Bio-Catalysis and Biomedical Perspectives of Magnetic Nanoparticles as Versatile Carriers
Abstract
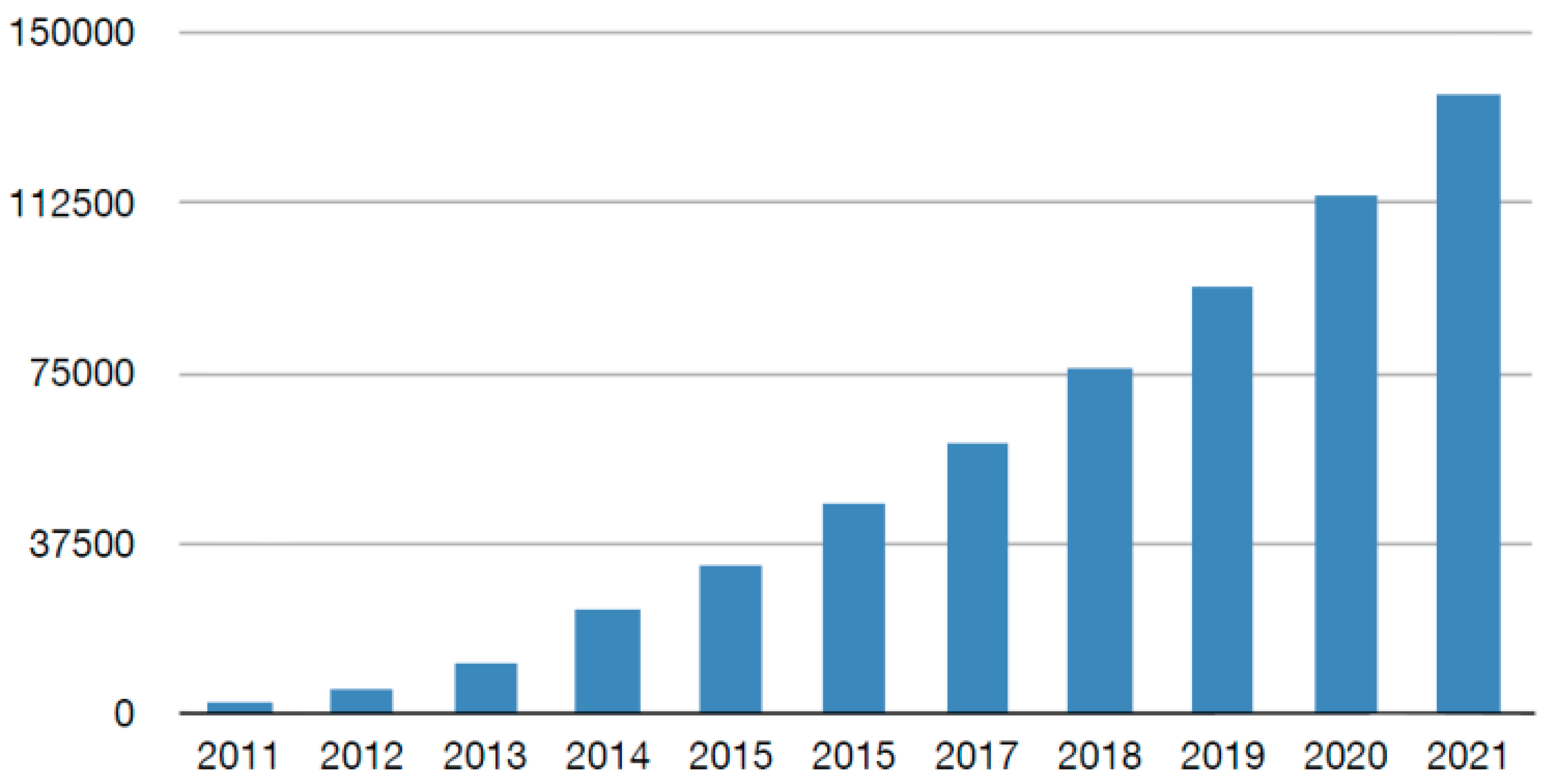
1. Introduction
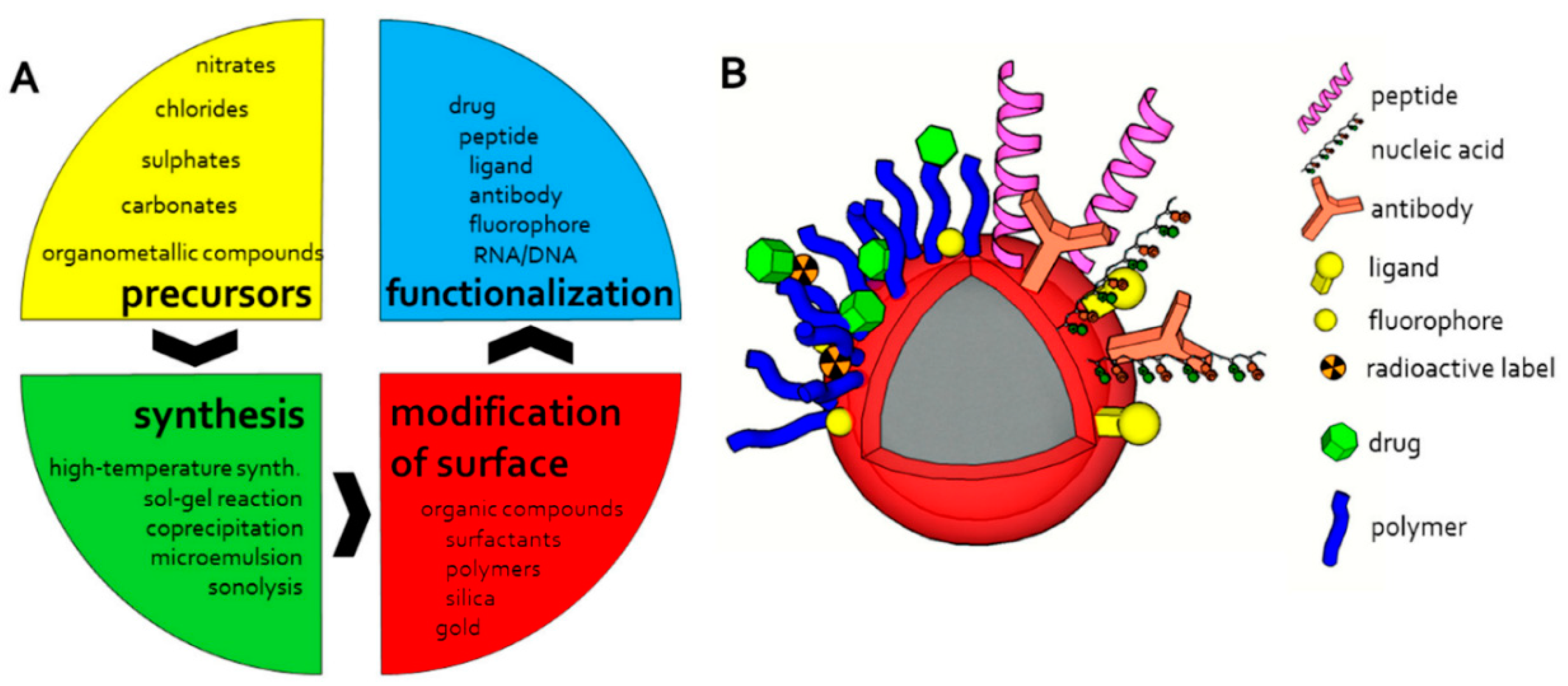
2. Magnetic Nanoparticles: Formation and Stabilization
3. Applications of Magnetic Nanoparticles
3.1. Bio-Catalysis Perspectives of Magnetic Nanoparticles
3.2. Degradation of Dye Pollutants
3.3. Fruit Juice Clarification
3.4. Biotransformation of Inulin to High Fructose Syrup
3.5. Other Applications
4. Biomedical Perspectives of Magnetic Nanomaterials
4.1. Efficacy of Nanoparticles-Based Drug Delivery
4.2. Iron-Based MNPs for Biomedical Applications
4.3. Cobalt-Based MNPs for Biomedical Applications
4.4. Other MNPs for Biomedical Applications
5. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xu, J.; Sun, J.; Wang, Y.; Sheng, J.; Wang, F.; Sun, M. Application of iron magnetic nanoparticles in protein immobilization. Molecules 2014, 19, 11465–11486. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Zhao, Y.; Rasheed, T.; Iqbal, H.M. Magnetic nanoparticles as versatile carriers for enzymes immobilization: A review. Int. J. Biol. Macromol. 2018, 120, 2530–2544. [Google Scholar] [CrossRef] [PubMed]
- Netto, C.G.; Toma, H.E.; Andrade, L.H. Superparamagnetic nanoparticles as versatile carriers and supporting materials for enzymes. J. Mol. Catal. B Enzym. 2013, 85, 71–92. [Google Scholar] [CrossRef]
- Abu-Dief, A.M.; Abdel-Fatah, S.M. Development and functionalization of magnetic nanoparticles as powerful and green catalysts for organic synthesis. Beni-Suef Univ. J. Basic Appl. Sci. 2018, 7, 55–67. [Google Scholar] [CrossRef]
- Jessie, A.; Guillaume, W. Tumor-Targeting Drug-Loaded Particles. U.S. Patent US8043631B2, 3 October 2012. [Google Scholar]
- Bazile, D.; Couvreur, P.; Lakkireddy, H.R.; MacKiewicz, N.; Nicolas, J. Functional PLA-PEG Copolymers, the Nanoparticles Thereof, Their Preparation and Use for Targeted Drug Delivery and Imaging. Patent EP2634179A1, 14 June 2016. [Google Scholar]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef] [PubMed]
- Taft, D.; Tzannis, S.; Dai, W.-G.; Ottensmann, S.; Bitler, S.; Zheng, Q.; Bell, A. Polymer Formulations for Delivery of Bioactive Materials. U.S. Patent US8784893B2, 14 February 2012. [Google Scholar]
- Angelova, A.; Angelov, B.; Drechsler, M.; Lesieur, S. Neurotrophin delivery using nanotechnology. Drug Discov. Today 2013, 18, 1263–1271. [Google Scholar] [CrossRef]
- Martins, P.; Rosa, D.; R Fernandes, A.; Baptista, P.V. Nanoparticle drug delivery systems: Recent patents and applications in nanomedicine. Recent Pat. Nanomed. 2013, 3, 105–118. [Google Scholar] [CrossRef]
- Ako-Adounvo, A.-M.; Marabesi, B.; Lemos, R.C.; Patricia, A.; Karla, P.K. Drug and Gene Delivery Materials and Devices. In Emerging Nanotechnologies for Diagnostics, Drug Delivery and Medical Devices; Elsevier: Amsterdam, The Netherlands, 2017; pp. 375–392. [Google Scholar]
- Nam, J.-M.; Thaxton, C.S.; Mirkin, C.A. Nanoparticle-based bio-bar codes for the ultrasensitive detection of proteins. Science 2003, 301, 1884–1886. [Google Scholar] [CrossRef]
- Jain, A.; Cheng, K. The principles and applications of avidin-based nanoparticles in drug delivery and diagnosis. J. Control. Release 2017, 245, 27–40. [Google Scholar] [CrossRef]
- Yin, C.; Hong, B.; Gong, Z.; Zhao, H.; Hu, W.; Lu, X.; Li, J.; Li, X.; Yang, Z.; Fan, Q. Fluorescent oligo (p-phenyleneethynylene) contained amphiphiles-encapsulated magnetic nanoparticles for targeted magnetic resonance and two-photon optical imaging in vitro and in vivo. Nanoscale 2015, 7, 8907–8919. [Google Scholar] [CrossRef]
