Investigation of Aqueous Delamination Processes for Lithium-Ion Battery Anodes
Abstract
1. Introduction
1.1. Background and Relevance
1.2. Direct Recycling as an Approach
1.3. Current Research Landscape
1.4. Research Needs and Objectives
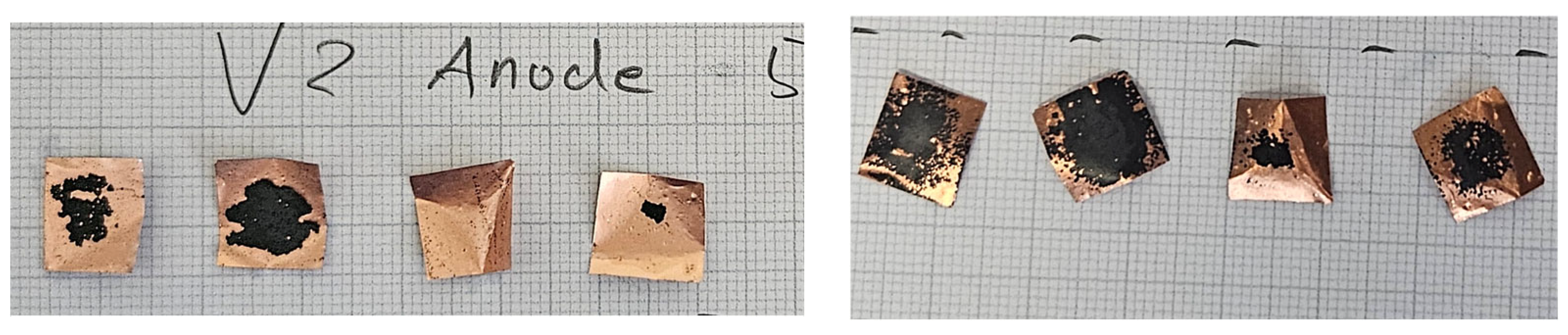
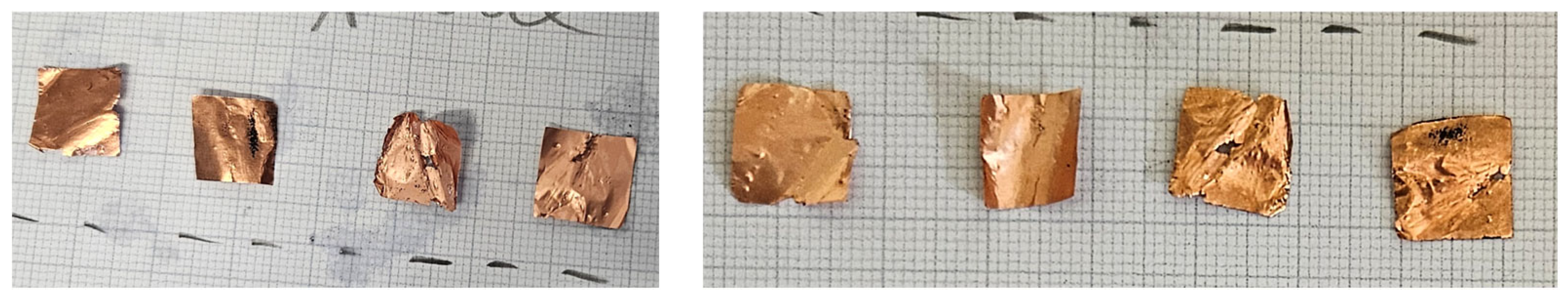
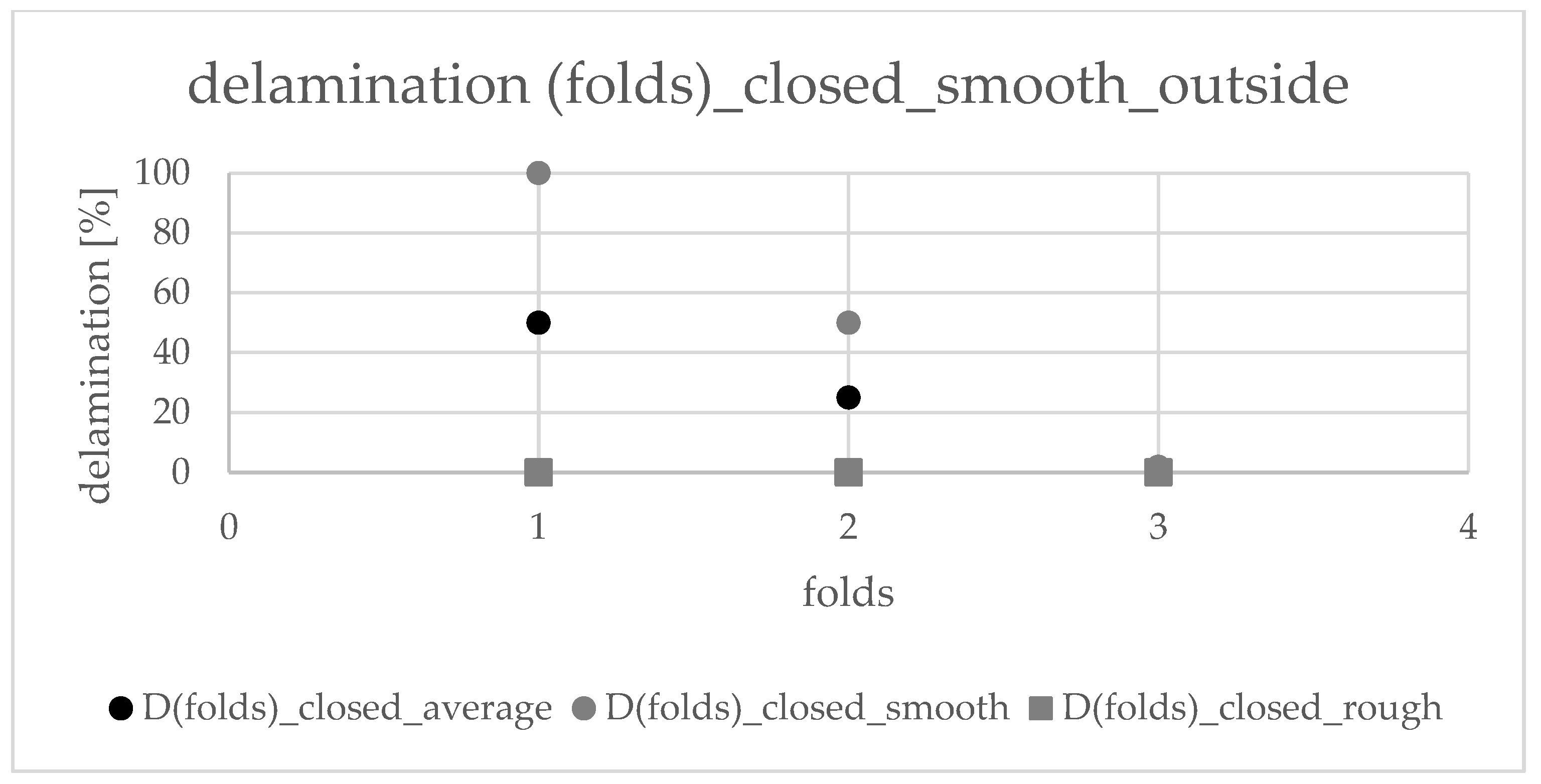
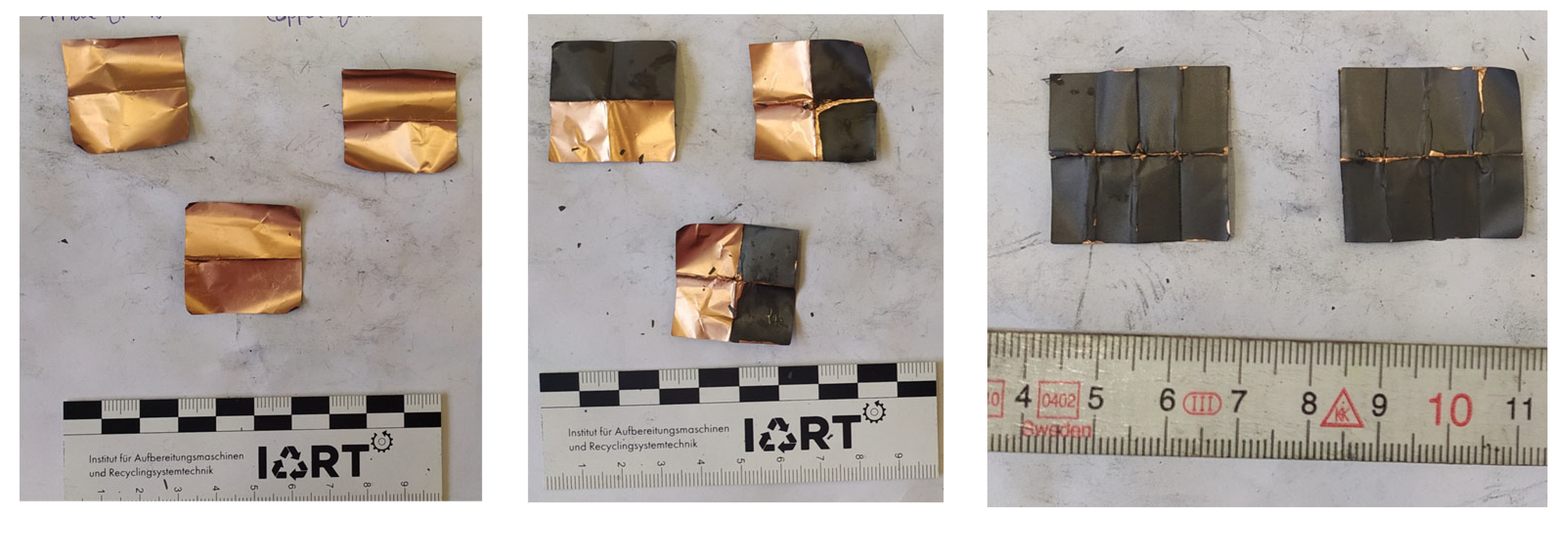
2. Results
2.1. Temperature
2.2. Particle Size
2.3. Ultrasonic Treatment
2.4. Precrushing and Deformation
3. Discussion
4. Materials and Methods
4.1. Experimental Series
4.1.1. Temperature
4.1.2. Particle Size
4.1.3. Ultrasonic Treatment
4.1.4. Precrushing and Deformation
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Connelly, E.; Aryanpur, V.; Paoli, L.; Teter, J. Global Electric Vehicle Outlook 2022. Securing Supplies for an Electric Future. 2022. Available online: https://iea.blob.core.windows.net/assets/ad8fb04c-4f75-42fc-973a-6e54c8a4449a/GlobalElectricVehicleOutlook2022.pdf (accessed on 6 October 2025).
- Curry, C. Lithium-ion Battery Costs and Market. Squeezed Margins Seek Technology Improvements & New Business Models. 2017. Available online: https://roar-assets-auto.rbl.ms/documents/36549/BNEF-Lithium-ion-battery-costs-and-market.pdf (accessed on 6 October 2025).
