High Yield Synthesis and Application of Magnetite Nanoparticles (Fe3O4)
Abstract
:1. Introduction
2. Results and Discussion
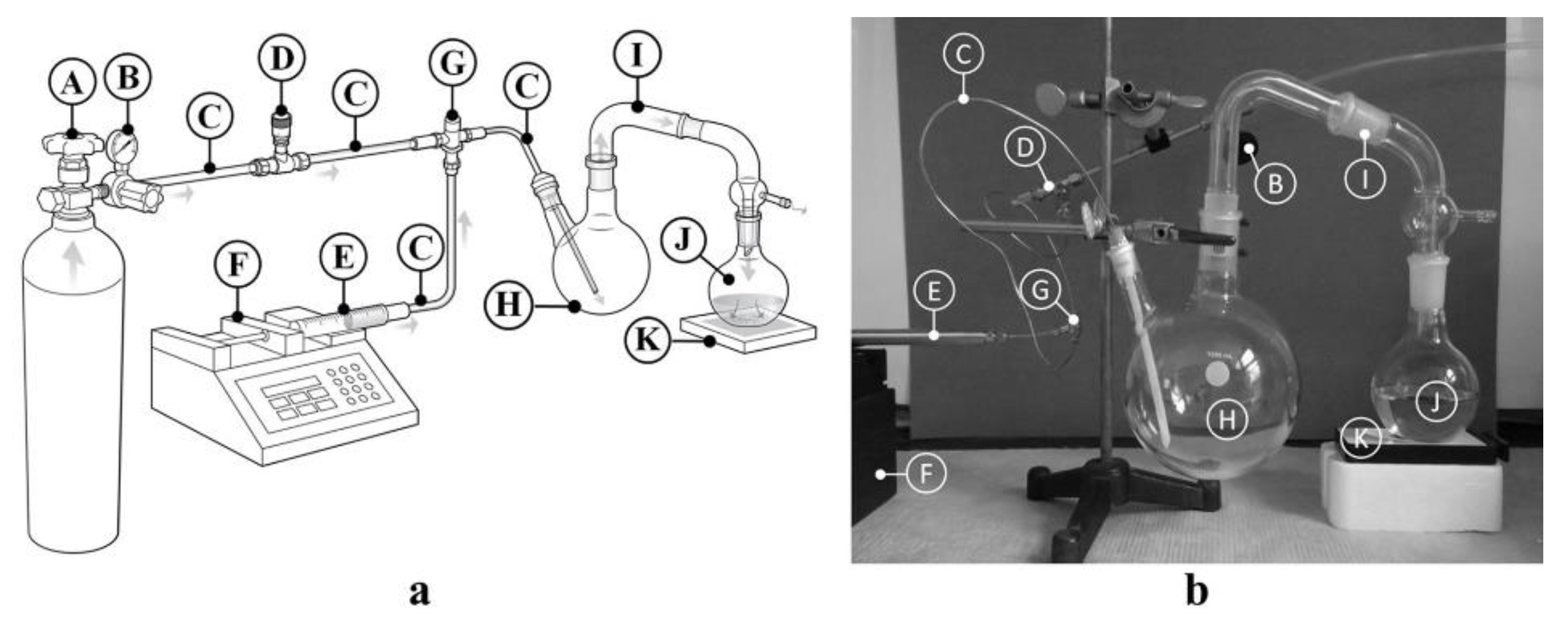
2.1. Apparatus
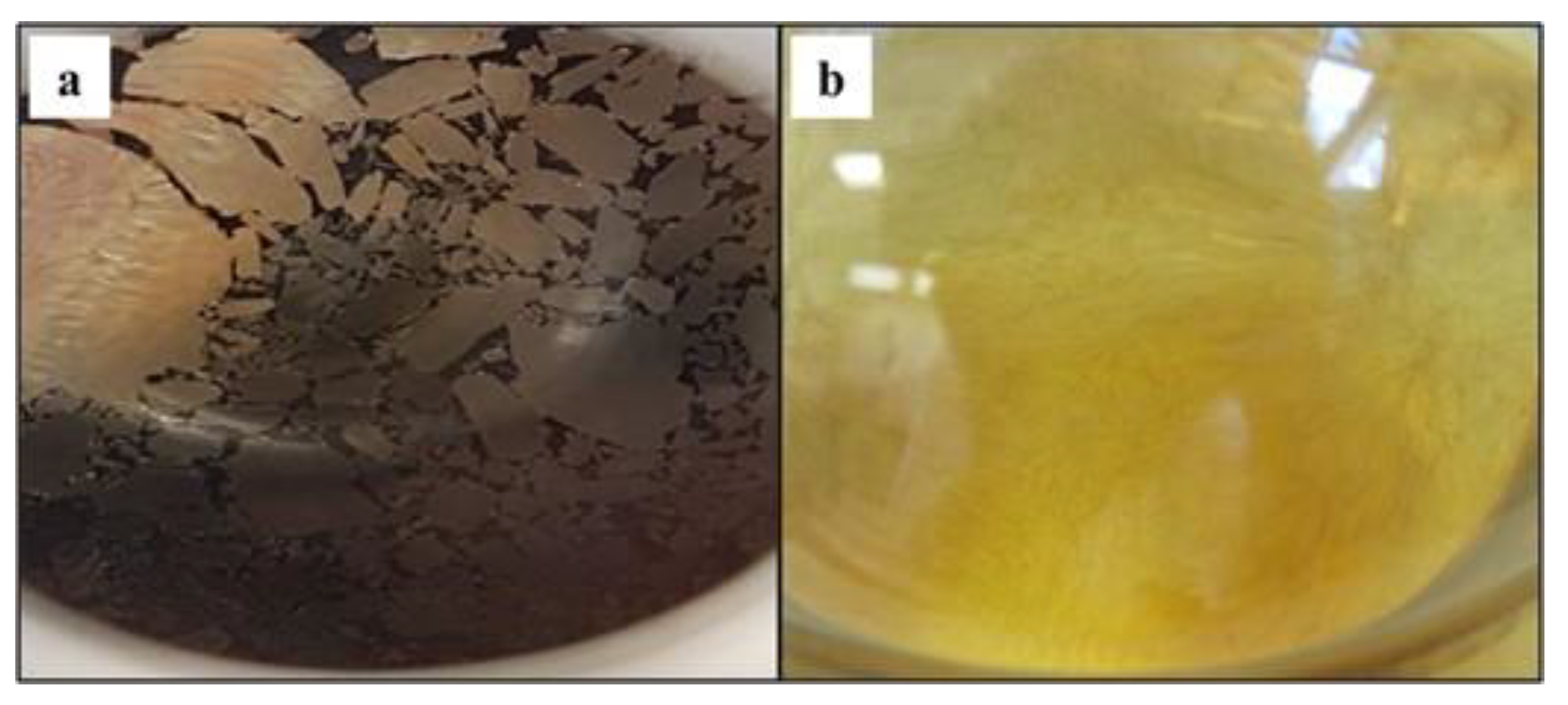
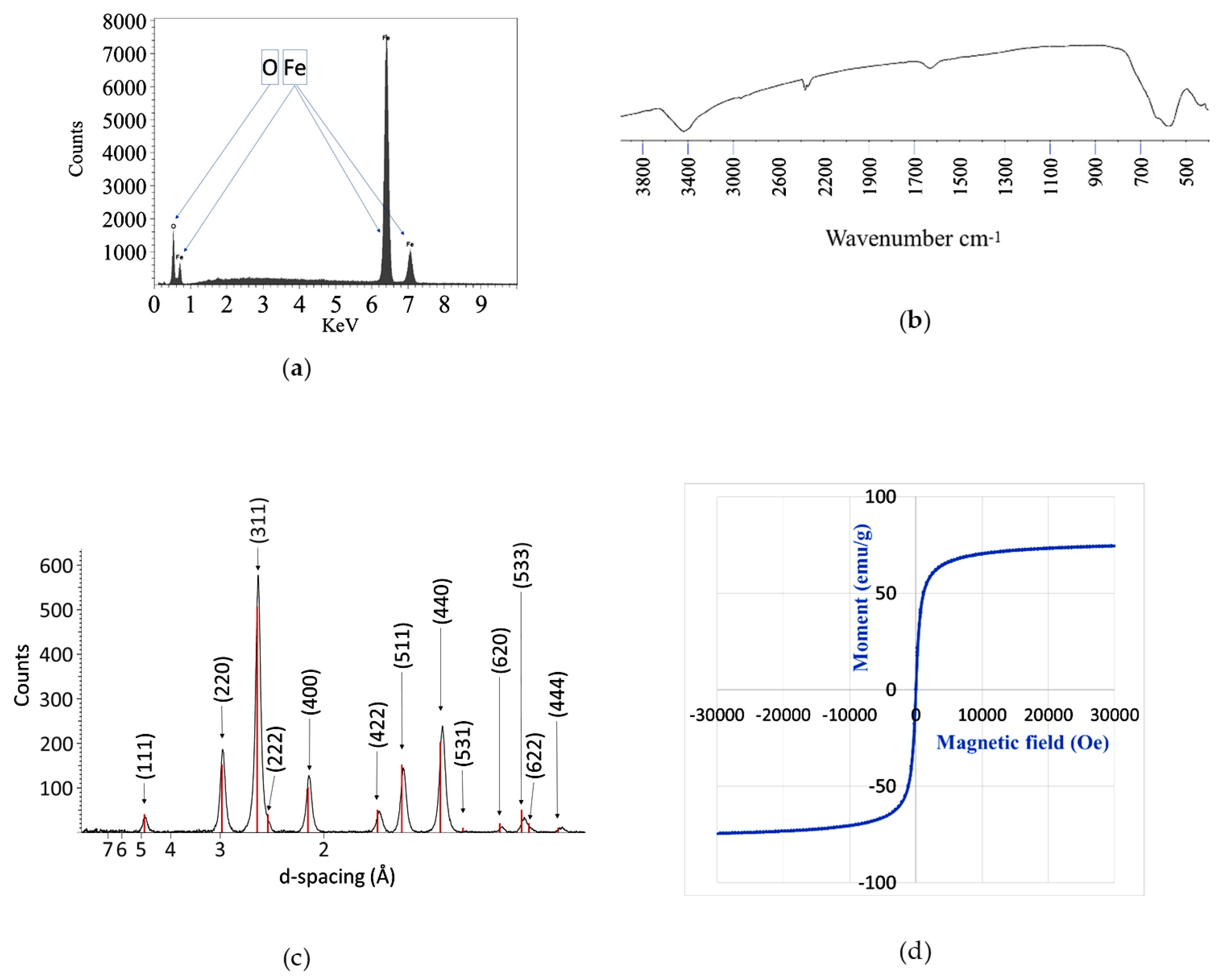
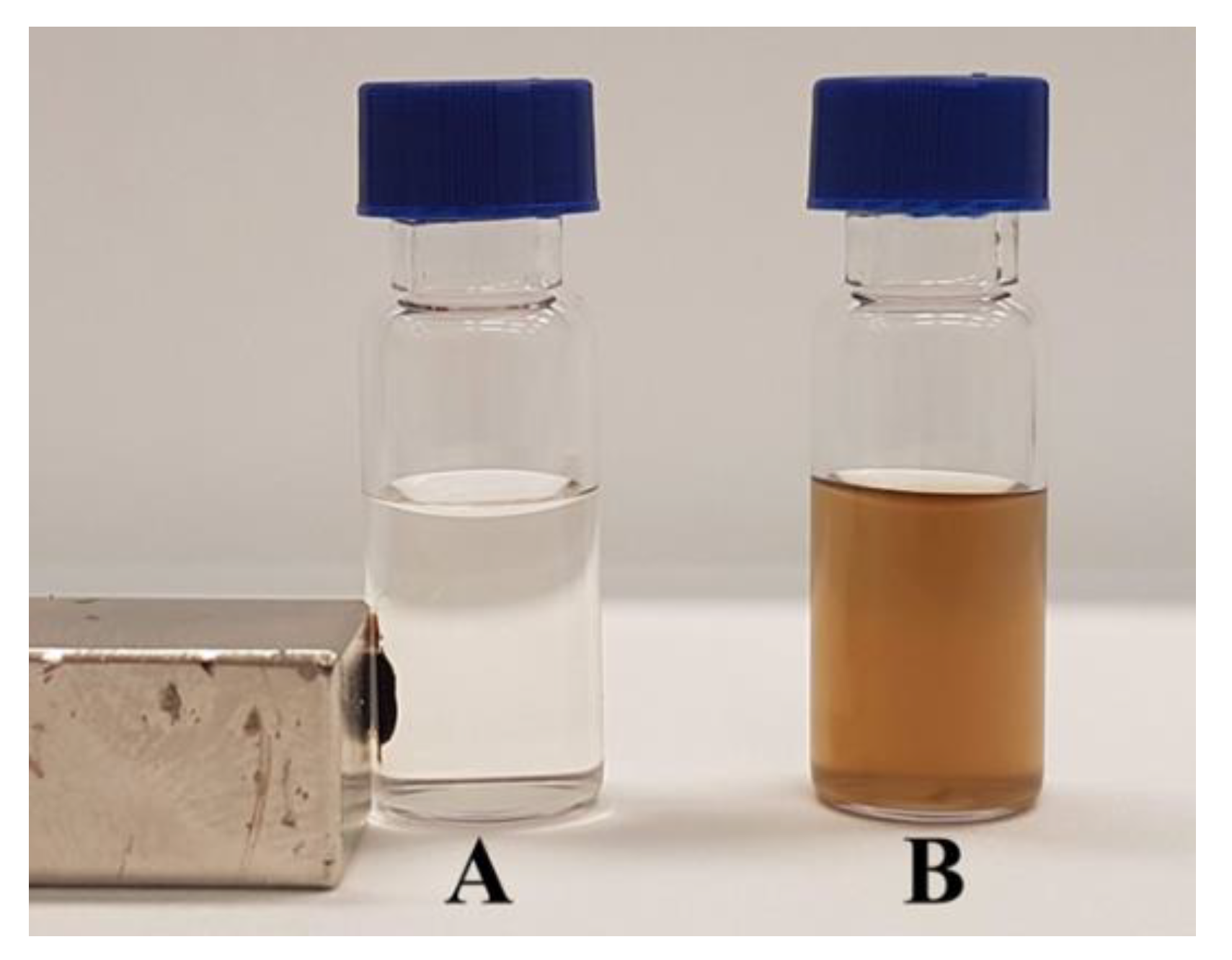
2.2. Material Analyses
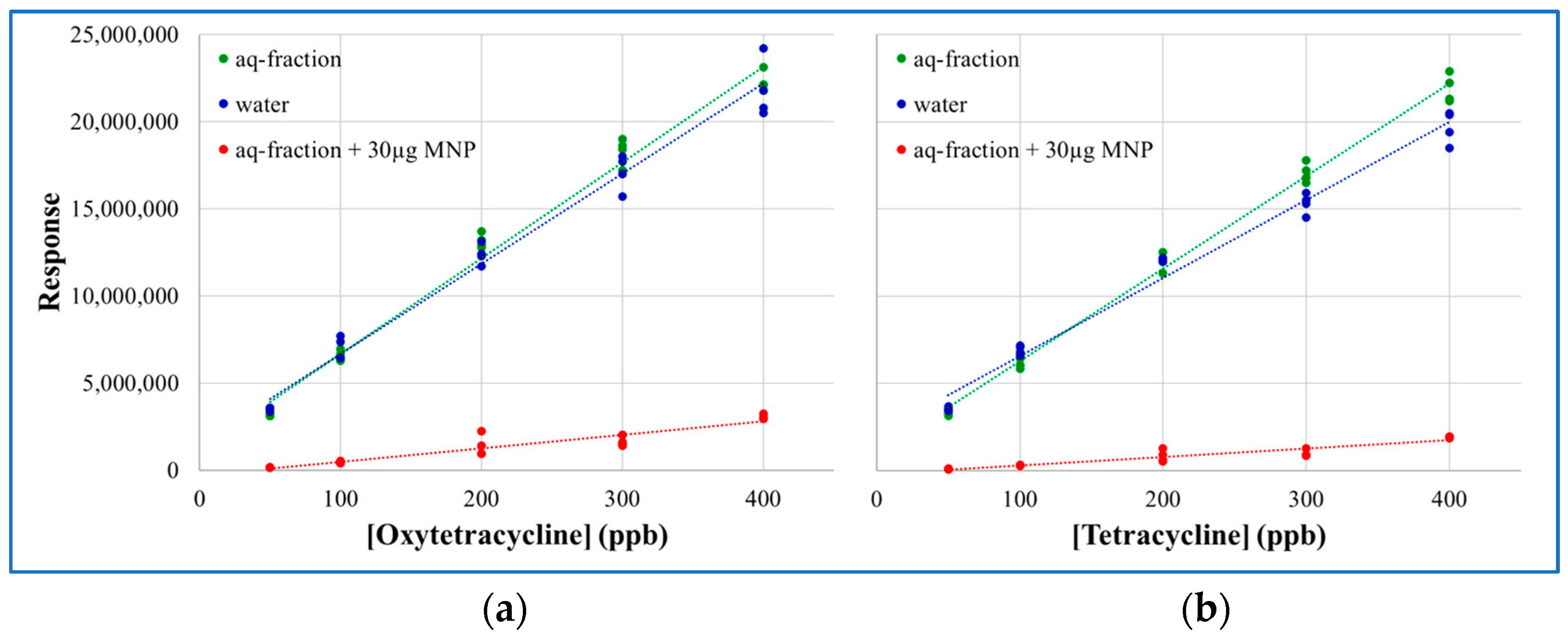
2.3. MNP Extraction of Tetracyclines
3. Materials and Methods
3.1. Materials and Instrumentation
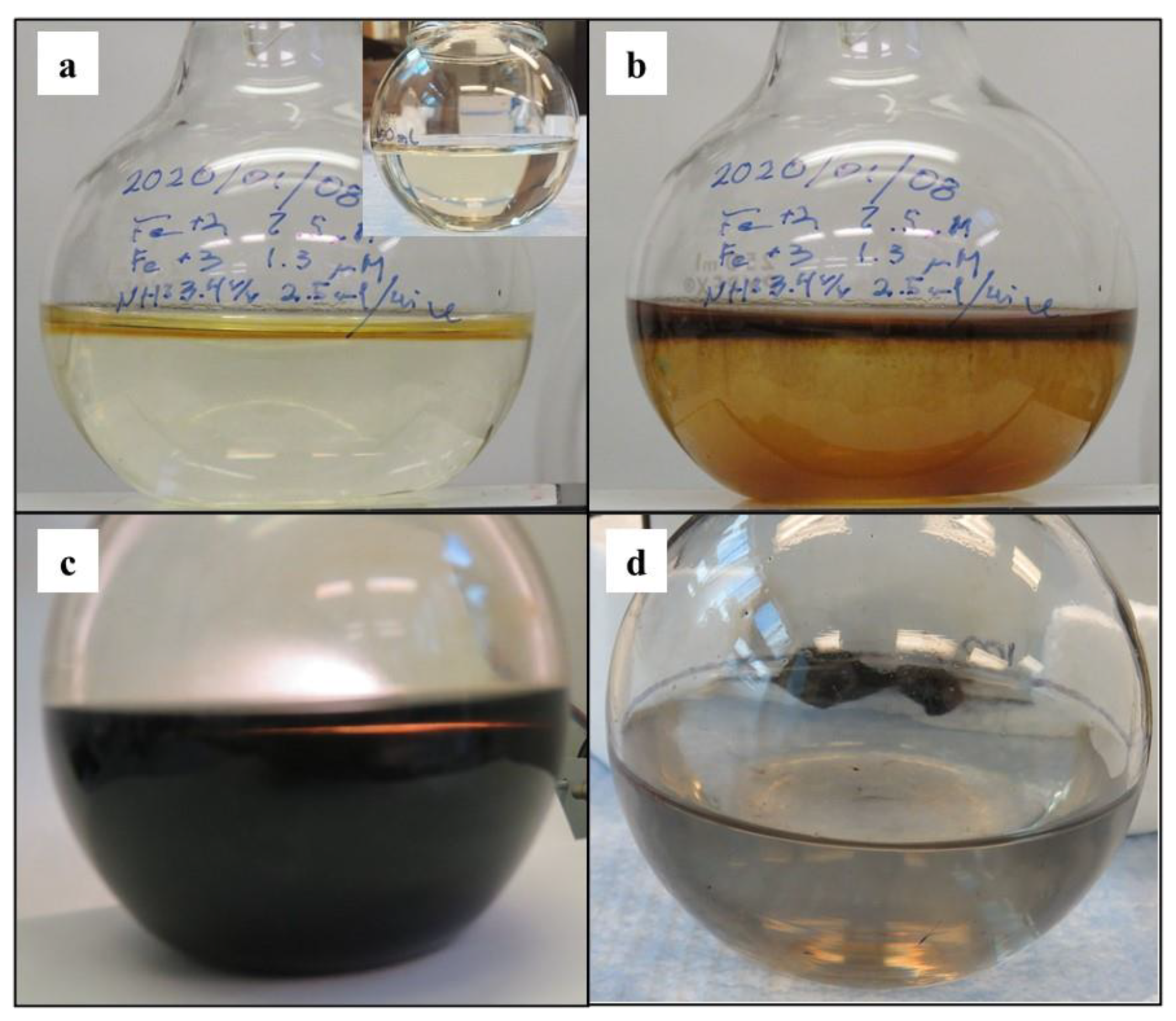
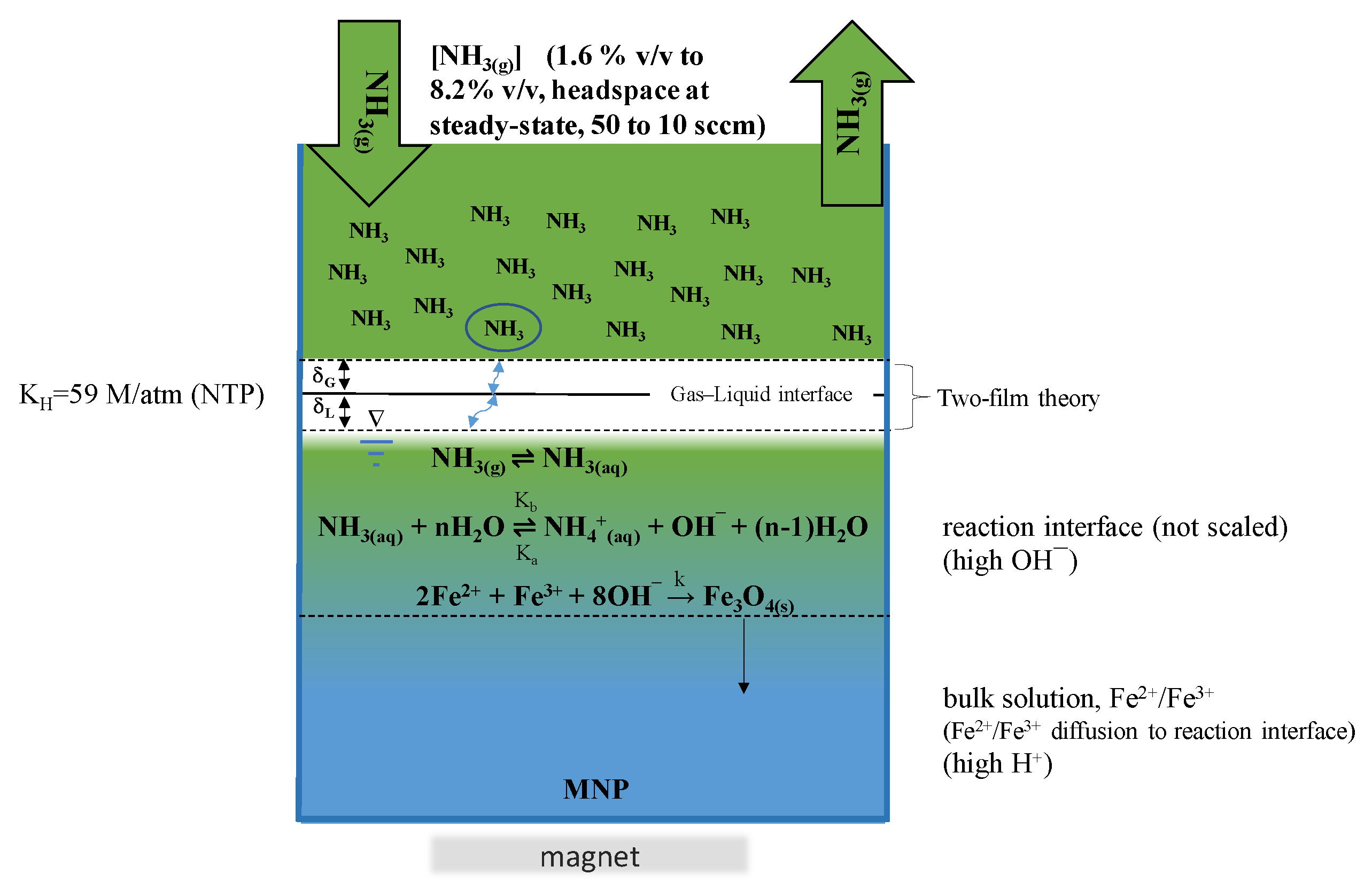
3.2. Magnetite Nanoparticle Synthesis
3.2.1. Chemical Co-Precipitation
3.2.2. Iron Salts Solutions (Fe2+ and Fe3+)
3.2.3. Steady State [NH3(g)]
3.2.4. Apparatus
3.3. Material Analyses
3.3.1. Preparation of Nanoparticles for Analyses
3.3.2. TEM
3.3.3. Particle Size Analyses
3.3.4. ICP-MS Analyses of Aqueous Fraction
3.3.5. EDXRF
3.3.6. FT-IR
3.3.7. XRD
3.3.8. Magnetization Saturation
3.3.9. Extraction of Tetracyclines with Magnetite Nanoparticles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhou, L.; Yuan, J.; Yuan, W.; Sui, X.; Wu, S.; Li, Z.; Shen, D. Synthesis, characterization, and controllable drug release of pH-sensitive hybrid magnetic nanoparticles. J. Magn. Magn. Mater. 2009, 321, 2799–2804. [Google Scholar] [CrossRef]
- Yang, J.; Park, S.B.; Yoon, H.G.; Huh, Y.M.; Haam, S. Preparation of poly ε-caprolactone nanoparticles containing magnetite for magnetic drug carrier. Int. J. Pharm. 2006, 324, 185–190. [Google Scholar] [CrossRef]
