Magnetic Nanoparticles as In Vivo Tracers for Alzheimer’s Disease
Abstract
1. Alzheimer’s Disease
2. Diagnostic Tracers for Targeting Brain Tissue in Alzheimer’s Disease
3. Modes of Brain Targeting for Detection of Alzheimer’s Disease
3.1. Across the Blood-Brain Barrier
3.2. Bypassing the Blood-Brain Barrier
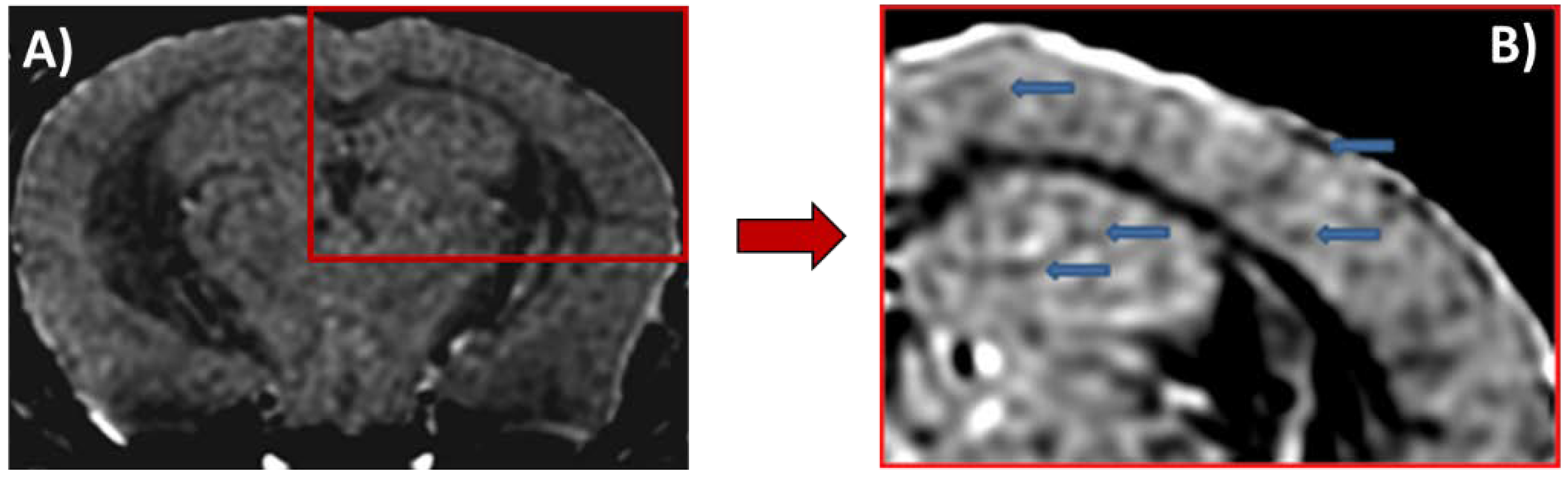
4. Magnetic Resonance Imaging in Alzheimer’s Disease
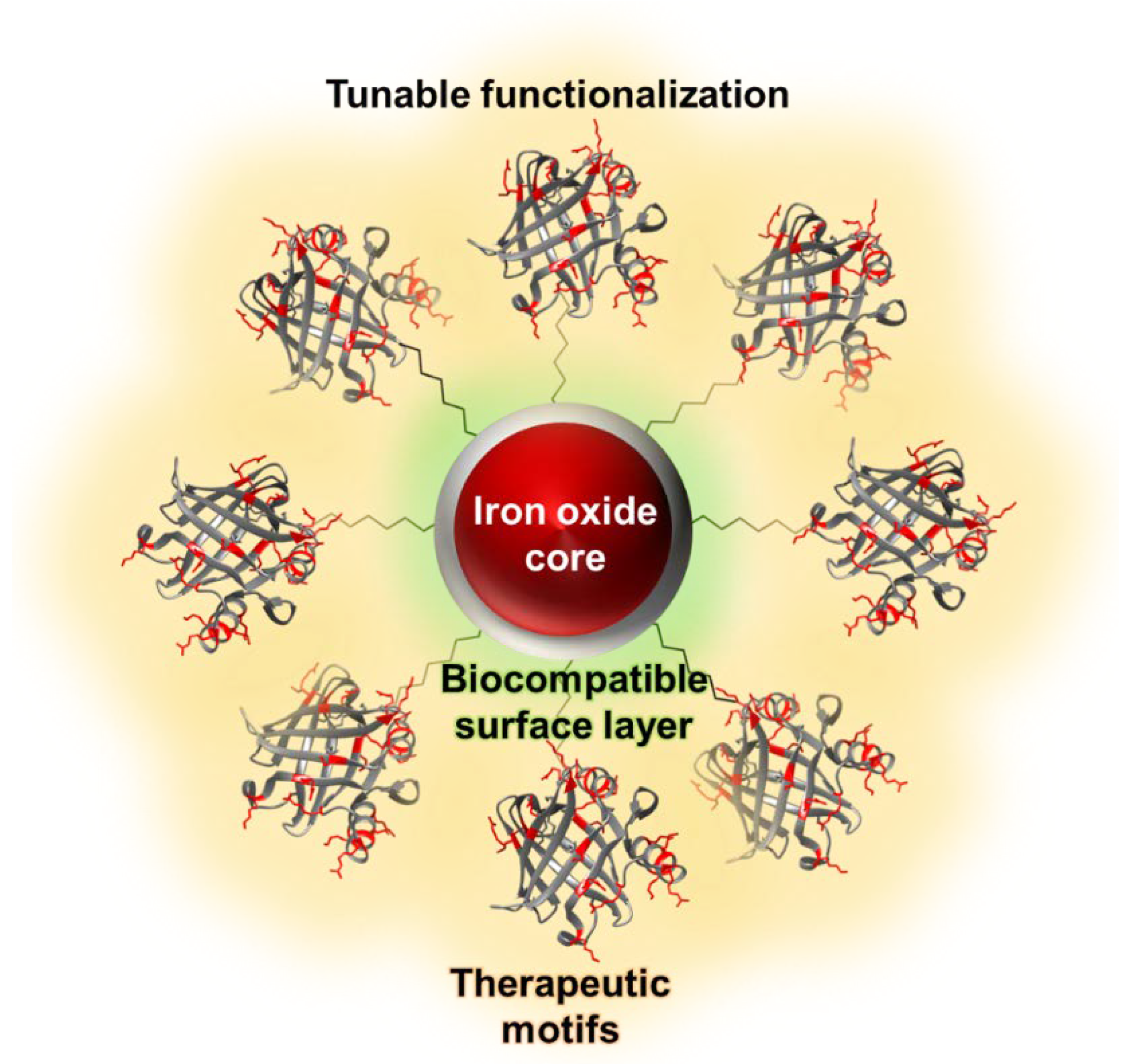
5. Iron Oxide Nanoparticles as Magnetic Resonance Imaging Probes
6. Magnetic Resonance Imaging Probes for Alzheimer’s Disease Therapeutics
7. The Future Outlook for Alzheimer’s Disease Tracers
Author Contributions
Funding
Conflicts of Interest
References
- Alzheimer’s Association. 2015 Alzheimer’s disease facts and figures. Alzheimers Dement. 2015, 11, 332–384. [Google Scholar] [CrossRef]
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Shui, B.; Tao, D.; Florea, A.; Cheng, J.; Zhao, Q.; Gu, Y.; Li, W.; Jaffrezic-Renault, N.; Mei, Y.; Guo, Z. Biosensors for Alzheimer’s disease biomarker detection: A review. Biochimie 2018, 147, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Vieira, D.B.; Gamarra, L.F. Getting into the brain: Liposome-based strategies for effective drug delivery across the blood-brain barrier. Int. J. Nanomed. 2016, 11, 5381–5414. [Google Scholar] [CrossRef] [PubMed]
- Masserini, M. Nanoparticles for brain drug delivery. ISRN Biochem 2013, 2013, 238428. [Google Scholar] [CrossRef]
- Vieira, D.B.; Gamarra, L.F. Multifunctional Nanoparticles for Successful Targeted Drug Delivery across the Blood-Brain Barrier. In Molecular Insight of Drug Design; Parikesit, A.A., Ed.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Dong, X. Current Strategies for Brain Drug Delivery. Theranostics 2018, 8, 1481–1493. [Google Scholar] [CrossRef]
- Eisenberg, D.; Jucker, M. The amyloid state of proteins in human diseases. Cell 2012, 148, 1188–1203. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Sagare, A.P.; Zlokovic, B.V. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 2018, 14, 133–150. [Google Scholar] [CrossRef]
