3.1. Material Information and Electrode Slice Characterization
The physicochemical and structural properties of electrode materials are summarized in
Table 1. The AC, synthesized via vapor activation, displays a median particle size (D
50) of 5.9 μm and possesses a high specific surface area (SSA) of ~1600 m
2 g
−1 with a well–defined mesoporous structure. In contrast, the NVP exhibits a D
50 of 5.1 μm and a much lower SSA of ~15 m
2 g
−1. The HC shows a D
50 of 5.4 μm and an SSA of ~7 m
2 g
−1. The surface of NVP is coated with ~2.0 wt.% amorphous carbon, improving electrical conductivity and facilitating homogenized interfacial contact with AC and conductive agents. Compared with AC and HC, NVP shows significantly higher electrical resistivity and tap density.
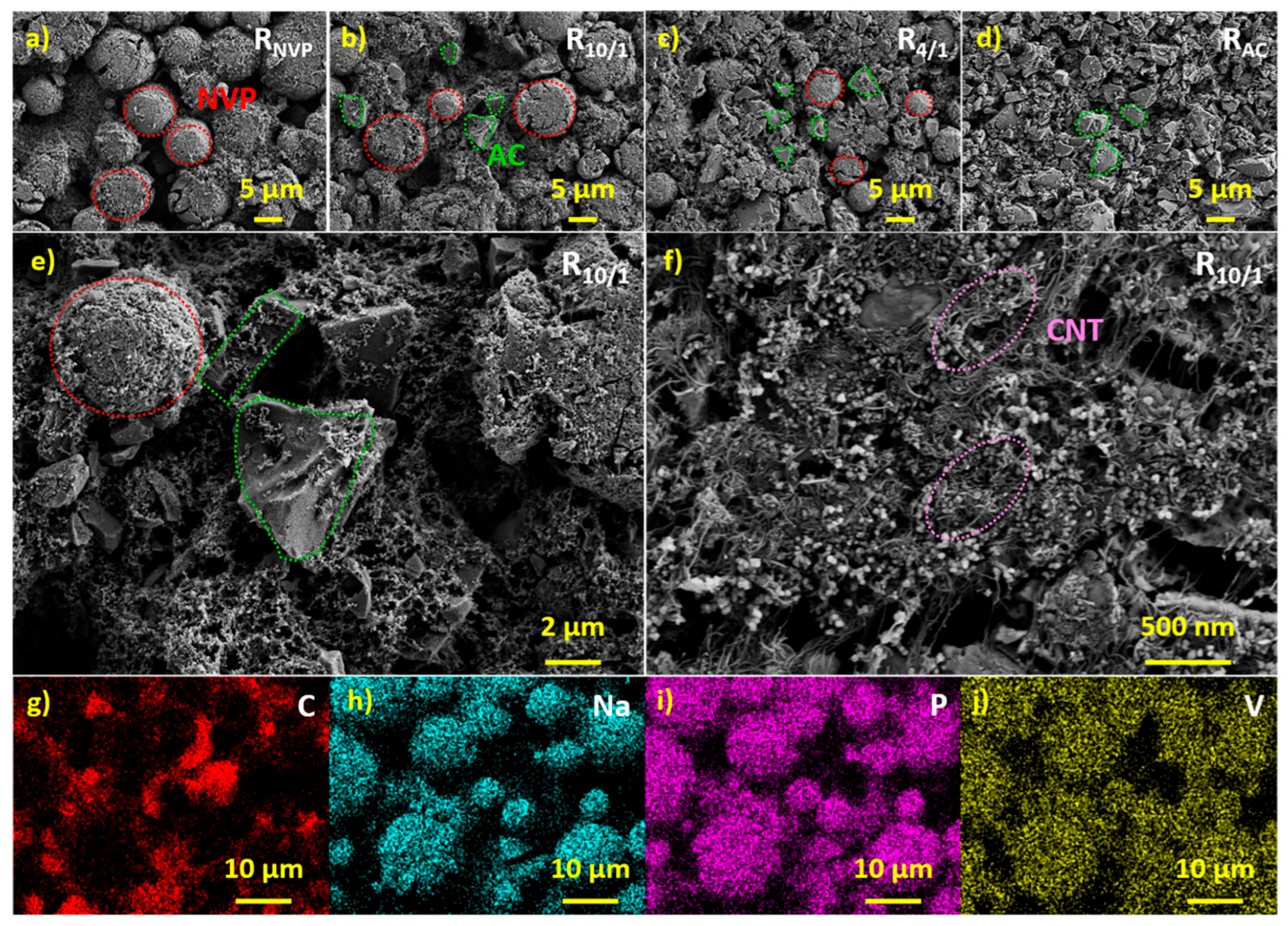
The surface morphologies of cathode slices were characterized via SEM.
Figure 1a–d display the images of R
NVP, R
10/1, R
4/1, and R
AC at a magnification of 2k×, where NVP appears as regular spherical particles, while AC presents as irregular lumpy particles. To optimize electrochemical performance, a dual conductive agent system was employed. Higher–magnification SEM images of R
10/1 (
Figure 1e,f) demonstrate that long–range conductive MWCNTs form percolating networks bridging AC and NVP particles, while short–range conductive SP fills interparticle voids. Their incorporation significantly improves electrical conductivity of both the horizontal and vertical surfaces of electrode slices. Elemental mapping (
Figure 1g–j) confirms the homogeneous distribution of C, Na, P, and V throughout the R
10/1 slice. Round NVP particles and irregularly shaped AC particles can be distinguished using their color–coded positions. The uniform element distribution across the slice not only confirms the homogeneous mixing of active materials, conductive agents, and binder but also validates the effectiveness of our slurry preparation.
