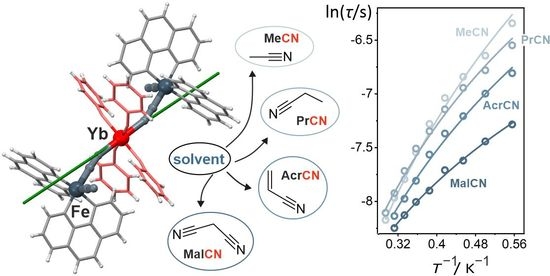
Near-Infrared Emissive Cyanido-Bridged {YbFe2} Molecular Nanomagnets Sensitive to the Nitrile Solvents of Crystallization
Abstract
1. Introduction
2. Results and Discussion
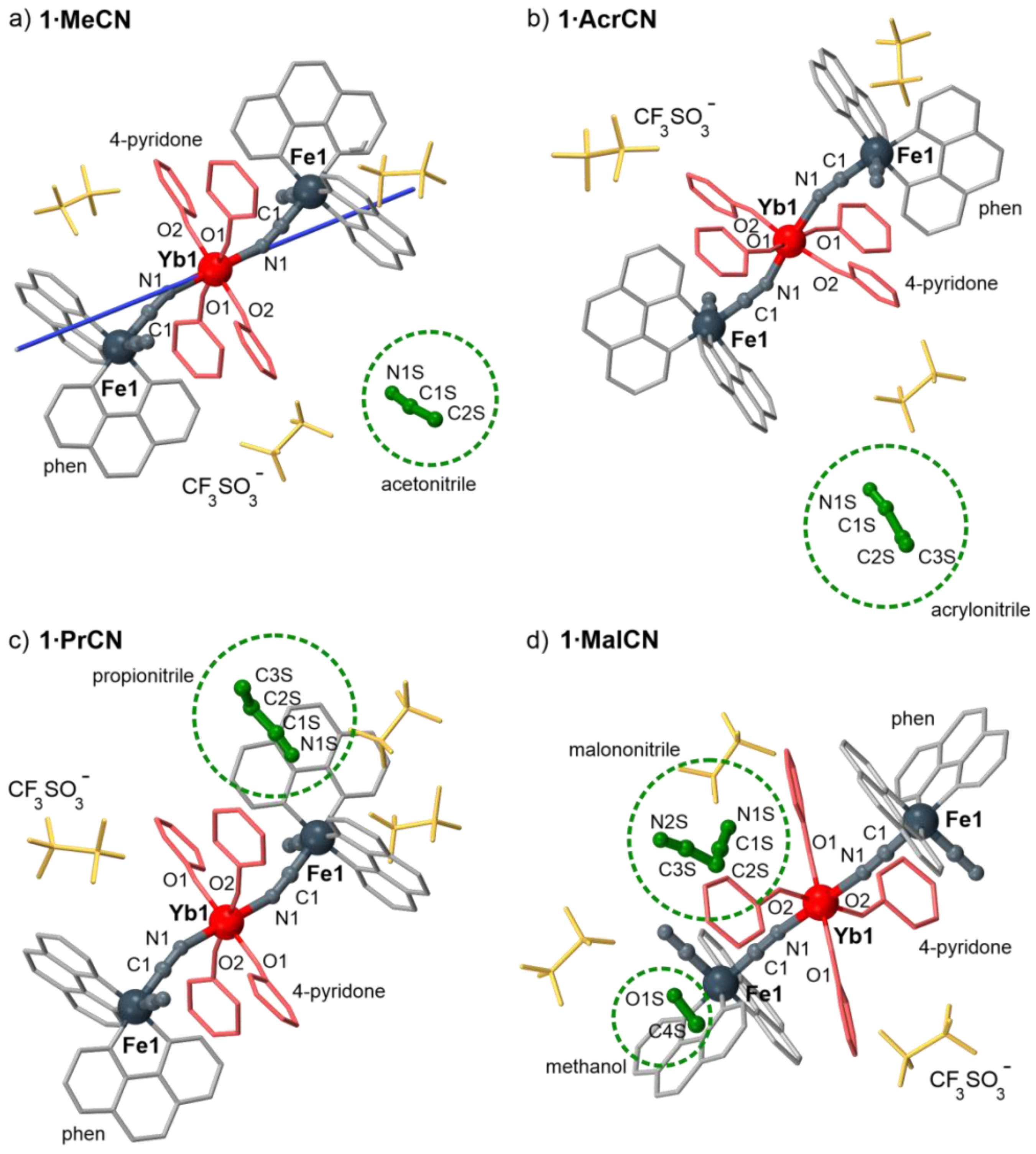
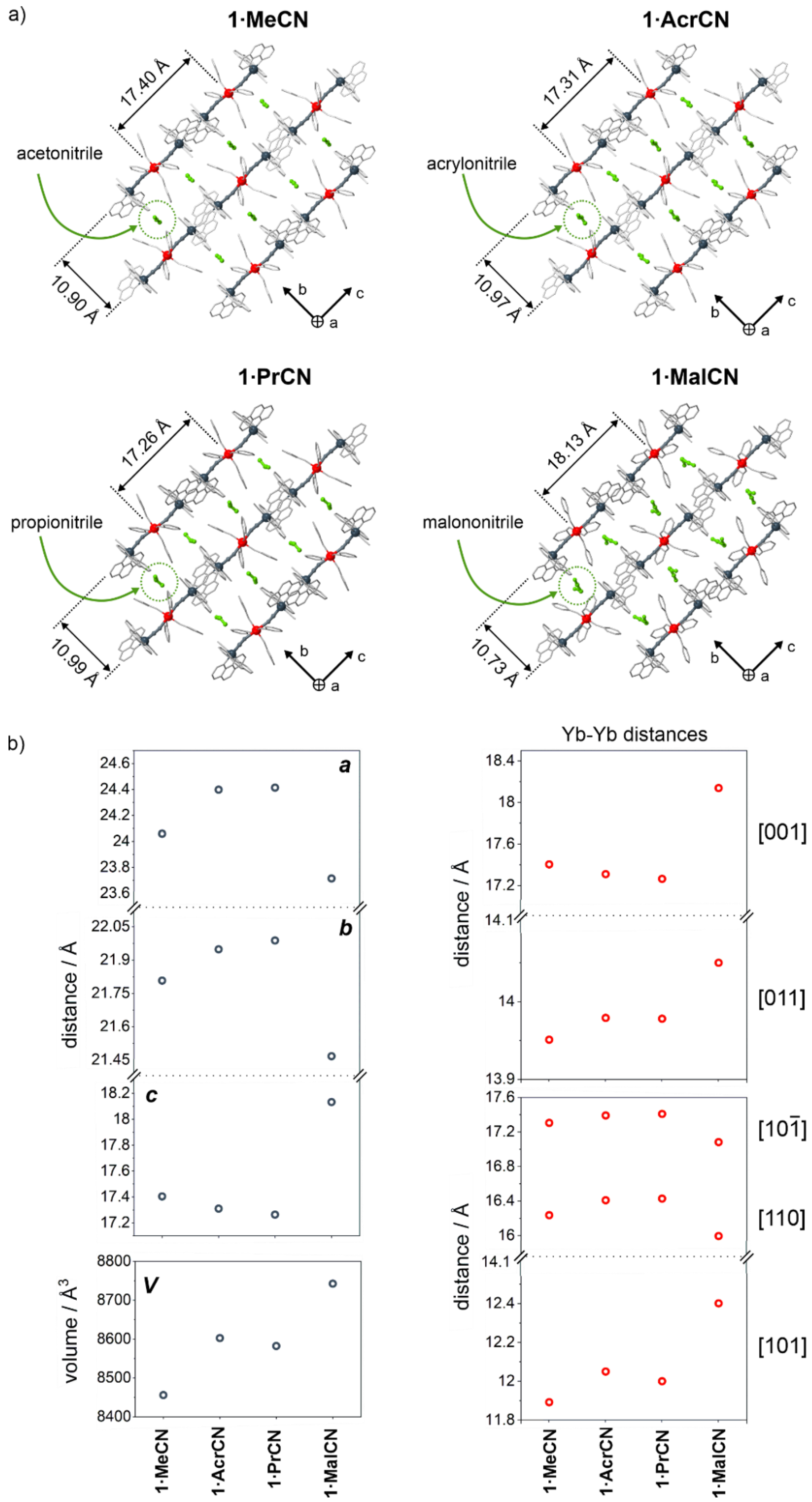
2.1. Structural Studies
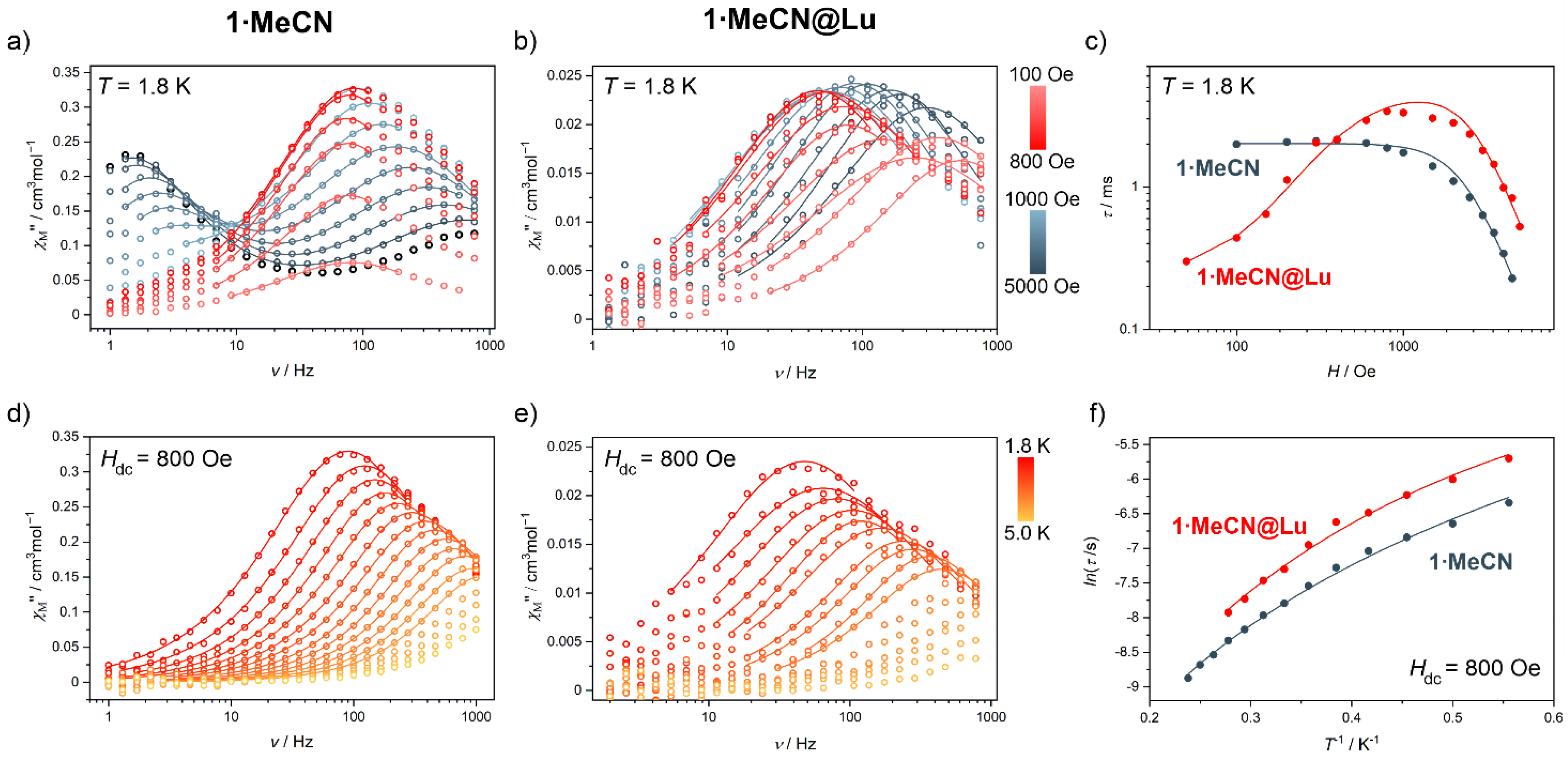
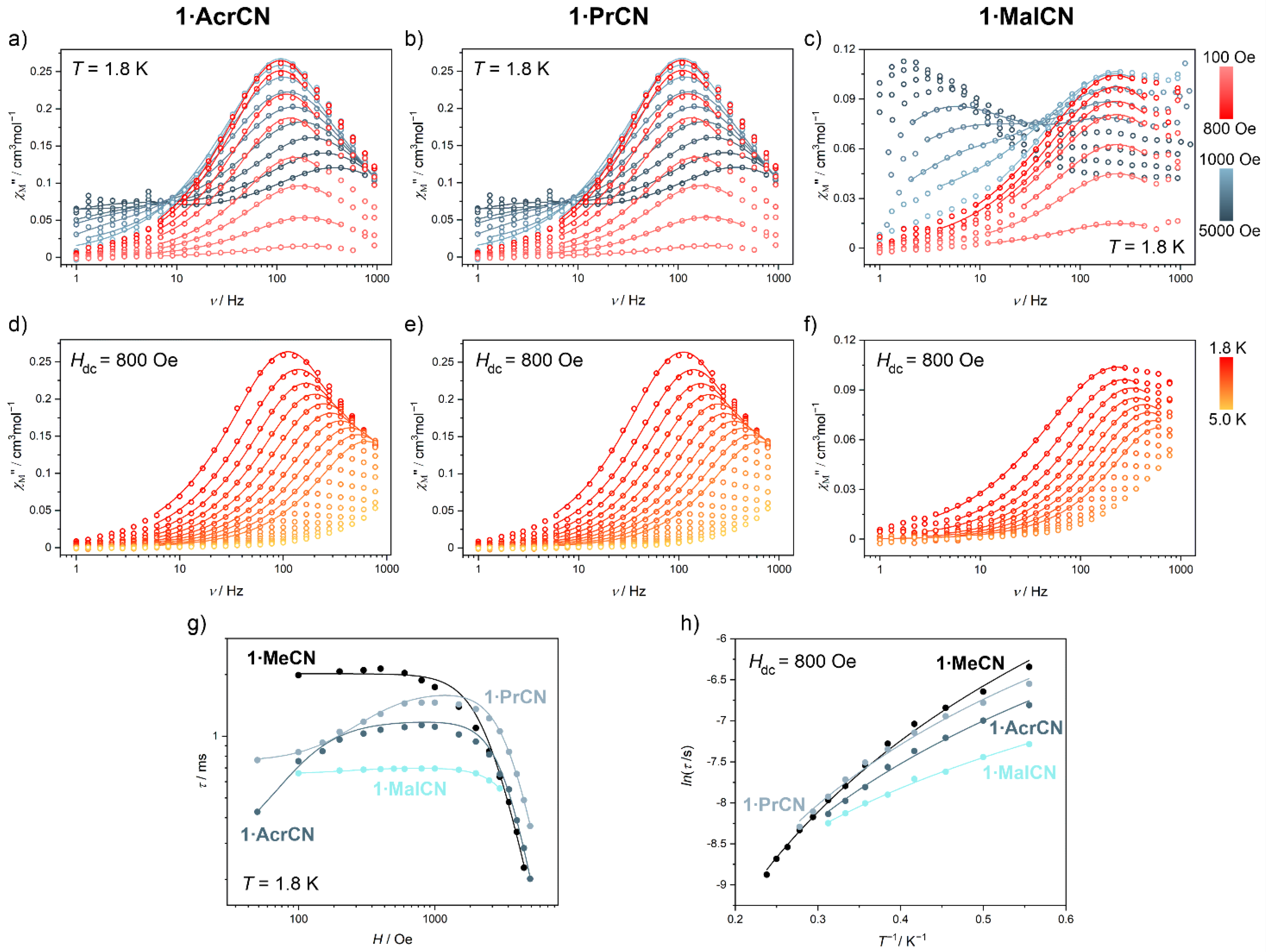
2.2. Magnetic Properties
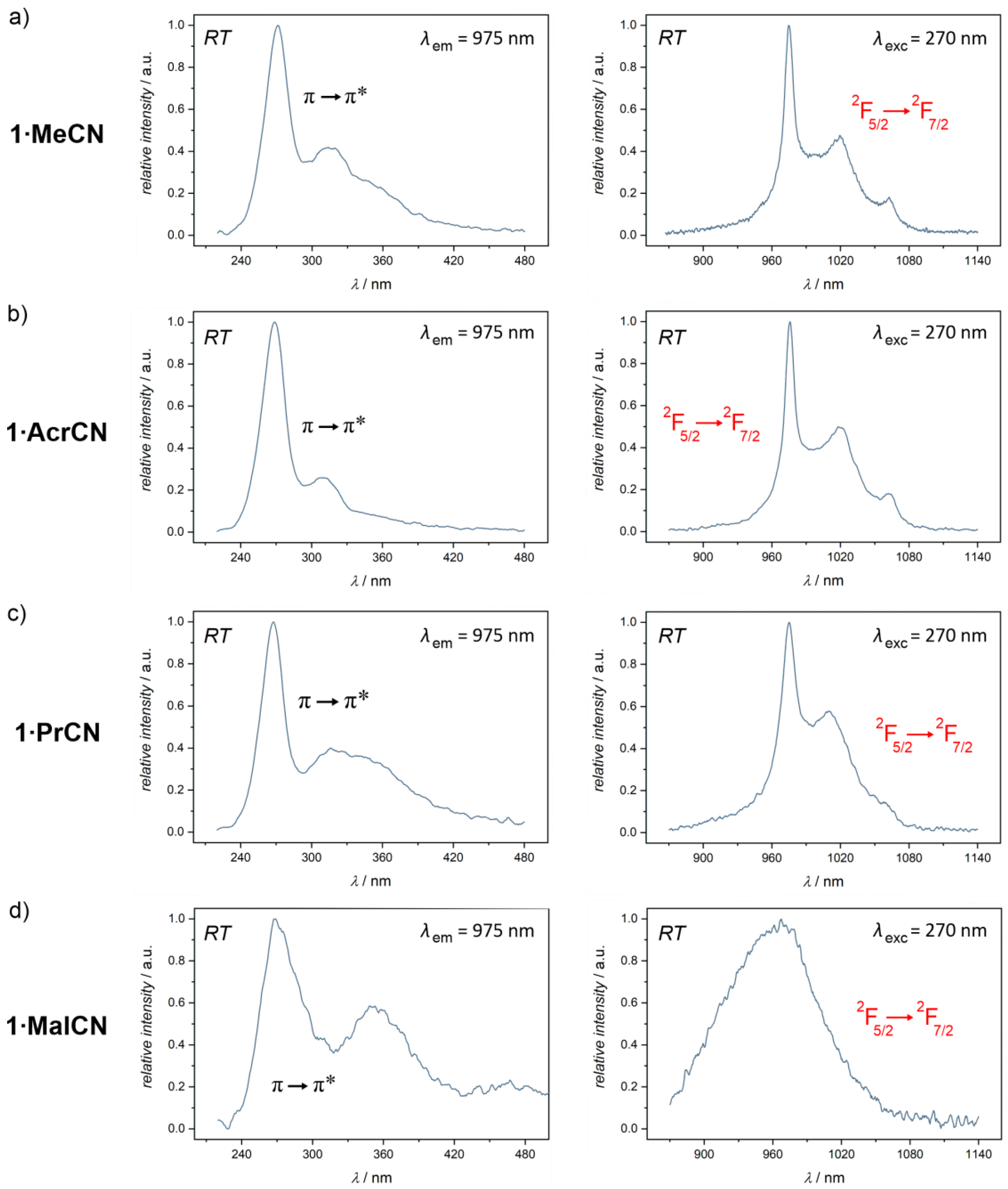
2.3. Optical Studies
3. Materials and Methods
3.1. Reagents and Materials
3.2. Synthetic Procedures and Basic Characterization
3.3. X-Ray Diffraction Analysis
3.4. Physical Techniques
3.5. Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Salonitis, K.; Pandremenos, J.; Paralikas, J.; Chryssolouris, G. Multifunctional materials: Engineering applications and processing challenges. Int. J. Adv. Manuf. Technol. 2010, 49, 803–826. [Google Scholar] [CrossRef]
- Pacchioni, G. Two–dimensional Oxides: Multifunctional Materials for Advanced Technologies. Chem. Eur. J. 2012, 18, 10144–10158. [Google Scholar] [CrossRef] [PubMed]
- Spaldin, N.A.; Ramesh, R. Advances in magnetoelectric multiferroics. Nat. Mater. 2019, 18, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.H.; Moon, H.; Kim, H.; Lee, G.H.; Kwon, W.; Yoo, S.; Myung, D.; Yun, S.H.; Bao, Z.; Hahn, S.K. Multifunctional materials for implantable and wearable photonic healthcare devices. Nat. Rev. Mater. 2020, 5, 149–165. [Google Scholar] [CrossRef]
- Allendorf, M.D.; Dong, R.; Feng, X.; Kaskel, S.; Matoga, D.; Stavila, V. Electronic Devices Using Open Framework Materials. Chem. Rev. 2020, 16, 8581–8640. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, G.; Zheng, X.; Chen, F.; Duan, H. Emerging miniaturized energy storage devices for microsystem application: From design to integration. Int. J. Extrem. Manuf. 2020, 2, 042001. [Google Scholar] [CrossRef]
- Meng, J.; Liu, X.; Niu, C.; Pang, Q.; Li, J.; Liu, F.; Liu, Z.; Mai, L. Advances in metal–organic framework coating: Versatile synthesis and broad applications. Chem. Soc. Rev. 2020, 49, 3142–3186. [Google Scholar] [CrossRef]
- Zhang, Y.; Liao, W.-Q.; Fu, D.-W.; Ye, H.-Y.; Liu, C.-M.; Chen, Z.-N.; Xiong, R.-G. The First Organic-Inorganic Hybrid Luminescent Multiferroic: (Pyrrolidinium)MnBr3. Adv. Mater. 2015, 27, 3942–3946. [Google Scholar] [CrossRef]
- Benaicha, B.; van Do, K.; Yangui, A.; Pittala, N.; Lusson, A.; Sy, M.; Bouchez, G.; Fourati, H.; Gomez-Garcia, C.J.; Triki, S.; et al. Interplat between spin-crossover and luminescence in a multifunctional single crystal iron(II) complex: Towards a new generation of molecular sensors. Chem. Sci. 2019, 10, 6791–6798. [Google Scholar] [CrossRef]