- Jain, T.K.; Morales, M.A.; Sahoo, S.K.; Leslie-Pelecky, D.L.; Labhasetwar, V. Iron oxide nanoparticles for sustained delivery of anticancer agents. Mol. Pharm. 2005, 2, 194–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Javed, Y.; Akhtar, K.; Anwar, H.; Jamil, Y. MRI based on iron oxide nanoparticles contrast agents: Effect of oxidation state and architecture. J. Nanopart. Res. 2017, 19, 366. [Google Scholar] [CrossRef]
- Corot, C.; Robert, P.; Idée, J.M.; Port, M. Recent advances in iron oxide nanocrystal technology for medical imaging. Adv. Drug Deliv. Rev. 2006, 58, 1471–1504. [Google Scholar] [CrossRef] [PubMed]
- Bruck, A.M.; Cama, C.A.; Gannett, C.N.; Marschilok, A.C.; Takeuchi, E.S.; Takeuchi, K.J. Nanocrystalline iron oxide based electroactive materials in lithium ion batteries: The critical role of crystallite size, morphology, and electrode heterostructure on battery relevant electrochemistry. Inorg. Chem. Front. 2016, 3, 26–40. [Google Scholar] [CrossRef]
- Katz, E. Synthesis, Properties and Applications of Magnetic Nanoparticles and Nanowires—A Brief Introduction. Magnetochemistry 2019, 5, 61. [Google Scholar] [CrossRef] [Green Version]
- Shipley, H.J.; Engates, K.E.; Guettner, A.M. Study of iron oxide nanoparticles in soil for remediation of arsenic. J. Nanopart. Res. 2011, 13, 2387–2397. [Google Scholar] [CrossRef]
