Sustainable Upcycling of Spent Battery Graphite into High-Performance PEG Anodes via Flash Joule Heating
Abstract
1. Introduction
2. Results and Discussion
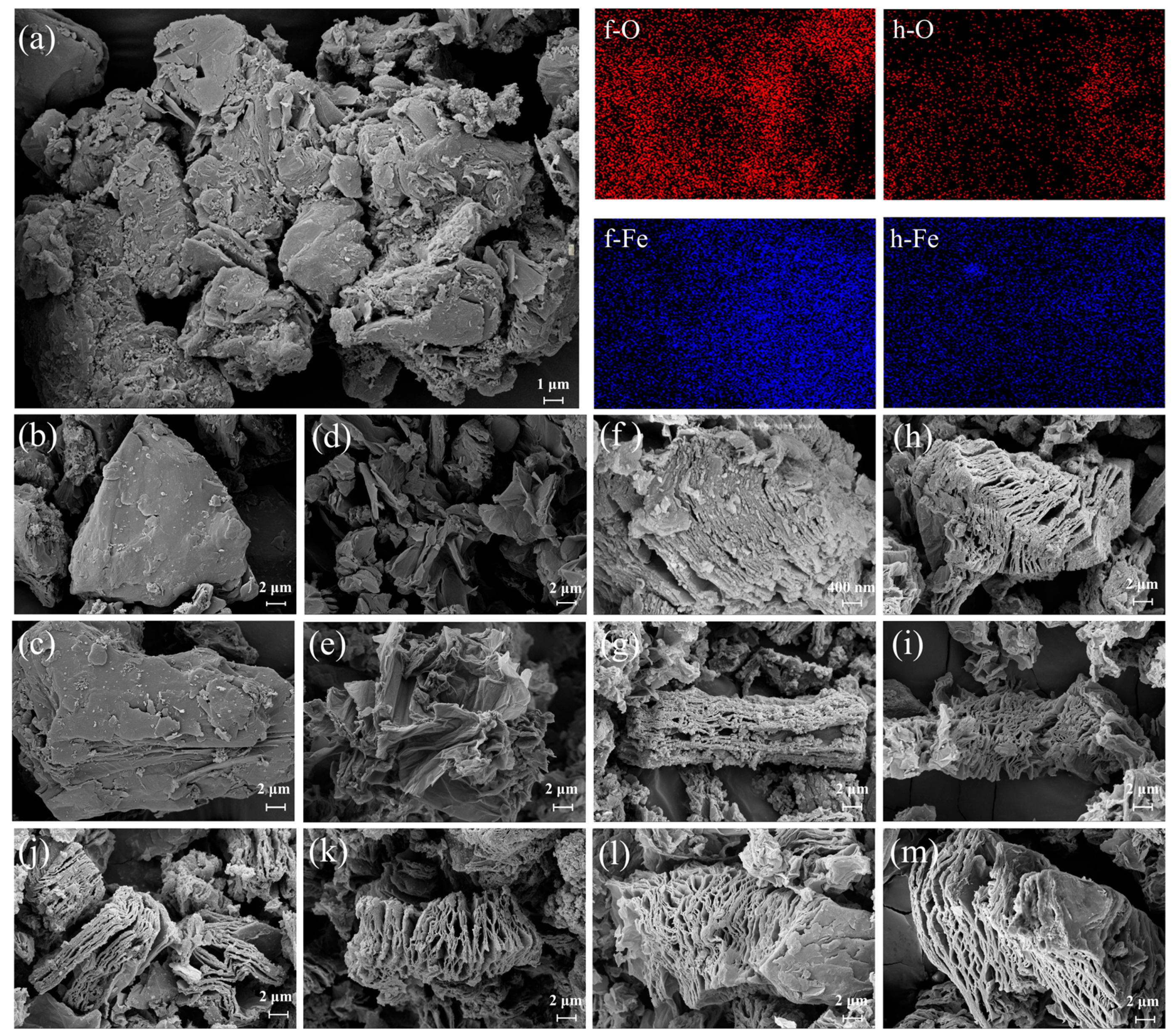
2.1. Characterization of Materials
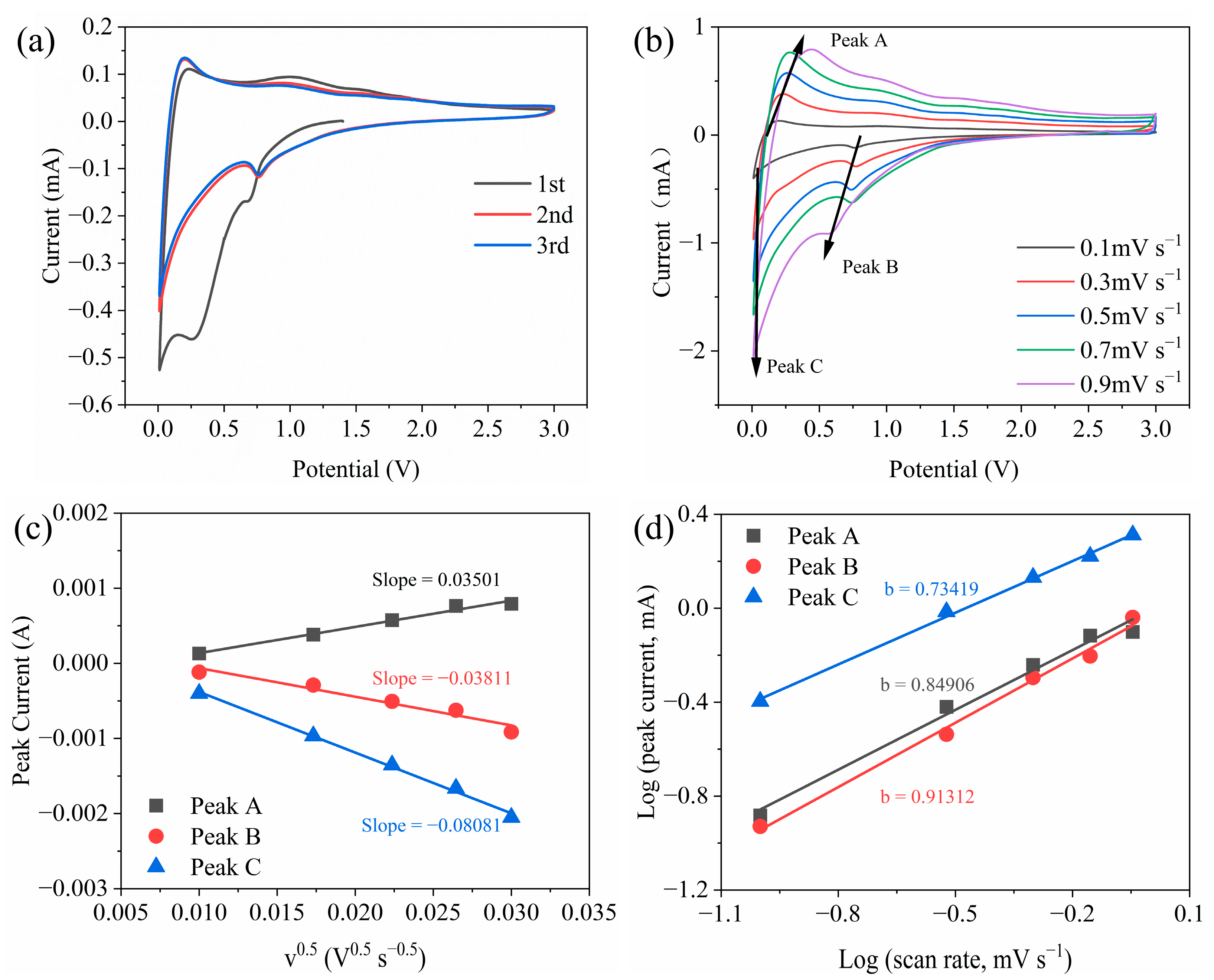
2.2. Electrochemical Characterization
3. Materials and Methods
3.1. Materials
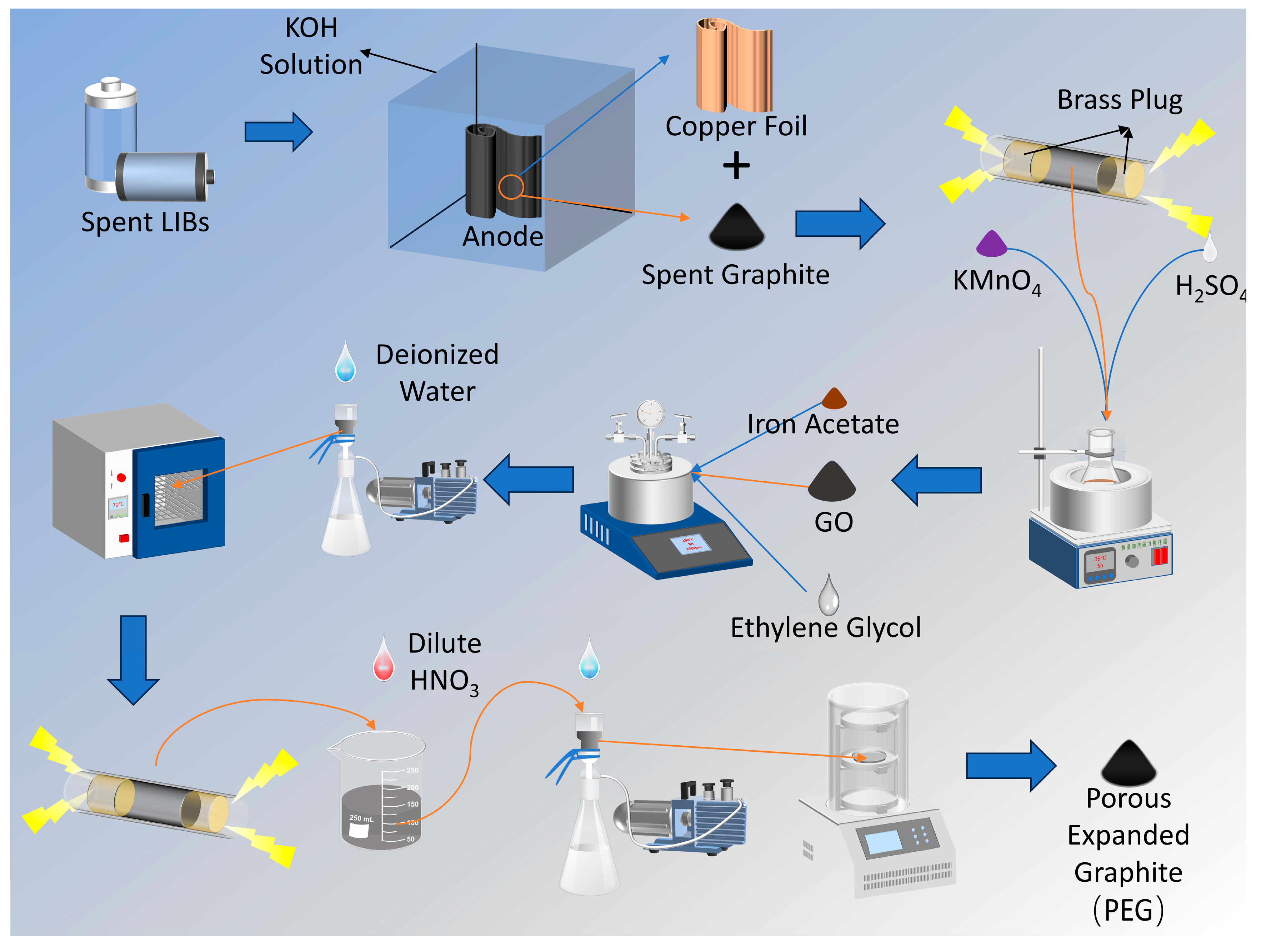
3.2. Acquisition of Spent Graphite
3.3. Preparation of Materials
3.4. Characterization and Electrochemical Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, W. Green energy and resources: Advancing green and low-carbon development. Green Energy Resour. 2023, 1, 100009. [Google Scholar] [CrossRef]
- Nguyen-Tien, V.; Zhang, C.; Strobl, E.; Elliott, R.J.R. The closing longevity gap between battery electric vehicles and internal combustion vehicles in Great Britain. Nat. Energy 2025, 10, 354–364. [Google Scholar] [CrossRef]
- Li, X.; Deng, C.; Liu, M.; Xiong, J.; Zhang, X.; Yan, Q.; Lin, J.; Chen, C.; Wu, F.; Zhao, Y.; et al. Reutilization and upcycling of spent graphite for sustainable lithium-ion batteries: Progress and perspectives. eScience 2025, 5, 100394. [Google Scholar] [CrossRef]
- Xiao, J.; Li, J.; Xu, Z. Challenges to Future Development of Spent Lithium Ion Batteries Recovery from Environmental and Technological Perspectives. Environ. Sci. Technol. 2020, 54, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Lecocq, A.; Eshetu, G.G.; Grugeon, S.; Martin, N.; Laruelle, S.; Marlair, G. Scenario-based prediction of Li-ion batteries fire-induced toxicity. J. Power Sources 2016, 316, 197–206. [Google Scholar] [CrossRef]
- Wang, Q.; Mao, B.; Stoliarov, S.I.; Sun, J. A review of lithium ion battery failure mechanisms and fire prevention strategies. Progress. Energy Combust. Sci. 2019, 73, 95–131. [Google Scholar] [CrossRef]