- Chorazy, S.; Zakrzewski, J.J.; Magott, M.; Korzeniak, T.; Nowicka, B.; Pinkowicz, D.; Podgajny, R.; Sieklucka, B. Octacyanidometallates for multifunctional molecule–based materials. Chem. Soc. Rev. 2020, 49, 5545–6001. [Google Scholar] [CrossRef]
- Rocha, J.; Carlos, L.D.; Paz, F.A.A.; Ananias, D. Luminescent multifunctional lanthanides-based metal-organic frameworks. Chem. Soc. Rev. 2011, 40, 926–940. [Google Scholar] [CrossRef]
- Ishikawa, N.; Sugita, M.; Ishikawa, T.; Koshihara, S.; Kaizu, Y. Lanthanide Double–Decker Complexes Functioning as Magnets at the Single–Molecular Level. J. Am. Chem. Soc. 2003, 125, 8694–8695. [Google Scholar] [CrossRef]
- Woodruff, D.N.; Winpenny, R.E.P.; Layfield, R.A. Lanthanide Single-Molecule Magnets. Chem. Rev. 2013, 113, 5110–5148. [Google Scholar] [CrossRef]
- Harriman, K.L.M.; Brosmer, J.L.; Ungur, L.; Diaconescu, P.L.; Murugesu, M. Pursuit of Record Breaking Energy Barriers: A Study of Magnetic Axiality in Diamide Ligated DyIII Single-Molecule Magnets. J. Am. Chem. Soc. 2017, 139, 1420–1423. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.-S.; Day, B.M.; Chen, Y.-C.; Tong, M.-L.; Mansikkamäki, A.; Layfield, R.A. Magnetic hysteresis up to 80 kelvin in a dysprosium metallocene single-molecule magnet. Science 2018, 362, 1400–1403. [Google Scholar] [CrossRef]
- Mannini, M.; Pineider, F.; Sainctavit, P.; Danieli, C.; Otero, E.; Sciancelepore, E.; Talarico, A.M.; Arrio, M.-A.; Cornia, A.; Gatteschi, D.; et al. Magnetic memory of a single–molecule quantum magnet wired to a gold surface. Nat. Mater. 2009, 8, 194–197. [Google Scholar] [CrossRef]
- Candini, A.; Klyatskaya, S.; Ruben, M.; Wernsdorfer, W.; Affronte, M. Graphene Spinctronics Devices with Molecular Nanomagnets. Nano Lett. 2011, 11, 2634–2639. [Google Scholar] [CrossRef]
- Thiele, S.; Balestro, F.; Ballou, R.; Klyatskaya, S.; Ruben, M.; Wernsdorfer, W. Electrically driven nuclear sing resonance in single–molecule magnets. Science 2014, 344, 1135–1138. [Google Scholar] [CrossRef]
- Bunzli, J.-C.G.; Piguet, C. Taking advantage of luminescent lanthanide ions. Chem. Soc. Rev. 2005, 34, 1048–1077. [Google Scholar] [CrossRef]
- Eliseeva, S.V.; Bünzli, J.-C.G. Lanthanide luminescence for functional materials and biosciences. Chem. Soc. Rev. 2010, 39, 189–227. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Han, S.; Huang, W.; Liu, X. Enhancing solar cell efficiency: The search for luminescent materials as spectral converters. Chem. Soc. Rev. 2013, 42, 173–201. [Google Scholar] [CrossRef]
- Zinna, F.; Pasini, M.; Galeotti, F.; Botta, C.; Di Bari, L.; Giovanella, U. Design of Lanthanide-Based OLEDs with Remarkable Circularly Polarized Electroluminescence. Adv. Funct. Mater. 2017, 27, 1603719. [Google Scholar] [CrossRef]