- Ren, X.; Tong, Z.; Dai, Y.; Ma, G.; Lv, Z.; Bu, X.; Bilal, M.; Vakylabad, A.B.; Hassanzadeh, A. Effects of Mechanical Stirring and Ultrasound Treatment on the Separation of Graphite Electrode Materials from Copper Foils of Spent LIBs: A Comparative Study. Separations 2023, 10, 246. [Google Scholar] [CrossRef]
- Gaines, L.; Zhang, J.; He, X.; Bouchard, J.; Melin, H.E. Tracking Flows of End-of-Life Battery Materials and Manufacturing Scrap. Batteries 2023, 9, 360. [Google Scholar] [CrossRef]
- Wiechers, P.; Hermann, A.; Koob, S.; Glaum, F.; Gleiß, M. Development of a Process for Direct Recycling of Negative Electrode Scrap from Lithium-Ion Battery Production on a Technical Scale and Its Influence on the Material Quality. Batteries 2024, 10, 218. [Google Scholar] [CrossRef]
- Jiang, M.; Li, B.; Liu, X. Global value chain restructuring driven by critical material trade: A case study of graphite. Resour. Conserv. Recycl. 2025, 222, 108489. [Google Scholar] [CrossRef]
- Park, J.; Cho, S.-J.; Shin, S.; Kim, R.; Shin, D.; Shin, Y. Overview of graphite supply chain and its challenges. Geosci. J. 2025, 29, 329–341. [Google Scholar] [CrossRef]
- Bobba, S.; Carrara, S.; Huisman, J.; Mathieux, F.; Pavel, C. Critical Raw Materials for Strategic Technologies and Sectors in the EU: A Foresight Study; European Union, Publications Office of the European Union: Luxembourg, 2020. [Google Scholar]