- Pardridge, W.M. Molecular Trojan horses for blood-brain barrier drug delivery. Curr. Opin. Pharmacol. 2006, 6, 494–500. [Google Scholar] [CrossRef]
- Wadghiri, Y.Z.; Li, J.; Wang, J.; Hoang, D.M.; Sun, Y.; Xu, H.; Tsui, W.; Li, Y.; Boutajangout, A.; Wang, A.; et al. Detection of amyloid plaques targeted by bifunctional USPIO in Alzheimer’s disease transgenic mice using magnetic resonance microimaging. PLoS ONE 2013, 8, e57097. [Google Scholar] [CrossRef]
- Paterson, R.W.; Slattery, C.F.; Poole, T.; Nicholas, J.M.; Magdalinou, N.K.; Toombs, J.; Chapman, M.D.; Lunn, M.P.; Heslegrave, A.J.; Foiani, M.S.; et al. Cerebrospinal fluid in the differential diagnosis of Alzheimer’s disease: Clinical utility of an extended panel of biomarkers in a specialist cognitive clinic. Alzheimers Res. Ther. 2018, 10, 32. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Delivery of Biologics Across the Blood-Brain Barrier with Molecular Trojan Horse Technology. BioDrugs 2017, 31, 503–519. [Google Scholar] [CrossRef] [PubMed]
- Wesson, D.W.; Levy, E.; Nixon, R.A.; Wilson, D.A. Olfactory dysfunction correlates with amyloid-beta burden in an Alzheimer’s disease mouse model. J. Neurosci. 2010, 30, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Amiri, H.; Saeidi, K.; Borhani, P.; Manafirad, A.; Ghavami, M.; Zerbi, V. Alzheimer’s disease: Pathophysiology and applications of magnetic nanoparticles as MRI theranostic agents. ACS Chem. Neurosci. 2013, 4, 1417–1429. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.Y.; Chen, T.W.; Zhang, X.M.; Huang, X.H. GRE T2 *-weighted MRI: Principles and clinical applications. Biomed. Res. Int. 2014, 2014, 312142. [Google Scholar] [CrossRef]
- Haase, A. FLASH MR imaging: A success story since 25 years. J. Magn. Reson. 2011, 213, 542–543. [Google Scholar] [CrossRef]
- Bruker. ParaVision 6.0.1; Müller-BBM GmbH, Ed.; Bruker Corporation: Ettlingen, Germany, 2015. [Google Scholar]