- Zhang, K.Y.; Yu, Q.; Wei, H.; Liu, S.; Zhao, Q.; Huang, W. Long-Lived Emissive Probes for Time-Resolved Photoluminescence Bioimaging and Biosensing. Chem. Rev. 2018, 118, 1770–1839. [Google Scholar] [CrossRef]
- Mako, T.L.; Racicot, J.M.; Levine, M. Supramolecular Luminescent Sensors. Chem. Rev. 2019, 119, 322–477. [Google Scholar] [CrossRef]
- Monteiro, J.H.S.K. Recent Advances in Luminescence Imaging of Biological Systems Using Lanthanide(III) Luminescent Complexes. Molecules 2020, 25, 2089. [Google Scholar] [CrossRef] [PubMed]
- Rocha, J.; Brites, C.D.S.; Carlos, L.D. Lanthanide Organic Framework Luminescent Thermometers. Chem. Eur. J. 2016, 22, 14782–14795. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Chorazy, S.; Nakabayashi, K.; Sato, H.; Sieklucka, B.; Ohkoshi, S. TbCo and Tb0.5Dy0.5Co layered cyanido-bridged frameworks for construction of colorimetric and ratiometric luminescent thermometers. J. Mater. Chem. C 2018, 6, 8372–8384. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Kitagawa, Y. Thermo-sensitive luminescence of lanthanide complexes, clusters, coordination polymers and metal-organic frameworks with organic photosensitizers. J. Mater. Chem. C 2019, 7, 7494–7511. [Google Scholar] [CrossRef]
- Pointillart, F.; Jung, J.; Berraud-Pache, R.; Le Guennic, B.; Dorcet, V.; Golhen, S.; Cador, O.; Maury, O.; Guyot, Y.; Decurtins, S.; et al. Luminescence and Single-Molecule Magnet Behavior in Lanthanide Complex Involving a Tetrathiafulvalene-Fused Dipyridophenazine Ligand. Inorg. Chem. 2015, 54, 5384–5397. [Google Scholar] [CrossRef]
- Chorazy, S.; Rams, M.; Nakabayashi, K.; Sieklucka, B.; Ohkoshi, S. White Light Emissive DyIII Single-Molecule Magnets Sensitized by Diamagnetic [CoIII(CN)6]3– Linkers. Chem. Eur. J. 2016, 22, 7371–7375. [Google Scholar] [CrossRef]
- Jia, J.-H.; Li, Q.-W.; Chen, Y.-C.; Liu, J.-L.; Tong, M.-L. Luminescent single—molecule magnets based on lanthanides: Design strategies, recent advances and magneto-luminescent studies. Coord. Chem. Rev. 2019, 378, 365–381. [Google Scholar]
- Xin, Y.; Wang, J.; Zychowicz, M.; Zakrzewski, J.J.; Nakabayashi, K.; Sieklucka, B.; Chorazy, S.; Ohkoshi, S. Dehydration–Hydration Switching of Single-Molecule Magnet Behavior and Visible Photoluminescence in a Cyanido-Bridged DyIIICoIII Framework. J. Am. Chem. Soc. 2019, 141, 18211–18220. [Google Scholar] [CrossRef] [PubMed]
- Gendron, F.; Di Pietro, S.; Galan, L.A.; Riobe, F.; Placide, V.; Guy, L.; Zinna, F.; Di Bari, L.; Bensalah-Ledoux, A.; Guyot, Y.; et al. Luminescence, chiroptical, magnetic and ab initio crystal-field characterizations of an enantiopure helicoidal Yb(III) complex. Inorg. Chem. Front. 2021, 8, 914–926. [Google Scholar] [CrossRef]
