Abstract
Many ingredients found within nail cosmetic products are capable of sensitizing patients’ immune systems and causing contact dermatitis (CD). These include but are not limited to tosylamide, (meth)acrylates, and formaldehyde. A clear temporal relationship between nail cosmetic procedures and an eczematous outbreak on the hands, face, or other ectopic body regions can be a key indicator of CD secondary to nail cosmetic exposure. Once an inciting allergen is identified through patch testing, elimination and avoidance becomes a mainstay of treatment alongside the use of emollients and topical anti-inflammatory therapies. Patients should be counselled to approach future nail cosmetic products and procedures with caution and careful attention to ingredients, regardless of whether or not it has a “hypoallergenic” label.
1. Introduction
Nail cosmetics have been a mainstay of grooming and beauty since the 17th century, with the earliest findings of nail coloring and enhancements dating back to ancient civilizations in Egypt and China [1]. Today, millions of people worldwide utilize nail cosmetic products in order to achieve aesthetically pleasing nails, with the nail cosmetic industry growing from a USD 3 billion industry in 2007 to a USD 45 billion industry by 2012 [2]. In the USA alone, spending on nail salon services exceeds USD 8.5 billion yearly [3]. While many of the products used to achieve this look have been shown to be relatively safe, there are several complications associated with their use. These complications include mechanical and traumatic complications, infection, exposure to carcinogenic ultraviolet (UV) radiation (used to cure and set gel nail polish), and contact dermatitis (CD) [4]. The latter complication will be the focus of this narrative review article.
CD, which can be further divided into allergic (ACD; 20% of cases) and irritant CD (ICD; 80% of cases), is an immune reaction that occurs in response to external compounds. This causative relationship between nail cosmetic products and CD has been well documented for over 50 years [5,6,7]. Specifically, a 1987 study showed that 4.2% of CD cases was due to the use of a cosmetic product, with approximately 8% of those cases attributed to nail cosmetics [6]. Since then, the number of patients diagnosed with CD secondary to use of a nail cosmetic product has grown along with the nail cosmetics industry, likely due to a combination of increased use and sensitization to the included ingredients [8,9,10,11]. In fact, the prevalence of contact allergies to one or more (meth)acrylates was shown to have significantly increased by more than 20% between 2009–2013 and 2014–2019 among nail technicians and product users [12]. A retrospective analysis of 38,775 patients who had previously undergone patch testing found that 769 (2%) had an allergic or irritant patch test reaction to a common ingredient in nail cosmetics [9]. This study was consistent with prior estimates that 1–3% of the population is sensitized to at least one ingredient found in nail cosmetics [5]. CD secondary to nail cosmetics is most prevalent among women aged 26–46 and nail technicians within their first year of work; however, it can develop within any demographic if nail products are used.
In order to consolidate the current body of literature on this growing problem and provide suggestions for improved diagnosis, management, and education, PubMed searches were conducted using the key phrases “nail contact dermatitis”, “nail contact allergy”, “nail cosmetics allergy”, and “acrylate allergy”. In the current narrative review article, we will discuss the most common causative ingredients, clinical presentation, evaluation, and management of contact dermatitis secondary to nail cosmetics.
2. Common Nail Cosmetic Products and Associated Allergens
According to modern beauty standards, aesthetically pleasing nails have a glossy, smooth surface, no overhanging or ragged cuticles, a tip extending beyond the nail plate, an oval contour to the nail plate, and a gentle curve when visualized from the side [1]. A variety of products can be used to achieve these looks, including liquid nail polish, nail wraps, gel nail polish, dipping powders, and acrylics (often referred to as “tips”) [4]. Other nail care products include nail strengthener, cuticle softener, and nail hydrating polishes, oils, and serums. More detailed descriptions of these products can be found in Table 1. Regular manicures using nail polish and gel manicures are the two most popular nail cosmetic procedures in the USA [3].

Table 1.
Description of common nail cosmetic products.
While the products discussed above are certainly useful in enhancing nail appearance, various ingredients within these products can produce both ACD and ICD. Table 2 displays the most common allergens and irritants in nail cosmetic products and which products/procedures they are found in. While the list of these ingredients is extensive, a recent retrospective analysis of patch testing results performed in those diagnosed with CD secondary to nail cosmetic ingredients most frequently revealed positive reactions in response to hydroxyethyl methacrylate (56.6%), tosylamide (36.2%), methylmethacrylate (27.8%), ethyl acrylate (25.2%), and ethyl-2-cyanoacrylate (6.9%) [9]. We discuss these and other common ingredients below:

Table 2.