- Iturrondobeitia, M.; Vallejo, C.; Berroci, M.; Akizu-Gardoki, O.; Minguez, R.; Lizundia, E. Environmental Impact Assessment of LiNi1/3Mn1/3Co1/3O2 Hydrometallurgical Cathode Recycling from Spent Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2022, 10, 9798–9810. [Google Scholar] [CrossRef]
- Natarajan, S.; Aravindan, V. An Urgent Call to Spent LIB Recycling: Whys and Wherefores for Graphite Recovery. Adv. Energy Mater. 2020, 10, 2002238. [Google Scholar] [CrossRef]
- Yi, C.; Zhou, L.; Wu, X.; Sun, W.; Yi, L.; Yang, Y. Technology for recycling and regenerating graphite from spent lithium-ion batteries. Chin. J. Chem. Eng. 2021, 39, 37–50. [Google Scholar] [CrossRef]
- Li, Z.; Li, J.; Wang, L. Renewed graphite for high-performance lithium-ion batteries: Catalytic graphitization approach. J. Mater. Sci. Mater. Electron. 2024, 35, 599. [Google Scholar] [CrossRef]
- Shang, Z.; Yu, W.; Zhou, J.; Zhou, X.; Zeng, Z.; Tursun, R.; Liu, X.; Xu, S. Recycling of spent lithium-ion batteries in view of graphite recovery: A review. eTransportation 2024, 20, 100320. [Google Scholar] [CrossRef]
- Yu, W.; Guo, Y.; Xu, S.; Yang, Y.; Zhao, Y.; Zhang, J. Comprehensive recycling of lithium-ion batteries: Fundamentals, pretreatment, and perspectives. Energy Storage Mater. 2023, 54, 172–220. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, W.; Fang, Q.; Xu, S. Improved recovery of valuable metals from spent lithium-ion batteries by efficient reduction roasting and facile acid leaching. Waste Manag. 2020, 102, 847–855. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, W.; Hu, J.; Zhang, T.; Xu, S. Stepwise Recovery of Valuable Metals from Spent Lithium Ion Batteries by Controllable Reduction and Selective Leaching and Precipitation. ACS Sustain. Chem. Eng. 2020, 8, 15496–15506. [Google Scholar] [CrossRef]