- Brunet, G.; Marin, R.; Monk, M.-J.; Resch-Genger, U.; Galico, D.A.; Sigoli, F.A.; Suturina, E.A.; Hemmer, E.; Murugesu, M. Exploring the dual functionality of an ytterbium complex for luminescence thermometry and slow magnetic relaxation. Chem. Sci. 2019, 10, 6799–6808. [Google Scholar] [CrossRef]
- Wang, J.; Zakrzewski, J.J.; Zychowicz, M.; Vieru, V.; Chibotaru, L.F.; Nakabyashi, K.; Chorazy, S.; Ohkoshi, S. Holmium(III) molecular nanomagnets for optical thermometry exploring the luminescence re-absorption effect. Chem. Sci. 2021, 12, 730–741. [Google Scholar] [CrossRef]
- Ren, M.; Bao, S.-S.; Ferreira, R.A.S.; Zheng, L.-M.; Carlos, L.D. A layered erbium phosphanate in pseudo-D5h symmetry exhibiting field-tunable magnetic relaxation and optical correlation. Chem. Commun. 2014, 50, 7621–7624. [Google Scholar] [CrossRef]
- Long, J.; Guari, Y.; Ferreira, R.A.S.; Carlos, L.D.; Larionova, J. Recent advances in luminescent lanthanide based Single-Molecule Magnets. Coord. Chem. Rev. 2019, 363, 57–70. [Google Scholar] [CrossRef]
- Jankowski, R.; Zakrzewski, J.J.; Surma, O.; Ohkoshi, S.; Chorazy, S.; Sieklucka, B. Near–infrared emissive Er(III) and Yb(III) molecular nanomagnets in metal–organic chains functionalized by octacyanidometallates(IV). Inorg. Chem. Front. 2019, 6, 2423–2434. [Google Scholar] [CrossRef]
- Weissleder, R.; Pittet, M.J. Imaging in the area of molecular oncology. Nature 2008, 452, 580–589. [Google Scholar] [CrossRef]
- Naczynski, D.J.; Tan, M.C.; Zevon, M.; Wall, B.; Kohl, J.; Kulesa, A.; Chen, S.; Roth, C.M.; Riman, R.E.; Moghe, P.V. Rare–earth–doped biological composites as in vivo shortwave infrared reports. Nat. Commun. 2013, 4, 2199. [Google Scholar] [CrossRef]
- Hildebrand, S.A.; Weissleder, R. Near–infrared fluorescence: Application to in vivo molecular imaging. Curr. Opin. Chem. Biol. 2010, 14, 71–79. [Google Scholar] [CrossRef]
- Pansare, V.J.; Hejazi, S.; Faenza, W.J.; Prud’homme, R.K. Review of Long-Wavelength Optical and NIR Imaging Materials Contrast Agents, Fluorophores, and Multifunctional Nano Carries. Chem. Mater. 2012, 24, 8812–8827. [Google Scholar] [CrossRef] [PubMed]
- Gai, S.; Li, C.; Yang, P.; Lin, J. Recent Progress in Rare Earth Micro/Nanocrystals: Soft Chemical Synthesis, Luminescence Properties, and Biomedical Applications. Chem. Rev. 2014, 4, 2343–2389. [Google Scholar] [CrossRef] [PubMed]
- Creutz, S.E.; Fainblat, R.; Kim, Y.; De Siena, M.C.; Gamelin, D.R. A Selective Cation Exchange Strategy for the Synthesis of Colloidal Yb3+-Doped Chalcogenide Nanocrystals with Strong Broadband Visible Absorption and Long-Lived Near-Infrared Emission. J. Am. Chem. Soc. 2017, 139, 11814–11824. [Google Scholar] [CrossRef] [PubMed]