- Gaiani, G.; O’Sullivan, C.K.; Campàs, M. Magnetic Beads in Marine Toxin Detection: A Review. Magnetochemistry 2019, 5, 62. [Google Scholar] [CrossRef] [Green Version]
- Cecchin, I.; Reddy, K.R.; Thomé, A.; Tessaro, E.F.; Schnaid, F. Nanobioremediation: Integration of nanoparticles and bioremediation for sustainable remediation of chlorinated organic contaminants in soils. Int. Biodeterior. Biodegrad. 2017, 119, 419–428. [Google Scholar] [CrossRef]
- Sarcletti, M.; Vivod, D.; Luchs, T.; Rejek, T.; Portilla, L.; Müller, L.; Dietrich, H.; Hirsch, A.; Zahn, D.; Halik, M. Superoleophilic Magnetic Iron Oxide Nanoparticles for Effective Hydrocarbon Removal from Water. Adv. Funct. Mater. 2019, 29, 1–7. [Google Scholar] [CrossRef]
- Huang, Y.F.; Wang, Y.F.; Yan, X.P. Amine-functionalized magnetic nanoparticles for rapid capture and removal of bacterial pathogens. Environ. Sci. Technol. 2010, 44, 7908–7913. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Wu, Z.; Yu, T.; Jiang, C.; Kim, W.S. Recent progress on magnetic iron oxide nanoparticles: Synthesis, surface functional strategies and biomedical applications. Sci. Technol. Adv. Mater. 2015, 16, 23501. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Fakhrullin, R.F.; Özmen, M.; Wang, H.; Wang, J.; Paunov, V.N.; Li, G.; Huang, W.E. Functionalization of whole-cell bacterial reporters with magnetic nanoparticles. Microb. Biotechnol. 2011, 4, 89–97. [Google Scholar] [CrossRef] [Green Version]