- Bao, G.; Mitragotri, S.; Tong, S. Multifunctional nanoparticles for drug delivery and molecular imaging. Annu. Rev. Biomed. Eng. 2013, 15, 253–282. [Google Scholar] [CrossRef]
- Li, L.; Jiang, W.; Luo, K.; Song, H.; Lan, F.; Wu, Y.; Gu, Z. Superparamagnetic iron oxide nanoparticles as MRI contrast agents for non-invasive stem cell labeling and tracking. Theranostics 2013, 3, 595. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hu, X.; Xiang, D. Nanoparticle drug delivery systems: An excellent carrier for tumor peptide vaccines. Drug Deliv. 2018, 25, 1319–1327. [Google Scholar] [CrossRef]
- Seenuvasan, M.; Kumar, K.; Kumar, M.A.; Iyyappan, J.; Suganthi, J. Response surface estimation and canonical quantification for the pectin degrading Fe3O4-SiO2 nanobiocatalyst fabrication. Int. J. ChemTech Res. 2014, 6, 3618–3627. [Google Scholar]
- Gao, F.; Guo, Y.; Fan, X.; Hu, M.; Li, S.; Zhai, Q.; Jiang, Y.; Wang, X. Enhancing the catalytic performance of chloroperoxidase by co-immobilization with glucose oxidase on magnetic graphene oxide. Biochem. Eng. J. 2019, 143, 101–109. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, H.; Wang, L.; Zhou, L.; Huang, Z.; Ma, L.; He, Y.; Shi, L.; Gao, J. Virus-like organosilica nanoparticles for lipase immobilization: Characterization and biocatalytic applications. Biochem. Eng. J. 2019, 144, 125–134. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Kim, S.M.; Sayed, M.; Younesi, H.; Bahramifar, N.; Park, J.H.; Pyo, S.-H. Lipase-immobilized chitosan-crosslinked magnetic nanoparticle as a biocatalyst for ring opening esterification of itaconic anhydride. Biochem. Eng. J. 2019, 143, 141–150. [Google Scholar] [CrossRef]
- Mangkorn, N.; Kanokratana, P.; Roongsawang, N.; Laobuthee, A.; Laosiripojana, N.; Champreda, V. Synthesis and characterization of Ogataea thermomethanolica alcohol oxidase immobilized on barium ferrite magnetic microparticles. J. Biosci. Bioeng. 2019, 127, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Mokarram, R.R.; Ghorbani, M.; Hamishehkar, H. Inulinase immobilized gold-magnetic nanoparticles as a magnetically recyclable biocatalyst for facial and efficient inulin biotransformation to high fructose syrup. Int. J. Biol. Macromol. 2019, 123, 846–855. [Google Scholar] [CrossRef]
- Suo, H.; Xu, L.; Xu, C.; Qiu, X.; Chen, H.; Huang, H.; Hu, Y. Graphene oxide nanosheets shielding of lipase immobilized on magnetic composites for the improvement of enzyme stability. ACS Sustain. Chem. Eng. 2019, 7, 4486–4494. [Google Scholar] [CrossRef]
- Torabizadeh, H.; Mahmoudi, A. Inulin hydrolysis by inulinase immobilized covalently on magnetic nanoparticles prepared with wheat gluten hydrolysates. Biotechnol. Rep. 2018, 17, 97–103. [Google Scholar] [CrossRef]
- Dal Magro, L.; Silveira, V.C.; de Menezes, E.W.; Benvenutti, E.V.; Nicolodi, S.; Hertz, P.F.; Klein, M.P.; Rodrigues, R.C. Magnetic biocatalysts of pectinase and cellulase: Synthesis and characterization of two preparations for application in grape juice clarification. Int. J. Biol. Macromol. 2018, 115, 35–44. [Google Scholar] [CrossRef]
- Mardani, T.; Khiabani, M.S.; Mokarram, R.R.; Hamishehkar, H. Immobilization of α-amylase on chitosan-montmorillonite nanocomposite beads. Int. J. Biol. Macromol. 2018, 120, 354–360. [Google Scholar] [CrossRef]