- Blasiak, B.; Barnes, S.; Foniok, T.; Rushforth, D.; Matyas, J.; Ponjevic, D.; Weglarz, W.P.; Tyson, R.; Iqbal, U.; Abulrob, A.; et al. Comparison of T 2 and T 2*-weighted MR molecular imaging of a mouse model of glioma. BMC Med. Imaging 2013, 13, 20. [Google Scholar] [CrossRef]
- Daldrup-Link, H.E. Ten Things You Might Not Know about Iron Oxide Nanoparticles. Radiology 2017, 284, 616–629. [Google Scholar] [CrossRef]
- Cardona, F.A.; Urquiza, E.S.; De la Presa, P.; Tobón, S.H.; Pal, U.; Fraijo, P.H.; Yacaman, M.J.; Lozada Ramírez, J.D.; Ivkov, R.; Angulo-Molina, A.; et al. Enhanced magnetic properties and MRI performance of bi-magnetic core–shell nanoparticles. RSC Adv. 2016, 6, 77558–77568. [Google Scholar] [CrossRef]
- Mohammadi, A.; Barikani, M. Synthesis and characterization of superparamagnetic Fe3O4 nanoparticles coated with thiodiglycol. Mater. Charact. 2014, 90, 88–93. [Google Scholar] [CrossRef]
- Arbab, A.S.; Bashaw, L.A.; Miller, B.R.; Jordan, E.K.; Lewis, B.K.; Kalish, H.; Frank, J.A. Characterization of Biophysical and Metabolic Properties of Cells Labeled with Superparamagnetic Iron Oxide Nanoparticles and Transfection Agent for Cellular MR Imaging. Radiology 2003, 229, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Hubert, V.; Dumot, C.; Ong, E.; Amaz, C.; Canet-Soulas, E.; Chauveau, F.; Wiart, M. MRI coupled with clinically-applicable iron oxide nanoparticles reveals choroid plexus involvement in a murine model of neuroinflammation. Sci. Rep. 2019, 9, 10046. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.K.; Chan, P.S.; Fan, S.; Kwan, S.M.; Yeung, K.L.; Wang, Y.X.; Chow, A.H.; Wu, E.X.; Baum, L. Curcumin-conjugated magnetic nanoparticles for detecting amyloid plaques in Alzheimer’s disease mice using magnetic resonance imaging (MRI). Biomaterials 2015, 44, 155–172. [Google Scholar] [CrossRef] [PubMed]
- Venkatesha, N.; Pudakalakatti, S.M.; Qurishi, Y.; Atreya, H.S.; Srivastava, C. MnFe2O4–Fe3O4 core–shell nanoparticles as a potential contrast agent for magnetic resonance imaging. RSC Adv. 2015, 5, 97807–97815. [Google Scholar] [CrossRef]