- Ahn, T.; Kim, J.H.; Yang, H.M.; Lee, J.W.; Kim, J.D. Formation pathways of magnetite nanoparticles by coprecipitation method. J. Phys. Chem. C 2012, 116, 6069–6076. [Google Scholar] [CrossRef]
- Wu, S.; Sun, A.; Zhai, F.; Wang, J.; Xu, W.; Zhang, Q.; Volinsky, A.A. Fe3O4 magnetic nanoparticles synthesis from tailings by ultrasonic chemical co-precipitation. Mater. Lett. 2011, 65, 1882–1884. [Google Scholar] [CrossRef]
- Abou-Hassan, A.; Dufrêchfer, J.F.; Sandre, O.; Mériguet, G.; Bernard, O.; Cabuil, V. Fluorescence confocal laser scanning microscopy for pH mapping in a coaxial flow microreactor: Application in the synthesis of superparamagnetic nanoparticles. J. Phys. Chem. C 2009, 113, 18097–18105. [Google Scholar] [CrossRef] [Green Version]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Elst, L.V.; Muller, R.N. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef]
- Tronc, E.; Belleville, P.; Jolivet, J.P.; Livage, J. Transformation of ferric hydroxide into spinel by Fe(II) adsorption. Langmuir 1992, 8, 313–319. [Google Scholar] [CrossRef]
- Jolivet, J.; Chanéac, C.; Tronc, E. Iron oxide chemistry. From molecular clusters to extended solid networks. Chem. Commun. 2004, 98, 477–483. [Google Scholar] [CrossRef]