- Sojitra, U.V.; Nadar, S.S.; Rathod, V.K. Immobilization of pectinase onto chitosan magnetic nanoparticles by macromolecular cross-linker. Carbohydr. Polym. 2017, 157, 677–685. [Google Scholar] [CrossRef]
- Sojitra, U.V.; Nadar, S.S.; Rathod, V.K. A magnetic tri-enzyme nanobiocatalyst for fruit juice clarification. Food Chem. 2016, 213, 296–305. [Google Scholar] [CrossRef]
- Wang, B.; Cheng, F.; Lu, Y.; Ge, W.; Zhang, M.; Yue, B. Immobilization of pectinase from Penicillium oxalicum F67 onto magnetic cornstarch microspheres: Characterization and application in juice production. J. Mol. Catal. B Enzym. 2013, 97, 137–143. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Jiang, X.-P.; Li, Y.; Zeng, S.; Zhang, Y.-W. Preparation Fe3O4@ chitosan magnetic particles for covalent immobilization of lipase from Thermomyces lanuginosus. Int. J. Biol. Macromol. 2015, 75, 44–50. [Google Scholar] [CrossRef]
- Bilal, M.; Rasheed, T.; Iqbal, H.M.; Yan, Y. Peroxidases-assisted removal of environmentally-related hazardous pollutants with reference to the reaction mechanisms of industrial dyes. Sci. Total Environ. 2018, 644, 1–13. [Google Scholar] [CrossRef]
- Rehman, S.; Bhatti, H.N.; Bilal, M.; Asgher, M. Cross-linked enzyme aggregates (CLEAs) of Pencilluim notatum lipase enzyme with improved activity, stability and reusability characteristics. Int. J. Biol. Macromol. 2016, 91, 1161–1169. [Google Scholar] [CrossRef]
- Rehman, S.; Wang, P.; Bhatti, H.N.; Bilal, M.; Asgher, M. Improved catalytic properties of Penicillium notatum lipase immobilized in nanoscale silicone polymeric films. Int. J. Biol. Macromol. 2017, 97, 279–286. [Google Scholar] [CrossRef]
- Amin, F.; Bhatti, H.N.; Bilal, M.; Asgher, M. Improvement of activity, thermo-stability and fruit juice clarification characteristics of fungal exo-polygalacturonase. Int. J. Biol. Macromol. 2017, 95, 974–984. [Google Scholar] [CrossRef]
- Amin, F.; Bhatti, H.N.; Bilal, M.; Asgher, M. Multiple parameter optimizations for enhanced biosynthesis of exo-polygalacturonase enzyme and its application in fruit juice clarification. Int. J. Food Eng. 2017, 13. [Google Scholar] [CrossRef]
- Asgher, M.; Noreen, S.; Bilal, M. Enhancement of catalytic, reusability, and long-term stability features of Trametes versicolor IBL-04 laccase immobilized on different polymers. Int. J. Biol. Macromol. 2017, 95, 54–62. [Google Scholar] [CrossRef]
- Asgher, M.; Noreen, S.; Bilal, M. Enhancing catalytic functionality of Trametes versicolor IBL-04 laccase by immobilization on chitosan microspheres. Chem. Eng. Res. Des. 2017, 119, 1–11. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M. Chemical, physical, and biological coordination: An interplay between materials and enzymes as potential platforms for immobilization. Coord. Chem. Rev. 2019, 388, 1–23. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M. Naturally-derived biopolymers: Potential platforms for enzyme immobilization. Int. J. Biol. Macromol. 2019, 130, 462–482. [Google Scholar] [CrossRef]
- Darwesh, O.M.; Matter, I.A.; Eida, M.F. Development of peroxidase enzyme immobilized magnetic nanoparticles for bioremediation of textile wastewater dye. J. Environ. Chem. Eng. 2019, 7, 102805. [Google Scholar] [CrossRef]
- Kashefi, S.; Borghei, S.M.; Mahmoodi, N.M. Superparamagnetic enzyme-graphene oxide magnetic nanocomposite as an environmentally friendly biocatalyst: Synthesis and biodegradation of dye using response surface methodology. Microchem. J. 2019, 145, 547–558. [Google Scholar] [CrossRef]