- Bai, G.; Yang, Z.; Lin, H.; Jie, W.; Hao, J. Lanthanide Yb/Er co-doped semiconductor layered WSe2 nanosheets with near-infrared luminescence at telecommunication wavelengths. Nanoscale 2018, 10, 9261–9267. [Google Scholar] [CrossRef] [PubMed]
- Shuvaev, S.; Parker, D. A near-IR luminescent ratiometric ytterbium pH probe. Dalton Trans. 2019, 48, 4471–4473. [Google Scholar] [CrossRef]
- Nguyen, T.N.; Eliseeva, S.V.; Gladysiak, A.; Petoud, S.; Stylianou, K.C. Design of lanthanide-based metal-organic frameworks with enhanced near-infrared emission. J. Mater. Chem. A 2020, 8, 10188–10192. [Google Scholar] [CrossRef]
- Soussi, K.; Jung, J.; Pointillart, F.; Le Guennic, B.; Lefeuvre, B.; Golhen, S.; Cador, O.; Guyot, Y.; Maury, O.; Ouahab, L. Magnetic and photo-physical investigations into DyIII and YbIII complexes involving tetrathiafulvalene ligand. Inorg. Chem. Front. 2015, 2, 1105–1117. [Google Scholar] [CrossRef]
- Lazarides, T.; Davies, G.M.; Adams, H.; Sabatini, C.; Barigelletti, F.; Barbieri, A.; Pope, S.J.A.; Faulkner, S.; Ward, M.D. Ligand-field excited states of hexacyanochromate and hexacyanocobaltate as sensitisers for near-infrared luminescence from Nd(III) and Yb(III) in cyanide-bridged d–f assemblies. Photochem. Photobiol. Sci. 2007, 6, 1152–1157. [Google Scholar] [CrossRef]
- Chorazy, S.; Sieklucka, B.; Ohkoshi, S. Near-Infrared Photoluminescence in Hexacyanido-Bridged Nd-Cr Layered Ferromagnet. Cryst. Growth Des. 2016, 16, 4918–4925. [Google Scholar] [CrossRef]
- Chorazy, S.; Charytanowicz, T.; Wang, J.; Ohkoshi, S.; Sieklucka, B. Hybrid organic-inoganic connectivity of NdIII(pyrazine–N,N′–dioxide)[CoIII(CN)6]3− coordination chains for crating near–infrared emissive Nd(III) showing field-induced slow magnetic relaxation. Dalton Trans. 2018, 47, 7870–7874. [Google Scholar] [CrossRef] [PubMed]
- Chorazy, S.; Zychowicz, M.; Ohkoshi, S.; Sieklucka, B. Wide-Range UV-to-Visible Excitation of Near-Infrared Emission and Slow Magnetic Relaxation in LnIII(4,4′-Azopyridine-1,1′-dioxide)[CoIII(CN)6]3− Layered Frameworks. Inorg. Chem. 2019, 58, 165–179. [Google Scholar] [CrossRef]
- Wang, J.; Zakrzewski, J.J.; Heczko, M.; Zychowicz, M.; Nakagawa, K.; Nakabayashi, K.; Sieklucka, B.; Chorazy, S.; Ohkoshi, S. Proton Conductive Luminescent Thermometer Based on Near–Infrared Emissive {YbCo2} Molecular Nanomagnets. J. Am. Chem. Soc. 2020, 142, 3970–3979. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewski, J.J.; Liberka, M.; Zychowicz, M.; Chorazy, S. Diverse physical functionalities of rare-earth hexacyanidometallate frameworks and their molecular analogues. Inorg. Chem. Front. 2021, 8, 452–483. [Google Scholar] [CrossRef]