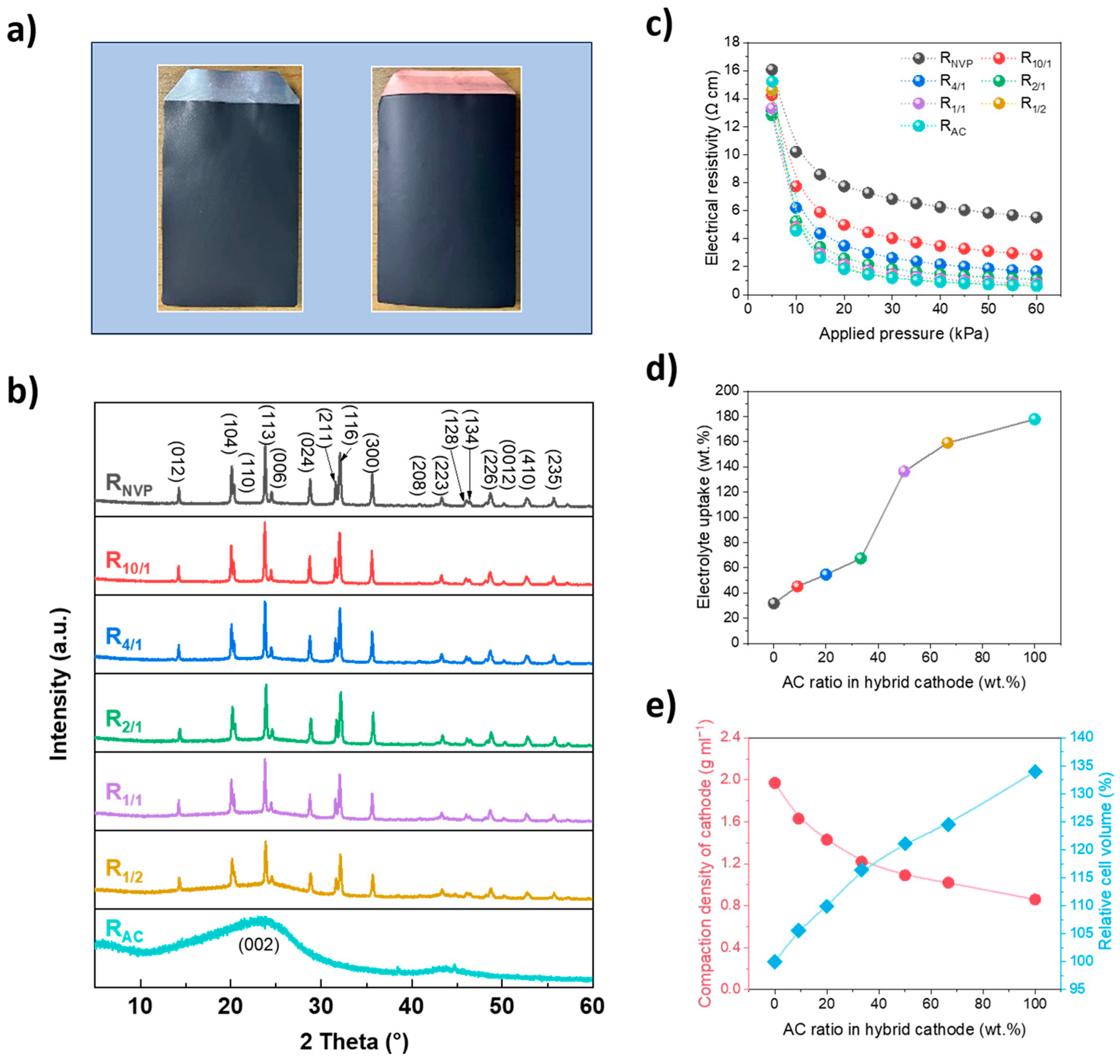
The digital images of cathode and anode slices are presented in
Figure 2a, where both samples exhibit a uniform, smooth, and dense morphology after the rolling process. XRD analysis (
Figure 2b) reveals the crystallographic features of cathode slices with varying NVP/AC mass ratios. The R
NVP slice exhibits characteristic diffraction peaks corresponding to a rhombohedral structure with an
R3c space group, consistent with that of pure NVP (JCPDS No. 53–0018) [
24]. For R
AC, the broad diffraction peaks centered at 23.4° (002) and 43.5° (100) exhibit typical amorphous carbon features [
25]. Hybrid cathodes demonstrate superimposed diffraction patterns containing both crystalline NVP and AC components, with AC–derived features being most pronounced in R
1/1 and R
1/2 configurations where AC ratio is ≥50 wt.%. Progressive attenuation of amorphous carbon signatures occurs with increasing NVP content, becoming negligible at AC ratios of ≤20 wt.% due to the dominant signal contribution from the highly crystalline NVP.
The incorporation of AC significantly modifies both electrical and electrochemical properties.
Figure 2c demonstrates a pronounced pressure–dependent resistivity behavior, where increasing applied pressure from 5 to 60 MPa reduces electrical resistivity across all cathode slices. This highlights the critical role of particle–to–particle contact in establishing effective conductive networks during slice preparation. However, the competing effects between enhanced interparticle contact versus reduced electrode porosity should be considered carefully, which would influence the electrode stability [
26]. The AC component contributes to both conductivity enhancement and electrolyte accessibility. At an applied pressure of 60 MPa, R
10/1 and R
4/1 exhibit significant resistivity reductions of 48.6% and 75.2%, respectively, compared with R
NVP. The conductivity enhancement nearly saturates at an NVP/AC mass ratio of 2:1, suggesting the threshold of AC addition.
The marked difference in SSA between AC and NVP fundamentally governs the electrolyte–uptake behavior. As shown in
Figure 2d, a strong correlation exists between AC ratio and electrolyte–uptake capability, with values ranging from 31.7 wt.% for R
NVP to 177.8 wt.% for the R
AC slice. This enhancement results from the tenability of slice porosity and AC’s hierarchical pore structure: micropores providing high surface areas for electrolyte wetting; mesopores facilitating rapid ion transport; macropores serving as electrolyte reservoirs [
27]. Improving electrolyte–uptake capability is generally conducive to mitigating polarization and preventing electrolyte depletion during long–term cycling.
