Abstract
A low-coordinate dinuclear dysprosium complex {[Dy(N3N)(THF)][LiCl(THF)]}2 (Dy2) with a double bridging ‘LiCl’ moiety and tris(amido)amine (N3N)3− anions as a blocking ligand is synthesized and characterized structurally and magnetically. Thanks to the use of the chelating blocking ligand (N3N)3− equipped with large steric –SiMe3 groups, the coordination sphere of both DyIII ions is restricted to only six donor atoms. The three amido nitrogen atoms determine the orientation of the easy magnetization axes of both DyIII centers. Consequently, Dy2 shows slow magnetic relaxation typical for single molecule magnets (SMMs). However, the effective energy barrier for magnetization reversal determined from the AC magnetic susceptibility measurements is much lower than the separation between the ground and the first excited Kramers doublet based on the CASSCF ab initio calculations. In order to better understand the possible influence of the anticipated intramolecular magnetic interactions in this dinuclear molecule, its GdIII-analog {[Gd(N3N)(THF)][LiCl(THF)]}2 (Gd2) is also synthesized and studied magnetically. Detailed magnetic measurements reveal very weak antiferromagnetic interactions in Gd2. This in turn suggests similar antiferromagnetic interactions in Dy2, which might be responsible for its peculiar SMM behavior and the absence of the magnetic hysteresis loop.
1. Introduction
Polynuclear lanthanide (Ln) complexes often exhibit slow magnetic relaxation and related single molecule magnet (SMM) behavior similar to their more famous mononuclear relatives [1,2,3] but show also the influence of the superexchange interactions. Similarly to mononuclear Ln-SMMs, the field of the polynuclear congeners is also dominated by DyIII [4,5,6,7,8]. Due to very weak intermolecular magnetic interactions between the lanthanide ions in such polynuclear compounds, the slow relaxation of the magnetization is usually dominated by single-ion effects which can be determined by performing experiments involving the diamagnetic dilution of the investigated compounds with Y or La [9]. Obviously, the employment of longer bridging ligands leads to a good separation of the magnetic centers and therefore the possible slow magnetic relaxation becomes a single-ion effect [10]. Due to the weakness of the magnetic interactions in lanthanide-based compounds, only short molecular bridges could provide sufficient communication between the magnetic centers that could influence the slow magnetic relaxation of the compound [3]. Still, the exchange coupling constants between the lanthanide ions in Ln-SMMs, even with very short bridges, are one or two orders of magnitude smaller than the typical values of the energy barrier for the magnetization reversal (J-values are typically in the range 0.1–3 cm−1 for diamagnetic bridging ligands). The highest exchange coupling constant for a polymetallic Ln-SMMs was determined by studying the GdIII congener of the famous [N2]3− bridged dinuclear Tb-SMM {[(Me3Si)2N]2(THF)TbIII}2(μ-η2:η2-N2)− [11]. The magnetic interaction between the GdIII and the paramagnetic [N2]3− molecular bridge was reported to be −27 cm−1 (Ĥ = −2JŜ1Ŝ2 Hamiltonian type) [12]. It is noteworthy that in a complex bridged by a radical bipyrimidyl ligand the exchange interaction (J = −10 cm−1; Ĥ = −2JŜ1Ŝ2 Hamiltonian type) [13] was a bit weaker than in the complex bridged by a significantly smaller [N2]3−.
The magnetic interactions between the lanthanide ions can lead to a shift of otherwise-degenerate mJ sublevels to different energies and reduce the probability of the QTM (quantum tunneling of magnetization) [3]. QTM is frequently the reason for a very fast relaxation acceleration under zero magnetic field, so its exclusion is crucial to improve single molecule magnet performance. Even weak exchange interaction (mostly dipolar in nature) between two lanthanide ions can be significant enough to hinder the quantum tunneling of magnetization at HDC = 0 [14,15,16,17,18,19,20]. Moreover, magnetic interactions are necessary to engineer universal qugates (quantum gates) as demonstrated for dinuclear LnLn′ molecules [21]. Therefore, searching for polynuclear Ln-SMMs with effective magnetic interactions is highly desired.
The design of polynuclear Ln-SMMs is challenging because of the difficulties in predicting how the lanthanides interact with each other and what would be the orientation of their easy magnetization axes in a particular chemical and geometrical environment [22]. The task becomes even more difficult when very small ligands, such as NO3−, H2O, OH−, THF, etc., are allowed to coordinate to the metal centers leading to complexes with high coordination numbers ≥8 and uncontrolled coordination geometries. This is why we have focused our efforts on obtaining Ln complexes with a bulky chelating ligand tris(N-trimethylsilyl-2-amidoethyl)amine (N3N)3− in the form of a lithium salt [23]. The (N3N)3− ligand was successfully used to obtain transition metal complexes with a strictly controlled coordination geometry such as (N3N)MoIVCl [24] showing extremely attractive chemical properties such as N2 activation or a family of [MII(N3N)Li(THF)] (M=Mn, Fe, Co, Ni) compounds with a significant magnetic anisotropy associated with their trigonal pyramidal geometry imposed by the (N3N)3− ligand [25].