- Burgess, J.; Radulovic, S. Solvatochromism and solvation of ternary iron-diimine-cyanide complexes. Transition Met. Chem. 1987, 12, 529–536. [Google Scholar] [CrossRef]
- Al-alousy, A.; Burgess, J. Bis(1,10-phenanthroline) dicyanoiron(II): An almost universal inorganic solvent polarity indicator. Inorg. Chim. Acta 1990, 169, 167–170. [Google Scholar] [CrossRef]
- Tłaczała, T.; Bartecki, A. Studies on the solvatochromism of Fe(CN)2(phen)2. Monatsch. Chem. 1997, 128, 225–234. [Google Scholar] [CrossRef]
- Georgieva, I.; Aquino, A.J.A.; Trendafilova, N.; Santos, P.S.; Lischka, H. Solvatochromic and Ionochromic Effects of Iron(II)bis(1,10-phenanhroline)dicyano: A Theoretical Study. Inorg. Chem. 2010, 49, 1634–1646. [Google Scholar] [CrossRef]
- Shavaleev, N.M.; Scopelliti, R.; Gumy, F.; Bünzli, J.-C.G. Surprisingly Bright Near-Infrared Luminescence and Short Radiative Lifetimes of Ytterbium in Hetero-Binuclear Yb–Na Chelates. Inorg. Chem. 2009, 48, 7937–7946. [Google Scholar] [CrossRef]
- Lu, H.; Peng, Y.; Ye, H.; Cui, X.; Hu, J.; Gu, H.; Khlobystov, A.N.; Green, M.A.; Blower, P.B.; Wyatt, P.B.; et al. S Sensitization, energy transfer and infra-red emission decay modulation in Yb3+-doped NaYF4 nanoparticles with visible light through a perfluoroanthraquinone chromophore. Sci. Rep. 2017, 7, 5066. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, K.S.; Dreiser, J.; Weihe, H.; Sibille, R.; Johannesen, H.V.; Sørensen, M.A.; Nielsen, B.E.; Sigrist, M.; Mutka, H.; Rols, S.; et al. Design of single-molecule magnets: Insufficiency of the anisotropy barrier as the sole criterion. Inorg. Chem. 2015, 54, 7600–7606. [Google Scholar] [CrossRef]
- Lunghi, A.; Totti, F.; Sessoli, R.; Sanvito, S. The role of anharmonic phonons in under-barrier spin relaxation of single molecule magnets. Nat. Commun. 2017, 8, 14620. [Google Scholar] [CrossRef]
- Giansiracusa, M.J.; Kostopoulos, A.K.; Collison, D.; Winpenny, R.E.P.; Chilton, N.F. Correlating blocking temperatures with relaxation mechanism in monometallic single-molecule magnets with high energy barrier (Ueff > 600 K). Chem. Commun. 2019, 55, 7025–7028. [Google Scholar] [CrossRef]
- Castro-Alvarez, A.; Gil, Y.; Llanos, L.; Aravena, D. High performance single-molecule magnets, Orbach or Raman relaxation suppression? Inorg. Chem. Front. 2020, 7, 2478–2486. [Google Scholar] [CrossRef]
- Ohkoshi, S.; Imoto, K.; Tsunobuchi, Y.; Takano, S.; Tokoro, H. Light-induced spin-crossover magnet. Nat. Chem. 2011, 3, 564–569. [Google Scholar] [CrossRef]