- Lee, S.J.; Jeong, J.R.; Shin, S.C.; Kim, J.C.; Kim, J.D. Synthesis and characterization of superparamagnetic maghemite nanoparticles prepared by coprecipitation technique. J. Magn. Magn. Mater. 2004, 282, 147–150. [Google Scholar] [CrossRef]
- Mo, Z.; Zhang, C.; Guo, R.; Meng, S.; Zhang, J. Synthesis of Fe3O4 nanoparticles using controlled ammonia vapor diffusion under ultrasonic irradiation. Ind. Eng. Chem. Res. 2011, 50, 3534–3539. [Google Scholar] [CrossRef]
- Mamani, J.B.; Gamarra, L.F.; De Souza Brito, G.E. Synthesis and characterization of Fe3O4 nanoparticles with perspectives in biomedical applications. Mater. Res. 2014, 17, 542–549. [Google Scholar] [CrossRef]
- Petrushevska, M.; Pavlovska, K.; Laskova, J.; Zdravkovski, P.; Dodov, M.G. Transmission Electron Microscopy: Novel Application of Established Technique in Characterization of Nanoparticles as Drug Delivery Systems. Prilozi 2019, 40, 67–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habila, M.A.; Alothman, Z.A.; El-Toni, A.M.; Labis, J.P.; Soylak, M. Synthesis and application of Fe3O4@SiO2@TiO2 for photocatalytic decomposition of organic matrix simultaneously with magnetic solid phase extraction of heavy metals prior to ICP-MS analysis. Talanta 2016, 154, 539–547. [Google Scholar] [CrossRef]