- Xu, P.; Tan, D.H.S.; Jiao, B.; Gao, H.; Yu, X.; Chen, Z. A Materials Perspective on Direct Recycling of Lithium-Ion Batteries: Principles, Challenges and Opportunities. Adv. Funct. Mater. 2023, 33, 2213168. [Google Scholar] [CrossRef]
- Sloop, S.; Crandon, L.; Allen, M.; Koetje, K.; Reed, L.; Gaines, L.; Sirisaksoontorn, W.; Lerner, M. A direct recycling case study from a lithium-ion battery recall. Sustain. Mater. Technol. 2020, 25, e00152. [Google Scholar] [CrossRef]
- Lefherz, H.; Ahuis, M.; Dilger, N.; Kwade, A.; Zellmer, S. Sustainability of electrode scrap recycling—Quantifying the environmental benefit of direct recycling routes for battery production scrap. J. Energy Storage 2025, 132, 117688. [Google Scholar] [CrossRef]
- Shin, Y.; Kim, S.; Park, S.; Lee, J.; Bae, J.; Kim, D.; Joo, H.; Ban, S.; Lee, H.; Kim, Y.; et al. A comprehensive review on the recovery of cathode active materials via direct recycling from spent Li-ion batteries. Renew. Sustain. Energy Rev. 2023, 187, 113693. [Google Scholar] [CrossRef]
- Recell Center. Direct Recycling of Materials. Available online: https://recellcenter.org/research/direct-recycling-of-materials/ (accessed on 6 October 2025).