Common allergens and irritants in nail cosmetic procedures.
- Tosylamide/formaldehyde resin (TSFR) has long been known to be the most common cause of ACD related to nail polish, with data from the North American Contact Dermatitis Group (NACDG) suggesting that 4% of all positive patch tests involved sensitivity to TSFR [13]. It has been found to sensitize both nail components as well as produce ectopic ACD in areas of touching, scratching, or rubbing [14]. Because of this, many nail polish brands have switched formulation to include tosylamide epoxy resin instead; however, this has also been shown to sensitize both locally and ectopically [15].
- Methacrylates (powder and liquid) are mixed to form a flexible polymer for acrylic manicures. These ingredients can also be found in smaller quantities in both regular and gel nail polish. The three most common allergenic forms of these ingredients are 2-hydroxyethyl methacrylate (2-HEMA), 2-hydroxypropyl methacrylate (2-HPMA), and ethylene glycol dimethacrylate (EGDMA) [16,17]. These ingredients are most sensitizing in their liquid form (during application) and rarely cause reactions once hardened and cured. In addition, methacrylates are extremely cross-reactive with one another, so people are often allergic to multiple [15].
- Acrylates are mainly used in gel manicure systems. These include 2-hydroxyethyl acrylate (2-HEA), 2-ethylhexyl acrylate (2-EHA), 2-hydroxypropyl acrylate (2-HPA), ethyl acrylate (EA), and triethylene glycol diacrylate (TREGDA) [18]. With the increasing popularity of and consumer access to “at-home” gel manicure kits, sensitization to acrylates has increased, and those that use such kits are also more likely to be sensitized [19,20].
- Dibutyl phthalate (DBP) is considered a “plasticizer” that increases nail polish flexibility [21]. It has not only been seen to cause ACD, but it has also been shown to interfere with male reproductive development in animal models [22].
- Benzophenone is an additive to regular and gel nail polishes that protects cosmetic products by absorbing UV light and preventing product degradation prior to use. Cases report both ACD and photocontact dermatitis as a result of contact with benzophenone [23].
- Formaldehyde (also listed as formalin or methylene glycol) is the main ingredient in nail strengthening products and has been shown to cause ACD [14].
- Solvents such as ethyl acetate and isopropyl alcohol have been shown to cause ICD and ACD, albeit rarely. However, more recent studies have led many to believe that reactions to isopropyl alcohol are more common than once thought [24]. These ingredients can be found in both nail polishes as well as nail dehydrators.
- Ethyl Cyanoacrylate (ECA) is an ingredient in nail glue that has been associated with ACD, paronychia, and nail dystrophy [25,26]. While similar in name to (meth)acrylates (see below), evidence shows that there is no cross-sensitization between the two ingredients.
- Methacrylic Acid (MAA) is an ingredient in acidic nail primer that is known to be an extremely corrosive chemical. It can cause ACD if it accidentally comes in contact with the skin or cuticle on application [10]. Non-acid primers, those that do not contain MAA, have become more popular in order to avoid this adverse reaction; however, these still contain other allergens and irritants.
3. Clinical Presentation
The classic clinical presentation of CD associated with nail cosmetic use (typically ACD) includes proximal nail fold, hyponychial or paronychial tenderness, edema, erythema, fissuring, and paresthesia [3]. The exact location of the reaction within the nail complex depends on where the aggravating product was applied. More severe cases of ACD, typically associated with gel and acrylic manicures, may also include paronychia, thickened and dry nail plates, and onycholysis, including hemorrhagic onycholysis (Figure 1) with total nail detachment. Nail technicians who are more frequently exposed to these ingredients can present with eczematous hand dermatitis [17].