- Tapre, A.; Jain, R. Pectinases: Enzymes for fruit processing industry. Int. Food Res. J. 2014, 21, 447–453. [Google Scholar]
- Dal Magro, L.; Hertz, P.F.; Fernandez-Lafuente, R.; Klein, M.P.; Rodrigues, R.C. Preparation and characterization of a Combi-CLEAs from pectinases and cellulases: A potential biocatalyst for grape juice clarification. RSC Adv. 2016, 6, 27242–27251. [Google Scholar] [CrossRef]
- Echavarría, A.; Torras, C.; Pagán, J.; Ibarz, A. Fruit juice processing and membrane technology application. Food Eng. Rev. 2011, 3, 136–158. [Google Scholar] [CrossRef]
- Jiménez-Sánchez, C.; Lozano-Sánchez, J.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Alternatives to conventional thermal treatments in fruit-juice processing. Part 1: Techniques and applications. Crit. Rev. Food Sci. Nutr. 2017, 57, 501–523. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-C.; Wang, W.; Xue, C.; Mao, X. Effective enzyme immobilization onto a magnetic chitin nanofiber composite. ACS Sustain. Chem. Eng. 2018, 6, 8118–8124. [Google Scholar] [CrossRef]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Glutaraldehyde in bio-catalysts design: A useful crosslinker and a versatile tool in enzyme immobilization. RSC Adv. 2014, 4, 1583–1600. [Google Scholar] [CrossRef]
- Mateo, C.; Palomo, J.M.; Van Langen, L.M.; Van Rantwijk, F.; Sheldon, R.A. A new, mild cross-linking methodology to prepare cross-linked enzyme aggregates. Biotechnol. Bioeng. 2004, 86, 273–276. [Google Scholar] [CrossRef]
- Zhen, Q.; Wang, M.; Qi, W.; Su, R.; He, Z. Preparation of β-mannanase CLEAs using macromolecular cross-linkers. Catal. Sci. Technol. 2013, 3, 1937–1941. [Google Scholar] [CrossRef]
- Flores-Gallegos, A.C.; Contreras-Esquivel, J.C.; Morlett-Chávez, J.A.; Aguilar, C.N.; Rodríguez-Herrera, R. Comparative study of fungal strains for thermostable inulinase production. J. Biosci. Bioeng. 2015, 119, 421–426. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Aguiar, L.M.; Marinha, M.I.; Jorge, R.C.; Ferreira, E.C. Economic analysis and environmental impact assessment of three different fermentation processes for fructooligosaccharides production. Bioresour. Technol. 2015, 198, 673–681. [Google Scholar] [CrossRef][Green Version]
- Singh, R.S.; Chauhan, K.; Kennedy, J.F. A panorama of bacterial inulinases: Production, purification, characterization and industrial applications. Int. J. Biol. Macromol. 2017, 96, 312–322. [Google Scholar] [CrossRef]
- Vandamme, E.J.; Derycke, D.G. Microbial inulinases: Fermentation process, properties, and applications. In Advances in Applied Microbiology; Elsevier: Amsterdam, The Netherlands, 1983; Volume 29, pp. 139–176. [Google Scholar]
- Barthomeuf, C.; Regerat, F.; Pourrat, H. Production of inulinase by a new mold of Penicillium rugulosum. J. Ferment. Bioeng. 1991, 72, 491–494. [Google Scholar] [CrossRef]
- Torabizadeh, H.; Habibi-Rezaei, M.; Safari, M.; Moosavi-Movahedi, A.A.; Sharifizadeh, A.; Azizian, H.; Amanlou, M. Endo-inulinase stabilization by pyridoxal phosphate modification: A kinetics, thermodynamics, and simulation approach. Appl. Biochem. Biotechnol. 2011, 165, 1661–1673. [Google Scholar] [CrossRef] [PubMed]
- Wilkerson, J.W.; Yang, S.-O.; Funk, P.J.; Stanley, S.K.; Bundy, B.C. Nanoreactors: Strategies to encapsulate enzyme biocatalysts in virus-like particles. New Biotechnol. 2018, 44, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Patterson, D.P.; Prevelige, P.E.; Douglas, T. Nanoreactors by programmed enzyme encapsulation inside the capsid of the bacteriophage P22. ACS Nano 2012, 6, 5000–5009. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, J.D.; Brown, S.D.; Lau, J.L.; Finn, M. RNA-directed packaging of enzymes within virus-like particles. Angew. Chem. Int. Ed. 2010, 49, 9648–9651. [Google Scholar] [CrossRef] [PubMed]