Herein, we describe a dinuclear SMM based on DyIII {[Dy(N3N)(THF)][LiCl(THF)]}2 (Dy2) and its GdIII analog (Gd2). Both compounds are obtained by reacting the aforementioned lithium salt of the tris(amido)amine with the respective anhydrous LnCl3 salts (Ln = Dy, Gd) in THF. Interestingly, the bridge between the two lanthanide ions consists of two Cl− anions and two Li+ cations stabilized by two THF molecules. The influence of the bridging LiCl on the magnetic properties of both Dy2 and Gd2 is discussed in light of the CASSCF calculations.
2. Results and Discussion
2.1. Synthesis and Crystal Structure
Both dinuclear compounds {[Dy(N3N)(THF)][LiCl(THF)]}2 (Dy2) and {[Gd(N3N)(THF)][LiCl(THF)]}2 (Gd2) (N3N = tris(N-trimethylsilyl-2-amidoethyl)amine; [(Me3SiNCH2CH2)3N]3−; Figure 1a) were obtained by reacting the respective anhydrous chlorides with Li3(N3N) in anhydrous THF followed by solvent removal and extraction with pentane. Noteworthy, the Gd2 and Dy2 presented in this work are formed under very similar reaction conditions to the monometallic ErIII complex reported by us recently [26].
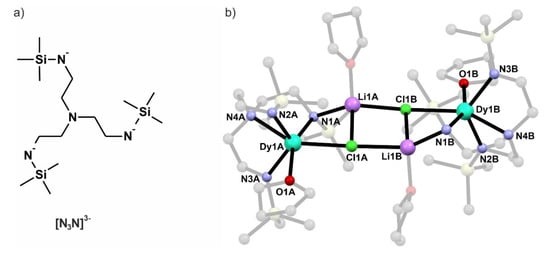
Figure 1.
Structural formula of the trianionic tris(N-trimethylsilyl-2-amidoethyl)amine ligand (a) and the crystal structure of {[Dy(N3N)(THF)][LiCl(THF)]}2 (Dy2) (b) with atom labeling scheme (the blocking ligands (N3N)3−, THF molecules and hydrogen atoms are dimmed for the sake of clarity).
Crystals of Dy2 and Gd2 are grown from pentane solutions at −40 °C. They crystallize in a triclinic system, space group P21/c as determined by single crystal X-ray diffraction (SCXRD) structural analysis. Both compounds are isostructural and therefore Dy2 will be discussed as the representative one. Selected crystallographic details are presented in Table 1. The asymmetric unit contains the whole molecule consisting of two metal centers blocked by (N3N)3− ligand and connected by double LiCl bridge with THF attached to it (Figure 1b). Both lanthanide centers within the molecule are coordinated by four nitrogen atoms of the (N3N)3− ligands, one chloride and one oxygen atom of the THF molecule leading to a pseudo-octahedral coordination geometry of both centers. The chelating (N3N)3− ligands do not block all of the coordination sites, unlike in many transition metal complexes [24,25], but leave enough space for the ‘LiCl’ bridge and coordinated THF molecule. The distance between the two lanthanides is 7.937 Å for Dy2 and 7.961 Å for Gd2. Such a long distance suggests that the intramolecular magnetic interactions between the two metal centers within the dinuclear ‘units’ should be very weak. Intermolecular Ln⋯Ln distances are also long (the shortest ones are 7.969 Å for Dy2 and 8.122 Å for Gd2). Figure 2 presents the packing diagram of the Dy(LiCl)2Dy cores with the shortest intermolecular distances highlighted as a dotted line. As aforementioned, the coordination spheres of the lanthanide ions in Dy2 and Gd2 are six-coordinate and resemble a strongly distorted octahedron. Table 2 provides the metric parameters of the first coordination spheres in both compounds. The distortion form the octahedral geometry is caused mainly by significantly elongated Ln-Cl bonds: 2.733 (1) Å, 2.740(1) Å for Dy2 and 2.763(1) Å, 2.769(1) Å for Gd2 as compared to the average 2.38(11) Å for Dy2 and 2.41(11) Å for Gd2. The angles presented in Table 2 also clearly show how strongly the coordination spheres of the metal centers in both compounds are distorted from the ideal six-coordinate octahedral geometry.

Table 1.
Selected crystallographic parameters for Dy2 and Gd2.