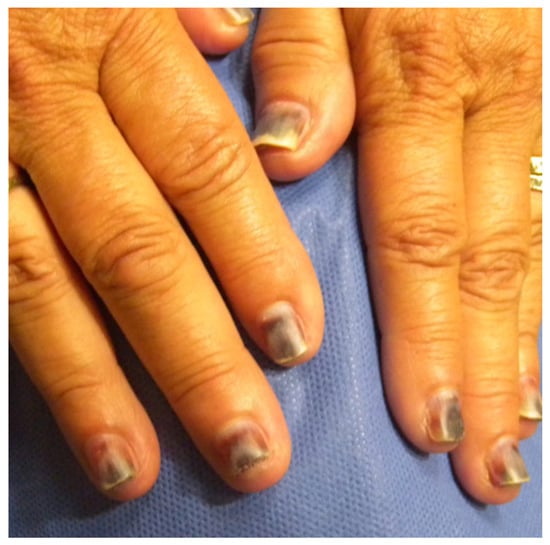
Figure 1.
Hemorrhagic onycholysis in patient after a gel manicure.
In addition to the nails, CD may develop on ectopic body sites that the nails frequently come in contact with through touching, itching, or rubbing. This includes the face, neck, eyes and presents similarly to ACD and ICD caused by other triggers—erythematous, indurated, scaly plaques with possible vesiculation, fissuring, and edema. Some ingredients, such as acrylates, are also capable of becoming airborne, producing a more generalized, symmetrical airborne contact dermatitis [27].
A recent NACDG retrospective analysis of data from 2001 to 2016 found that the face was actually more commonly involved in nail-cosmetic-related CD compared to the hands and nails themselves (43% vs. 27.6% of cases, respectively) [9]. Tosylamide allergy alone was more associated with face involvement, whereas (meth)acrylate allergy was more highly associated with hand involvement. In addition, 14.4% of cases involved the eyelids, and 12.1% of cases were considered to have scattered/generalized distribution [9]. A separate retrospective review of European data from 2013 to 2015 found greater involvement of the hands (88.9%) than the face (36.8%, including eyelids, lips, and cheeks) [8].
4. Evaluation and Workup
The diagnosis of CD secondary to nail cosmetic use can often be made through a thorough patient history and physical exam, with special attention paid to the temporal relationship of nail cosmetic use/procedures to the onset of skin findings [28]. ACD will typically present 7–10 days after first/current exposure to the allergen, whereas ICD will present much sooner, often immediately or within a few days. Additionally, ICD tends to occur more on the dorsal plane of the hands, compared to the palmar aspect in ACD, and less frequently presents with vesicles and/or pain [29]. The differential diagnosis may also include atopic dermatitis and dyshidrotic eczema. The diagnosis of ACD can be confirmed by patch testing, although treatment and counselling may begin in the interim with a strong clinical suspicion of CD.
Patch testing is the standard diagnostic and confirmatory test for determining causative allergens in ACD and has both a sensitivity and specificity around 70–80% [30]. Up to 10% of all patients with ACD have a positive result for one or more ingredients in nail cosmetics [31]. However, as many commercially available patch testing kits do not include many of the antigens found in nail cosmetic products, additional customized testing using diluted versions of these ingredients should be performed for the most thorough results [3]. In fact, close to one-fifth of nail care product-associated allergens may be missed without additional screenings beyond the NACDG screening [9]. Suggested patch testing protocols for these ingredients can be found in Table 3. While broad-spectrum testing is useful for both identifying the current causative agent and future planning, suspected causative agents can be narrowed based on the procedure experienced and its included products (Table 2).

Table 3.
Patch testing protocols for nail cosmetic ingredients [3].
5. Treatment
5.1. Behavioral Interventions
Causative substance identification, removal, and avoidance is a mainstay of treatment for CD of any etiology. Ingredients that are more specific to certain products and/or nail procedures are likely easier to eliminate and, if desired, substitute. However, caution must still be taken. Patients must be aware that most polishes labeled “hypoallergenic” do not contain TSFR; however, these polishes typically still contain (meth)acrylates [38]. Additionally, some allergic to (meth)acrylates may find regular nail polish (that contains (meth)acrylates in much lower concentrations) to be a suitable substitution, while others continue to react. Some brands, such as CND Shellac and BRISA, claim to have created “acrylate-free” gel polishes, which have anecdotally been nonreactive in some with proven acrylate allergies. However, since cosmetics do not have ingredient regulating bodies, anyone with a history of CD secondary to nail cosmetic use should try new products with extreme caution [16].