- Yu, H.; Dai, H.; Zhu, Y.; Hu, H.; Zhao, R.; Wu, B.; Chen, D. Mechanistic insights into the lattice reconfiguration of the anode graphite recycled from spent high-power lithium-ion batteries. J. Power Sources 2021, 481, 229159. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, X.; Hou, H.; Tang, L.; Xiao, J.; Zhong, Q. Regeneration and utilization of graphite from the spent lithium-ion batteries by modified low-temperature sulfuric acid roasting. Waste Manag. 2022, 150, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, J.; Chen, Y.; Wang, L.; Wang, C. Graphite regenerating from retired (LFP) lithium-ion battery: Phase transformation mechanism of impurities in low-temperature sulfation roasting process. Renew. Energy 2023, 204, 290–299. [Google Scholar] [CrossRef]
- Xie, X.; Zhang, J.; Chen, Y.; Wang, C. A method for the preparation of graphene from spent graphite of retired lithium-ion batteries. J. Power Sources 2024, 594, 234023. [Google Scholar] [CrossRef]
- Zhang, J.; Lei, Y.; Lin, Z.; Xie, P.; Lu, H.; Xu, J. A novel approach to recovery of lithium element and production of holey graphene based on the lithiated graphite of spent lithium ion batteries. Chem. Eng. J. 2022, 436, 135011. [Google Scholar] [CrossRef]
- Xiao, F.; Chen, X.; Zhang, J.; Huang, C.; Hu, T.; Hong, B.; Xu, J. Large-scale production of holey graphite as high-rate anode for lithium ion batteries. J. Energy Chem. 2020, 48, 122–127. [Google Scholar] [CrossRef]
- Ma, X.; Li, S.; Tang, W.; Liu, R.; Fu, Z.; Wang, S. Laser-Induced Coal-Based Porous Graphene as Anode Toward Advanced Lithium-Ion Battery. Adv. Sci. 2025, 12, e2504592. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Zhao, Y.; Li, Y. A facile solution-free etching preparation of porous graphene nanosheets with high performances for lithium storage. Chem. Eng. J. 2017, 320, 283–289. [Google Scholar] [CrossRef]
- Xiao, Y.; Li, J.; Huang, W.; Wang, L.; Luo, J. Green & efficient regeneration of graphite anode from spent lithium ion batteries enabled by asphalt coating. J. Mater. Sci. Mater. Electron. 2022, 33, 16740–16752. [Google Scholar] [CrossRef]