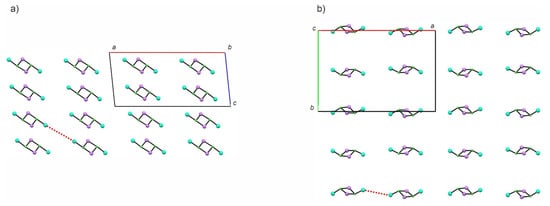
Figure 2.
Structural packing diagram for Dy2 presented along the b (a) and c (b) crystallographic direction. The red dotted lines indicate the shortest intermolecular Dy⋯Dy distances.

Table 2.
Selected bond lengths and angles of the coordination spheres of Dy in Dy2 and Gd in Gd2.
2.2. DC Magnetic Properties
The direct-current (DC) magnetic properties of Dy2 and Gd2 in the form of χT(T) (χ—molar magnetic susceptibility) and M(H) (M—molar magnetization, H—magnetic field strength) are presented in Figure 3. Gd2 shows magnetic properties typical for a completely isotropic magnetic system composed of two GdIII ions with a constant χT value of 16.0 cm3·K·mol−1 down to the lowest temperatures where only a slight decrease occurs due to the Zeeman effect and the antiferromagnetic Gd⋯Gd interactions. M(H) curve reaches the saturation value of 13.9 μB already at ca. 55 kOe and remains constant up to 70 kOe. Both experimental curves (black points and blue circles in Figure 3) match well the best fit with gGd = 2.00 (1) and JGdGd = −0.004 (1) cm−1 (PHI software [27]). Dy2, on the other hand, shows a gradual decrease of the χT(T) related to the thermal depopulation of the mJ states. The room temperature χT value of 27.7 cm3·K·mol−1 corresponds well with the expected 28.34 cm3·K·mol−1 for two isolated DyIII ions (6H15/2, gJ = 4/3) [28]. Both experimental dependences χT(T) and M(H) for Dy2 (black points and red circles, respectively, in Figure 3) are well reproduced by the CASSCF calculations (red solid lines in Figure 3; for details see section Ab initio calculations) which confirm the significant magnetic anisotropy of Dy2. A very steep decrease of the χT at around 1.8 K, which is not reproduced by the CASSCF calculations (red line in Figure 3a), suggests non-negligible antiferromagnetic superexchange coupling between DyIII ions in Dy2, most probably of intramolecular character rather than intermolecular. Intermolecular magnetic coupling in the case of Dy2 should have a through-space dipole–dipole character due to the absence of other intermolecular contacts between the neighboring Dy2 molecules—except the van der Waals contacts. Such contacts usually yield much weaker magnetic coupling than the superexchange even through several atoms as in the case of the double LiCl bridge within the Dy2 dimer.
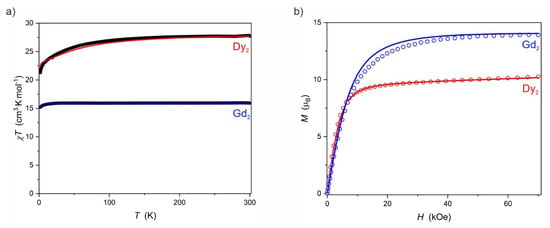
Figure 3.
χT(T) recorded at 1000 Oe (a) and M(H) recorded at 2.0 K (b) for Dy2 (red) and Gd2 (blue). Black overlapping points in (a) represent the experimental data and the colored solid lines correspond to the CASSCF ab initio calculations (for details see below) in the case of Dy2 and the best fit (PHI software) in the case of Gd2 with g = 2.00 and JGdGd = −0.004 (1).
2.3. AC Magnetic Properties
Alternating-current (AC) magnetic susceptibility (χ) measurements revealed slow magnetic relaxation for Dy2 under an applied DC field. The frequency (ν) dependence in the 1–1000 Hz range under varied magnetic fields 100–7000 Oe recorded at 3.5 K is presented in Figure 4. In the 200–3000 Oe DC field, only one major maximum can be observed in the out-of-phase magnetic susceptibility (χ″). Only under DC fields larger than 3000 Oe a second maximum reveals itself at lower frequency range, which was not analyzed. The main maximum shifts from around 15 Hz at HDC = 200 Oe to around 7 Hz at the magnetic field in the 800–1400 Oe range and then shifts towards higher frequencies under HDC > 2000 Oe.
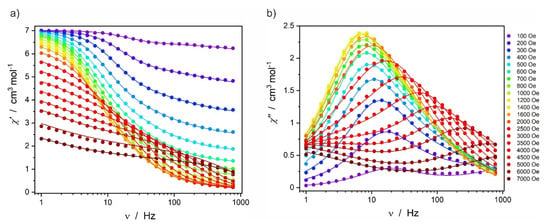
Figure 4.
