Preparation and Application of Iron Oxide Nanoclusters
Abstract
1. Introduction
2. Preparation of Iron Oxide Nanoclusters
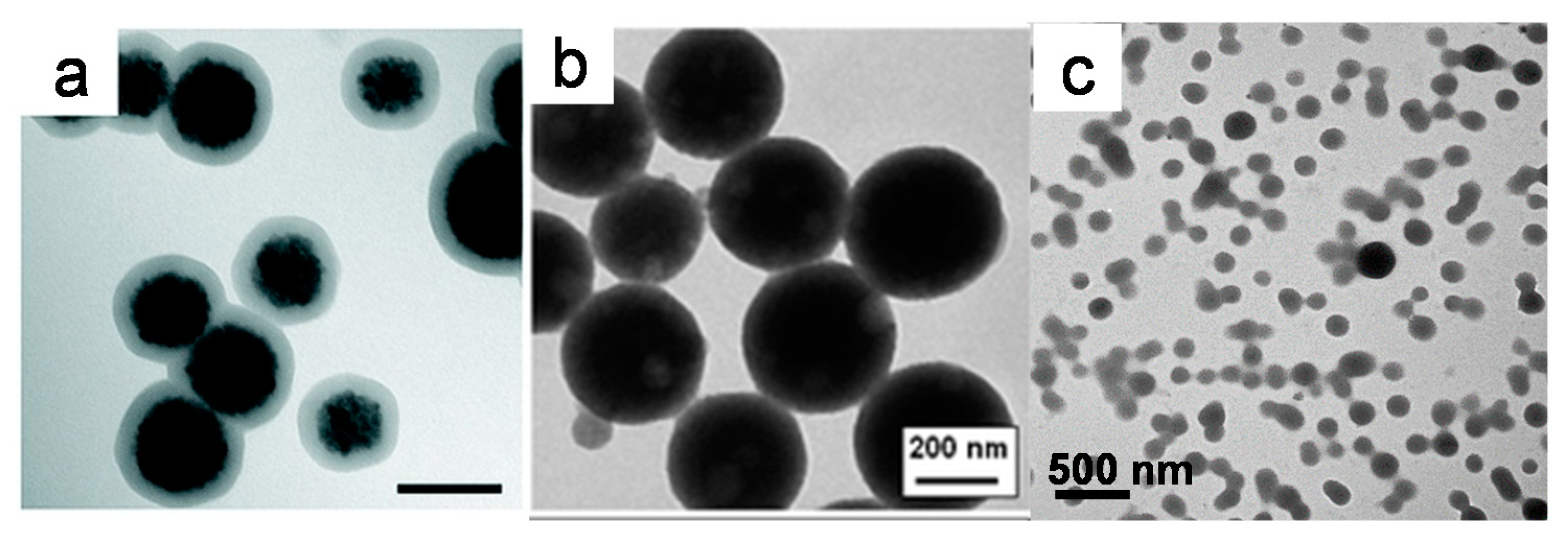
2.1. Controlled Aggregation of Nanoparticles during Synthesis
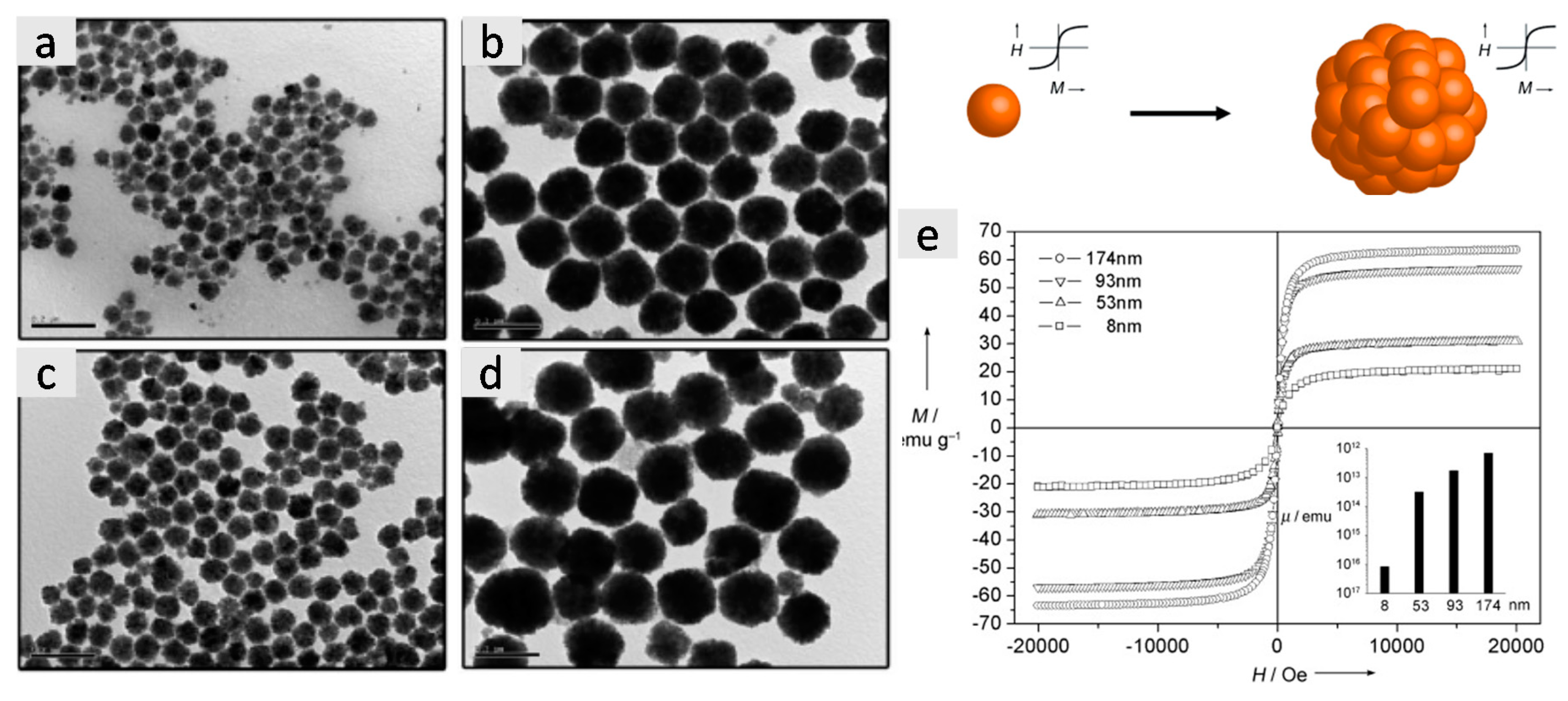
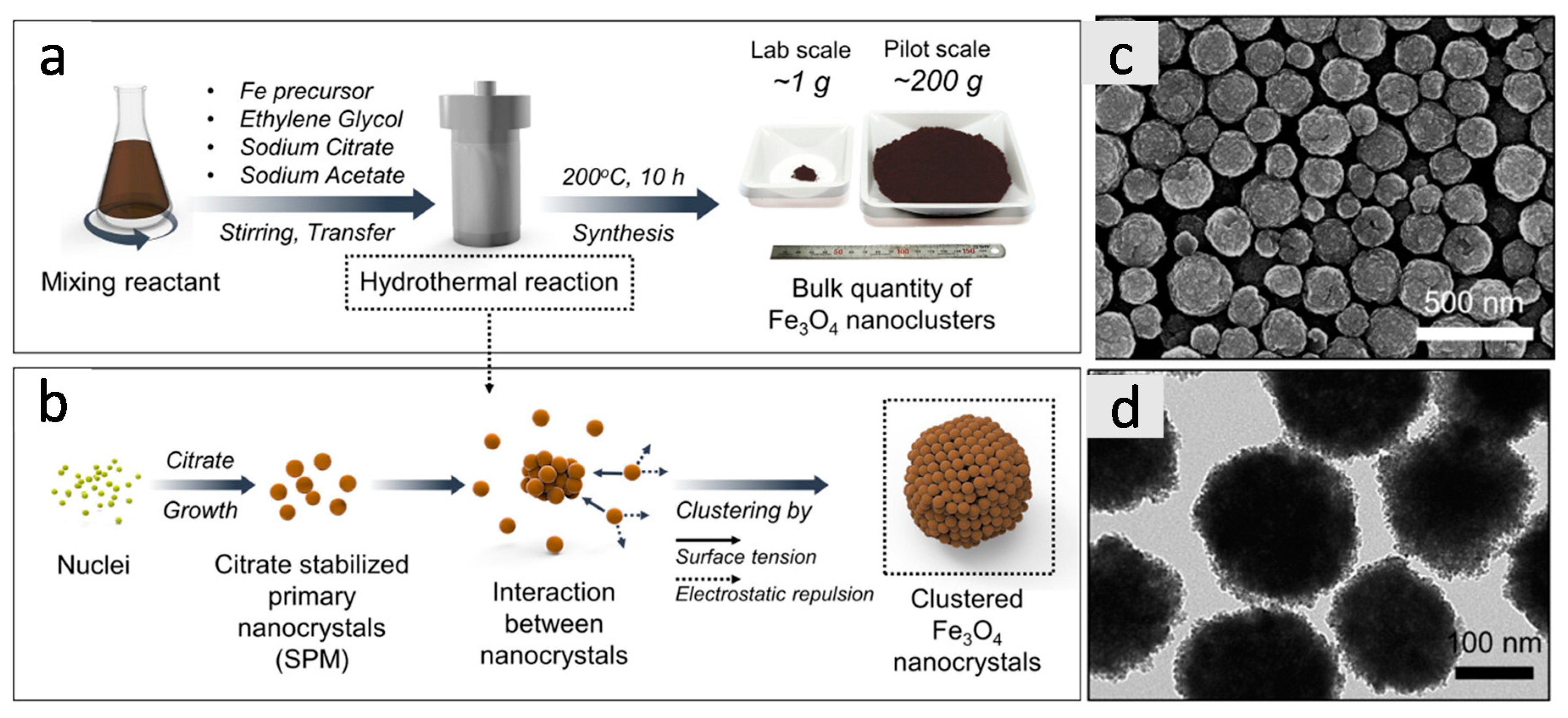
2.1.1. Polyol Method
2.1.2. Solvothermal Synthesis
2.2. Controlled Assembly of Ligand-Capped Nanoparticles
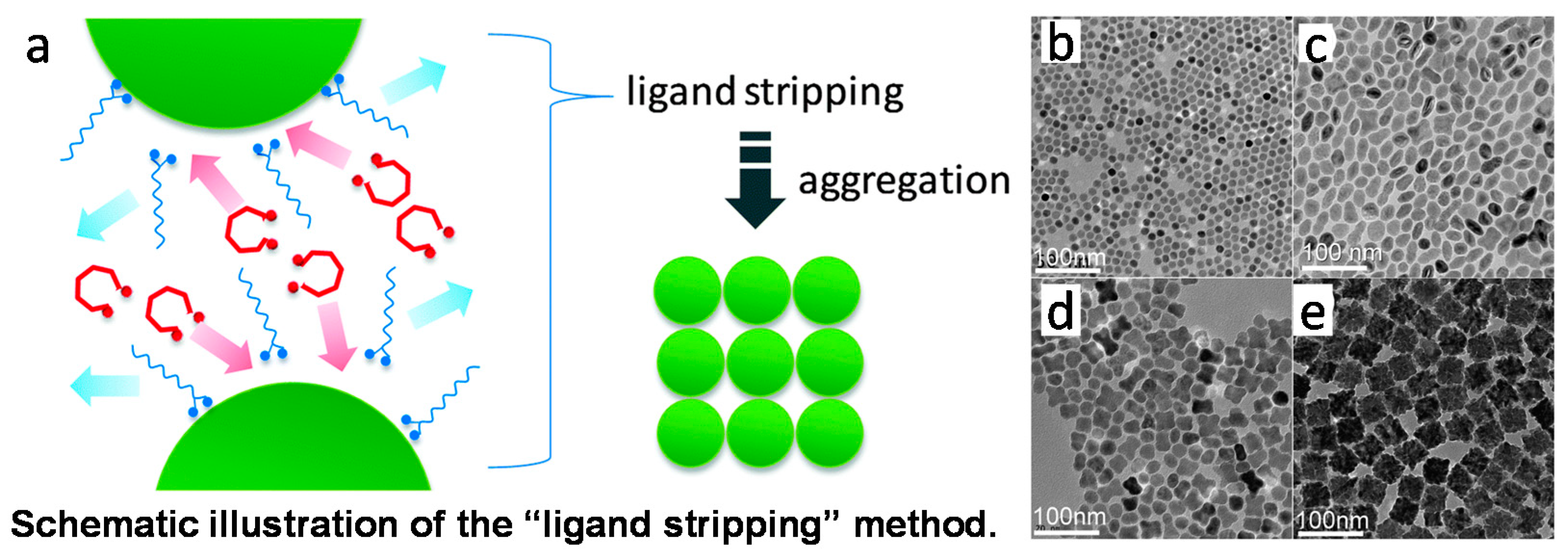
2.2.1. Ligand Etching
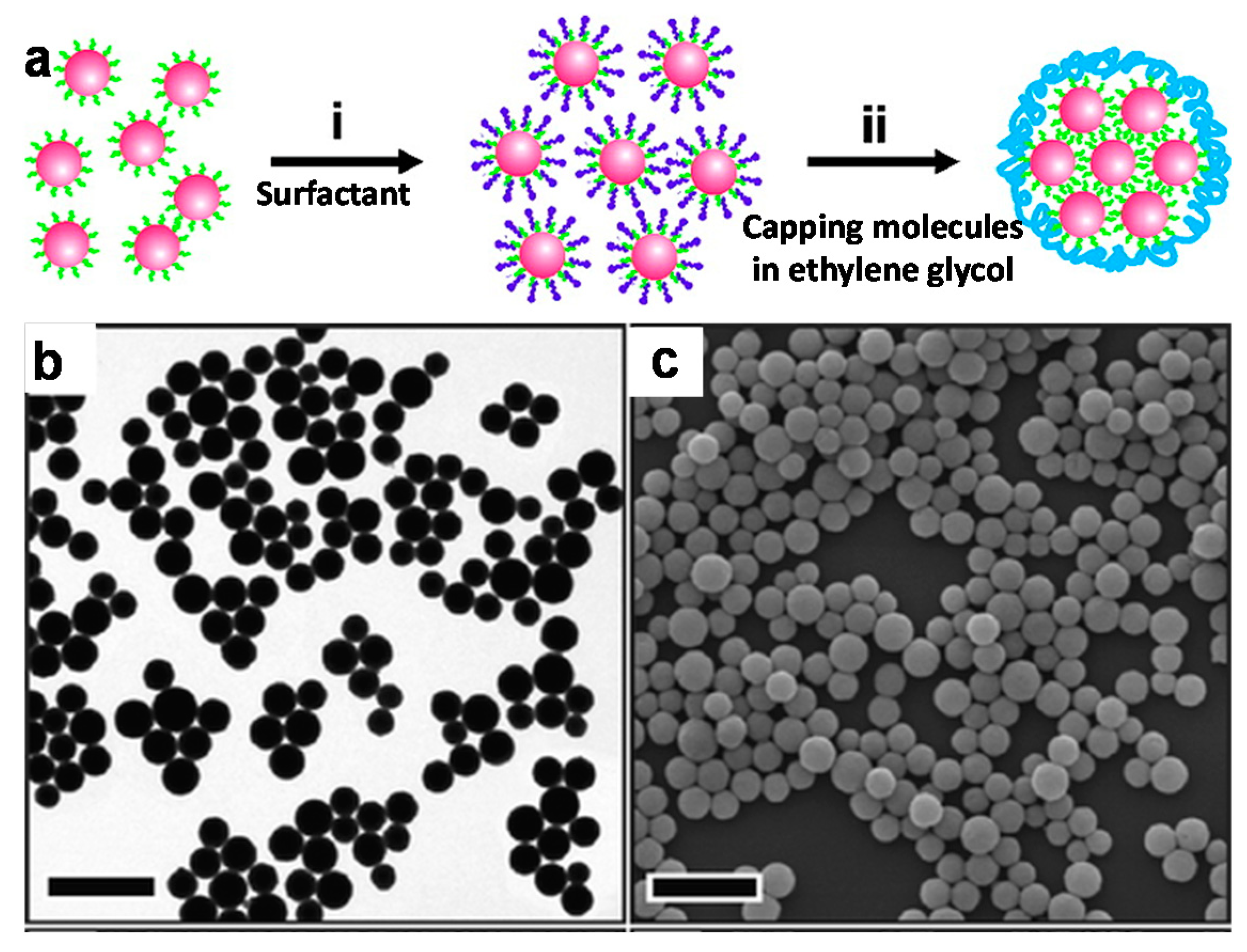
2.2.2. Solvophobic Interactions
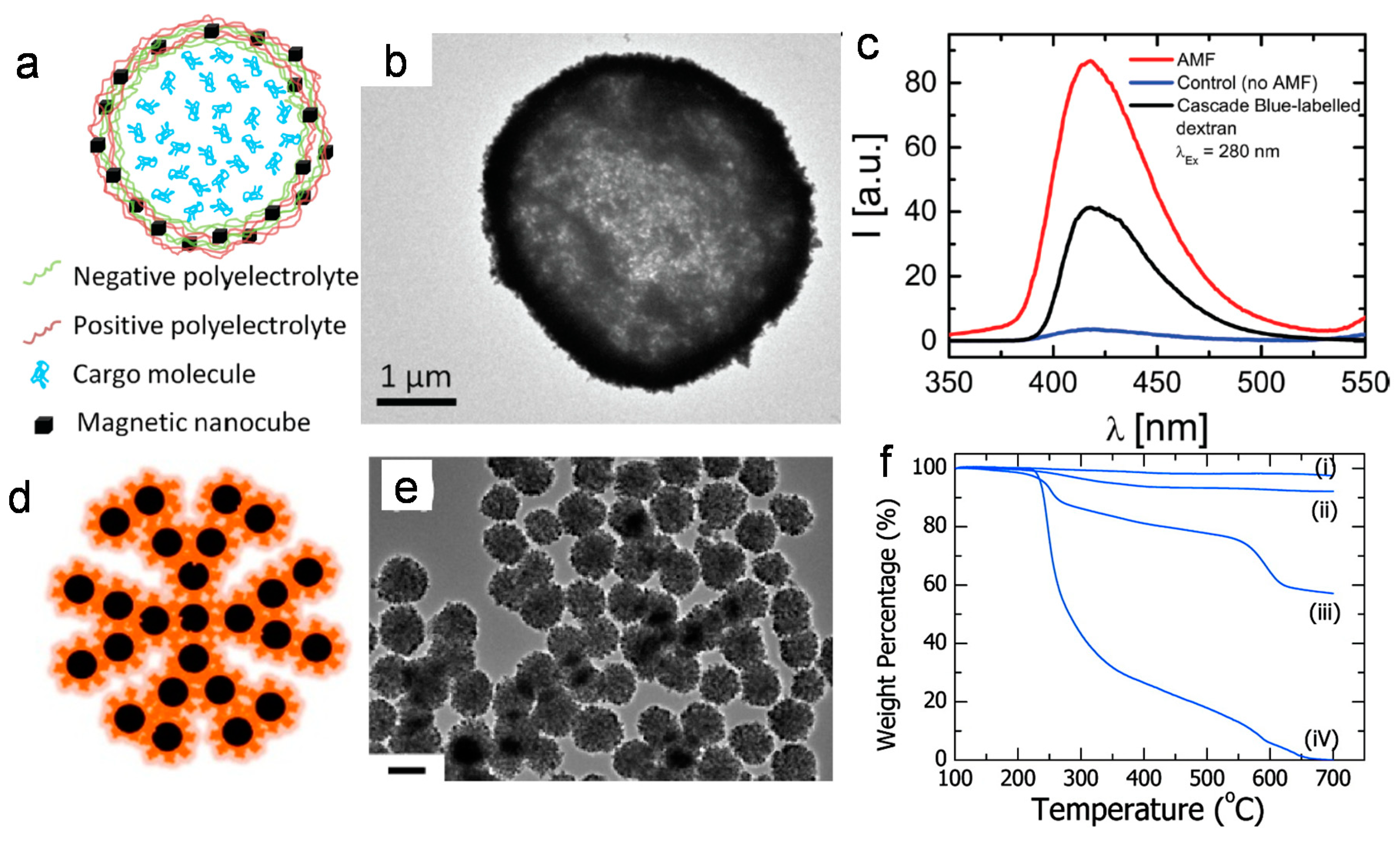
2.3. Matrix Encapsulation of Nanoparticles
3. Applications of Iron Oxide Nanoclusters
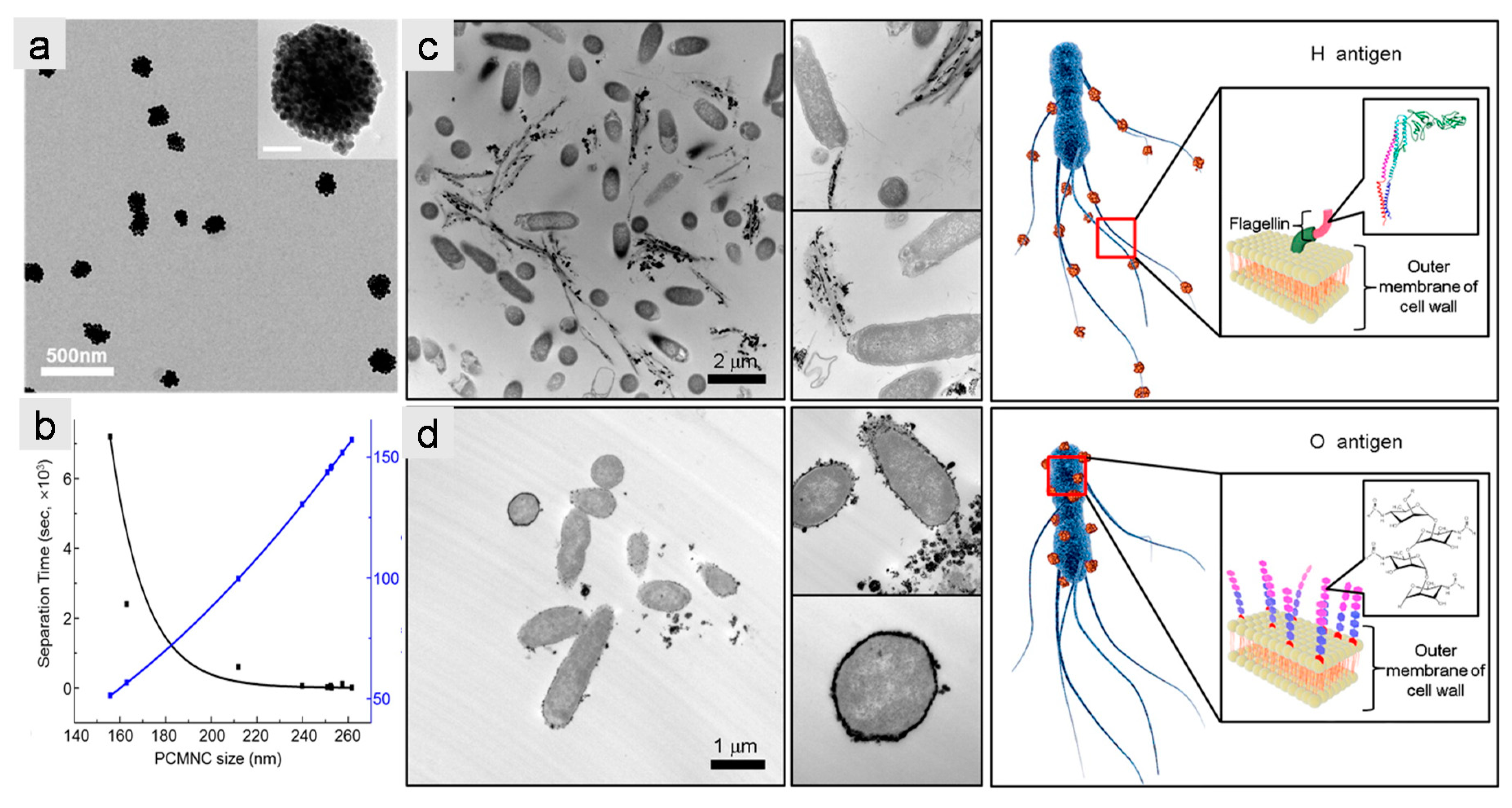
3.1. Iron Oxide Nanoclusters for Magnetic Separation
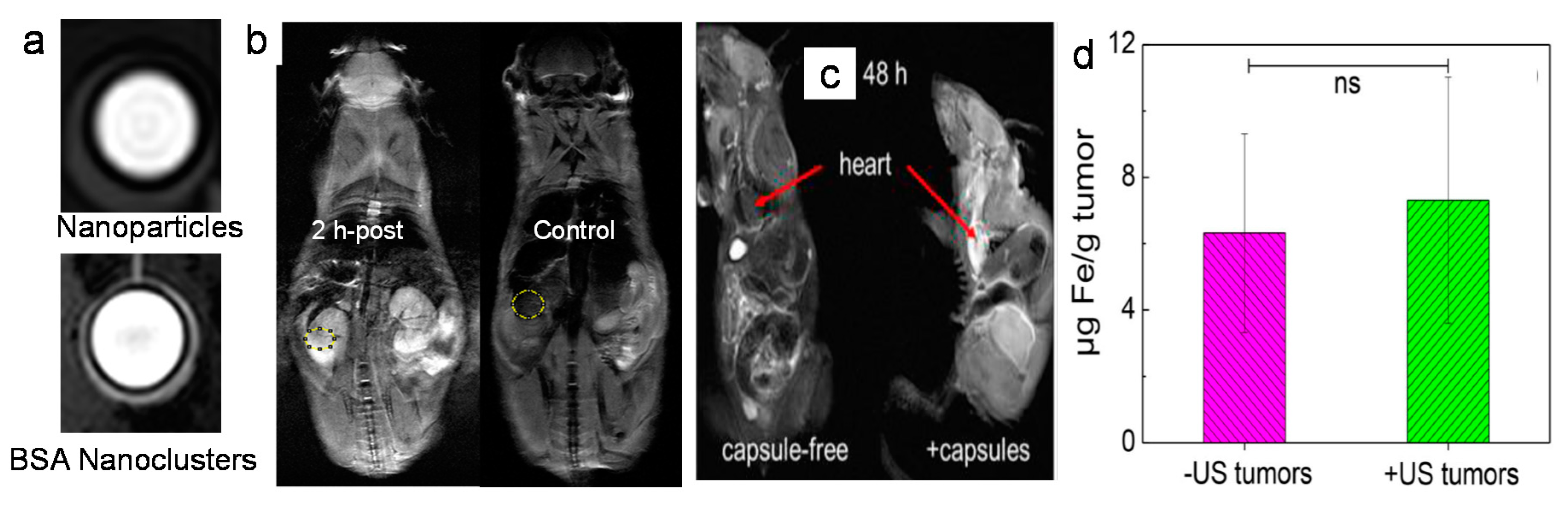
3.2. Biomedical Applications of Iron Oxide Nanoclusters
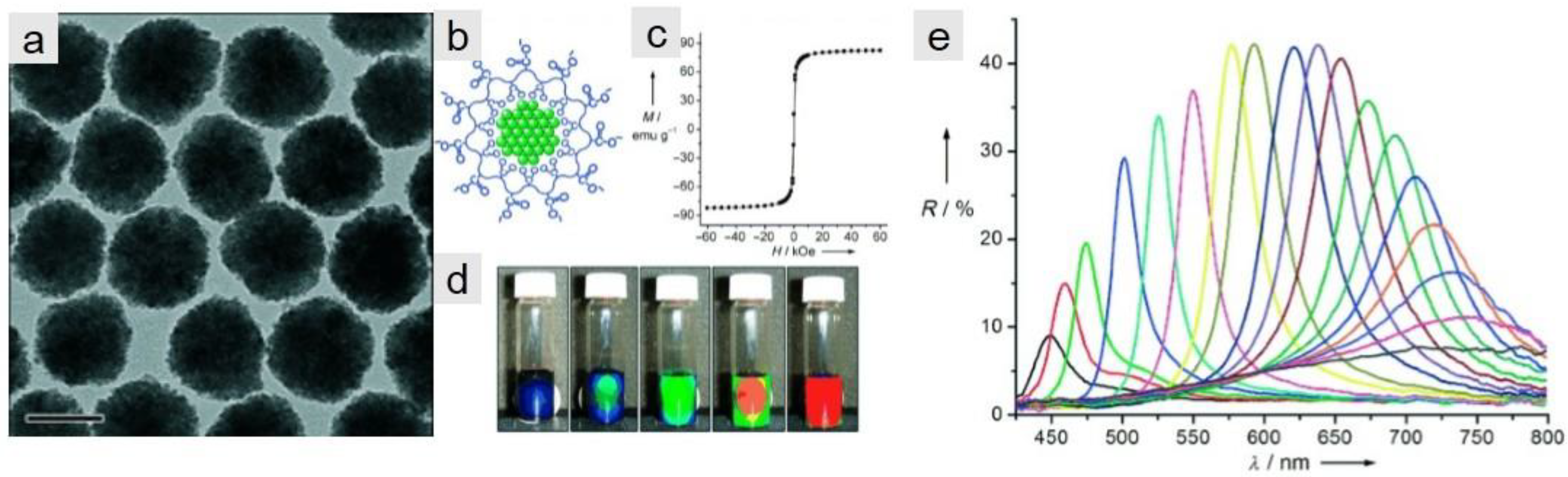
3.3. Optical Applications of Iron Oxide Nanoclusters
4. Summary and Outlook
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, J.S.; Cha, J.M.; Yoon, H.Y.; Lee, J.K.; Kim, Y.K. Magnetic multi-granule nanoclusters: A model system that exhibits universal size effect of magnetic coercivity. Sci. Rep. 2015, 5, 12135. [Google Scholar] [CrossRef]
- Lu, Z.; Yin, Y. Colloidal nanoparticle clusters: Functional materials by design. Chem. Soc. Rev. 2012, 41, 6874–6887. [Google Scholar] [CrossRef] [PubMed]
- Kostopoulou, A.; Lappas, A. Colloidal magnetic nanocrystal clusters: Variable length-scale interaction mechanisms, synergetic functionalities and technological advantages. Nanotechnol. Rev. 2015, 4, 595–624. [Google Scholar] [CrossRef]
- Krishnan, K.M.; Pakhomov, A.B.; Bao, Y.; Blomqvist, P.; Chun, Y.; Gonzales, M.; Griffin, K.; Ji, X.; Roberts, B.K. Nanomagnetism and spin electronics: Materials, microstructure and novel properties. J. Mater. Sci. 2006, 41, 793–815. [Google Scholar] [CrossRef]
- Ge, J.; Hu, Y.; Biasini, M.; Beyermann, W.P.; Yin, Y. Superparamagnetic magnetite colloidal nanocrystal clusters. Angew. Chem. Int. Ed. 2007, 46, 4342–4345. [Google Scholar] [CrossRef]
- Kostopoulou, A.; Tsiaoussis, I.; Lappas, A. Magnetic iron oxide nanoclusters with tunable optical response. Photonics Nanostruct. Fundam. Appl. 2011, 9, 201–206. [Google Scholar] [CrossRef]