For nail technicians and beauticians who occupationally come in contact with these allergens, latex gloves should be avoided as (meth)acrylates have been shown to penetrate through this material [39]. Rather, nitrile gloves should be used and switched every hour, as after this time frame, protection can diminish. Trilaminated polyethylene gloves are the most effective at protecting underlying skin from (meth)acrylates and other ingredients, but these are typically impractical [40].
5.2. Topical Anti-Inflammatory Treatment
For immediate treatment of CD skin lesions, topical steroids can be prescribed. Caution should be taken to prescribe a steroid of appropriate potency for the affected region, such as higher potency steroids for hand lesions and lower potency steroids for the more delicate the face [41]. Ointments may be chosen over creams as they are less likely to contain allergenic preservatives [28]. Alternatively, topical calcineurin inhibitors, such as pimecrolimus, tacrolimus, and cyclosporine, can be useful for ACD of the eyelids [35].
5.3. Emollients and Barrier Creams
Emollients and barrier creams, such as petrolatum, dimethicone, and paraffin, should be recommended in both treatment and prevention of CD by providing an extra layer of protection from allergens above the stratum corneum [42]. These creams have also been shown to have some anti-inflammatory properties as well as the ability to restore the skin barrier [41]. Hyaluronic acids can enhance intracellular lipid production, while ceramides help to retain moisture [33]. Occlusive dressings with emollients placed over affected areas (when practical) may help emollient adherence to skin and decrease subsequent exposure to allergens prior to healing.
6. Conclusions
As the popularity of nail cosmetic products and procedures continues to increase, nail-cosmetic-related CD has increasingly become a significant burden to patients, clinicians, and nail technicians. The high number of potentially allergenic ingredients in combination with the lack of a cosmetic regulatory body makes identification and elimination of triggering substances particularly challenging. As such, careful diagnosis, causative agent identification, treatment, and counseling are pertinent for positive outcomes.
Author Contributions
All authors were equally responsible for the conceptualization, methodology, resources and data curation, writing, revisions/editing, and visualization of this project. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
Zoe M. Lipman has no conflict of interest to declare. Antonella Tosti has served as a consultant or advisor for DS Laboratories, Monat Global, Almirall, Thirty Madison, Lilly, Leo Pharmaceuticals, Bristol Myers Squibb, and Procter & Gamble.
References
- Madnani, N.; Khan, K. Nail cosmetics. Indian J. Dermatol. Venereol. Leprol. 2012, 78, 309. [Google Scholar] [CrossRef]
- Reinecke, J.; Hinshaw, M.A. Nail health in women. Int. J. Women Dermatol. 2020, 6, 73–79. [Google Scholar] [CrossRef]
- Chou, M.; Dhingra, N.; Strugar, T.L. Contact sensitization to allergens in nail cosmetics. Dermatitis 2017, 28, 231–240. [Google Scholar] [CrossRef]
- Dinani, N.; George, S. Nail cosmetics: A dermatological perspective. Clin. Exp. Dermatol. 2019, 44, 599–605. [Google Scholar] [CrossRef] [Green Version]
- De Groot, A.C.; Nater, J.P.; Weyland, J.W. Unwanted Effects of Cosmetics and Drugs Used in Dermatology; No. 282; Elsevier: Amsterdam, The Netherlands, 1994. [Google Scholar]
- Groot, A.C. Contact allergy to cosmetics: Causative ingredients. Contact Dermat. 1987, 17, 26–34. [Google Scholar] [CrossRef]
- Tosti, A.; Guerra, L.; Vincenzi, C.; Piraccini, B.M.; Peluso, A.M. Contact sensitization caused by toluene sulfona-mide-formaldehyde resin in women who use nail cosmetics. Dermatitis 1993, 4, 150–153. [Google Scholar] [CrossRef]
