, A. Contact-allergic reactions to cosmetics. J. Allergy 2011, 2011, 467071. [Google Scholar] [CrossRef] [Green Version]
- Salverda, J.G.W.; Bragt, P.J.C.; de Wit-Bos, L.; Rustemeyer, T.; Coenraads, P.J.; Tupker, R.A.; Kunkeler, L.C.M.; Laheij-de Boer, A.-M.; Stenveld, H.J.; van Ginkel, C.J.W.; et al. Results of a cosmetovigilance survey in The Netherlands. Contact Dermat. 2013, 68, 139–148. [Google Scholar] [CrossRef] [Green Version]
- Heisterberg, M.V.; Menné, T.; Johansen, J.D. Contact allergy to the 26 specific fragrance ingredients to be declared on cosmetic products in accordance with the EU cosmetics directive. Contact Dermat. 2011, 65, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Warshaw, E.M.; Buchholz, H.J.; Belsito, D.V.; Maibach, H.I.; Fowler, J.F.; Rietschel, R.L.; Zug, K.A.; Mathias, C.G.T.; Pratt, M.D.; Sasseville, D.; et al. Allergic patch test reactions associated with cosmetics: Retrospective analysis of cross-sectional data from the North American Contact Dermatitis Group, 2001–2004. J. Am. Acad. Dermatol. 2009, 60, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Berne, B.; Tammela, M.; Färm, G.; Inerot, A.; Lindberg, M. Can the reporting of adverse skin reactions to cosmetics be improved? A prospective clinical study using a structured protocol. Contact Dermat. 2008, 58, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Berne, B.; Boström, A.; Grahnén, A.F.; Tammela, M. Adverse effects of cosmetics and toiletries reported to the Swedish Medical Products Agency 1989–1994. Contact Dermat. 1996, 34, 359–362. [Google Scholar] [CrossRef]
- Caruso, J.C.; Cliff, N. Empirical Size, Coverage, and Power of Confidence Intervals for Spearman’s Rho. Educ. Psychol. Meas. 1997, 57, 637–654. [Google Scholar] [CrossRef]
- Kim, H.-Y. Statistical notes for clinical researchers: Assessing normal distribution (2) using skewness and kurtosis. Restor. Dent. Endod. 2013, 38, 52–54. [Google Scholar] [CrossRef]
- Mohd Razali, N.; Bee Wah, Y. Power comparisons of Shapiro-Wilk, Kolmogorov-Smirnov, Lilliefors and Anderson-Darling tests. J. Stat. Modeling Anal. 2011, 2, 21–23. [Google Scholar]
- Shapiro, S.S.; Wilk, M.B. An Analysis of Variance Test for Normality (Complete Samples). Biometrika 1965, 52, 591. [Google Scholar] [CrossRef]
- Brandwein, M.; Katz, I.; Katz, A.; Kohen, R. Beyond the gut: Skin microbiome compositional changes are associated with BMI. Hum. Microbiome J. 2019, 13, 100063. [Google Scholar] [CrossRef]
- Ellis, S.R.; Nguyen, M.; Vaughn, A.R.; Notay, M.; Burney, W.A.; Sandhu, S.; Sivamani, R.K. The skin and gut microbiome and its role in common dermatologic conditions. Microorganisms 2019, 7, 550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Pessemier, B.; Grine, L.; Debaere, M.; Maes, A.; Paetzold, B.; Callewaert, C. Gut–skin axis: Current knowledge of the interrelationship between microbial dysbiosis and skin conditions. Microorganisms 2021, 9, 353. [Google Scholar] [CrossRef] [PubMed]
- Lack, G.; Fox, D.; Northstone, K.; Golding, J. Factors Associated with the Development of Peanut Allergy in Childhood. N. Engl. J. Med. 2003, 348, 977–985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- BIOVIS. Intestinal Flora and Stool Diagnostics. Available online: https://www.biovis-diagnostik.eu/wp-content/uploads/Intestinal-Flora-EN.pdf (accessed on 2 September 2021).
