Effect of Terbium Ion Substitution in Inverse Spinel Nickel Ferrite: Structural and Magnetic Study
Abstract
1. Introduction
2. Experimental
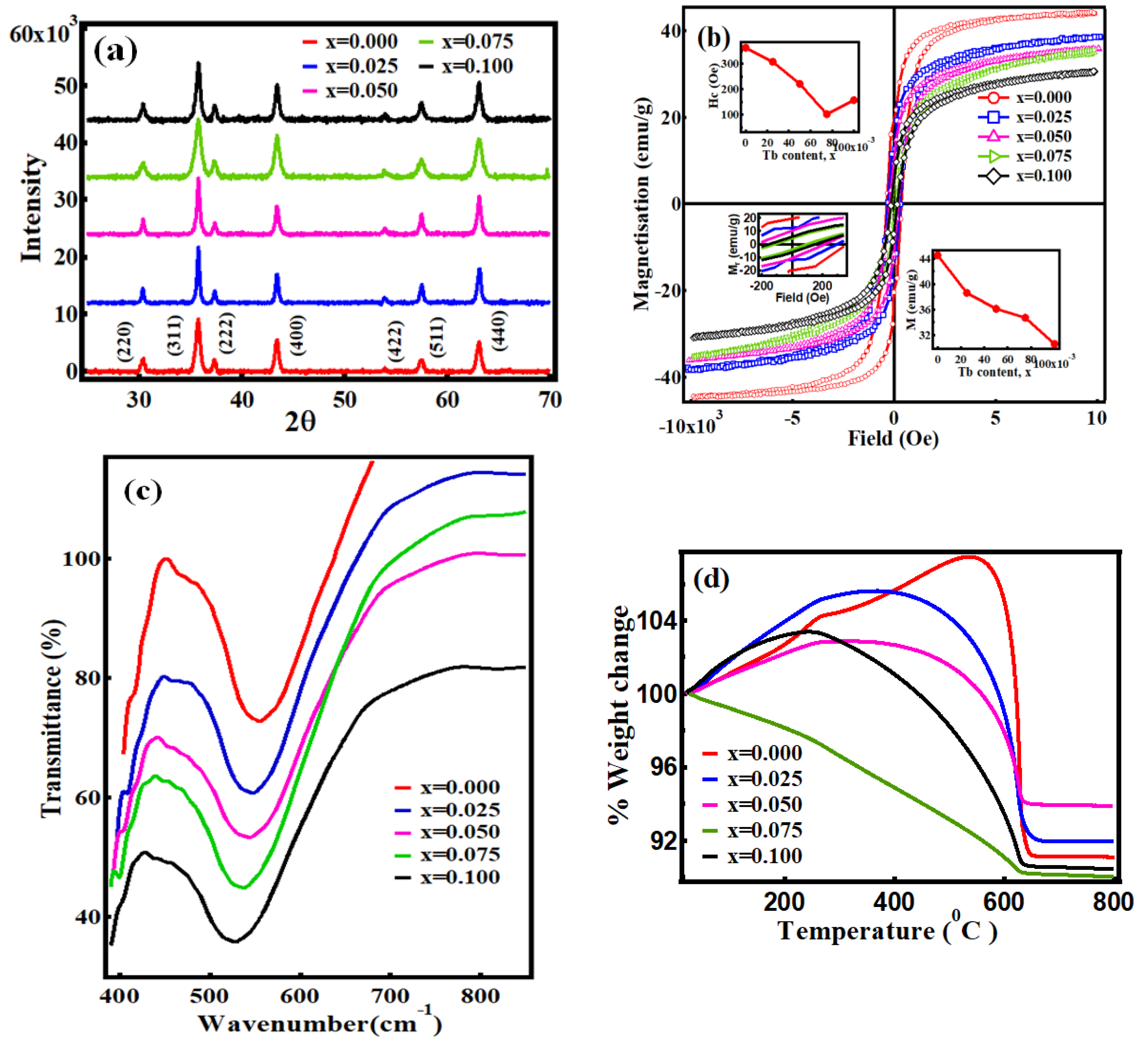
3. Results and Discussion
4. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Pérez, E.; Marquez, G.; Sagredo, V. Effect of calcination on characteristics of nickel ferrite nanoparticles synthesized by sol-gel method. Iraqi J. Appl. Phys. 2019, 15, 13–17. [Google Scholar]
- Dixit, G.; Negi, P.; Singh, J.P.; Srivastava, R.C.; Agrawal, H.M. Effect of Ce Doping on the Magnetic Properties of NiFe2O4 Nanoparticles. J. Supercond. Nov. Magn. 2013, 26, 1015–1019. [Google Scholar] [CrossRef]
- Singhal, S.; Chandra, K. Cation distribution and magnetic properties in chromium-substituted nickel ferrites prepared using aerosol route. J. Sol. State Chem. 2007, 180, 296–300. [Google Scholar] [CrossRef]
- Krishna, K.R.; Ravinder, D.; Kumar, K.V.; Lincon, C.A. Synthesis, XRD & SEM studies of zinc substitution in nickel ferrites by citrate gel technique. World J. Cond. Matt. Phys. 2012, 2, 153. [Google Scholar]
- Dormann, J.L.; Nogues, M. Magnetic structures in substituted ferrites. J. Phys. Cond. Matt. 1990, 2, 1223. [Google Scholar] [CrossRef]
- Rezlescu, N.; Rezlescu, E.; Pasnicu, C.; Craus, M.L. Effects of the rare-earth ions on some properties of a nickel-zinc ferrite. J. Phys. Cond. Matt. 1994, 6, 5707. [Google Scholar] [CrossRef]
- Virden, A.E.; O’Grady, K. Structure and magnetic properties of NiZn ferrite nanoparticles. J. Magn. Magn. Mater. 2005, 290, 868–870. [Google Scholar] [CrossRef]
- Batoo, K.M. Microstructural and Mössbauer properties of low temperature synthesized Ni-Cd-Al ferrite nanoparticles. Nanoscale Res. Let. 2011, 6, 499. [Google Scholar] [CrossRef]
- Sharma, R.K.; Suwalka, O.P.; Lakshmi, N.; Venugopalan, K. Hyperfine fields in nano particles of Cr0.25Co0.25Zn0.5Fe2O4. Indian J. Pure Appl. Phys. 2007, 45, 830–833. [Google Scholar]
- Wang, L.; Rai, B.K.; Mishra, S.R. Structural and magnetic study of Al3+ doped Ni0.75Zn0.25Fe2−xAlxO4 nanoferrites. Mater. Res. Bull. 2015, 65, 183–194. [Google Scholar] [CrossRef]
- Borhan, A.I.; Iordan, A.R.; Palamaru, M.N. Correlation between structural, magnetic and electrical properties of nanocrystalline Al3+ substituted zinc ferrite. Mater. Res. Bull. 2013, 48, 2549–2556. [Google Scholar] [CrossRef]
- Hashim, M.; Kumar, S.; Ali, S.; Koo, B.H.; Chung, H.; Kumar, R. Structural, magnetic and electrical properties of Al3+ substituted Ni–Zn ferrite nanoparticles. J. Alloy. Compd. 2012, 511, 107–114. [Google Scholar] [CrossRef]