- Zhuang, J.; Wu, H.; Yang, Y.; Cao, Y. Controlling colloidal superparticle growth through solvophobic interactions. Angew. Chem. Int. Ed. 2008, 47, 2208–2212. [Google Scholar] [CrossRef]
- Ge, J.P.; Hu, Y.X.; Biasini, M.; Dong, C.L.; Guo, J.H.; Beyermann, W.P.; Yin, Y.D. One-step synthesis of highly water-soluble magnetite colloidal nanocrystals. Chem. Eur. J. 2007, 13, 7153–7161. [Google Scholar] [CrossRef]
- Ge, J.; Hu, Y.; Yin, Y. Highly tunable superparamagnetic colloidal photonic crystals. Angew. Chem. Int. Ed. 2007, 46, 7428–7431. [Google Scholar] [CrossRef]
- Xuan, S.; Wang, Y.; Yu, J.; Leung, K.C.F. Tuning the grain size and particle size of superparamagnetic Fe3O4 microparticles. Chem. Mater. 2009, 21, 5079–5087. [Google Scholar] [CrossRef]
- Gao, J.; Ran, X.; Shi, C.; Cheng, H.; Cheng, T.; Su, Y. One-step solvothermal synthesis of highly water-soluble, negatively charged superparamagnetic Fe3O4 colloidal nanocrystal clusters. Nanoscale 2013, 5, 7026–7033. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, T.; Tang, R.; Qiu, H.; Wang, C.; Zhou, Z. Solvothermal synthesis and characterization of monodisperse superparamagnetic iron oxide nanoparticles. J. Magn. Magn. Mater. 2015, 379, 226–231. [Google Scholar] [CrossRef]
- Wang, W.; Tang, B.; Wu, S.; Gao, Z.; Ju, B.; Teng, X.; Zhang, S. Controllable 5-sulfosalicylic acid assisted solvothermal synthesis of monodispersed superparamagnetic Fe3O4 nanoclusters with tunable size. J. Magn. Magn. Mater. 2017, 423, 111–117. [Google Scholar] [CrossRef]
- Kim, J.; Tran, V.T.; Oh, S.; Kim, C.S.; Hong, J.C.; Kim, S.; Joo, Y.S.; Mun, S.; Kim, M.H.; Jung, J.W.; et al. scalable solvothermal synthesis of superparamagnetic Fe3O4 nanoclusters for bioseparation and theragnostic probes. ACS Appl. Mater. Interfaces 2018, 10, 41935–41946. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Ma, H.; Luo, W.; Wang, S. Synthesis of magnetite submicrospheres with tunable size and superparamagnetism by a facile polyol process. Mater. Chem. Phys. 2013, 139, 383–388. [Google Scholar] [CrossRef]
- Luo, B.; Xu, S.; Luo, A.; Wang, W.; Wang, S.; Guo, J.; Lin, Y.; Zhao, D.; Wang, C. Mesoporous biocompatible and acid-degradable magnetic colloidal nanocrystal clusters with sustainable stability and high hydrophobic drug loading capacity. ACS Nano 2011, 5, 1428–1435. [Google Scholar] [CrossRef] [PubMed]