- Xu, C.; Ma, G.; Yang, W.; Che, S.; Li, Y.; Jia, Y.; Liu, H.; Chen, F.; Zhang, G.; Liu, H.; et al. One-step reconstruction of acid treated spent graphite for high capacity and fast charging lithium-ion batteries. Electrochim. Acta 2022, 415, 140198. [Google Scholar] [CrossRef]
- Gong, H.; Xiao, H.; Ye, L.; Ou, X. High-performance expanded graphite regenerated from spent lithium-ion batteries by integrated oxidation and purification method. Waste Manag. 2023, 171, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Liu, G.; Hu, J.; Su, G.; Liu, M.; Li, X.; Xu, B. Low-temperature molten salt ion regeneration strategy towards green and efficient spent graphite recycling. Green. Chem. 2025, 27, 6145–6155. [Google Scholar] [CrossRef]
- Dong, S.; Song, Y.; Ye, K.; Yan, J.; Wang, G.; Zhu, K.; Cao, D. Ultra-fast, low-cost, and green regeneration of graphite anode using flash joule heating method. EcoMat 2022, 4, e12212. [Google Scholar] [CrossRef]
- Yi, C.; Ge, P.; Wu, X.; Sun, W.; Yang, Y. Tailoring carbon chains for repairing graphite from spent lithium-ion battery toward closed-circuit recycling. J. Energy Chem. 2022, 72, 97–107. [Google Scholar] [CrossRef]
- Yang, J.-L.; Zhao, X.-X.; Li, W.-H.; Liang, H.-J.; Gu, Z.-Y.; Liu, Y.; Du, M.; Wu, X.-L. Advanced cathode for dual-ion batteries: Waste-to-wealth reuse of spent graphite from lithium-ion batteries. eScience 2022, 2, 95–101. [Google Scholar] [CrossRef]
- Li, L.; Ruan, M.; Tian, D.; Zhang, X.; Hou, X.; Zhong, K.; Cheng, F.; Tong, Y.; Fang, Z. Dual regulation mechanism of MoS2/MoO3 heterostructure for polysulfide and volume effect enables stable and durable lithium storage. Appl. Surf. Sci. 2024, 646, 158949. [Google Scholar] [CrossRef]