- Lakhani, V.K.; Zhao, B.; Wang, L.; Trivedi, U.N.; Modi, K.B. Negative magnetization, magnetic anisotropy and magnetic ordering studies on Al3+-substituted copper ferrite. J. Alloy. Compd. 2011, 509, 4861–4867. [Google Scholar] [CrossRef]
- Lakhani, V.K.; Pathak, T.K.; Vasoya, N.H.; Modi, K.B. Structural parameters and X-ray Debye temperature determination study on copper-ferrite-aluminates. Solid State Sci. 2011, 13, 539–547. [Google Scholar] [CrossRef]
- Sharifi, I.; Shokrollahi, H. Structural, magnetic and Mössbauer evaluation of Mn substituted Co–Zn ferrite nanoparticles synthesized by co-precipitation. J. Magn. Magn. Mater. 2013, 334, 36–40. [Google Scholar] [CrossRef]
- Poudel, T.P.; Rai, B.K.; Yoon, S.; Guragain, D.; Neupane, D.; Mishra, S.R. The effect of gadolinium substitution in inverse spinel nickel ferrite: Structural, Magnetic, and Mössbauer study. J. Alloy. Compd. 2019, 802, 609–619. [Google Scholar] [CrossRef]
- Jacob, B.P.; Kumar, A.; Pant, R.P.; Singh, S.; Mohammed, E.M. Influence of preparation method on structural and magnetic properties of nickel ferrite nanoparticles. B. Mater. Sci. 2011, 34, 1345–1350. [Google Scholar] [CrossRef]
- Sontu, U.B.; Yelasani, V.; Musugu, V.R.R. Structural, electrical and magnetic characteristics of nickel substituted cobalt ferrite nano particles, synthesized by self combustion method. J. Magn. Magn. Mater. 2015, 374, 376–380. [Google Scholar] [CrossRef]
- Almessiere, M.A.; Slimani, Y.; Güngüneş, H.; Ali, S.; Manikandan, A.; Ercan, I.; Baykal, A.; Trukhanov, A.V. Magnetic attributes of NiFe2O4 nanoparticles: Influence of dysprosium Ions (Dy3+) substitution. Nanomaterials 2019, 9, 820. [Google Scholar] [CrossRef]
- Cheng, F.X.; Jia, J.T.; Xu, Z.G.; Zhou, B.; Liao, C.S.; Yan, C.H.; Chen, L.Y.; Zhao, H.B. Microstructure, magnetic, and magneto-optical properties of chemical synthesized Co–RE (RE= Ho, Er, Tm, Yb, Lu) ferrite nanocrystalline films. J. Appl. Phys. 1999, 86, 2727–2732. [Google Scholar] [CrossRef]
- Morrish, A.H. The physical principles of magnetism. In The Physical Principles of Magnetism; Morrish, A.H., Ed.; Wiley-VCH: Hoboken, NJ, USA, 2001; p. 696. [Google Scholar]
- Bharathi, K.K.; Chelvane, J.A.; Markandeyulu, G. Magnetoelectric properties of Gd and Nd-doped nickel ferrite. J. Magn. Magn. Mater. 2009, 321, 3677–3680. [Google Scholar] [CrossRef]
- Şabikoğlu, İ.; Paralı, L.; Malina, O.; Novak, P.; Kaslik, J.; Tucek, J.; Pechousek, J.; Navarik, J.; Schneeweiss, O. The effect of neodymium substitution on the structural and magnetic properties of nickel ferrite. Prog. Nat. Sci. Mater. 2015, 25, 215–221. [Google Scholar] [CrossRef]