- Nikitin, A.A.; Shchetinin, I.V.; Tabachkova, N.Y.; Soldatov, M.A.; Soldatov, A.V.; Sviridenkova, N.V.; Beloglazkina, E.K.; Savchenko, A.G.; Fedorova, N.D.; Abakumov, M.A.; et al. Synthesis of iron oxide nanoclusters by thermal decomposition. Langmuir 2018, 34, 4640–4650. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Yu, J.; Zhu, X.; Chan, K.; Wang, Y. Ultra-fast method to synthesize mesoporous magnetite nanoclusters as highly sensitive magnetic resonance probe. J. Colloid Interface Sci. 2012, 379, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; He, L.; Xu, W.; Zhuang, J.; Yang, X.; Zhang, X.; Wu, M.; Yin, Y. Formation of colloidal nanocrystal clusters of iron oxide by controlled ligand stripping. Chem. Commun. 2016, 52, 128–131. [Google Scholar] [CrossRef]
- Zhuang, J.; Wu, H.; Yang, Y.; Cao, Y. Supercrystalline colloidal particles from artificial atoms. J. Am. Chem. Soc. 2007, 129, 14166–14167. [Google Scholar] [CrossRef]
- Ninjbadgar, T.; Brougham, D.F. Epoxy ring opening phase transfer as a general route to water dispersible superparamagnetic Fe3O4 nanoparticles and their application as positive MRI contrast agents. Adv. Funct. Mater. 2011, 21, 4769–4775. [Google Scholar] [CrossRef]
- Sherwood, J.; Rich, M.; Lovas, K.; Warram, J.; Bolding, M.S.; Bao, Y. T1-Enhanced MRI-visible nanoclusters for imaging-guided drug delivery. Nanoscale 2017, 9, 11785–11792. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wei, Z.; Lv, H.; Wu, L.; Cui, Y.; Yao, H.; Li, J.; Zhang, H.; Yang, B.; Jiang, J. Iron oxide nanoparticles promote the migration of mesenchymal stem cells to injury sites. Int. J. Nanomed. 2019, 14, 573–589. [Google Scholar] [CrossRef]
- Paquet, C.; de Haan, H.W.; Leek, D.; Lin, H.; Xiang, B.; Tian, G.H.; Kell, A.; Simard, B. Clusters of superparamagnetic iron oxide nanoparticles encapsulated in a hydrogel: A particle architecture generating a synergistic enhancement of the T2 relaxation. ACS Nano 2011, 5, 3104–3112. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Zhang, C. Controllable assembly of sydrophobic superparamagnetic iron oxide nanoparticle with mPEG-PLA copolymer and its effect on MR transverse relaxation rate. J. Nanomater. 2011. [Google Scholar] [CrossRef]