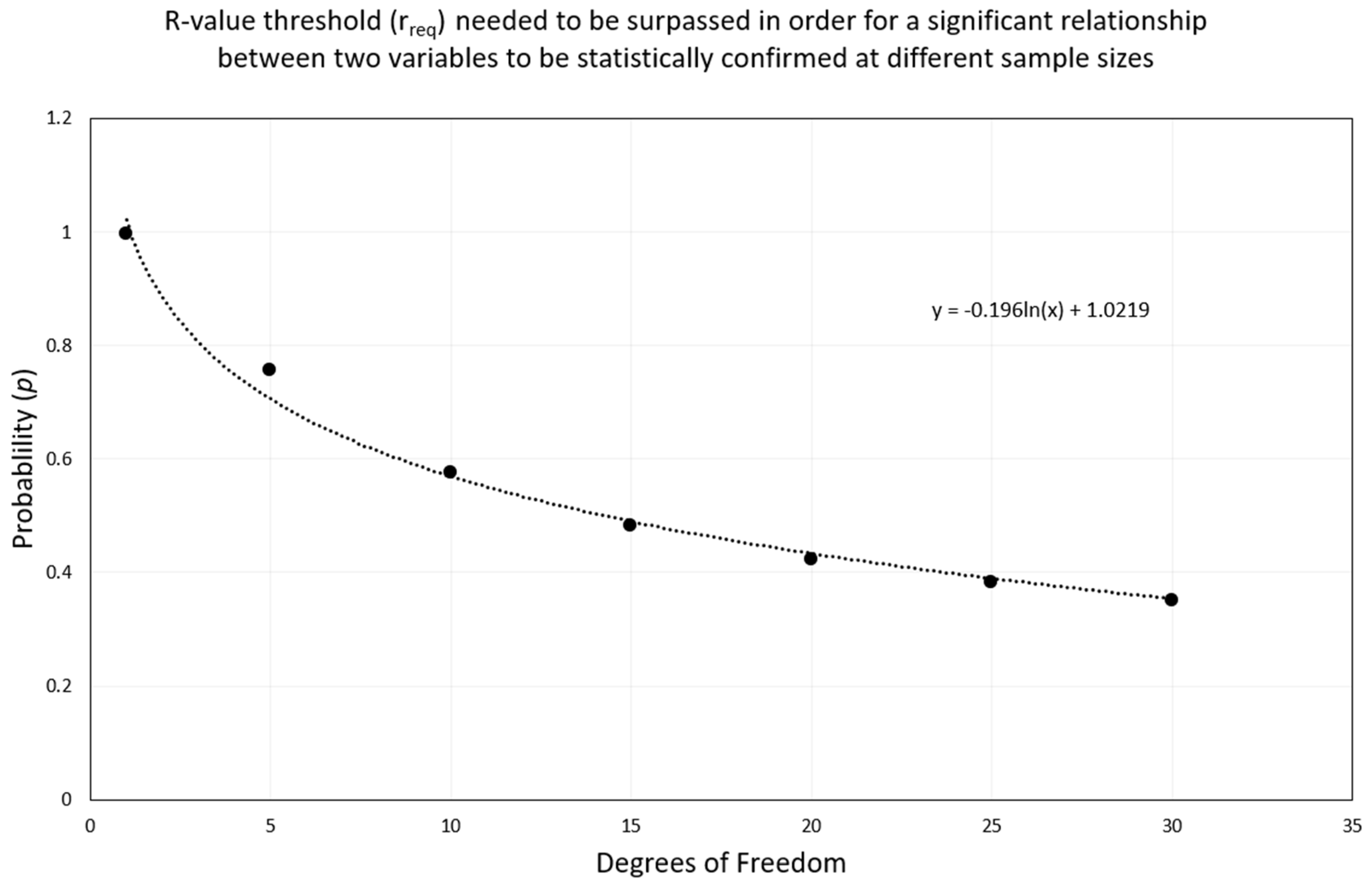



| Degrees of Freedom | Probability, p | |
|---|---|---|
| 0.05 | 0.01 | |
| 1 | 0.997 | 1 |
| 5 | 0.755 | 0.875 |
| 10 | 0.576 | 0.708 |
| 15 | 0.482 | 0.606 |
| 20 | 0.423 | 0.457 |
| 25 | 0.381 | 0.487 |
| 30 | 0.349 | 0.449 |
| 90 | 0.205 | 0.267 |
| 100 | 0.195 | 0.254 |
| Patient Number | Sex | Time Topical Steroids Taken For (Yrs) | Time TSW (Years) | Initial Skin Problem(s) | Other Drugs | Other Health Issues | Completed Study? |
|---|---|---|---|---|---|---|---|
| 1 | F | 48 | 4.5 | Eczema as a baby | - | - | Yes |
| 2 | F | 35 | 4.5 | Eczema when she was born | Antibiotics, Antifungal Creams | Pregnant, food, dust mites, and pollen allergies | Yes |
| 3 | F | 20 | 0.019 | Eczema 20+ years | - | Food allergies | Yes |
| 4 | F | 15 | 1.8 | Eczema as a baby | - | Food allergies | Yes |
| 5 | F | 20 | 4.5 | Eczema for 18+ years | Ventolin/Salbutamol | Asthma | Yes |
| 6 | F | - | - | Eczema | Unknown | No | |
| 7 | M | - | - | Eczema | - | No |
| Patient 1 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Deep Burning/Itching | Split Skin | Flares | Flaking Skin | Itch Attacks | Sleep Disruption | Skin Dryness | Red and Inflamed Skin | Elephant Skin | Average | |
| Before | 7 | 2 | 2 | 7 | 8 | 8 | 7 | 3 | 5 | |
| After | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | |
| % Change | 100% | 100% | 100% | 100% | 100% | 100% | 86% | 67% | 80% | 92% |
| Patient 2 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Deep Burning | Deep Itching | Red and Inflamed Skin | Split Skin | Oozing | Flaking Skin | Itch Attacks | Skin Dryness | Soreness Applying | Average | |
| Before | 6 | 6 | 0 | 7 | 7 | 7 | 7 | 9 | 7 | |
| After | 0 | 3 | 3 | 3 | 4 | 6 | 2 | 6 | 2 | |
| % Change | 100% | 50% | 30% | 57% | 43% | 14% | 71% | 33% | 71% | 52% |
| Patient 3 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Deep Burning/Itching | Red and Inflamed Skin | Split Skin | Oozing | Flaking Skin | Thinned Skin | Day Pain | Night Pain | |