- Lueken, H. Course of Lectures on Magnetism of Lanthanide Ions under Varying Ligand and Magnetic Fields; Institute of Inorganic Chemistry, RWTH Aachen: Aachen, Germany, 2008. [Google Scholar]
- Jacob, B.P.; Thankachan, S.; Xavier, S.; Mohammed, E.M. Effect of Tb3+ substitution on structural, electrical and magnetic properties of sol–gel synthesized nanocrystalline nickel ferrite. J. Alloy. Compd. 2013, 578, 314–319. [Google Scholar] [CrossRef]
- Smith, J.; Wijn, H.P.J. Ferrites; John Wiley: New York, NY, USA, 1959. [Google Scholar]
- Pandit, A.A.; More, S.S.; Dorik, R.G.; Jadhav, K.M. Structural and magnetic properties of Co1+ySnyFe2-2y-xCrxO4 ferrite system. B Mater. Sci. 2003, 26, 517–521. [Google Scholar] [CrossRef]
- Abbas, T.J. X-ray Diffraction and Site Preference Analysis of Ni-Substituted MgFe2O4 Ferrites. J. Appl. Sci. 2002, 2, 1110–1114. [Google Scholar]
- Wohlfarth, E.P. Ferromagnetic Materials; North-Holland Publication Co.: Amsterdam, The Netherlands, 1982. [Google Scholar]
- Dhiman, R.L.; Taneja, S.P.; Reddy, V.R. Preparation and Characterization of Manganese Ferrite Aluminates. Adv. Condens. Matter Phys. 2008, 7. [Google Scholar] [CrossRef]
- Waldron, R.D. Infrared spectra of ferrites. Phys. Rev. 1955, 99, 1727. [Google Scholar] [CrossRef]
- Hemeda, O.M.; Barakat, M.M.; Hemeda, D.M. Structural, Electrical and Spectral Studies on Double Rare-Earth Orthoferrites La{1-x} NdxFeO3. J. Turk. Phys. 2003, 27, 537. [Google Scholar]
- Shinde, T.J.; Gadkari, A.B.; Vasambekar, P.N. Influence of Nd3+ substitution on structural, electrical and magnetic properties of nanocrystalline nickel ferrites. J. Alloy. Compd. 2012, 513, 80–85. [Google Scholar] [CrossRef]
- Evans, B.J.; Hafner, S.S. Mössbauer resonance of Fe57 in oxidic spinels containing Cu and Fe. J. Phys. Chem. Solids 1968, 29, 1573–1588. [Google Scholar] [CrossRef]
- Modi, K.B.; Rangolia, M.K.; Chhantbar, M.C.; Joshi, H.H. Study of infrared spectroscopy and elastic properties of fine and coarse grained nickel–cadmium ferrites. J. Mater. Sci. 2006, 41, 7308–7318. [Google Scholar] [CrossRef]
- Kodama, R.H.; Berkowitz, A.E.; McNiff, E.J., Jr.; Foner, S. Surface spin disorder in NiFe2O4 nanoparticles. Phys. Rev. Lett. 1996, 77, 394–397. [Google Scholar] [CrossRef] [PubMed]
- Berkowitz, A.E.; Kodama, R.H.; Makhlouf, S.A.; Parker, F.T.; Spada, F.E.; McNiff, E.J., Jr.; Foner, S. Anomalous properties of magnetic nanoparticles. J. Magn. Magn. Mater. 1999, 196, 591–594. [Google Scholar] [CrossRef]
- Hemeda, O.M.; Said, M.Z.; Barakat, M.M. Spectral and transport phenomena in Ni ferrite-substituted Gd2O3. J. Magn. Magn. Mater. 2001, 224, 132–142. [Google Scholar] [CrossRef]