Moreover, the intrinsic tap density difference between active materials affects the electrode slice compaction behavior. As shown in
Figure 2e, R
NVP achieves the highest compaction density of 1.97 g cm
−3, approximately 2.3 times that of the R
AC slice (0.86 g cm
−3). With increasing AC content, the compaction density progressively decreases from 1.63 g cm
−3 for R
10/1 to 1.02 g cm
−3 for R
1/2. Notably, under equivalent areal loading conditions, R
AC (SIC) exhibits a 34.0% larger cell volume than R
NVP (SIB). In contrast, hybrid cathodes demonstrate tunable volume efficiency, with R
10/1 and R
4/1 only showing 5.6% and 9.9% relative volume increases relative to R
NVP, respectively. This hybrid design with balance between volume density and slice porosity offers a critical advantage for space–constrained applications compared with traditional SICs.
3.2. Electrochemical Performance Evaluation in Half Cells
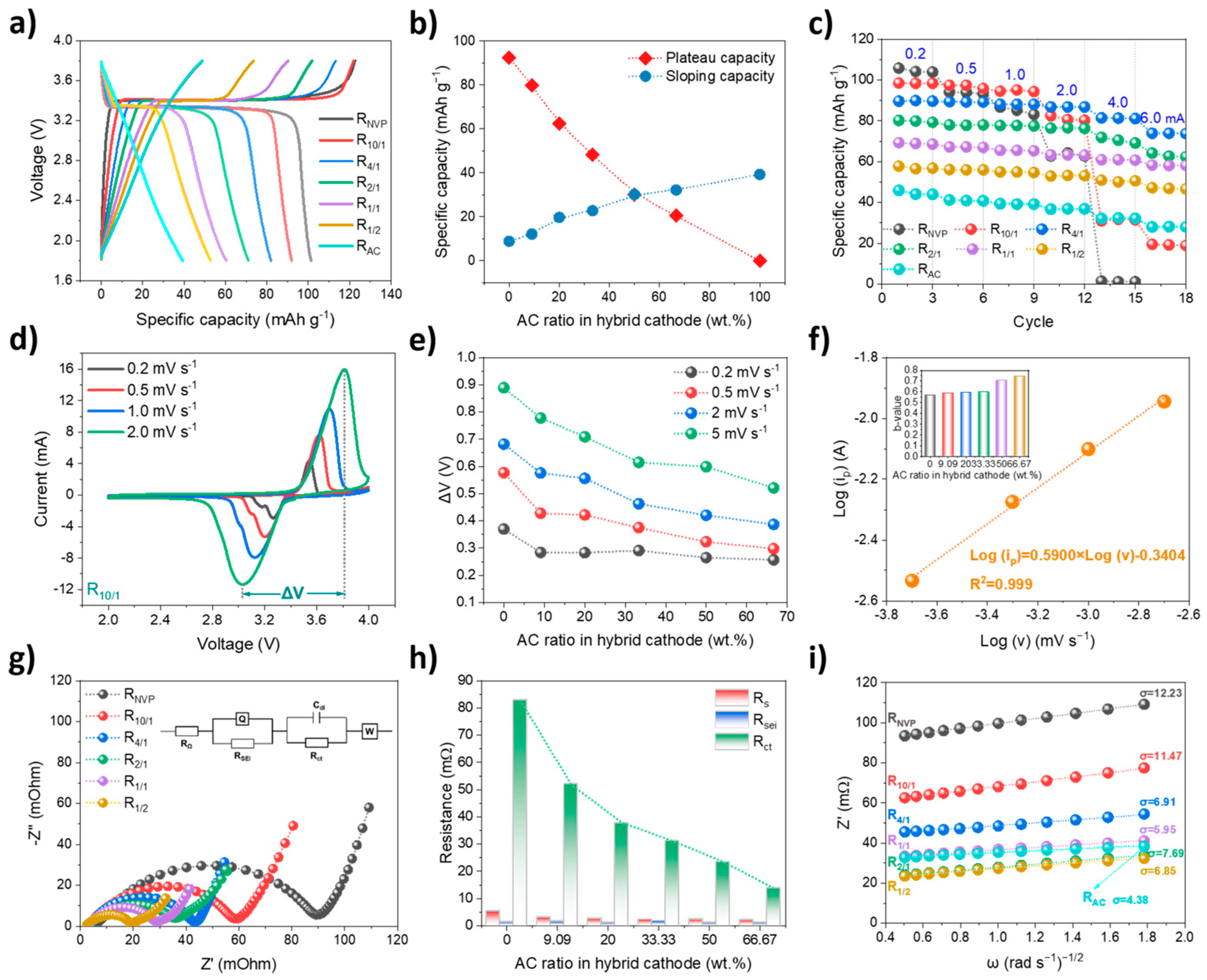
Prior to full–cell assembly, the electrochemical properties of various cathodes were evaluated in half cells within 1.8–3.8 V. As shown in
Figure 3a, R
AC exhibits near–liner GCD profiles, indicative of dominant capacitive behavior. In contrast, NVP–containing cathodes display a combination of flat redox plateaus and sloping regions, suggesting a hybrid EES mechanism involving both Faradaic Na
+ intercalation/deintercalation from NVP and non–Faradaic ion adsorption/desorption on AC. A clear correlation is observed between cathode composition and specific capacity (
Figure 3b). R
NVP and R
AC deliver specific capacities of 101.4 and 39.3 mAh g
−1, with capacitive contributions (quantified via sloping regions) accounting for 5.8% and 100% of the total capacity, respectively. As the NVP/AC mass ratio decreases from 10:1 to 1:2, the cathodic capacity declines from 92.1 to 52.8 mAh g
−1, with the capacitive contribution increasing from 8.4% to 35.4%, highlighting the growing role of capacitive controlled processes. Note that the corresponding N/P capacity ratios are 1.46, 1.61, 1.80, 2.08, 2.44, 2.80, and 3.76, respectively, according to the specific capacities of the various cathodes and hard carbon anode (300 mAh g
−1). A near–linear relationship between the sloping capacity and AC ratio confirms the tunability of the storage mechanism through compositional design. The influence of AC on electrochemical kinetics was investigated through rate tests, as shown in
Figure 3c. Increasing the AC content significantly improves capacity retention at high currents, particularly in cells with AC ratios of ≥20 wt.%. This trend underscores the superior rate capability of capacitive–dominated materials. Under a symmetric charge–discharge current regime, R
NVP exhibits a sharp capacity drop to negligible values even at a moderate current of 0.4 mA (6.45 A g
−1), with retention far below other cathodes. Although R
10/1 shows improved performance over R
NVP—delivering 19.2 mAh g
−1 with 19.5% retention at an elevated current of 0.6 mA (9.68 A g
−1)—it is substantially outperformed by R
4/1, which achieves the highest capacity of 73.9 mAh g
−1 with 82.2% retention. This result emphasizes the limited effectiveness of insufficient AC addition in improving rate performance. The hybrid cathodes demonstrate a clear synergistic effect, significantly exceeding pure AC, pure NVP, and their capacity sum at high currents.