| Before | 9 | 5 | 7 | 9 | 5 | 9 | 6 | 9 |
| After | 6 | 7 | 3 | 7 | 3 | 9 | 5 | 7 |
| % Change | 33% | −40% | 57% | 22% | 40% | 0% | 17% | 22% |
| Patient 4 | |||
|---|---|---|---|
| Deep Burning/Itching | Flaking Skin | Itch Attacks | |
| Before | 7 | 7 | 7 |
| After | 6 | 6 | 6 |
| % Change | 14% | 14% | 14% |
| Patient 5 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Deep Burning/Itching | Red and Inflamed Skin | Split Skin | Oozing | Flaking Skin | Itch Attacks | Secondary Infections | Metallic Smelling Ooze | Elephant Skin | Skin Dryness | Avg | |
| Before | 10 | 6 | 7 | 7 | 10 | 10 | 7 | 6 | 8 | 9 | - |
| After | 8 | 6 | 7 | 5 | 8 | 8 | 0 | 6 | 8 | 6 | - |
| % Change | 20% | 0% | 0% | 29% | 20% | 20% | 100% | 0% | 0% | 33% | 22% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wallen-Russell, C.; Gijsberts-Veens, A.; Wallen-Russell, S. Could Modifying the Skin Microbiome, Diet, and Lifestyle Help with the Adverse Skin Effects after Stopping Long-Term Topical Steroid Use? Allergies 2022, 2, 1-15. https://doi.org/10.3390/allergies2010001
Wallen-Russell C, Gijsberts-Veens A, Wallen-Russell S. Could Modifying the Skin Microbiome, Diet, and Lifestyle Help with the Adverse Skin Effects after Stopping Long-Term Topical Steroid Use? Allergies. 2022; 2(1):1-15. https://doi.org/10.3390/allergies2010001
Chicago/Turabian StyleWallen-Russell, Christopher, Anja Gijsberts-Veens, and Samuel Wallen-Russell. 2022. "Could Modifying the Skin Microbiome, Diet, and Lifestyle Help with the Adverse Skin Effects after Stopping Long-Term Topical Steroid Use?" Allergies 2, no. 1: 1-15. https://doi.org/10.3390/allergies2010001
APA StyleWallen-Russell, C., Gijsberts-Veens, A., & Wallen-Russell, S. (2022). Could Modifying the Skin Microbiome, Diet, and Lifestyle Help with the Adverse Skin Effects after Stopping Long-Term Topical Steroid Use? Allergies, 2(1), 1-15. https://doi.org/10.3390/allergies2010001