- Gonçalo, M.; Pinho, A.; Agner, T.; Andersen, K.E.; Bruze, M.; Diepgen, T.; Foti, C.; Giménez-Arnau, A.; Goossens, A.; Johanssen, J.D.; et al. Allergic contact dermatitis caused by nail acrylates in Europe. An EECDRG study. Contact Dermat. 2017, 78, 254–260. [Google Scholar] [CrossRef]
- Warshaw, E.M.; Voller, L.M.; Silverberg, J.I.; Dekoven, J.G.; Atwater, A.R.; Maibach, H.I.; Reeder, M.J.; Sasseville, D.; Belsito, D.V.; DeLeo, V.A.; et al. Contact dermatitis associated with nail care products: Retrospective analysis of North American contact dermatitis group data, 2001–2016. Dermatitis 2020, 31, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Uter, W.; Geier, J. Contact allergy to acrylates and methacrylates in consumers and nail artists—Data of the Information Network of Departments of Dermatology, 2004–2013. Contact Dermat. 2015, 72, 224–228. [Google Scholar] [CrossRef]
- Montgomery, R.; Stocks, S.J.; Wilkinson, S.M. Contact allergy resulting from the use of acrylate nails is increasing in both users and those who are occupationally exposed. Contact Dermat. 2016, 74, 120–122. [Google Scholar] [CrossRef] [PubMed]
- Gregoriou, S.; Tagka, A.; Velissariou, E.; Tsimpidakis, A.; Hatzidimitriou, E.; Platsidaki, E.; Kedikoglou, S.; Chatziioannou, A.; Katsarou, A.; Nicolaidou, E.; et al. The rising incidence of allergic contact dermatitis to acrylates. Dermatitis 2020, 31, 140–143. [Google Scholar] [CrossRef]
- Adams, R.M.; Maibach, H.I.; Clendenning, W.; Fisher, A.; Jordan, W.; Kanof, N.; Larsen, W.; Mitchell, J.; Rudner, E.; Schorr, W.; et al. A five-year study of cosmetic reactions. J. Am. Acad. Dermatol. 1985, 13, 1062–1069. [Google Scholar] [CrossRef]
- Sainio, E.-L.; Engström, K.; Henriks-Eckerman, M.-L.; Kanerva, L. Allergenic ingredients in nail polishes. Contact Dermat. 1997, 37, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.N.; Pokorny, C.D.; Law, S.; Pratt, M.; Sasseville, D.; Storrs, F.J. Cross-reactivity among epoxy acrylates and bisphenol F epoxy resins in patients with bisphenol A epoxy resin sensitivity. Arch. Phys. Med. Rehabilit. 2002, 13, 108–115. [Google Scholar]
- Dahlin, J.; Berne, B.; Dunér, K.; Hosseiny, S.; Matura, M.; Nyman, G.; Tammela, M.; Isaksson, M. Several cases of undesirable effects caused by methacrylate ultraviolet-curing nail polish for non-professional use. Contact Dermat. 2016, 75, 151–156. [Google Scholar] [CrossRef]
- Roche, E.; de la Cuadra, J.; Alegre, V. Sensitization to acrylates caused by artificial acrylic nails: Review of 15 cases. Actas Dermo. Sifiliogr. 2008, 99, 788–794. [Google Scholar] [CrossRef]
- Scher, R.K. Cosmetics and ancillary preparations for the care of nails: Composition, chemistry, and adverse reactions. J. Am. Acad. Dermatol. 1982, 6, 523–528. [Google Scholar] [CrossRef]
- Sánchez-Pujol, M.J.; Docampo-Simón, A.; Sánchez-Herrero, A.; García-Martínez, E.; Silvestre-Salvador, J.F. Allergic contact dermatitis caused by an acrylic nails kit for domestic use. Dermatitis 2020, 31, e27–e28. [Google Scholar] [CrossRef] [PubMed]
- Gatica-Ortega, M.E.; Pastor-Nieto, M.A.; Gil-Redondo, R.; Martínez-Lorenzo, E.R.; Schöendorff-Ortega, C. Non-occupational allergic contact dermatitis caused by long-lasting nail polish kits for home use: ‘The tip of the iceberg’. Contact Dermat. 2018, 78, 261–265. [Google Scholar] [CrossRef]
- Chowdhury, M.; Statham, B.N. Allergic contact dermatitis from dibutyl phthalate and benzalkonium chloride in Timodine® cream. Contact Dermat. 2002, 46, 57. [Google Scholar] [CrossRef]
- Fisher, J.S.; MacPherson, S.; Marchetti, N.; Sharpe, R.M. Human ‘testicular dysgenesis syndrome’: A possible model using in-utero exposure of the rat to dibutyl phthalate. Hum. Reprod. 2003, 18, 1383–1394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goossens, A. Contact-allergic reactions to cosmetics. J. Allergy 2011, 2011, 467071. [Google Scholar] [CrossRef] [Green Version]