- Baum, Z.J.; Bird, R.E.; Yu, X.; Ma, J. Lithium-Ion Battery Recycling—Overview of Techniques and Trends. ACS Energy Lett. 2022, 7, 712–719. [Google Scholar] [CrossRef]
- Sliz, R.; Valikangas, J.; Vilmi, P.; Hu, T.; Lassi, U.; Fabritius, T. Replacement of NMP solvent for more sustainable, high-capacity, printed Li-ion battery cathodes. In Proceedings of the IEEE 16th Nanotechnology Materials and Devices Conference (NMDC), Vancouver, BC, Canada, 12–15 December 2021. [Google Scholar]
- Bai, Y.; Muralidharan, N.; Li, J.; Essehli, R.; Belharouak, I. Sustainable Direct Recycling of Lithium-Ion Batteries via Solvent Recovery of Electrode Materials. ChemSusChem 2020, 13, 5664–5670. [Google Scholar] [CrossRef]
- Tong, Z.; Ren, X.; Ni, M.; Bu, X.; Dong, L. Review of Ultrasound-Assisted Recycling and Utilization of Cathode Materials from Spent Lithium-Ion Batteries: State-of-the-Art and Outlook. Energy Fuels 2023, 37, 14574–14588. [Google Scholar] [CrossRef]
- Gupta, V.; Appleberry, M.; Li, W.; Chen, Z. Direct recycling industrialization of Li-ion batteries: The pre-processing barricade. Next Energy 2024, 2, 100091. [Google Scholar] [CrossRef]
- Bai, Y.; Li, M.; Jafta, C.J.; Dai, Q.; Essehli, R.; Polzin, B.J.; Belharouak, I. Direct recycling and remanufacturing of anode scraps. Sustain. Mater. Technol. 2023, 35, e00542. [Google Scholar] [CrossRef]














| Nr. | T [°C] | t [min] | P [%] | D [%] |
|---|---|---|---|---|
| V1 | 20 | 5 | 100 | 29.04 |
| V2 | 20 | 5 | 50 | 64.69 |
| V5 | 60 | 5 | 50 | 92.65 |
| V6 | 60 | 3 | 50 | 71.69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trebeck, E.; Grams, A.; Talkenberger, J.; Prakash, S.; Grimmenstein, J.E.; Krampitz, T.; Lieberwirth, H.; Valenas, A. Investigation of Aqueous Delamination Processes for Lithium-Ion Battery Anodes. Recycling 2025, 10, 189. https://doi.org/10.3390/recycling10050189
Trebeck E, Grams A, Talkenberger J, Prakash S, Grimmenstein JE, Krampitz T, Lieberwirth H, Valenas A. Investigation of Aqueous Delamination Processes for Lithium-Ion Battery Anodes. Recycling. 2025; 10(5):189. https://doi.org/10.3390/recycling10050189
Chicago/Turabian StyleTrebeck, Eric, Anting Grams, Jan Talkenberger, Sricharana Prakash, Julius Eik Grimmenstein, Thomas Krampitz, Holger Lieberwirth, and Adrian Valenas. 2025. "Investigation of Aqueous Delamination Processes for Lithium-Ion Battery Anodes" Recycling 10, no. 5: 189. https://doi.org/10.3390/recycling10050189
APA StyleTrebeck, E., Grams, A., Talkenberger, J., Prakash, S., Grimmenstein, J. E., Krampitz, T., Lieberwirth, H., & Valenas, A. (2025). Investigation of Aqueous Delamination Processes for Lithium-Ion Battery Anodes. Recycling, 10(5), 189. https://doi.org/10.3390/recycling10050189