In-phase χ′ (a) and out-of-phase χ″ (b) AC magnetic susceptibility for Dy2 recorded at 3.5 K under various magnetic fields HDC in the 100–7000 Oe range.
The AC magnetic susceptibility was then studied under the optimal magnetic field of 1000 Oe at various temperatures in the 2.8–8.3 K range (Figure 5). The maxima in the χ″(ν) plots show a clear shift towards higher frequencies when the temperature is increased.
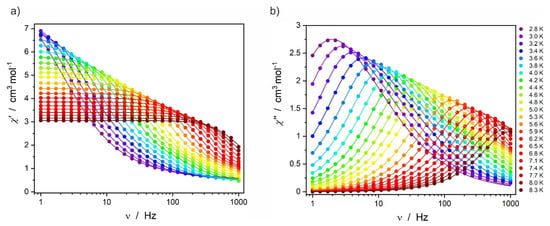
Figure 5.
In-phase χ′ (a) and out-of-phase χ″ (b) AC magnetic susceptibility for Dy2 recorded under 1000 Oe magnetic field at various temperatures in the 2.8–8.3 K range.
The AC data presented in Figure 4 and Figure 5 were fitted using a modified Debye model [29] and the resulting best fits are shown in the respective Figures as colored solid lines. The extracted relaxation times of the magnetization were then plotted vs. the magnetic field τ−1(H) (Figure 6a) and vs. the temperature lnτ(T−1) (Figure 6b). Equation (1) was used to fit the magnetic field dependence of the relaxation times τ extracted from the χ′,χ″(ν) under various magnetic fields [30]:
where the first part is the quantum tunneling of magnetization (QTM), the second one is related to the direct relaxation process and the constant value A4 stands for the contribution of the field-independent processes (Orbach and Raman) (Figure 6). The best fit parameters are gathered in Table 3 (top part).
τ −1(H) = A1/(1 + A2H2) + A3H4 + A4
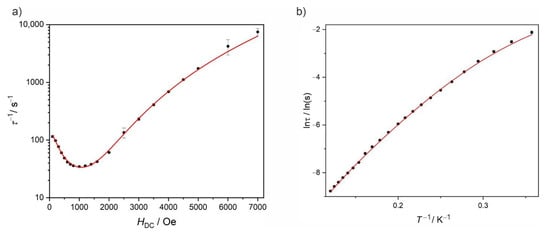
Figure 6.
Field dependence of τ−1 at 3.5 K in the 100–7000 Oe range (a) and temperature dependence of lnτ under 1000 Oe in the 2.8–8.3 K range (b) for Dy2. Red solid lines are the best fits to Equation (1) (a) or Equation (2) (b) (for details see text).

The values of the relaxation time τ extracted from the temperature dependence of the χ′,χ″(ν) were fitted using Equation (2) [30]:
where the constant value C0 stands for the temperature-independent QTM, the second part is related to the direct process, C2 and n describe the Raman process and the last part characterizes the contribution of the Orbach relaxation. The best fit parameters are gathered in Table 3 (bottom part). The QTM contribution under the applied magnetic field of 1000 Oe was fixed to zero in this equation, as the analysis of the field dependence of τ did not show significant contribution of QTM process under this optimal applied DC magnetic field. The contribution of the direct process was calculated from Equation (1) and then inserted into Equation (2) as a fixed value (C1 = 0.75 s−1·K−1) (Figure 6b).
lnτ(T−1) = ln[(C0 + C1T + C2T n + τ0−1exp(-Ueff/kBT))−1]
The fit of the temperature dependence of lnτ shows a significant contribution of the Raman relaxation process (C2 = 0.015 (7) s−1·K−n, n = 5.81 (33)) and a small contribution of the Orbach process, especially visible at lower temperatures (τ0 = 4.9 (1.9)·10−6 s and Ueff/kB = 34 (2) K or 23.6 cm−1 which might be significantly underestimated).
2.4. Ab Initio Calculations
Theoretical calculations CASSCF were performed separately for both DyIII centers based on the SCXRD structural models using the OpenMolcas software [31] (for details, see Table S1 in the Supplementary Materials; SM). The ground state Kramers doublet (KD) for both centers is composed mainly of the |±15/2 > mJ state with a significantly axial character (Tables S2 and S3 in the SM) and nearly parallel easy magnetization axes (Figure 7a). The first excited KD doublet is located ca. 130 cm−1 above the ground KD, which is significantly larger than the experimentally estimated energy barrier for the magnetization reversal Ueff = 23.6 cm−1 (Figure 7b) This suggests that the relaxation of the magnetization in Dy2 is controlled by the Raman relaxation mechanism (not Orbach), as already pointed out in the section discussing the AC magnetic data.