- Fan, J.; Yang, J.; Li, H.; Tian, J.; Wang, M.; Zhao, Y. Cryogenic mechanical properties of graphene oxide/epoxy nanocomposites: Influence of graphene oxide with different oxidation degrees. Polym. Test. 2021, 96, 107074. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, H.; Xie, F.; Ma, X.; Niu, B.; Chen, M.; Zhang, H.; Zhang, Y.; Long, D. General synthesis of ultrafine metal oxide/reduced graphene oxide nanocomposites for ultrahigh-flux nanofiltration membrane. Nat. Commun. 2022, 13, 471. [Google Scholar] [CrossRef]
- Al-Tabbakh, A.A.; Karatepe, N.; Al-Zubaidi, A.B.; Benchaabane, A.; Mahmood, N.B. Crystallite size and lattice strain of lithiated spinel material for rechargeable battery by X-ray diffraction peak-broadening analysis. Int. J. Energy Res. 2019, 43, 1903–1911. [Google Scholar] [CrossRef]
- Sun, N.; Li, Z.; Zhang, X.; Qin, W.; Zhao, C.; Zhang, H.; Ng, D.H.L.; Kang, S.; Zhao, H.; Wang, G. Hierarchical Porous Carbon Materials Derived from Kelp for Superior Capacitive Applications. ACS Sustain. Chem. Eng. 2019, 7, 8735–8743. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, X. In situ activation graphitization to fabricate hierarchical porous graphitic carbon for supercapacitor. Sci. Rep. 2021, 11, 6825. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Q.; Li, Y.; Sun, G.; Yu, X.; Li, H. Crack-induced abrupt capacity degradation in commercial LiNi(0.8)Co(0.1)Mn(0.1)O(2) (NCM811)/SiO (x) -graphite pouch batteries. RSC Adv. 2024, 14, 19116–19123. [Google Scholar] [CrossRef] [PubMed]
- Jia, P.; Sun, J.; Li, S.; Wang, W.; Song, Z.; Zhao, X.; Mao, Y. A feasible recycling route for spent graphite: Microwave-assisted puffing with Fe2O3 loading to construct high-performance anode for lithium-ion batteries. Mater. Today Sustain. 2024, 25, 100620. [Google Scholar] [CrossRef]
- Rahman, M.A.; Wong, Y.C.; Song, G.; Wen, C. A review on porous negative electrodes for high performance lithium-ion batteries. J. Porous Mater. 2015, 22, 1313–1343. [Google Scholar] [CrossRef]