A detailed electrochemical investigation of various cathodes was conducted via CV measurements. R
AC exhibits a nearly rectangular CV profile—consistent with typical capacitive charge storage behavior. While NVP–containing cathodes display pronounced redox peaks, corresponding to reversible Na
+ deintercalation/intercalation in a NVP lattice [
22]. Representative CV curves at scan rates ranging from 0.2 to 2 mV s
−1 for the R
10/1 cathode are presented in
Figure 3d. With increasing scan rate, the oxidation peak shifts toward higher potentials, while the reduction peak shifts towards lower potentials, indicating enhanced cell polarization. The potential difference (ΔV) between the oxidation and reduction peaks, as derived from CV curves in
Figure 3d, is used to evaluate kinetic limitations within cathodes [
28]. As summarized in
Figure 3e, R
NVP exhibits the largest ΔV across all scan rates, indicating pronounced polarization and slow reaction kinetics. In contrast, hybrid cathodes demonstrate progressively smaller ΔV values as the AC ratio increases, confirming that AC incorporation significantly reduces electrode polarization. This improvement is particularly evident when the NVP/AC mass ratio falls below 4:1. The current response (i
p) as a function of scan rate (ν) is analyzed using the power–law relationship [
29], where the b–value derived from the fitting analysis serves as an indicator of the charge storage mechanism. As shown in
Figure 3f, the b–value (inset) for the redox peak increases from 0.569 to 0.743 with increasing AC ratio from 0 to 66.67 wt.%, confirming enhanced contributions from the capacitive controlled processes. The b–values are comparable across samples with NVP/AC mass ratios of 10:1–2:1. However, a pronounced b–value increase occurs when the NVP/AC mass ratio decreases from 2:1 (b = 0.597) to 1:1 (b = 0.706), marking the point where capacitive contributions become more prominent.
Additionally, EIS was employed to investigate the resistance behavior, with Nyquist plots presented in
Figure 3g, along with an inner equivalent circuit model for quantitative fitting. The circuit includes series resistance (R
s), solid–electrolyte interphase resistance (R
sei), charge–transfer resistance (R
ct), and Warburg impedance (Z
w). Clearly, the semicircle (combined R
sei + R
ct) in the Nyquist plot is noticeably compressed as the AC content increases, indicating exceptionally favorable charge–transfer kinetics. This trend is corroborated by subsequent detailed EIS fitting results, as summarized in
Figure 3h. Compared with R
s and R
sei, R
ct constitutes the dominant component of the total resistance. The observed R
ct reduction with increasing AC ratio highlights the enhanced charge–transfer kinetics, attributable to improved ionic transport at electrode–electrolyte interface and more efficient electronic percolation throughout hybrid cathode. Specifically, R
NVP exhibits the highest R
ct of 83.0 mΩ, with this value decreasing substantially to 52.2 mΩ for R
10/1, and, ultimately, dropping to 13.8 mΩ for R
1/2. This progressive R
ct reduction aligns with the decreased polarization observed in CV measurements, further underscoring the beneficial role of AC in facilitating faster kinetics. Beyond mitigating charge–transfer impedance, the AC incorporation exerts a significant impact on ion diffusion behavior. As illustrated in
Figure 3i, the real part of impedance (Z′) exhibits a linear dependence on the inverse square root of angular frequency (ω
−1/2), allowing for the determination of Warburg diffusion factor (σ) and subsequent calculation of sodium–ion diffusion coefficients (D
Na+) for each cathode [
30]. With increasing AC content, σ shows a clearly decreasing trend, with R
4/1 representing a critical optimization point. Specifically, σ decreases from 12.23 for R
NVP to 6.91 for R
4/1, corresponding to a D
Na+ increase from 1.31 × 10
−13 to 4.10 × 10
−13 cm
2 s
−1. Further increasing AC ratios beyond 20 wt.% has a negligible effect on D
Na+, indicating the saturated AC–facilitated diffusion–enhancing effect. Overall, the simultaneous reduction in R
ct and enhancement of D
Na+ are attributed to the synergistic effects arising from the NVP/AC hybrid design, including improved electrical conductivity, greater electrolyte permeability, and facilitated ion transport. The NVP/AC mass ratio of 4:1 is identified as optimal in half–cell system, as it delivers satisfactory capacity alongside superior rate performance—benefiting from significantly decreased R
ct and improved D
Na+. These results underscore that the strategic integration of NVP and AC creates a complementary interplay between diffusion–controlled and capacitive mechanisms, enabling hybrid cathodes with low polarization and superior kinetic performance.