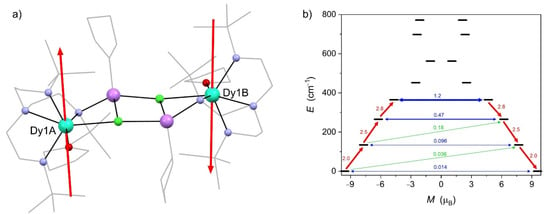
Figure 7.
Structural diagram of Dy2 with the red sticks indicating the orientation of the easy magnetization axes of the ground state Kramers doublet (KD) for both DyIII centers obtained from the CASSCF calculations (the direction indicated by the arrows is hypothetical, assuming intramolecular antiferromagnetic interactions between the DyIII centers within the dimer based on the analysis of the DC magnetic properties of Dy2 and its Gd2 analogue) (a). Energy diagram of the calculated Kramers doublets within the 6H15/2 multiplet of one of the DyIII centers in Dy2 (b). Arrows correspond to the most important magnetization relaxation pathways.
3. Materials and Methods
3.1. General Considerations
The syntheses of the reported compounds as well as their preparation for measurements were performed inside the Inert PureLab HE glovebox filled with argon gas due to their sensitivity to air (predominantly moisture). Solvents (HPLC grade) were passed through the Inert PureSolv EN7 solvent purification system under inert atmosphere. Anhydrous DyCl3 (99.99%) was purchased from Sigma Aldrich and GdCl3 (99.9%) was purchased from Alfa Aesar. Both chemicals were used without further purification. Li3(N3N) was prepared according to the previously published method [23].
3.2. Preparation of Dy2
Solid DyCl3 (0.68 mmol, 183 mg) was added in portions into the solution of Li3(N3N) (0.71 mmol, 272 mg) in 5.90 g of anhydrous THF. The reaction mixture was stirred for 4 days and then the solvent was removed under vacuum and replaced with pentane. The off-white suspension was stirred for 20 min and then filtered using a P4 fritted funnel (gravitational filtering). The solid was extracted with three more portions of pentane. The yellow filtrates were combined and left at −40 °C for crystallization. After 3 days colorless crystals were collected. Yield: 70 mg (15%). The identity and purity of the compound was confirmed by powder X-ray diffraction (PXRD) measurements (Figure 8).
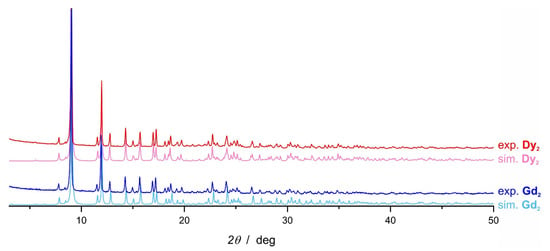
Figure 8.
Powder X-ray diffraction patterns: Dy2 experimental at room temperature (red); Dy2 simulated from SCXRD data collected at 296 K (pink); Gd2 experimental at room temperature (blue); Gd2 simulated from SCXRD data collected at 296 K (bright blue).
3.3. Preparation of Gd2
Solid GdCl3 (0.67 mmol, 178 mg) was added in portions into the solution of Li3(N3N) (0.70 mmol, 267 mg) in 5.90 g of anhydrous THF. The reaction mixture was stirred for 3 h and then the solvent was removed under vacuum and replaced with pentane. The suspension was filtered (P4 fritted funnel; gravitational filtering) and the solid was extracted with three more portions of pentane. The yellowish filtrates were combined and left in the freezer at −40 °C for crystallization. After 3 days colorless crystals were collected. Yield: 70 mg (15%). The identity and purity of the compound was confirmed by powder X-ray diffraction (PXRD) measurements (Figure 8, dark blue and light blue solid lines).
3.4. Single Crystal X-ray Diffraction
Data collection was performed using Bruker D8 Quest Eco (Photon50) diffractometer (Mo Kα radiation source: sealed tube with Triumph® monochromator). The crystals were transferred from the mother liquor directly into the Paratone-N oil and mounted using MiTeGen cryomounts. The data for each compound were collected first at 100 K (complete data for publication) and then at room temperature (296 K; fast data collection for comparison with experimental PXRD patterns). Data processing was performed using Apex3 suite of programs. The structures were solved using direct methods and refined anisotropically (weighted full-matrix least-squares on F2 [32]). Hydrogen atoms were placed in the calculated positions and refined as riding on the parent atoms. Structural diagrams were prepared using Mercury software. CCDC 2099063 (Dy2 at 100 K), 2107188 (Dy2 at 296 K), 2099064 (Gd2 at 100 K) and 2107189 (Gd2 at 296 K) contain the supplementary crystallographic data for this paper, which can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif (accessed on 2 September 2021). The cif files can also be found in the Supplementary Materials.