- García-Gavín, J.; Lissens, R.; Timmermans, A.; Goossens, A. Allergic contact dermatitis caused by isopropyl alcohol: A missed allergen? Contact Dermat. 2011, 65, 101–106. [Google Scholar] [CrossRef]
- Kanerva, L.; Estlander, T. Allergic onycholysis and paronychia caused by cyanoacrylate nail glue, but not by photo-bonded methacrylate nails. Eur. J. Dermatol. 2000, 10, 223–225. [Google Scholar]
- Sachse, M.M.; Junghans, T.; Rose, C.; Wagner, G. Allergic contact dermatitis caused by topical 2-octyl-cyanoacrylate. Contact Dermat. 2013, 68, 317–319. [Google Scholar] [CrossRef] [PubMed]
- Baran, R.; Andre, J. Side effects of nail cosmetics. J. Cosmet. Dermatol. 2005, 4, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Usatine, R.P.; Riojas, M. Diagnosis and management of contact dermatitis. Am. Fam. Physician 2010, 82, 249–255. [Google Scholar]
- English, J.S.C. Current concepts of irritant contact dermatitis. Occup. Environ. Med. 2004, 61, 722–726. [Google Scholar] [CrossRef] [Green Version]
- Bourke, J.; Coulson, I.; English, J. Guidelines for the management of contact dermatitis: An update. Br. J. Dermatol. 2009, 160, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Orton, D.I.; Wilkinson, J.D. Cosmetic allergy. Am. J. Clin. Dermatol. 2004, 5, 327–337. [Google Scholar] [CrossRef]
- Walter, J.F.; Kelsey, W.H.; Voorhees, J.J.; Duell, E.A. Psoralen plus black light inhibits epidermal DNA synthesis. Arch. Dermatol. 1973, 107, 861–865. [Google Scholar] [CrossRef]
- Takahashi, A.; Kirst, A.; Heinrich, U.; Kiyomine, A.; Ishida, K.; Tronnier, H.; Theis, H.; Nishizaka, T.; Tanabe, H. Evaluation of a barrier repair cream containing pseudo-ceramide for practical use by hairdressers with hand skin disorders due to daily exposure to chemical irritants. J. Cosmet. Dermatol. Sci. Appl. 2013, 3, 263–270. [Google Scholar] [CrossRef] [Green Version]
- Coven, T.R.; Walters, I.B.; Cardinale, I.; Krueger, J.G. PUVA-induced lymphocyte apoptosis: Mechanism of action in psoriasis. Photodermatol. Photoimmunol. Photomed. 1999, 15, 22–27. [Google Scholar] [CrossRef]
- Katsarou, A.; Armenaka, M.; Vosynioti, V.; Lagogianni, E.; Kalogeromitros, D.; Katsambas, A. Tacrolimus ointment 0.1% in the treatment of allergic contact eyelid dermatitis. J. Eur. Acad. Dermatol. Venereol. 2009, 23, 382–387. [Google Scholar] [CrossRef]
- Jensen, L.; Stensgaard, A.; Andersen, K.E. Psoralen plus ultraviolet A (PUVA) soaks and UVB TL01 treatment for chronic hand dermatoses. Dermatol. Rep. 2012, 4, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bickers, D.R. Position paper—PUVA therapy. J. Am. Acad. Dermatol. 1983, 8, 265–270. [Google Scholar] [CrossRef]
- Ramos, L.; Cabral, R.; Gonçalo, M. Allergic contact dermatitis caused by acrylates and methacrylates—A 7-year study. Contact Dermat. 2014, 71, 102–107. [Google Scholar] [CrossRef]
- Muttardi, K.; White, I.R.; Banerjee, P. The burden of allergic contact dermatitis caused by acrylates. Contact Dermat. 2016, 75, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Spencer, A.; Gazzani, P.; Thompson, D.A. Acrylate and methacrylate contact allergy and allergic contact disease: A 13-year review. Contact Dermat. 2016, 75, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Qin, R.; Lampel, H.P. Review of occupational contact dermatitis—Top allergens, best avoidance measures. Curr. Treat. Options Allergy 2015, 2, 349–364. [Google Scholar] [CrossRef] [Green Version]
- Harcharik, S.; Emer, J. Steroid-sparing properties of emollients in dermatology. Ski. Ther. Lett. 2014, 19, 5–10. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).