- Li, L.; Choo, E.S.G.; Yi, J.; Ding, J.; Tang, X.; Xue, J. Superparamagnetic silica composite nanospheres (SSCNs) with ultrahigh loading of iron oxide nanoparticles via an oil-in-DEG microemulsion route. Chem. Mater. 2008, 20, 6292–6294. [Google Scholar] [CrossRef]
- Alford, A.; Rich, M.; Kozlovskaya, V.; Chen, J.; Sherwood, J.; Bolding, M.; Warram, J.; Bao, Y.; Kharlampieva, E. Ultrasound-triggered delivery of anticancer therapeutics from MRI-visible multilayer microcapsules. Adv. Therap. 2018, 1800051. [Google Scholar] [CrossRef]
- Maity, D.; Chandrasekharan, P.; Pradhan, P.; Chuang, K.; Xue, J.; Feng, S.; Ding, J. Novel synthesis of superparamagnetic magnetite nanoclusters for biomedical applications. J. Mater. Chem. 2011, 21, 14717–14724. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, J.; Wang, X.; Li, X.; Wu, M.; Liang, F.; Yang, Y. One-pot solvothermal synthesis of Carboxylatopillar 5 arene-modified Fe3O4 magnetic nanoparticles for ultrafast separation of cationic dyes. Dyes Pigm. 2019, 162, 512–516. [Google Scholar] [CrossRef]
- Li, M.; Gu, H.; Zhang, C. Highly sensitive magnetite nano clusters for MR cell imaging. Nanoscale Res. Lett. 2012, 7, 204. [Google Scholar] [CrossRef]
- Yang, P.; Li, H.; Zhang, S.; Chen, L.; Zhou, H.; Tang, R.; Zhou, T.; Bao, F.; Zhang, Q.; He, L.; et al. Gram-scale synthesis of superparamagnetic Fe3O4 nanocrystal clusters with long-term charge stability for highly stable magnetically responsive photonic crystals. Nanoscale 2016, 8, 19036–19042. [Google Scholar] [CrossRef] [PubMed]
- Borlido, L.; Azevedo, A.M.; Roque, A.C.A.; Aires-Barros, M.R. Magnetic separations in biotechnology. Biotechnol. Adv. 2013, 31, 1374–1385. [Google Scholar] [CrossRef] [PubMed]
- Ditsch, A.; Lindenmann, S.; Laibinis, P.E.; Wang, D.I.C.; Hatton, T.A. High-gradient magnetic separation of magnetic nanoclusters. Ind. Eng. Chem. Res. 2005, 44, 6824–6836. [Google Scholar] [CrossRef]
- Ezzaier, H.; Marins, J.A.; Claudet, C.; Hemery, G.; Sandre, O.; Kuzhir, P. Kinetics of aggregation and magnetic separation of multicore iron oxide nanoparticles: Effect of the grafted layer thickness. Nanomaterials 2018, 8, 623. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhu, M.; Yang, Y.; Cao, J.; Shi, F. Extraction of genomic DNA via superparamagnetic Fe3O4 magnetic colloidal nanocrystal clusters. J. Nanosci. Nanotechnol. 2018, 18, 8105–8110. [Google Scholar] [CrossRef] [PubMed]