- Fan, K.; Cao, C.; Pan, Y.; Lu, D.; Yang, D.; Feng, J.; Song, L.; Liang, M.; Yan, X. Magnetoferritin nanoparticles for targeting and visualizing tumour tissues. Nat. Nanotechnol. 2012, 7, 459–464. [Google Scholar] [CrossRef]
- Uchida, M.; Terashima, M.; Cunningham, C.H.; Suzuki, Y.; Willits, D.A.; Willis, A.F.; Yang, P.C.; Tsao, P.S.; McConnell, M.V.; Young, M.J.; et al. A human ferritin iron oxide nano-composite magnetic resonance contrast agent. Magn. Reson. Med. Off. J. Soc. Magn. Reson. Med. Soc. Magn. Reson. Med. 2008, 60, 1073–1081. [Google Scholar] [CrossRef]
- Sana, B.; Johnson, E.; Lim, S. The unique self-assembly/disassembly property of Archaeoglobus fulgidus ferritin and its implications on molecular release from the protein cage. Biochim. Biophys. Acta 2015, 1850, 2544–2551. [Google Scholar] [CrossRef]
- Sana, B.; Johnson, E.; Sheah, K.; Poh, C.L.; Lim, S. Iron-based ferritin nanocore as a contrast agent. Biointerphases 2010, 5, FA48–FA52. [Google Scholar] [CrossRef]
- Viola, K.L.; Sbarboro, J.; Sureka, R.; De, M.; Bicca, M.A.; Wang, J.; Vasavada, S.; Satpathy, S.; Wu, S.; Joshi, H.; et al. Towards non-invasive diagnostic imaging of early-stage Alzheimer’s disease. Nat. Nanotechnol. 2015, 10, 91–98. [Google Scholar] [CrossRef]
- Hu, B.; Dai, F.; Fan, Z.; Ma, G.; Tang, Q.; Zhang, X. Nanotheranostics: Congo Red/Rutin-MNPs with Enhanced Magnetic Resonance Imaging and H2O2-Responsive Therapy of Alzheimer’s Disease in APPswe/PS1dE9 Transgenic Mice. Adv. Mater. 2015, 27, 5499–5505. [Google Scholar] [CrossRef]
- Chiu, M.J.; Chen, Y.F.; Chen, T.F.; Yang, S.Y.; Yang, F.P.; Tseng, T.W.; Chieh, J.J.; Chen, J.C.; Tzen, K.Y.; Hua, M.S.; et al. Plasma tau as a window to the brain-negative associations with brain volume and memory function in mild cognitive impairment and early Alzheimer’s disease. Hum. Brain Mapp. 2014, 35, 3132–3142. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Pervushin, K. Neuroprotective Function of Non-Proteolytic Amyloid-β Chaperones in Alzheimer’s Disease. In Amyloid Diseases; Kurouski, D., Ed.