- Minten, I.J.; Hendriks, L.J.; Nolte, R.J.; Cornelissen, J.J. Controlled encapsulation of multiple proteins in virus capsids. J. Am. Chem. Soc. 2009, 131, 17771–17773. [Google Scholar] [CrossRef] [PubMed]
- Minten, I.J.; Claessen, V.I.; Blank, K.; Rowan, A.E.; Nolte, R.J.; Cornelissen, J.J. Catalytic capsids: The art of confinement. Chem. Sci. 2011, 2, 358–362. [Google Scholar] [CrossRef]
- Kim, K.; Lee, O.; Lee, E. Nano-immobilized biocatalysts for biodiesel production from renewable and sustainable resources. Catalysts 2018, 8, 68. [Google Scholar]
- Zhang, C.; Liu, Y.; Sun, Y. Lipase immobilized to a short alkyl chain-containing zwitterionic polymer grafted on silica nanoparticles: Moderate activation and significant increase of thermal stability. Biochem. Eng. J. 2019, 146, 124–131. [Google Scholar] [CrossRef]
- Frimpong, R.A.; Hilt, J.Z. Magnetic nanoparticles in biomedicine: Synthesis, functionalization and applications. Nanomedicine 2010, 5, 1401–1414. [Google Scholar] [CrossRef] [PubMed]
- Bárcena, C.; Sra, A.K.; Gao, J. Applications of magnetic nanoparticles in biomedicine. In Nanoscale Magnetic Materials and Applications; Springer: New York, NY, USA, 2009; pp. 591–626. [Google Scholar]
- Pankhurst, Q.A.; Connolly, J.; Jones, S.; Dobson, J. Applications of magnetic nanoparticles in biomedicine. J. Phys. D Appl. Phys. 2003, 36, R167. [Google Scholar] [CrossRef]
- Jha, S.; Sharma, P.K.; Malviya, R. Hyperthermia: Role and risk factor for cancer treatment. Achiev. Life Sci. 2016, 10, 161–167. [Google Scholar] [CrossRef]
- Neuberger, T.; Schöpf, B.; Hofmann, H.; Hofmann, M.; Von Rechenberg, B. Superparamagnetic nanoparticles for biomedical applications: Possibilities and limitations of a new drug delivery system. J. Magn. Magn. Mater. 2005, 293, 483–496. [Google Scholar] [CrossRef]
- Huber, D.L. Synthesis, properties, and applications of iron nanoparticles. Small 2005, 1, 482–501. [Google Scholar] [CrossRef] [PubMed]
- Bomatí-Miguel, O.; Morales, M.P.; Tartaj, P.; Ruiz-Cabello, J.; Bonville, P.; Santos, M.; Zhao, X.; Veintemillas-Verdaguer, S. Fe-based nanoparticulate metallic alloys as contrast agents for magnetic resonance imaging. Biomaterials 2005, 26, 5695–5703. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.M.; Jeong, H.J.; Kim, E.M.; Kim, D.W.; Lim, S.T.; Kim, H.T.; Park, I.K.; Jeong, Y.Y.; Kim, J.W.; Sohn, M.H. Superparamagnetic iron oxide nanoparticles as a dual imaging probe for targeting hepatocytes in vivo. Magn. Reson. Med. Off. J. Int. Soc. Magn. Reson. Med. 2009, 62, 1440–1446. [Google Scholar] [CrossRef]
- Yean, S.; Cong, L.; Yavuz, C.T.; Mayo, J.; Yu, W.; Kan, A.; Colvin, V.; Tomson, M. Effect of magnetite particle size on adsorption and desorption of arsenite and arsenate. J. Mater. Res. 2005, 20, 3255–3264. [Google Scholar] [CrossRef]
- Yokoyama, T.; Masuda, H.; Suzuki, M.; Ehara, K.; Nogi, K.; Fuji, M.; Fukui, T.; Suzuki, H.; Tatami, J.; Hayashi, K. Basic properties and measuring methods of nanoparticles. In Nanoparticle Technology Handbook; Elsevier: Amsterdam, The Netherlands, 2008; pp. 3–48. [Google Scholar]