- Meerod, S.; Deepuppha, N.; Rutnakornpituk, B.; Rutnakornpituk, M. Reusable magnetic nanocluster coated with poly (acrylic acid) and its adsorption with an antibody and an antigen. J. Appl. Polym. Sci. 2018, 135, 46160. [Google Scholar] [CrossRef]
- Long, X.; Li, J.; Sheng, D.; Lian, H. Low-cost iron oxide magnetic nanoclusters affinity probe for the enrichment of endogenous phosphopeptides in human saliva. RSC Adv. 2016, 6, 96210–96222. [Google Scholar] [CrossRef]
- Wen, C.; Jiang, Y.; Li, X.; Tang, M.; Wu, L.; Hu, J.; Pang, D.; Zeng, J. Efficient enrichment and analyses of bacteria at ultralow concentration with quick-response magnetic nanospheres. ACS Appl. Mater. Interfaces 2017, 9, 9416–9425. [Google Scholar] [CrossRef]
- Kim, Y.T.; Kim, K.H.; Kang, E.S.; Jo, G.; Ahn, S.Y.; Park, S.H.; Kim, S.I.; Mun, S.; Baek, K.; Kim, B.; et al. Synergistic effect of detection and separation for pathogen using magnetic clusters. Bioconjug. Chem. 2016, 27, 59–65. [Google Scholar] [CrossRef]
- Ma, W.; Sha, X.; Gao, L.; Cheng, Z.; Meng, F.; Cai, J.; Tan, D.; Wang, R. Effect of iron oxide nanocluster on enhanced removal of molybdate from surface water and pilot scale test. Colloids Surf. A Physicochem. Eng. Asp. 2015, 478, 45–53. [Google Scholar] [CrossRef]
- Lee, S.H.; Cha, J.; Sim, K.; Lee, J.K. Efficient removal of arsenic using magnetic multi-granule nanoclusters. Bull. Korean Chem. Soc. 2014, 35, 605–609. [Google Scholar] [CrossRef][Green Version]
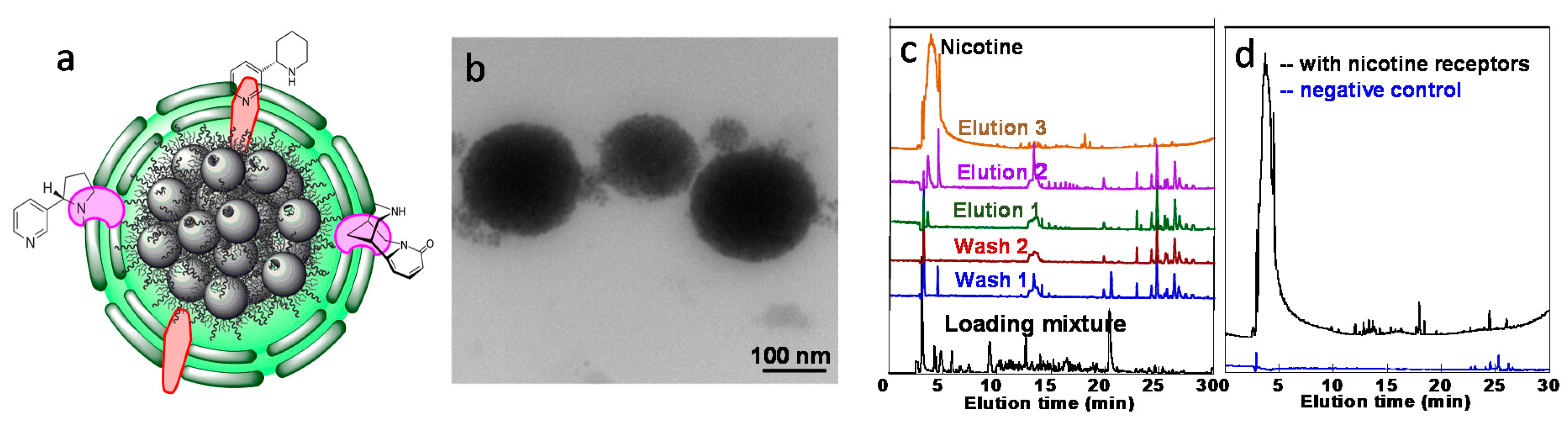
- Sherwood, J.; Sowell, J.; Beyer, N.; Irvin, J.; Stephen, C.; Antone, A.J.; Bao, Y.P.; Ciesla, L.M. Cell-membrane coated iron oxide nanoparticles for isolation and specific identification of drug leads from complex matrices. Nanoscale 2019, 11, 6352–6359. [Google Scholar] [CrossRef]
- Bu, Y.; Hu, Q.; Ke, R.; Sui, Y.; Xie, X.; Wang, S. Cell membrane camouflaged magnetic nanoparticles as a biomimetic drug discovery platform. Chem. Comm. 2018, 54, 13427–13430. [Google Scholar] [CrossRef]
- Hu, Q.; Bu, Y.; Zhen, X.; Xu, K.; Ke, R.; Xie, X.; Wang, S. Magnetic carbon nanotubes camouflaged with cell membrane as a drug discovery platform for selective extraction of bioactive compounds from natural products. Chem. Eng. J. 2019, 364, 269–279. [Google Scholar] [CrossRef]