- Farrell, D.; Majetich, S.A.; Wilcoxon, J.P. Preparation and characterization of monodisperse Fe nanoparticles. J. Phys. Chem. B 2003, 107, 11022–11030. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T.K. Characterization techniques for nanoparticles: Comparison and complementarity upon studying nanoparticle properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef] [Green Version]
- Mahdavi, M.; Namvar, F.; Bin Ahmad, M.; Mohamad, R. Green biosynthesis and characterization of magnetic iron oxide (Fe3O4) nanoparticles using seaweed (Sargassum muticum) aqueous extract. Molecules 2013, 18, 5954–5964. [Google Scholar] [CrossRef]
- Kim, W.; Suh, C.Y.; Cho, S.W.; Roh, K.M.; Kwon, H.; Song, K.; Shon, I.J. A new method for the identification and quantification of magnetite-maghemite mixture using conventional X-ray diffraction technique. Talanta 2012, 94, 348–352. [Google Scholar] [CrossRef]
- Silva, V.A.J.; Andrade, P.L.; Silva, M.P.C.; Bustamante, A.D.; De Los Santos Valladares, L.; Albino Aguiar, J. Synthesis and characterization of Fe3O4 nanoparticles coated with fucan polysaccharides. J. Magn. Magn. Mater. 2013, 343, 138–143. [Google Scholar] [CrossRef] [Green Version]
- Alibeigi, S.; Vaezi, M.R. Phase transformation of iron oxide nanoparticles by varying the molar ratio of Fe2+:Fe3+. Chem. Eng. Technol. 2008, 31, 1591–1596. [Google Scholar] [CrossRef]
- Petcharoen, K.; Sirivat, A. Synthesis and characterization of magnetite nanoparticles via the chemical co-precipitation method. Mater. Sci. Eng. B Solid State Mater. Adv. Technol. 2012, 177, 421–427. [Google Scholar] [CrossRef]