- Wiekhorst, F.; Seliger, C.; Jurgons, R.; Steinhoff, U.; Eberbeck, D.; Trahms, L.; Alexiou, C. Quantification of magnetic nanoparticles by magnetorelaxometry and comparison to histology after magnetic drug targeting. J. Nanosci. Nanotechnol. 2006, 6, 3222–3225. [Google Scholar] [CrossRef]
- Namdeo, M.; Saxena, S.; Tankhiwale, R.; Bajpai, M.; Mohan, Y.; Bajpai, S. Magnetic nanoparticles for drug delivery applications. J. Nanosci. Nanotechnol. 2008, 8, 3247–3271. [Google Scholar] [CrossRef]
- Lu, A.H.; Salabas, E.E.; Schüth, F. Magnetic nanoparticles: Synthesis, protection, functionalization, and application. Angew. Chem. Int. Ed. 2007, 46, 1222–1244. [Google Scholar] [CrossRef]
- Arruebo, M.; Galán, M.; Navascués, N.; Téllez, C.; Marquina, C.; Ibarra, M.R.; Santamaría, J. Development of magnetic nanostructured silica-based materials as potential vectors for drug-delivery applications. Chem. Mater. 2006, 18, 1911–1919. [Google Scholar] [CrossRef]
- Rosengart, A.J.; Kaminski, M.D.; Chen, H.; Caviness, P.L.; Ebner, A.D.; Ritter, J.A. Magnetizable implants and functionalized magnetic carriers: A novel approach for noninvasive yet targeted drug delivery. J. Magn. Magn. Mater. 2005, 293, 633–638. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, W.; Zhong, Y.; Huo, Y.; Fan, P.; Zhan, S.; Xiao, J.; Jin, X.; Gou, S.; Yin, T. Overexpression of G protein-coupled receptor GPR87 promotes pancreatic cancer aggressiveness and activates NF-κB signaling pathway. Mol. Cancer 2017, 16, 61. [Google Scholar] [CrossRef] [PubMed]
- Blake, A.; Dragan, M.; Tirona, R.G.; Hardy, D.B.; Brackstone, M.; Tuck, A.B.; Babwah, A.V.; Bhattacharya, M. G protein-coupled KISS1 receptor is overexpressed in triple negative breast cancer and promotes drug resistance. Sci. Rep. 2017, 7, 46525. [Google Scholar] [CrossRef] [PubMed]
- Boyd, B. Drug Delivery Report Autumn/Winter; PharmaVentures Ltd.: Oxford, UK, 2005. [Google Scholar]
- Tomalia, D.A. Birth of a new macromolecular architecture: Dendrimers as quantized building blocks for nanoscale synthetic polymer chemistry. Prog. Polym. Sci. 2005, 30, 294–324. [Google Scholar] [CrossRef]
- Vo, T.N.; Kasper, F.K.; Mikos, A.G. Strategies for controlled delivery of growth factors and cells for bone regeneration. Adv. Drug Deliv. Rev. 2012, 64, 1292–1309. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Saltzman, M. Engineering biodegradable nanoparticles for drug and gene delivery. Chem. Eng. Prog. 2013, 109, 25. [Google Scholar] [PubMed]
- Kohane, D.S. Microparticles and nanoparticles for drug delivery. Biotechnol. Bioeng. 2007, 96, 203–209. [Google Scholar] [CrossRef] [PubMed]
- D. Friedman, A.; E. Claypool, S.; Liu, R. The smart targeting of nanoparticles. Curr. Pharm. Des. 2013, 19, 6315–6329. [Google Scholar] [CrossRef]
- Osakwe, O.; Rizvi, S.A. Social Aspects of Drug Discovery, Development and Commercialization; Academic Press: San Diego, CA, USA, 2016. [Google Scholar]
- Onoue, S.; Yamada, S.; Chan, H.-K. Nanodrugs: Pharmacokinetics and safety. Int. J. Nanomed. 2014, 9, 1025. [Google Scholar] [CrossRef]
- Kudr, J.; Haddad, Y.; Richtera, L.; Heger, Z.; Cernak, M.; Adam, V.; Zitka, O. Magnetic nanoparticles: From design and synthesis to real world applications. Nanomaterials 2017, 7, 243. [Google Scholar] [CrossRef]
- Sun, C.; Lee, J.S.; Zhang, M. Magnetic nanoparticles in MR imaging and drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1252–1265. [Google Scholar] [CrossRef] [PubMed]