- Chen, H.; Fang, Z.; Chen, Y.; Chen, Y.; Yao, B.; Cheng, J.; Chien, C.; Chang, Y.; Hu, C. Targeting and enrichment of viral pathogen by cell membrane cloaked magnetic nanoparticles for enhanced detection. ACS Appl. Mater. Interfaces 2017, 9, 39953–39961. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, L.; Ma, W.; Zhang, Y.; Yu, M.; Guo, J.; Lu, H.; Wang, C. Two-in-one strategy for effective enrichment of phosphopeptides using magnetic mesoporous gamma-Fe2O3 nanocrystal clusters. ACS Appl. Mater. Interfaces 2013, 5, 614–621. [Google Scholar] [CrossRef]
- Yang, Q.; Lan, F.; Yi, Q.; Wu, Y.; Gu, Z. A colloidal assembly approach to synthesize magnetic porous composite nanoclusters for efficient protein adsorption. Nanoscale 2015, 7, 17617–17622. [Google Scholar] [CrossRef]
- Bueno, P.V.A.; Hilamatu, K.C.P.; Carmona-Ribeiro, A.M.; Petri, D.F.S. Magnetically triggered release of amoxicillin from xanthan/Fe3O4/albumin patches. Int. J. Biol. Macromol. 2018, 115, 792–800. [Google Scholar] [CrossRef]
- Kuo, C.; Liu, T.; Wang, K.; Hardiansyah, A.; Lin, Y.; Chen, H.; Chiu, W.Y. Magnetic and thermal-sensitive poly(N-isopropylacrylamide)-based microgels for magnetically triggered Controlled release. J. Vis. Exp. 2017. [Google Scholar] [CrossRef]
- Benyettou, F.; Flores, J.A.O.; Ravaux, F.; Rezgui, R.; Jouiad, M.; Nehme, S.I.; Parsapur, R.K.; Olsen, J.C.; Selvam, P.; Trabolsi, A. Mesoporous gamma-iron oxide nanoparticles for magnetically triggered release of doxorubicin and hyperthermia treatment. Chem. Eur. J. 2016, 22, 17018–17026. [Google Scholar] [CrossRef]
- Hua, X.; Yang, Q.; Dong, Z.; Zhang, J.; Zhang, W.; Wang, Q.; Tan, S.; Smyth, H.D.C. Magnetically triggered drug release from nanoparticles and its applications in anti-tumor treatment. Drug Deliv. 2017, 24, 511–518. [Google Scholar] [CrossRef]
- Xu, F.; Cheng, C.; Chen, D.; Gu, H. Magnetite nanocrystal clusters with ultra-high sensitivity in magnetic resonance imaging. ChemPhysChem 2012, 13, 336–341. [Google Scholar] [CrossRef]