- Singh, H.; Yadav, K.L. Structural, dielectric, vibrational and magnetic properties of Sm doped BiFeO3 multiferroic ceramics prepared by a rapid liquid phase sintering method. Ceram. Int. 2015, 41, 9285–9295. [Google Scholar] [CrossRef]
- Dar, M.I.; Shivashankar, S.A. Single crystalline magnetite, maghemite, and hematite nanoparticles with rich coercivity. RSC Adv. 2014, 4, 4105–4113. [Google Scholar] [CrossRef]
- Kolen’Ko, Y.V.; Bañobre-López, M.; Rodríguez-Abreu, C.; Carbó-Argibay, E.; Sailsman, A.; Piñeiro-Redondo, Y.; Cerqueira, M.F.; Petrovykh, D.Y.; Kovnir, K.; Lebedev, O.I.; et al. Large-scale synthesis of colloidal Fe3O4 nanoparticles exhibiting high heating efficiency in magnetic hyperthermia. J. Phys. Chem. C 2014, 118, 8691–8701. [Google Scholar] [CrossRef]
- He, X.; Zhong, W.; Au, C.T.; Du, Y. Size dependence of the magnetic properties of Ni nanoparticles prepared by thermal decomposition method. Nanoscale Res. Lett. 2013, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.L.; Li, N.; Cui, L.; Wang, X.; Zhao, R.S. Recent application of magnetic solid phase extraction for food safety analysis. TrAC Trends Anal. Chem. 2019, 120, 115632. [Google Scholar] [CrossRef]
- Neuvonen, P.J. Interactions with the Absorption of Tetracyclines. Drugs 1976, 11, 45–54. [Google Scholar] [CrossRef]