- Skalickova, S.; Nejdl, L.; Kudr, J.; Ruttkay-Nedecky, B.; Jimenez Jimenez, A.; Kopel, P.; Kremplova, M.; Masarik, M.; Stiborova, M.; Eckschlager, T. Fluorescence characterization of gold modified liposomes with antisense N-myc DNA bound to the magnetisable particles with encapsulated anticancer drugs (doxorubicin, ellipticine and etoposide). Sensors 2016, 16, 290. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Sant, S.; Wang, B.; Laurent, S.; Sen, T. Superparamagnetic iron oxide nanoparticles (SPIONs): Development, surface modification and applications in chemotherapy. Adv. Drug Deliv. Rev. 2011, 63, 24–46. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Gupta, M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 2005, 26, 3995–4021. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, J.R.; Weissleder, R. Multifunctional magnetic nanoparticles for targeted imaging and therapy. Adv. Drug Deliv. Rev. 2008, 60, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- Heger, Z.; Zitka, J.; Cernei, N.; Krizkova, S.; Sztalmachova, M.; Kopel, P.; Masarik, M.; Hodek, P.; Zitka, O.; Adam, V. 3D-printed biosensor with poly (dimethylsiloxane) reservoir for magnetic separation and quantum dots-based immunolabeling of metallothionein. Electrophoresis 2015, 36, 1256–1264. [Google Scholar] [CrossRef] [PubMed]
- Zitka, O.; Cernei, N.; Heger, Z.; Matousek, M.; Kopel, P.; Kynicky, J.; Masarik, M.; Kizek, R.; Adam, V. Microfluidic chip coupled with modified paramagnetic particles for sarcosine isolation in urine. Electrophoresis 2013, 34, 2639–2647. [Google Scholar] [CrossRef] [PubMed]
- Pileni, M.P. Magnetic fluids: Fabrication, magnetic properties, and organization of nanocrystals. Adv. Funct. Mater. 2001, 11, 323–336. [Google Scholar] [CrossRef]
- Tartaj, P.; del Puerto Morales, M.; Veintemillas-Verdaguer, S.; González-Carreño, T.; Serna, C.J. The preparation of magnetic nanoparticles for applications in biomedicine. J. Phys. D Appl. Phys. 2003, 36, R182. [Google Scholar] [CrossRef]
- Sun, Y.-k.; Ma, M.; Zhang, Y.; Gu, N. Synthesis of nanometer-size maghemite particles from magnetite. Colloids Surf. A Physicochem. Eng. Asp. 2004, 245, 15–19. [Google Scholar] [CrossRef]
- Ma, M.; Zhang, Y.; Yu, W.; Shen, H.-Y.; Zhang, H.-Q.; Gu, N. Preparation and characterization of magnetite nanoparticles coated by amino silane. Colloids Surf. A Physicochem. Eng. Asp. 2003, 212, 219–226. [Google Scholar] [CrossRef]
- Park, J.; An, K.; Hwang, Y.; Park, J.-G.; Noh, H.-J.; Kim, J.-Y.; Park, J.-H.; Hwang, N.-M.; Hyeon, T. Ultra-large-scale syntheses of monodisperse nanocrystals. Nat. Mater. 2004, 3, 891. [Google Scholar] [CrossRef] [PubMed]
- Zitka, O.; Krizkova, S.; Krejcova, L.; Hynek, D.; Gumulec, J.; Masarik, M.; Sochor, J.; Adam, V.; Hubalek, J.; Trnkova, L. Microfluidic tool based on the antibody-modified paramagnetic particles for detection of 8-hydroxy-2′-deoxyguanosine in urine of prostate cancer patients. Electrophoresis 2011, 32, 3207–3220. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.J.; Jeun, M.; Jang, G.H.; Song, S.H.; Jeong, I.G.; Kim, C.-S.; Searson, P.C.; Lee, K.H. Diagnosis of prostate cancer via nanotechnological approach. Int. J. Nanomed. 2015, 10, 6555. [Google Scholar]
- Patel, D.; Moon, J.Y.; Chang, Y.; Kim, T.J.; Lee, G.H. Poly (D, L-lactide-co-glycolide) coated superparamagnetic iron oxide nanoparticles: Synthesis, characterization and in vivo study as MRI contrast agent. Colloids Surf. A Physicochem. Eng. Asp. 2008, 313, 91–94. [Google Scholar] [CrossRef]