- Šepelák, V.; Baabe, D.; Mienert, D.; Schultze, D.; Krumeich, F.; Litterst, F.J.; Becker, K.D. Evolution of structure and magnetic properties with annealing temperature in nanoscale high-energy-milled nickel ferrite. J. Magn. Magn. Mater. 2003, 257, 377–386. [Google Scholar] [CrossRef]
- Bødker, F.; Mørup, S.; Linderoth, S. Surface effects in metallic iron nanoparticles. Rev. Lett. 1994, 72, 282. [Google Scholar] [CrossRef]
- Pfeiffer, H. Determination of anisotropy field distribution in particle assemblies taking into account thermal fluctuations. Phys. Status solidi A 1990, 118, 295–306. [Google Scholar] [CrossRef]
- Vestal, C.R.; Zhang, Z.J. Synthesis and magnetic characterization of Mn and Co spinel ferrite-silica nanoparticles with tunable magnetic core. Nano. Lett. 2003, 3, 1739–1743. [Google Scholar] [CrossRef]
- Stoner, E.C.; Wohlfarth, E.P. Philos. Handbook of Nanomaterials for Industrial Applications. T. R. Soc. S-A 1948, 240, 599–642. [Google Scholar]
- Jiang, J.Z.; Goya, G.F.; Rechenberg, H.R. Magnetic properties of nanostructured CuFe2O4. J. Phys. Condens Mat. 1999, 11, 4063. [Google Scholar] [CrossRef]
- Robert, C.O.; Handley, O. Modern Magnetic Materials: Principles and Applications; John Wiley & Sons, Inc.: New York, NY, USA, 2000. [Google Scholar]
- Merer, A.J. Spectroscopy of the diatomic 3d transition metal oxides. Annu. Rev. Phys. Chem. 1989, 40, 407–438. [Google Scholar] [CrossRef]
- Ham, F.S. Dynamical Jahn-Teller effect in paramagnetic resonance spectra: Orbital reduction factors and partial quenching of spin-orbit interaction. Phys. Rev. 1965, 138, A1727–A1740. [Google Scholar] [CrossRef]
- Kim, J.Y.; Koo, T.Y.; Park, J.H. Orbital and bonding anisotropy in a half-filled GaFeO3 magnetoelectric ferrimagnet. Phys. Rev. Lett. 2006, 96, 047205–047209. [Google Scholar] [CrossRef] [PubMed]
- Arima, T.; Higashiyama, D.; Kaneko, Y.; He, J.P.; Goto, T.; Miyasaka, S.; Kimura, T.; Oikawa, K.; Kamiyama, T.; Kumai, R.; et al. Structural and magnetoelectric properties of Ga2−xFexO3 single crystals grown by a floating-zone method. Phys. Rev. B 2004, 70, 064426. [Google Scholar] [CrossRef]
- Yoon, S.; Krishnan, K.M. Temperature dependence of magnetic anisotropy constant in manganese ferrite nanoparticles at low temperature. J. Appl. Phys. 2011, 109, 07B534. [Google Scholar] [CrossRef]
- Shenker, H. Magnetic anisotropy of cobalt ferrite (Co1.01Fe2.00O3.62) and nickel cobalt ferrite (Ni0.72 Fe0.20Co0.08Fe2O4). Phys. Rev. 1957, 107, 1246. [Google Scholar] [CrossRef]
- Gao, R.W.; Feng, W.C.; Liu, H.Q.; Wang, B.; Chen, W.; Han, G.B.; Zhang, P.; Li, H.; Li, W.; Guo, Y.Q.; et al. Exchange-coupling interaction, effective anisotropy and coercivity in nanocomposite permanent materials. J. Appl. Phys. 2003, 94, 664–668. [Google Scholar] [CrossRef]