- Smith, C.E.; Ernenwein, D.; Shkumatov, A.; Clay, N.E.; Lee, J.Y.; Melhem, M.; Misra, S.; Zimmerman, S.C.; Kong, H. Hydrophilic packaging of iron oxide nanoclusters for highly sensitive imaging. Biomaterials 2015, 69, 184–190. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, D.; Liu, X. Nanoclusters of superparamagnetic iron oxide nanoparticles coated with poly(dopamine) for magnetic field-directed, ultrasensitive MRI-guided photothermal cancer therapy. J. Control. Release 2015, 213, 78. [Google Scholar] [CrossRef]
- Carregal-Romero, S.; Guardia, P.; Yu, X.; Hartmann, R.; Pellegrino, T.; Parak, W.J. Magnetically triggered release of molecular cargo from iron oxide nanoparticle loaded microcapsules. Nanoscale 2015, 7, 570–576. [Google Scholar] [CrossRef]
- Dong, F.; Guo, W.; Bae, J.; Kim, S.H.; Ha, C. Highly porous, water-soluble, superparamagnetic, and biocompatible magnetite nanocrystal clusters for targeted drug delivery. Chem. Eur. J. 2011, 17, 12802–12808. [Google Scholar] [CrossRef]
- Lai, P.; Huang, R.; Lin, S.; Lin, Y.; Chang, C. Biomimetic stem cell membrane-camouflaged iron oxide nanoparticles for theranostic applications. RSC Adv. 2015, 5, 98222–98230. [Google Scholar] [CrossRef]
- Meng, Q.; Rao, L.; Zan, M.; Chen, M.; Yu, G.; Wei, X.; Wu, Z.; Sun, Y.; Guo, S.; Zhao, X.; et al. Macrophage membrane-coated iron oxide nanoparticles for enhanced photothermal tumor therapy. Nanotechnology 2018, 29. [Google Scholar] [CrossRef]
- Yu, G.; Rao, L.; Wu, H.; Yang, L.; Bu, L.; Deng, W.; Wu, L.; Nan, X.; Zhang, W.; Zhao, X.; et al. Myeloid-derived suppressor cell membrane-coated magnetic nanoparticles for cancer theranostics by Inducing macrophage polarization and synergizing immunogenic cell death. Adv. Funct. Mater. 2018, 28, 1801389. [Google Scholar] [CrossRef]
- Bu, L.; Rao, L.; Yu, G.; Chen, L.; Deng, W.; Liu, J.; Wu, H.; Meng, Q.; Guo, S.; Zhao, X.; et al. Cancer stem cell-platelet hybrid membrane-coated magnetic nanoparticles for enhanced photothermal therapy of head and neck squamous cell carcinoma. Adv. Funct. Mater. 2019, 29. [Google Scholar] [CrossRef]
- Bao, Y.; Sherwood, J.A.; Sun, Z. Magnetic iron oxide nanoparticles as T1 contrast agents for magnetic resonance imaging. J. Mater. Chem. C 2018, 6, 1280–1290. [Google Scholar] [CrossRef]
- Lu, Y.; Xu, Y.; Zhang, G.; Ling, D.; Wang, M.; Zhou, Y.; Wu, Y.; Wu, T.; Hackett, M.J.; Hyo Kim, B.; et al. Iron oxide nanoclusters for T1 magnetic resonance imaging of non-human primates. Nat. Biomed. Eng. 2017, 1, 637–643. [Google Scholar] [CrossRef]