3.5. Powder X-ray Diffraction
PXRD measurements were performed at room temperature using Bruker D8 Advance Eco diffractometer equipped with the CuKα radiation source (sealed tube), the Lynxeye silicon strip detector and a capillary stage. The samples were ground using the agate mortar inside the glovebox and loaded into 0.7 mm glass capillaries.
3.6. Magnetic Measurements
Magnetic measurements were performed using a Quantum Design MPMS-3 magnetometer in the −7 to 7 T magnetic field range and in the 1.8–300 K temperature range. The samples were prepared inside the glovebox due to their sensitivity to air. We loaded 25–30 mg of each compound into a custom-made Delrin sample holder [33] and sealed it. The experimental data were corrected for the diamagnetism of the sample and the contribution of the sample holder.
4. Conclusions
A new dinuclear dysprosium(III) single molecule magnet comprising six-coordinate DyIII centers shows slow relaxation of the magnetization controlled mainly by QTM at zero applied magnetic field and by Raman relaxation process at HDC > 0. The lack of the SMM behavior at zero field is most probably caused by the intramolecular antiferromagnetic interactions transmitted through a double LiCl bridge (similar antiferromagnetic interactions were found for the gadolinium(III) analog of Dy2). The field-induced SMM behavior, on the other hand, is controlled mainly by the Raman relaxation. This is partly confirmed by the CASSCF calculations, which indicate that Dy2 should be a much better-performing SMM than it is actually observed with effective energy barrier for magnetization reversal exceeding 130 cm−1. Further studies involving the diamagnetic dilution of the dinuclear Dy2 with yttrium(III) that would switch off the possible antiferromagnetic interactions are necessary for the complete understanding of the slow relaxation of the magnetization in this compound.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/magnetochemistry7090125/s1, Table S1: Description and contractions of the basis sets (two models: S—smaller, L—larger) employed in ab initio calculations of the DyIII crystal field, Table S2: Summary of the energy splitting of the 6H15/2 multiplet of the Dy1A in models: L, S with pseudo-g-tensors of each Kramers doublet and composition in the basis of ground state, Table S3: Summary of the energy splitting of the 6H15/2 multiplet of the Dy1B in models: L, S with pseudo-g-tensors of each Kramers doublet and composition in the basis of ground state.
Author Contributions
Conceptualization, D.P.; methodology, D.P.; investigation, M.B. and G.H.; resources, D.P.; writing—original draft preparation, G.H. and M.B.; writing—review and editing, D.P.; visualization, D.P., M.B. and G.H.; supervision, D.P.; project administration, D.P.; funding acquisition, D.P.; calculations, M.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Science Center within the Sonata Bis project no. 2016/22/E/ST5/00055.
Data Availability Statement
CCDC 2099063 (Dy2 at 100 K), 2107188 (Dy2 at 296 K), 2099064 (Gd2 at 100 K) and 2107189 (Gd2 at 296 K) contain the supplementary crystallographic data for this paper, which can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Goodwin, C.A.P.; Ortu, F.; Reta, D.; Chilton, N.F.; Mills, D.P. Molecular magnetic hysteresis at 60 kelvin in dysprosocenium. Nature 2017, 548, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Canaj, A.B.; Dey, S.; Wilson, C.; Céspedes, O.; Rajaraman, G.; Murrie, M. Engineering macrocyclic high performance pentagonal bipyramidal Dy(iii) single-ion magnets. Chem. Commun. 2020, 56, 12037–12040. [Google Scholar] [CrossRef]