- Kobylarczyk, J.; Liberka, M.; Konieczny, P.; Baran, S.; Kubicki, M.; Korzeniak, T.; Podgajny, R. Bulky ligad shape the separation between large spin carries to condition field–induced slow magnetic relaxation. Dalton Trans. 2020, 49, 300–311. [Google Scholar] [CrossRef]
- Pointillart, F.; Cador, O.; Le Guennic, B.; Ouahab, L. Uncommon lanthanide ions in purely 4f single molecule magnets. Coord. Chem. Rev. 2017, 346, 150–175. [Google Scholar] [CrossRef]
- Chorazy, S.; Zakrzewski, J.J.; Reczyński, M.; Nakabayashi, K.; Ohkoshi, S.; Sieklucka, B. Humidity driven molecular switch based on photoluminescent DyIIICoIII single-molecule magnets. J. Mater. Chem. C 2019, 7, 4164–4172. [Google Scholar] [CrossRef]
- Ding, Y.-S.; Yu, K.-X.; Reta, D.; Ortu, F.; Winpenny, R.E.P.; Zheng, Y.-Z.; Chilton, N.F. Field- and temperature-dependent quantum tunneling of the magnetization in a large barrier single-molecule magnet. Nat. Commun. 2018, 9, 3134. [Google Scholar] [CrossRef]
- Aravena, D.; Ruiz, E. Spin dynamics in single-molecule magnets and molecular qubits. Dalton Trans. 2020, 49, 9916–9928. [Google Scholar] [CrossRef]
- Rinehart, J.D.; Long, J.R. Exploiting single-ion anisotropy in the design of f-element single-molecule magnets. Chem. Sci. 2011, 2, 2078–2085. [Google Scholar] [CrossRef]
- Gu, L.; Wu, R. Origin of the anomalously low Raman exponents in single–molecule magnets. Phys. Rev. 2021, 103, 014401. [Google Scholar] [CrossRef]
- Reta, D.; Kragskow, J.G.C.; Chilton, N.F. Ab Initio Prediction of High-Temperature Magnetic Relaxation Rates in Single-Molecule Magnets. J. Am. Chem. Soc. 2021, 143, 5943–5950. [Google Scholar] [CrossRef] [PubMed]
- Armaroli, N.; De Cola, L.; Balzani, V.; Sauvage, J.-P.; Dietrich-Buchecker, C.O.; Kern, J.-M. Adsorption and luminescence properties of 1,10-phenantroline, 2,9-diphenyl-1,10-phenantroline, 2,9-dianisyl-1,10-phenantroline and their protonated forms in dichloromethane solution. J. Chem. Soc. Faraday Trans. 1992, 88, 553–556. [Google Scholar] [CrossRef]
- Schilt, A.A. Mixed Ligand Complexes of Iron(II) and (III) with Cyanide and Aromatic Di-imines. J. Am. Chem. Soc. 1960, 82, 3000–3005. [Google Scholar] [CrossRef]
- Maddock, A.G. The complex [Fe(phen)2(CN)2] (phen = 1,10–phenantroline) and its solutions. J. Chem. Soc. Dalton Trans. 1986, 5, 2349–2350. [Google Scholar] [CrossRef]
- Sheldrick, G.M. TWINABS; University of Göttingen: Göttingen, Germany, 2009. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystalstructure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Llunell, M.; Casanova, D.; Cirera, J.; Bofill, J.; Alemany, P.; Alvarez, S.; Pinsky, M.; Avnir, D. Program for the Calculation of Continuous Shape Measures of Polygonal and Polyhedral Molecular Fragments; SHAPE v. 2.1.; University of Barcelona: Barcelona, Spain, 2013. [Google Scholar]