- Macher, T.; Totenhagen, J.; Sherwood, J.; Qin, Y.; Gurler, D.; Bolding, M.S.; Bao, Y. Ultrathin iron oxide nanowhiskers as positive contrast agents for magnetic resonance imaging. Adv. Funct. Mater. 2015, 25, 490–494. [Google Scholar] [CrossRef]
- Sherwood, J.; Lovas, K.; Rich, M.; Yin, Q.; Lackey, K.; Bolding, M.S.; Bao, Y. Shape-dependent cellular behaviors and relaxivity of iron oxide-based T-1 MRI contrast agents. Nanoscale 2016, 8, 17506–17515. [Google Scholar] [CrossRef]
- Kostopoulou, A.; Brintakis, K.; Fragogeorgi, E.; Anthousi, A.; Manna, L.; Begin-Colin, S.; Billotey, C.; Ranella, A.; Loudos, G.; Athanassakis, I.; et al. Iron oxide colloidal nanoclusters as theranostic vehicles and their interactions at the cellular level. Nanomaterials 2018, 8, 315. [Google Scholar] [CrossRef]
- Lartigue, L.; Hugounenq, P.; Alloyeau, D.; Clarke, S.P.; Levy, M.; Bacri, J.C.; Bazzi, R.; Brougham, D.F.; Wilhelm, C.; Gazeau, F. Cooperative organization in iron oxide multi-core nanoparticles potentiates their efficiency as heating mediators and MRI contrast agents. ACS Nano 2012, 6, 10935–10949. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, Y.; Li, W.; Xie, Y.; Li, Y.; Wu, J.; Wang, S.; Tian, Y.; Tian, W.; Teng, Z.; et al. Synthesis of sub-100 nm biocompatible superparamagnetic Fe3O4 colloidal nanocrystal clusters as contrast agents for magnetic resonance imaging. RSC Adv. 2016, 6, 62550–62555. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antone, A.J.; Sun, Z.; Bao, Y. Preparation and Application of Iron Oxide Nanoclusters. Magnetochemistry 2019, 5, 45. https://doi.org/10.3390/magnetochemistry5030045
Antone AJ, Sun Z, Bao Y. Preparation and Application of Iron Oxide Nanoclusters. Magnetochemistry. 2019; 5(3):45. https://doi.org/10.3390/magnetochemistry5030045
Chicago/Turabian StyleAntone, Angelo J., Zaicheng Sun, and Yuping Bao. 2019. "Preparation and Application of Iron Oxide Nanoclusters" Magnetochemistry 5, no. 3: 45. https://doi.org/10.3390/magnetochemistry5030045
APA StyleAntone, A. J., Sun, Z., & Bao, Y. (2019). Preparation and Application of Iron Oxide Nanoclusters. Magnetochemistry, 5(3), 45. https://doi.org/10.3390/magnetochemistry5030045