- Xu, Y.-J.; Song, X.-H.; Chang, Q.; Hou, X.-L.; Sun, Y.; Feng, X.-Y.; Wang, X.-R.; Zhan, M.; Xiang, H.-F.; Yu, Y. The regeneration of graphite anode from spent lithium-ion batteries by washing with a nitric acid/ethanol solution. New Carbon. Mater. 2022, 37, 1011–1020. [Google Scholar] [CrossRef]
- Fan, W.; Zhang, J.; Ma, R.; Chen, Y.; Wang, C. Regeneration of graphite anode from spent lithium-ion batteries via microwave calcination. J. Electroanal. Chem. 2022, 908, 116087. [Google Scholar] [CrossRef]
- Lai, Y.; Zhu, X.; Li, J.; Gou, Q.; Li, M.; Xia, A.; Huang, Y.; Zhu, X.; Liao, Q. Recovery and regeneration of anode graphite from spent lithium-ion batteries through deep eutectic solvent treatment: Structural characteristics, electrochemical performance and regeneration mechanism. Chem. Eng. J. 2023, 457, 141196. [Google Scholar] [CrossRef]
- Kim, J.; Eum, J.H.; Kang, J.; Kwon, O.; Kim, H.; Kim, D.W. Tuning the hierarchical pore structure of graphene oxide through dual thermal activation for high-performance supercapacitor. Sci. Rep. 2021, 11, 2063. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Y.; Sun, J.; Chen, W.; Lu, S.; Wang, Z. Sustainable Upcycling of Spent Battery Graphite into High-Performance PEG Anodes via Flash Joule Heating. Recycling 2025, 10, 171. https://doi.org/10.3390/recycling10050171
Luo Y, Sun J, Chen W, Lu S, Wang Z. Sustainable Upcycling of Spent Battery Graphite into High-Performance PEG Anodes via Flash Joule Heating. Recycling. 2025; 10(5):171. https://doi.org/10.3390/recycling10050171
Chicago/Turabian StyleLuo, Yihan, Jing Sun, Wenxin Chen, Shuo Lu, and Ziliang Wang. 2025. "Sustainable Upcycling of Spent Battery Graphite into High-Performance PEG Anodes via Flash Joule Heating" Recycling 10, no. 5: 171. https://doi.org/10.3390/recycling10050171
APA StyleLuo, Y., Sun, J., Chen, W., Lu, S., & Wang, Z. (2025). Sustainable Upcycling of Spent Battery Graphite into High-Performance PEG Anodes via Flash Joule Heating. Recycling, 10(5), 171. https://doi.org/10.3390/recycling10050171