3.3. Electrochemical Performance Evaluation in Full Cells (SIBatCs)
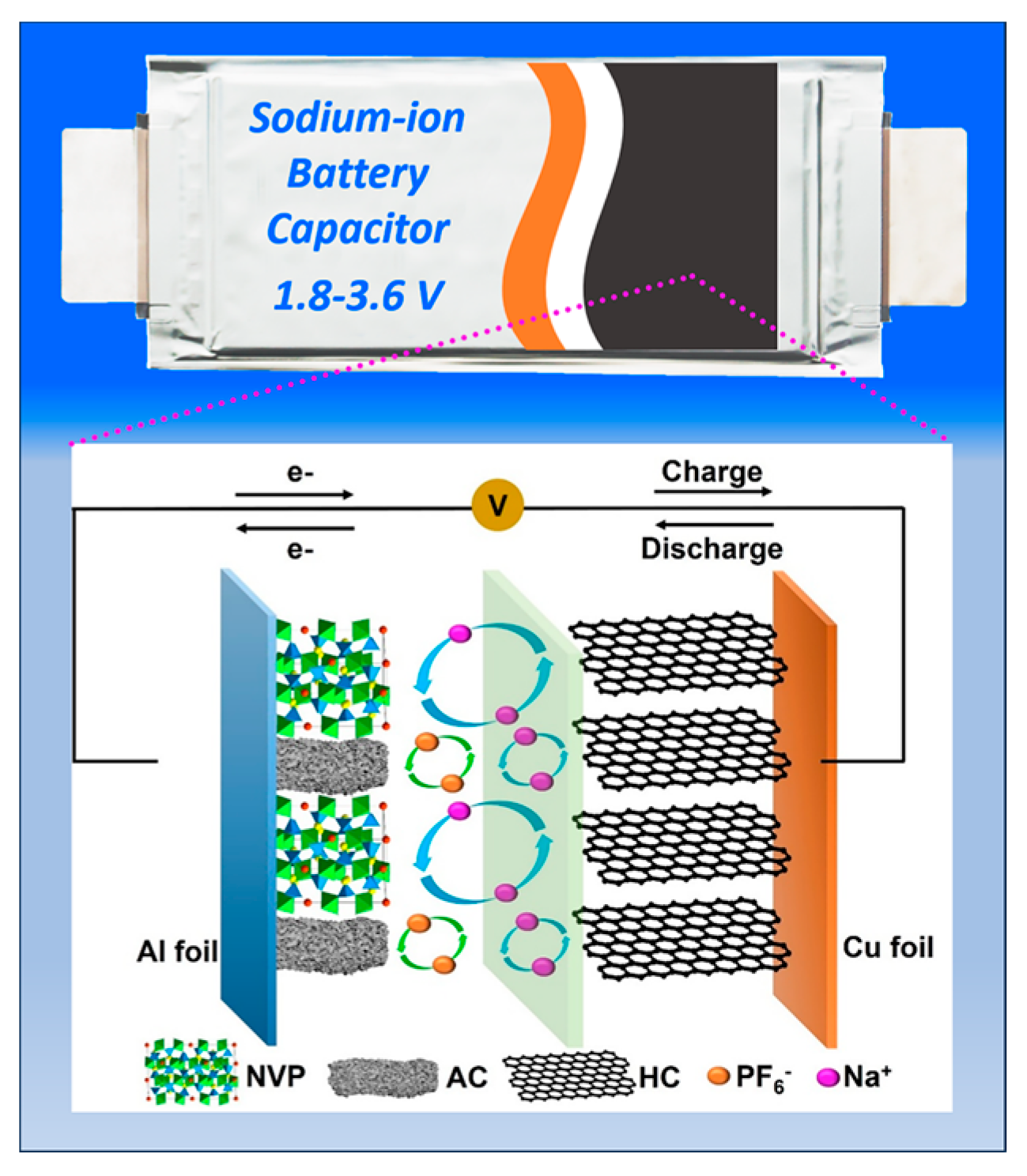
Compared with half cells, full cells yield more convincing electrochemical data, as their performance more closely reflects the real–world operating conditions. As schematically illustrated in
Figure 4, SIBatC is a hybrid EES device that integrates SIC and SIB components in an inter–parallel configuration at the electrode level. Owing to the complementary nature of NVP and AC, both Faradaic and non–Faradaic reactions occur concurrently during the entire energy storage operation [
22]. Specifically, during charging and discharging, PF
6− anions are adsorbed/desorbed on the AC surface, while Na
+ cations are reversibly deintercalated/intercalated within the NVP framework. To enhance performance, an optimized cellulose/PET composite separator was incorporated, improving electrolyte retention and facilitating rapid ion transport [
31].
Owing to the utilization of hard carbon anodes, the electrochemical behavior of each cathode may differ between full cells and half cells, and the formation process plays a critical role in activating SIBatCs. As shown in
Figure 5a, increasing AC content results in a decline in both the discharge capacity and initial coulombic efficiency (ICE). R
NVP demonstrates the highest performance, with a cathodic specific capacity of 93.1 mAh g
−1 and an ICE of 80.3%. As the NVP/AC mass ratio decreases from 10:1 to 1:2, the cathodic capacity drops from 88.0 to 31.3 mAh g
−1, while the anodic capacity decreases from 193.0 to 68.1 mAh g
−1, with the corresponding ICE declining from 77.9% to 44.1%. In contrast, R
AC exhibits particularly poor performance, with a discharge capacity of only 13.2 mAh g
−1 and an ICE of 7.5%, along with noticeable gas evolution. The reduced anodic capacity from 193.3 mAh g
−1 for R
NVP to 30.3 mAh g
−1 for R
AC demonstrates limited anode utilization. This imbalance forces the cathode to operate at higher potentials as the AC ratio increases within the fixed voltage window, promoting side reactions and accelerating performance degradation [
32].