- Wang, J.; Li, Q.-W.; Wu, S.-G.; Chen, Y.-C.; Wan, R.-C.; Huang, G.-Z.; Liu, Y.; Liu, J.-L.; Reta, D.; Giansiracusa, M.J.; et al. Opening Magnetic Hysteresis by Axial Ferromagnetic Coupling: From Mono-Decker to Double-Decker Metallacrown. Angew. Chem. Int. Ed. 2021, 60, 5299–5306. [Google Scholar] [CrossRef]
- Habib, F.; Murugesu, M. Lessons learned from dinuclear lanthanide nano-magnets. Chem. Soc. Rev. 2013, 42, 3278–3288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodruff, D.N.; Winpenny, R.E.P.; Layfield, R.A. Lanthanide Single-Molecule Magnets. Chem. Rev. 2013, 113, 5110–5148. [Google Scholar] [CrossRef]
- Xin, Y.; Wang, J.; Zychowicz, M.; Zakrzewski, J.J.; Nakabayashi, K.; Sieklucka, B.; Chorazy, S.; Ohkoshi, S.-I. Dehydration–Hydration Switching of Single-Molecule Magnet Behavior and Visible Photoluminescence in a Cyanido-Bridged DyIIICoIII Framework. J. Am. Chem. Soc. 2019, 141, 18211–18220. [Google Scholar] [CrossRef]
- Li, X.-L.; Wu, J.; Tang, J.; Le Guennic, B.; Shi, W.; Cheng, P. A planar triangular Dy3 + Dy3 single-molecule magnet with a toroidal magnetic moment. Chem. Commun. 2016, 52, 9570–9573. [Google Scholar] [CrossRef]
- Hewitt, I.J.; Tang, J.; Madhu, N.T.; Anson, C.E.; Lan, Y.; Luzon, J.; Etienne, M.; Sessoli, R.; Powell, A.K. Coupling Dy3 Triangles Enhances Their Slow Magnetic Relaxation. Angew. Chem. Int. Ed. 2010, 49, 6352–6356. [Google Scholar] [CrossRef]
- Habib, F.; Lin, P.-H.; Long, J.; Korobkov, I.; Wernsdorfer, W.; Murugesu, M. The Use of Magnetic Dilution To Elucidate the Slow Magnetic Relaxation Effects of a Dy2 Single-Molecule Magnet. J. Am. Chem. Soc. 2011, 133, 8830–8833. [Google Scholar] [CrossRef] [PubMed]
- Habib, F.; Long, J.; Lin, P.-H.; Korobkov, I.; Ungur, L.; Wernsdorfer, W.; Chibotaru, L.F.; Murugesu, M. Supramolecular architectures for controlling slow magnetic relaxation in field-induced single-molecule magnets. Chem. Sci. 2012, 3, 2158–2164. [Google Scholar] [CrossRef]
- Rinehart, J.D.; Fang, M.; Evans, W.J.; Long, J.R. A N23– Radical-Bridged Terbium Complex Exhibiting Magnetic Hysteresis at 14 K. J. Am. Chem. Soc. 2011, 133, 14236–14239. [Google Scholar] [CrossRef]
- Rinehart, J.D.; Fang, M.; Evans, W.J.; Long, J.R. Strong exchange and magnetic blocking in N23−-radical-bridged lanthanide complexes. Nat. Chem. 2011, 3, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Demir, S.; Zadrozny, J.M.; Nippe, M.; Long, J.R. Exchange Coupling and Magnetic Blocking in Bipyrimidyl Radical-Bridged Dilanthanide Complexes. J. Am. Chem. Soc. 2012, 134, 18546–18549. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.-H.; Sun, W.-B.; Yu, M.-F.; Li, G.-M.; Yan, P.-F.; Murugesu, M. An unsymmetrical coordination environment leading to two slow relaxation modes in a Dy2 single-molecule magnet. Chem. Commun. 2011, 47, 10993–10995. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.-N.; Xu, G.-F.; Wernsdorfer, W.; Ungur, L.; Guo, Y.; Tang, J.; Zhang, H.-J.; Chibotaru, L.F.; Powell, A.K. Strong Axiality and Ising Exchange Interaction Suppress Zero-Field Tunneling of Magnetization of an Asymmetric Dy2 Single-Molecule Magnet. J. Am. Chem. Soc. 2011, 133, 11948–11951. [Google Scholar] [CrossRef] [PubMed]
- Sakaue, S.; Fuyuhiro, A.; Fukuda, T.; Ishikawa, N. Dinuclear single-molecule magnets with porphyrin–phthalocyanine mixed triple-decker ligand systems giving SAP and SP coordination polyhedra. Chem. Commun. 2012, 48, 5337–5339. [Google Scholar] [CrossRef]
- Zou, L.; Zhao, L.; Chen, P.; Guo, Y.-N.; Guo, Y.; Li, Y.-H.; Tang, J. Phenoxido and alkoxido-bridged dinuclear dysprosium complexes showing single-molecule magnet behaviour. Dalton Trans. 2012, 41, 2966–2971. [Google Scholar] [CrossRef]
- Long, J.; Habib, F.; Lin, P.-H.; Korobkov, I.; Enright, G.; Ungur, L.; Wernsdorfer, W.; Chibotaru, L.F.; Murugesu, M. Single-Molecule Magnet Behavior for an Antiferromagnetically Superexchange-Coupled Dinuclear Dysprosium (III) Complex. J. Am. Chem. Soc. 2011, 133, 5319–5328. [Google Scholar] [CrossRef]