- Gilleo, M.A. Superexchange interaction energy for Fe3+-O2−-Fe3+ linkages. Phys. Rev. 1958, 109, 777. [Google Scholar] [CrossRef]
- Goldman, A. Modern Ferrite Technology; Van Nostrand Reinhold: New York, NY, USA, 1990; p. 145. [Google Scholar]

| x | Ms (emu/g) | Mr (emu/g) | Mr/Ms | Hc (Oe) | K1 × 105 (5K) (J/m3) | K1 × 105 (300K) (J/m3) | Tc (K) |
|---|---|---|---|---|---|---|---|
| 0.000 | 44.5 | 19.8 | 0.44 | 363 | 3.9 | 2.5 | 938 |
| 0.025 | 38.6 | 11.9 | 0.30 | 308 | 8.3 | 5.7 | 923 |
| 0.050 | 36.1 | 10.8 | 0.29 | 221 | 6.3 | 5.6 | 913 |
| 0.075 | 34.7 | 8.6 | 0.25 | 102 | 10.1 | 6.5 | 903 |
| 0.100 | 30.6 | 6.2 | 0.20 | 157 | 10.9 | 6.3 | 899 |
| x | Lattice Parameter (Å) | Avg. Crystallite size (nm) | X-ray Density (g/cm3) | dAL (Å) | dBL (Å) | dAE (Å) | dBE (Å) | dBEU (Å) | LA (Å) | LB (Å) |
|---|---|---|---|---|---|---|---|---|---|---|
| 0.000 | 8.3039(06) | 36.41 | 5.44 | 1.8855 | 2.0265 | 3.0791 | 2.7926 | 2.9376 | 3.5956 | 2.9358 |
| 0.025 | 8.3076(04) | 25.42 | 5.49 | 1.8864 | 2.0275 | 3.0805 | 2.7938 | 2.9389 | 3.5973 | 2.9371 |
| 0.050 | 8.3089(04) | 22.49 | 5.55 | 1.8867 | 2.0278 | 3.0809 | 2.7942 | 2.9393 | 3.5979 | 2.9376 |
| 0.075 | 8.3093(02) | 17.50 | 5.61 | 1.8868 | 2.0279 | 3.0811 | 2.7944 | 2.9395 | 3.5980 | 2.9378 |
| 0.100 | 8.3105 (05) | 16.00 | 5.66 | 1.8870 | 2.0281 | 3.0815 | 2.7948 | 2.9399 | 3.5985 | 2.9382 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guragain, D.; Rai, B.K.; Yoon, S.; Poudel, T.P.; Bhandari, S.C.; Mishra, S.R. Effect of Terbium Ion Substitution in Inverse Spinel Nickel Ferrite: Structural and Magnetic Study. Magnetochemistry 2020, 6, 14. https://doi.org/10.3390/magnetochemistry6010014
Guragain D, Rai BK, Yoon S, Poudel TP, Bhandari SC, Mishra SR. Effect of Terbium Ion Substitution in Inverse Spinel Nickel Ferrite: Structural and Magnetic Study. Magnetochemistry. 2020; 6(1):14. https://doi.org/10.3390/magnetochemistry6010014
Chicago/Turabian StyleGuragain, Deepa, Binod Kumar Rai, Sunghyun Yoon, Tej Prasad Poudel, Subash Chandra Bhandari, and Sanjay R Mishra. 2020. "Effect of Terbium Ion Substitution in Inverse Spinel Nickel Ferrite: Structural and Magnetic Study" Magnetochemistry 6, no. 1: 14. https://doi.org/10.3390/magnetochemistry6010014
APA StyleGuragain, D., Rai, B. K., Yoon, S., Poudel, T. P., Bhandari, S. C., & Mishra, S. R. (2020). Effect of Terbium Ion Substitution in Inverse Spinel Nickel Ferrite: Structural and Magnetic Study. Magnetochemistry, 6(1), 14. https://doi.org/10.3390/magnetochemistry6010014