The cathodic specific capacity (
C) and cell energy density (
E) are highly consistent with Equations (1) and (2) through fitting analysis, where
r denotes the AC ratio (wt.%) in the hybrid cathode. The units of
C and
E are mAh g
−1 and Wh kg
−1, respectively. This provides valuable insight for estimating the capacity and energy of SIBatCs incorporating dual–functional hybrid cathodes.
The GCD curves within 1.8–4.0 V at a current of 100 mA are presented in
Figure 5b. R
AC exhibits a fully linear profile, whereas NVP–containing curves consist of sloping segments and a flat voltage plateau. The capacity contribution derived from sloping regions correlates positively with the AC content. For R
2/1, the voltage plateau is barely discernible, suggesting dominant capacitive behavior in this hybrid configuration. Raising the operating voltage significantly improves energy output, particularly in AC–rich cathodes. As shown in
Figure 5c, elevating the upper cutoff voltage from 3.4 to 4.0 V results in an 6.3% energy enhancement for R
NVP, compared with 11.6–39.7% for R
10/1–R
1/1, in order. This underscores the voltage–sensitive energy storage behavior derived from capacitive materials, exceeding the limited voltage–dependent capacity increase in battery–type NVP. However, R
1/2 and R
AC exhibited severe gassing behavior above 3.6 V, mainly attributed to cathode overpotential under presodiation–free conditions [
39]. To ensure comprehensive performance, an optimal voltage window of 1.8–3.6 V is selected for SIBatCs.
Rapid self–discharge is a well–known limitation for supercapacitors, while the incorporation of battery–type material effectively suppresses this behavior. As illustrated in
Figure 5d, the self–discharge rate increases from 0.156 to 0.354 mV h
−1 as the AC ratio rises from 0 to 66.7 wt.%, with R
AC exhibiting the most rapid voltage decay. This is closely linked to the energy storage mechanism—Faradaic materials provide greater thermodynamic stability [
40]. The judicious inclusion of AC also beneficially influences resistance. As observed in
Figure 5e, R
10/1 and R
4/1 achieve the lowest DCR values of 58.9 and 50.1 mΩ, respectively, representing reductions of 6.4% and 20.3% relative to R
NVP (62.9 mΩ). Notably, the DCR increases sharply from 64.7 mΩ for R
2/1 to 90.7 mΩ for R
1/1—marking a critical threshold (33.3 wt.% AC). Beyond this ratio, the conductivity gains from AC are offset by the increased charge–transfer resistance, as illustrated in
Figure 6.
Rate capability—encompassing both rapid charge and discharge—was evaluated under different regimes. The constant–current charge ratio (CCCR) during CC–CV process serves as a key indicator of fast–charging capability. Under fast charge–slow discharge conditions (
Figure 5f), CCCR declines with increasing current, yet cells with higher AC content consistently outperform those dominated by NVP, confirming superior charge kinetics of hybrid cathodes. However, when the AC ratio is ≥50 wt.%, the capacity deteriorates and the overall competitiveness diminishes, indicating a trade–off between fast–charge acceptance and energy delivery. In the slow charge–fast discharge mode (
Figure 5g,h), R
NVP delivers the highest capacities at low–to–medium currents yet suffers significant degradation under high–current conditions. At a current of 50 A (329C), R
10/1 delivers the highest discharge capacity and retention, with values of 82.5 mAh and 44.9%, respectively. Both metrics outperform those of the R
NVP counterpart, demonstrating the excellent compatibility of R
10/1 with high–power operations. Notably, even under significantly higher current rates, R
4/1 and R
2/1 exhibit capacity retentions of 37.9% and 36.9%, respectively—values comparable to that of R
NVP (37.8%). Ragone plots derived from slow charge–fast discharge mode are presented in
Figure 5i. R
10/1 and R
4/1 achieve energy densities of 148.9 and 120.6 Wh kg
−1 at power densities of 81.0 and 79.3 W kg
−1, respectively. Even at ultrahigh power densities of 30,526.6 and 29,810.6 W kg
−1, they still retain energy densities of 50.4 and 39.6 Wh kg
−1, outperforming reported sodium–based EES devices [
12,
23,
33,
34,
35,
36,
37,
38]. Note that the mass of active materials accounts for 23.4% and 23.0% of the total mass of R
10/1 and R
4/1 configurations, respectively. This superior energy–power trade–off is particularly evident within the NVP/AC mass ratio range of 10:1 to 2:1, underscoring the efficacy of hybrid cathode design. The rate enhancement predominantly stems from the multifunctional role of the hybrid cathode design: it improves electrical conductivity, reduces internal resistance and polarization, increases electrolyte accessibility and retention, and facilitates rapid ion transport. Together, these contributions enable simultaneous high energy and power densities, positioning SIBatCs as competitive EES candidates.
To elucidate the influence of NVP/AC mass ratio on SIBatC kinetics, CV and EIS were carried out.
Figure 6a,b shows representative CV curves of R
2/1 and R
1/1 at varying scan rates. The presence of a presodiation–free HC anode significantly affects the CV shapes, which differs from those observed in half cells. As the AC content increases, the CV curves evolve continuously, with the coupling behaviors of NVP, AC, and HC becoming particularly evident in the SIBatC incorporating R
1/1 (
Figure 6b). Additionally, ΔV values for various SIBatCs are summarized in
Figure 6c. As the AC ratio increases, ΔV decreases monotonically until the system reaches an optimal NVP/AC mass ratio of 1:1, demonstrating the continued polarization. This kinetic enhancement serves as the foundation for the improved rate capability in hybrid cathode configurations. However, further increasing the AC content leads to a rise in ΔV—even larger than that of pure NVP—a behavior not observed in half cells, which is attributed to N/P capacity mismatch in full–cell configurations. Such an imbalance forces the cathode to operate at higher potentials, which promote the over–deintercalation of Na
+ from the NVP framework, ultimately resulting in structural degradation and accelerated failure. The anodic log(i
p) vs. log(ν) curves for SIBatCs incorporating NVP–containing cathodes are illustrated in
Figure 6d. Linear fitting results indicate that the b–value increases with rising AC ratios, ranging from 0.558 for R
NVP to 1.081 for R
1/1. A pronounced b–value increase is observed as the NVP/AC mass ratio shifts from 4:1 to 2:1, suggesting a notable enhancement in capacitive contribution. However, further increasing the AC ratio results in a markedly decreased b–value, indicating reduced kinetics associated with increased polarization as shown in
Figure 6c. These findings highlight the critical need to optimize NVP/AC mass ratios—not only to minimize polarization and enhance kinetics but also to maintain electrode balancing in full–cell configurations.