- Shen, F.-X.; Pramanik, K.; Brandão, P.; Zhang, Y.-Q.; Jana, N.C.; Wang, X.-Y.; Panja, A. Macrocycle supported dimetallic lanthanide complexes with slow magnetic relaxation in Dy2 analogues. Dalton Trans. 2020, 49, 14169–14179. [Google Scholar] [CrossRef]
- He, M.; Guo, F.-S.; Tang, J.; Mansikkamäki, A.; Layfield, R.A. Fulvalene as a platform for the synthesis of a dimetallic dysprosocenium single-molecule magnet. Chem. Sci. 2020, 11, 5745–5752. [Google Scholar] [CrossRef]
- Aguilà, D.; Barrios, L.A.; Velasco, V.; Roubeau, O.; Repollés, A.; Alonso, P.J.; Sesé, J.; Teat, S.J.; Luis, F.; Aromí, G. Heterodimetallic [LnLn’] Lanthanide Complexes: Toward a Chemical Design of Two-Qubit Molecular Spin Quantum Gates. J. Am. Chem. Soc. 2014, 136, 14215–14222. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.-N.; Chen, X.-H.; Xue, S.; Tang, J. Modulating Magnetic Dynamics of Three Dy2 Complexes through Keto–Enol Tautomerism of the o-Vanillin Picolinoylhydrazone Ligand. Inorg. Chem. 2011, 50, 9705–9713. [Google Scholar] [CrossRef] [PubMed]
- Cummins, C.C.; Schrock, R.R.; Davis, W.M. Synthesis of Terminal Vanadium (V) Imido, Oxo, Sulfido, Selenido, and Tellurido Complexes by Imido Group or Chalcogenide Atom Transfer to Trigonal Monopyramidal V[N3N] (N3N = [(Me3SiNCH2CH2)3N]3−). Inorg. Chem. 1994, 33, 1448–1457. [Google Scholar] [CrossRef]
- Schrock, R.R.; Seidel, S.W.; Mösch-Zanetti, N.C.; Shih, K.-Y.; O’Donoghue, M.B.; Davis, W.M.; Reiff, W.M. Synthesis and Decomposition of Alkyl Complexes of Molybdenum (IV) That Contain a [(Me3SiNCH2CH2)3N]3− Ligand. Direct Detection of α-Elimination Processes That Are More than Six Orders of Magnitude Faster than β-Elimination Processes. J. Am. Chem. Soc. 1997, 119, 11876–11893. [Google Scholar] [CrossRef]
- Pinkowicz, D.; Birk, F.J.; Magott, M.; Schulte, K.; Dunbar, K.R. Systematic Study of Open-Shell Trigonal Pyramidal Transition-Metal Complexes with a Rigid-Ligand Scaffold. Chem.—A Eur. J. 2017, 23, 3548–3552. [Google Scholar] [CrossRef]
- Brzozowska, M.; Handzlik, G.; Kurpiewska, K.; Zychowicz, M.; Pinkowicz, D. Pseudo-tetrahedral vs. pseudo-octahedral ErIII single molecule magnets and the disruptive role of coordinated TEMPO radical. Inorg. Chem. Front. 2021, 8, 2817–2828. [Google Scholar] [CrossRef]
- Chilton, N.F.; Anderson, R.P.; Turner, L.D.; Soncini, A.; Murray, K.S. PHI: A powerful new program for the analysis of anisotropic monomeric and exchange-coupled polynuclear d- and f-block complexes. J. Comput. Chem. 2013, 34, 1164–1175. [Google Scholar] [CrossRef]
- Liu, J.-L.; Chen, Y.-C.; Tong, M.-L. Symmetry strategies for high performance lanthanide-based single-molecule magnets. Chem. Soc. Rev. 2018, 47, 2431–2453. [Google Scholar] [CrossRef]
- Guo, Y.-N.; Xu, G.-F.; Guo, Y.; Tang, J. Relaxation dynamics of dysprosium (iii) single molecule magnets. Dalton Trans. 2011, 40, 9953–9963. [Google Scholar] [CrossRef] [PubMed]
- Liddle, S.T.; van Slageren, J. Improving f-element single molecule magnets. Chem. Soc. Rev. 2015, 44, 6655–6669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aquilante, F.; Autschbach, J.; Carlson, R.K.; Chibotaru, L.F.; Delcey, M.G.; De Vico, L.; Fdez Galván, I.; Ferré, N.; Frutos, L.M.; Gagliardi, L.; et al. Molcas 8: New capabilities for multiconfigurational quantum chemical calculations across the periodic table. J. Comput. Chem. 2016, 37, 506–541. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Arczyński, M.; Stanek, J.; Sieklucka, B.; Dunbar, K.R.; Pinkowicz, D. Site-Selective Photoswitching of Two Distinct Magnetic Chromophores in a Propeller-Like Molecule To Achieve Four Different Magnetic States. J. Am. Chem. Soc. 2019, 141, 19067–19077. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).