- Chemical, A.; National, S. 254th American Chemical Society National Meeting and Exposition; American Chemical Society: Washington, DC, USA, 2017; ISBN 2022494016. [Google Scholar]


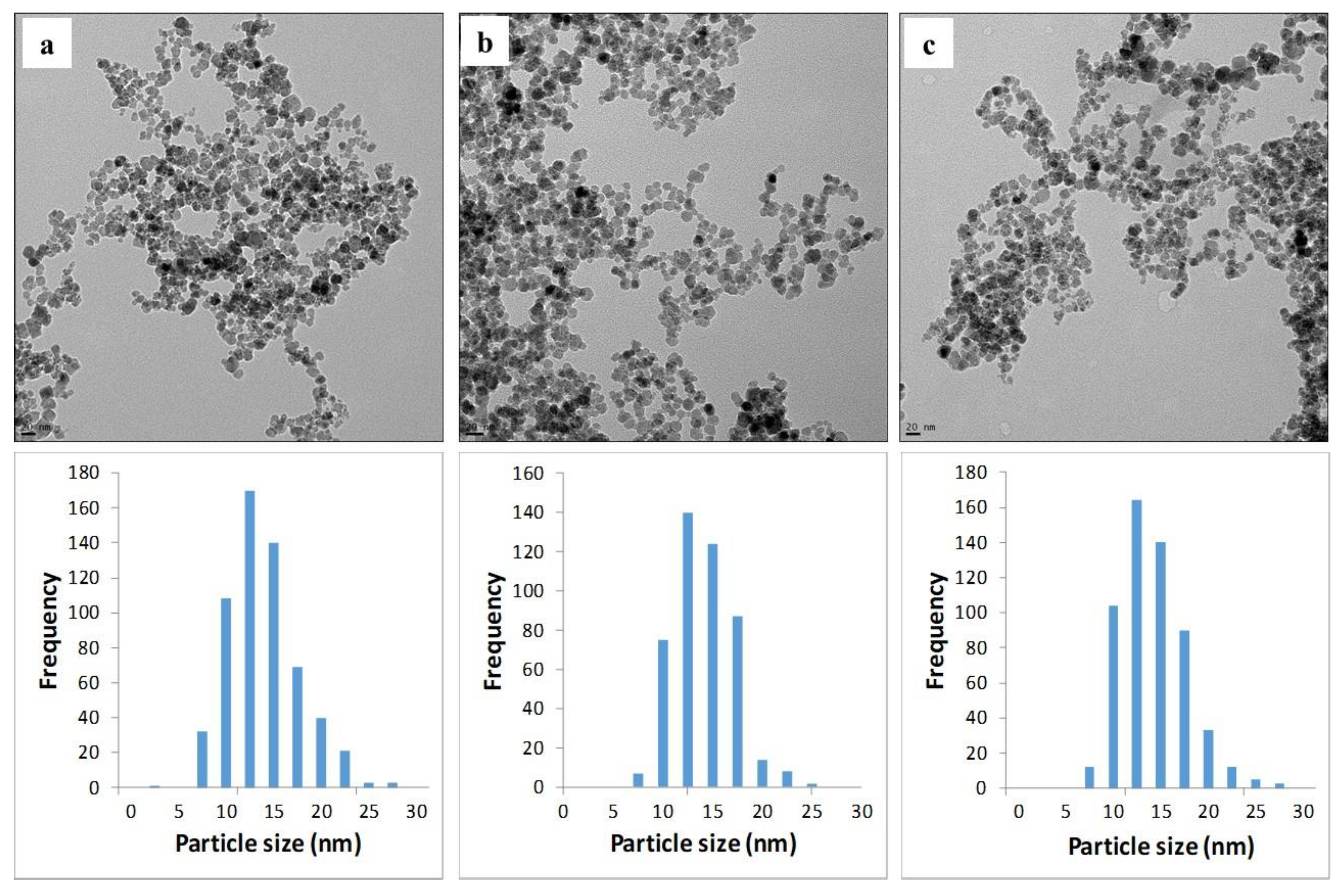
| [NH3(g)], %v/v | n | Mean (nm) | Median (nm) | S | ±1 S, % | Kurtosis | Skewness |
|---|---|---|---|---|---|---|---|
| 1.6 | 587 | 12.8 | 12.3 | ±3.7 | 70 | 0.7 | 0.6 |
| 3.3 | 457 | 12.9 | 12.6 | ±3.0 | 67 | 0.2 | 0.5 |
| 8.4 | 563 | 12.9 | 12.5 | ±3.5 | 70 | 1.0 | 0.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wroblewski, C.; Volford, T.; Martos, B.; Samoluk, J.; Martos, P. High Yield Synthesis and Application of Magnetite Nanoparticles (Fe3O4). Magnetochemistry 2020, 6, 22. https://doi.org/10.3390/magnetochemistry6020022
Wroblewski C, Volford T, Martos B, Samoluk J, Martos P. High Yield Synthesis and Application of Magnetite Nanoparticles (Fe3O4). Magnetochemistry. 2020; 6(2):22. https://doi.org/10.3390/magnetochemistry6020022
Chicago/Turabian StyleWroblewski, Charles, Tunde Volford, Blake Martos, Jurek Samoluk, and Perry Martos. 2020. "High Yield Synthesis and Application of Magnetite Nanoparticles (Fe3O4)" Magnetochemistry 6, no. 2: 22. https://doi.org/10.3390/magnetochemistry6020022
APA StyleWroblewski, C., Volford, T., Martos, B., Samoluk, J., & Martos, P. (2020). High Yield Synthesis and Application of Magnetite Nanoparticles (Fe3O4). Magnetochemistry, 6(2), 22. https://doi.org/10.3390/magnetochemistry6020022