The Nyquist plots of SIBatCs are shown in
Figure 6e, with the semicircle in the high–to–medium frequency region corresponding to the combined interfacial film resistance (R
f) and charge–transfer resistance (R
ct). The AC addition significantly reduces combined resistance (R
f + R
ct), with the most pronounced decrease observed for R
10/1 and R
4/1. However, as the NVP/AC mass ratio reaches 2:1, the (R
f + R
ct) exceeds that of R
NVP, and further increasing the AC ratio results in a dramatical rise in (R
f + R
ct). Both full cells and half cells demonstrate that the hybrid cathode design significantly reduces polarization and resistance. Notably, distinct behaviors in CV and EIS results are observed between the two configurations, which mainly stem from the coupling effect of the presodiation–free HC anode.
Distribution of relaxation times (DRT) analysis is a powerful deconvolution tool that resolves overlapping impedance processes into distinct, physically meaningful relaxation times, thereby overcoming the limitations of traditional equivalent circuit models for complex systems like ours. To quantify polarization contributions from individual electrochemical processes, DRT analysis was employed, with results illustrated in
Figure 6f. The DRT profiles reveal distinct time–constant regions corresponding to specific impedance sources: the peak in the range of 10
−4–10
−2 s is associated with the solid–electrolyte interphase (R
f); the peak within 10
−2–1 s represents charge–transfer resistance (R
ct); the peak within 1–10
2 s is related to solid–state ion transport in active materials (Z
w) [
41,
42]. For R
NVP and R
10/1, two discernible peaks are observed near 0.03 s and 12.5 s, with charge–transfer resistance dominating the overall polarization. However, when the AC ratio increases to ≥20 wt.%, the peak near 0.03 s splits into two peaks centered near 0.01 s and 0.045 s, corresponding to R
f and R
ct, respectively. Apparently, the AC incorporation reduces both R
ct and Z
w particularly at an NVP/AC mass ratio range of 10:1–4:1, suggesting enhanced interfacial ion transport and ion diffusion, which is attributed to AC’s superior electrical conductivity and improved electrolyte accessibility [
43]. When the AC ratio further increases to ≥33.3 wt.%, τ shifts to a larger value—signaling an increased R
ct, which hinders high–power operation. For R
1/1 and R
1/2, the larger τ values, along with markedly elevated R
ct and Z
w, confirm severely hindered charge––transfer and ion diffusion, explaining their inferior electrochemical performance. These findings highlight the importance of optimizing component ratios in hybrid cathodes to balance interfacial properties, charge–transfer, and diffusion—ultimately achieving lower internal resistance and reduced polarization, facilitating an enhanced rate capability [
44].
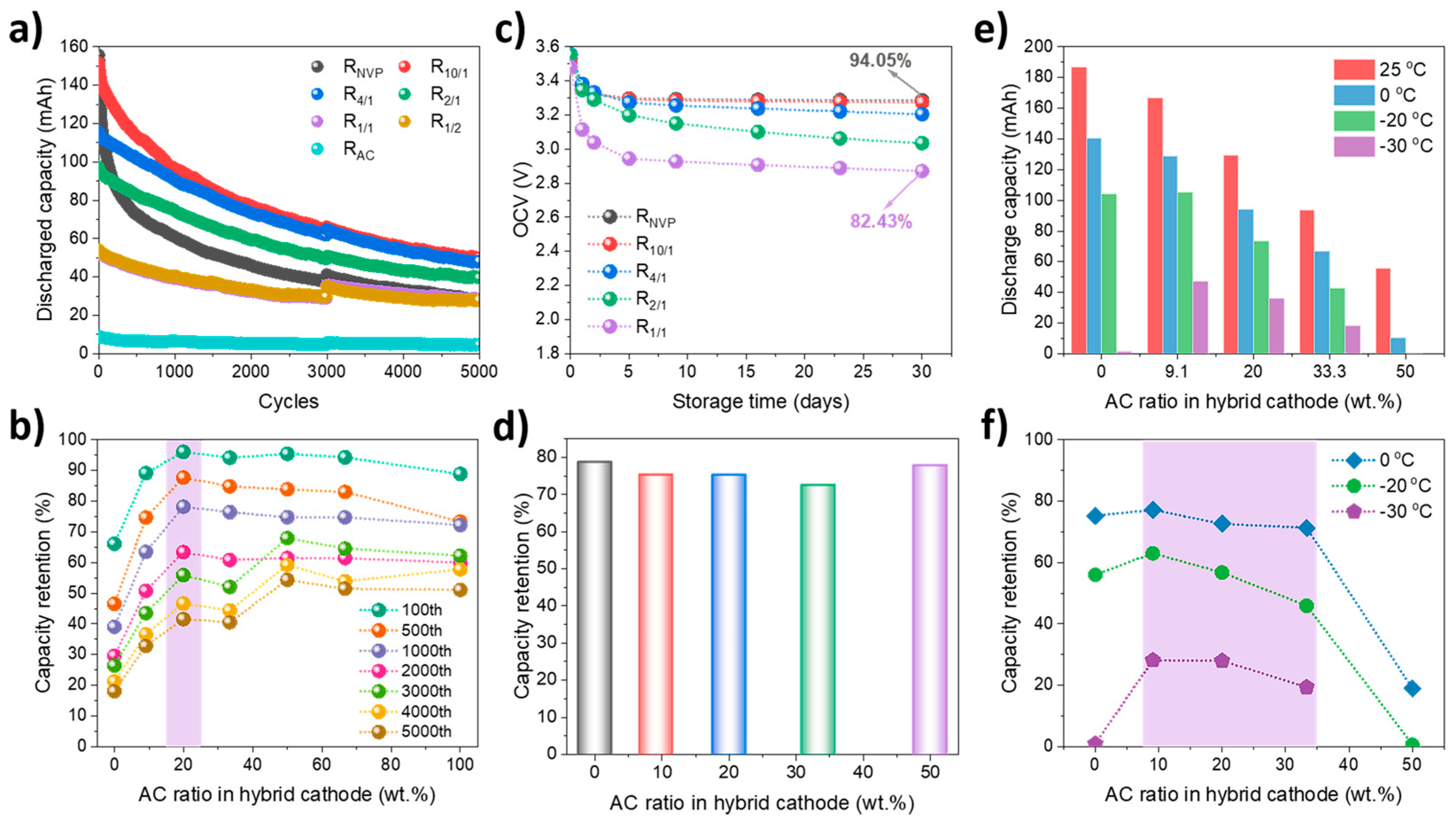
Cycling stability under high–rate conditions represents a critical advantage of SIBatCs over SIBs. As shown in
Figure 7a,b, at the current of 2 A, R
NVP and R
10/1 deliver comparable initial discharge capacities of 155.8 and 151.9 mAh, respectively. However, R
NVP exhibits a significantly accelerated capacity that fades during extended cycling compared with hybrid cathodes. Although R
4/1 displays a lower initial capacity, it surpasses R
10/1 after 1000 cycles due to its superior stability. After 5000 cycles, R
10/1, R
4/1, and R
2/1 maintain discharge capacities and retentions of (49.8 mAh, 32.8%), (48.2 mAh, 41.6%), and (39.5 mAh, 40.5%), respectively, significantly outperforming both R
NVP (28.1 mAh, 18.0%) and R
AC (4.6 mAh, 51.1%). A positive correlation between capacity retention and AC addition can be observed with AC ratios of ≤33.3 wt.%, underscoring the critical role of capacitive materials in stabilizing long–term, high–rate performance. Among samples, R