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Carver, J.A.; Grosas, A.B.; Ecroyd, H.; Quinlan, R.A. The functional roles of the unstructured N- and C-terminal regions in alphaB-crystallin and other mammalian small heat-shock proteins. Cell Stress Chaperones 2017, 22, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Kanekiyo, T.; Ban, T.; Aritake, K.; Huang, Z.L.; Qu, W.M.; Okazaki, I.; Mohri, I.; Murayama, S.; Ozono, K.; Taniike, M.; et al. Lipocalin-type prostaglandin D synthase/beta-trace is a major amyloid beta-chaperone in human cerebrospinal fluid. Proc. Natl. Acad. Sci. USA 2007, 104, 6412–6417. [Google Scholar] [CrossRef] [PubMed]
- Kannaian, B.; Sharma, B.; Phillips, M.; Chowdhury, A.; Manimekalai, M.S.S.; Adav, S.S.; Ng, J.T.Y.; Kumar, A.; Lim, S.; Mu, Y.; et al. Abundant neuroprotective chaperone Lipocalin-type prostaglandin D synthase (L-PGDS) disassembles the Amyloid-beta fibrils. Sci. Rep. 2019, 9, 12579. [Google Scholar] [CrossRef] [PubMed]
- Portioli, C.; Bovi, M.; Benati, D.; Donini, M.; Perduca, M.; Romeo, A.; Dusi, S.; Monaco, H.L.; Bentivoglio, M. Novel functionalization strategies of polymeric nanoparticles as carriers for brain medications. J. Biomed. Mater. Res. A 2016. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, B.; Pervushin, K. Magnetic Nanoparticles as In Vivo Tracers for Alzheimer’s Disease. Magnetochemistry 2020, 6, 13. https://doi.org/10.3390/magnetochemistry6010013
Sharma B, Pervushin K. Magnetic Nanoparticles as In Vivo Tracers for Alzheimer’s Disease. Magnetochemistry. 2020; 6(1):13. https://doi.org/10.3390/magnetochemistry6010013
Chicago/Turabian StyleSharma, Bhargy, and Konstantin Pervushin. 2020. "Magnetic Nanoparticles as In Vivo Tracers for Alzheimer’s Disease" Magnetochemistry 6, no. 1: 13. https://doi.org/10.3390/magnetochemistry6010013
APA StyleSharma, B., & Pervushin, K. (2020). Magnetic Nanoparticles as In Vivo Tracers for Alzheimer’s Disease. Magnetochemistry, 6(1), 13. https://doi.org/10.3390/magnetochemistry6010013