- Casanova, D.; Cirera, J.; Llunell, M.; Alemany, P.; Avnir, D.; Alvarez, S. Minimal Distortion Pathways in Polyhedral Rearrangements. J. Am. Chem. Soc. 2004, 126, 1755–1763. [Google Scholar] [CrossRef]
| Compound | 1∙MeCN | 1∙MeCN@Lu | 1∙AcrCN | 1∙PrCN | 1∙MalCN |
|---|---|---|---|---|---|
| field–dependence of relaxation time (Figure 3 and Figure 4, Figures S15, S17, S19, S21 and S23) | |||||
| T/K | 1.8 | 1.8 | 1.8 | 1.8 | 1.8 |
| Adirect/s–1K–1Oe–m | 1.14(1)∙10–8 | 1.38(3)∙10–9 | 5.25(5)∙10–9 | 1.65(2)∙10–10 | 3.12(3)∙10–9 |
| m | 3.15(1) | 3.26(3) | 3.75(1) | 3.54(1) | 3.19(2) |
| a/s–1 | 4∙103 (fixed) | 3.91(2)∙103 | 3.28(7)∙103 | 7.32(3)∙102 | 1.13(1)∙102 |
| b/Oe–2 | 2∙10–1 (fixed) | 9.48(9)∙10–5 | 4.81(2)∙10–5 | 2.14(3)∙10–5 | 2.21(6)∙10–5 |
| temperature–dependence of relaxation time (Figure 3 and Figure 4, Figures S16, S18, S20, S22 and S24) | |||||
| Hdc/Oe | 800 | 800 | 800 | 800 | 800 |
| BRaman/s–1K–n | 81.68(5) | 25.32(3) | 208.28(2) | 128.09(1) | 527.08(2) |
| n | 3.07(1) | 3.61(1) | 2.37(1) | 2.61(1) | 1.68(4) |
| temperature–dependence of relaxation time—Arrhenius law (Figures S16, S18, S20, S22 and S24) | |||||
| τ0/s | 7.28(3)∙10–6 | 1.48(1)∙10–5 | 2.51(7)∙10–5 | 1.46(7)∙10–5 | 5.82(9)∙10–5 |
| Ueff/K | 12.50(58) | 11.53(1) | 7.86(27) | 10.28(22) | 4.83(31) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liberka, M.; Boidachenko, K.; Zakrzewski, J.J.; Zychowicz, M.; Wang, J.; Ohkoshi, S.-i.; Chorazy, S. Near-Infrared Emissive Cyanido-Bridged {YbFe2} Molecular Nanomagnets Sensitive to the Nitrile Solvents of Crystallization. Magnetochemistry 2021, 7, 79. https://doi.org/10.3390/magnetochemistry7060079
Liberka M, Boidachenko K, Zakrzewski JJ, Zychowicz M, Wang J, Ohkoshi S-i, Chorazy S. Near-Infrared Emissive Cyanido-Bridged {YbFe2} Molecular Nanomagnets Sensitive to the Nitrile Solvents of Crystallization. Magnetochemistry. 2021; 7(6):79. https://doi.org/10.3390/magnetochemistry7060079
Chicago/Turabian StyleLiberka, Michal, Kseniia Boidachenko, Jakub J. Zakrzewski, Mikolaj Zychowicz, Junhao Wang, Shin-ichi Ohkoshi, and Szymon Chorazy. 2021. "Near-Infrared Emissive Cyanido-Bridged {YbFe2} Molecular Nanomagnets Sensitive to the Nitrile Solvents of Crystallization" Magnetochemistry 7, no. 6: 79. https://doi.org/10.3390/magnetochemistry7060079
APA StyleLiberka, M., Boidachenko, K., Zakrzewski, J. J., Zychowicz, M., Wang, J., Ohkoshi, S.-i., & Chorazy, S. (2021). Near-Infrared Emissive Cyanido-Bridged {YbFe2} Molecular Nanomagnets Sensitive to the Nitrile Solvents of Crystallization. Magnetochemistry, 7(6), 79. https://doi.org/10.3390/magnetochemistry7060079