10/1 and R
4/1 exhibit the highest energy efficiency of 88.2% and 87.4%, respectively, exceeding 85.8% for R
NVP and ~60% for R
AC. This enhanced cyclability predominantly stems from the synergistic effect of the hybrid cathode design and further validates that hybrid cathodes with NVP/AC mass ratios of 10:1 and 4:1 offer an optimal balance.
Given the performance degradation associated with excessive AC addition, the high–temperature stability of SIBatCs with AC ratios of ≤50 wt.% was systematically evaluated. As shown in
Figure 7c, after 28–day storage at 60 °C, R
NVP, R
10/1, and R
4/1 demonstrated excellent OCV retention, maintaining 94.0%, 92.7%, and 90.1% of their initial values, respectively. In contrast, R
1/1 exhibits rapid voltage decay, dropping from 3.483 V to 2.944 V within the first five days. Post–storage capacity retention is summarized in
Figure 7d. As the AC ratio increases, the capacity retention decreases from 78.8% for R
NVP to 72.5% for R
2/1. Interestingly, R
1/1 shows an improved capacity retention of 77.8%, which is attributed to its lower end–of–storage OCV resulting from higher self–discharge rates. It is well established that the capacity retention of the EES device is inversely related to both storage temperature and the operational OCV, as elevated voltages and temperatures accelerate parasitic reactions such as electrolyte decomposition and electrode passivation [
45]. These results reaffirm that hybrid cathodes with AC ratios of ≤20 wt.% effectively balance energy storage and high–temperature resilience.
Low–temperature discharge capability was evaluated under a discharge current of 1 A. As shown in
Figure 7e,f, R
10/1 exhibits capacity retentions of 77.2%, 63.1%, and 28.3% at 0 °C, −20 °C, and −30 °C, respectively. In comparison, R
NVP demonstrates retentions of 75.3% and 56.0% at 0 °C and −20 °C but fails to operate at −30 °C due to excessive internal resistance (IR) drop and sluggish ion diffusion. R
4/1 and R
2/1 also maintain notable capacity retentions of 28.0% and 19.5% at –30 °C, underscoring the beneficial role of moderate AC addition in enhancing kinetics under extreme conditions. To ensure balanced performance at low–temperature environments, the AC ratio should be limited to a maximum of 33.3 wt.%, which effectively mitigates the trade–off between energy retention and polarization resistance, enabling stable operation over a wide temperature range.
Regarding the role of AC in hybrid cathodes and SIBatCs, its multifunctional contributions can be summarized as follows: firstly, as a capacitive material, AC provides adsorption/desorption capacity with superior power performance; secondly, the AC incorporation enhances the electrical conductivity of the electrode slice, thereby facilitating efficient electron transfer; thirdly, owing to the ultrahigh SSA—analogous to a sponge—AC exhibits enhanced electrolyte–uptake capability, ensuring the battery–type NVP is “immersed” in an electrolyte–rich environment and enabling faster ion transport during high–power output; and finally, the presence of AC can regulate the porosity of the electrode slice, promoting electrolyte wetting and ion diffusion. These synergistic effects collectively migrate polarization and reduce resistance, thus enhancing SIBatC power performance and cycling stability. However, excessive AC content may lead to accelerated capacity fade, as well as high self–discharge rate and gas generation in full cells without presodiation. NVP/AC mass ratios within the range of 10:1–2:1 are identified as optimal to balance power density, energy density, and cycling durability. Furthermore, proper N/P capacity matching is also essential to minimize polarization and achieve stable long–term performance in SIBatC configurations, which is in progress.