- Prabha, G.; Raj, V. Formation and characterization of β-cyclodextrin (β-CD)–polyethyleneglycol (PEG)–polyethyleneimine (PEI) coated Fe3O4 nanoparticles for loading and releasing 5-Fluorouracil drug. Biomed. Pharmacother. 2016, 80, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Karimzadeh, I.; Aghazadeh, M.; Doroudi, T.; Ganjali, M.R.; Kolivand, P.H. Superparamagnetic iron oxide (Fe3O4) nanoparticles coated with PEG/PEI for biomedical applications: A facile and scalable preparation route based on the cathodic electrochemical deposition method. Adv. Phys. Chem. 2017, 2017. [Google Scholar] [CrossRef]
- Li, J.; Zheng, L.; Cai, H.; Sun, W.; Shen, M.; Zhang, G.; Shi, X. Polyethyleneimine-mediated synthesis of folic acid-targeted iron oxide nanoparticles for in vivo tumor MR imaging. Biomaterials 2013, 34, 8382–8392. [Google Scholar] [CrossRef]
- Rodrigo, M.A.M.; Krejcova, L.; Kudr, J.; Cernei, N.; Kopel, P.; Richtera, L.; Moulick, A.; Hynek, D.; Adam, V.; Stiborova, M. Fully automated two-step assay for detection of metallothionein through magnetic isolation using functionalized γ-Fe2O3 particles. J. Chromatogr. B 2016, 1039, 17–27. [Google Scholar] [CrossRef]
- Jian, P.; Fen, Z.; Lu, L.; Liang, T.; Li, Y.; Wei, C.; Hui, L.; TANG, J.-b.; WU, L.-x. Preparation and characterization of PEG-PEI/Fe3O4 nano-magnetic fluid by co-precipitation method. Trans. Nonferrous Metals Soc. China 2008, 18, 393–398. [Google Scholar]
- Lee, J.-H.; Huh, Y.-M.; Jun, Y.-W.; Seo, J.-W.; Jang, J.-T.; Song, H.-T.; Kim, S.; Cho, E.-J.; Yoon, H.-G.; Suh, J.-S. Artificially engineered magnetic nanoparticles for ultra-sensitive molecular imaging. Nat. Med. 2007, 13, 95. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, N.; Hyeon, T. Recent development of nanoparticles for molecular imaging. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2017, 375, 20170022. [Google Scholar] [CrossRef]
- Dutz, S.; Clement, J.H.; Eberbeck, D.; Gelbrich, T.; Hergt, R.; Müller, R.; Wotschadlo, J.; Zeisberger, M. Ferrofluids of magnetic multicore nanoparticles for biomedical applications. J. Magn. Magn. Mater. 2009, 321, 1501–1504. [Google Scholar] [CrossRef]
- Seo, W.S.; Lee, J.H.; Sun, X.; Suzuki, Y.; Mann, D.; Liu, Z.; Terashima, M.; Yang, P.C.; McConnell, M.V.; Nishimura, D.G. FeCo/graphitic-shell nanocrystals as advanced magnetic-resonance-imaging and near-infrared agents. Nat. Mater. 2006, 5, 971. [Google Scholar] [CrossRef] [PubMed]
- Kainz, Q.M.; Fernandes, S.; Eichenseer, C.M.; Besostri, F.; Körner, H.; Müller, R.; Reiser, O. Synthesis of functionalized, dispersible carbon-coated cobalt nanoparticles for potential biomedical applications. Faraday Discuss. 2015, 175, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, J.; Rutnakornpituk, M.; Vadala, M.; Esker, A.; Charles, S.; Wells, S.; Dailey, J.; Riffle, J. Magnetic cobalt dispersions in poly (dimethylsiloxane) fluids. J. Magn. Magn. Mater. 2001, 225, 47–58. [Google Scholar] [CrossRef]
- Rutnakornpituk, M.; Baranauskas, V.; Riffle, J.; Connolly, J.; St Pierre, T.; Dailey, J. Polysiloxane fluid dispersions of cobalt nanoparticles in silica spheres for use in ophthalmic applications. Eur. Cells Mater 2002, 3, 102–105. [Google Scholar]
- Osorio-Cantillo, C.; Santiago-Miranda, A.; Perales-Perez, O.; Xin, Y. Size-and phase-controlled synthesis of cobalt nanoparticles for potential biomedical applications. J. Appl. Phys. 2012, 111, 07B324. [Google Scholar] [CrossRef]
- Joubert, J. Magnetic micro composites as vectors for bioactive agents: The state of art. Anales de Quimica 1997, 93, 70–76. [Google Scholar]
- Grasset, F.; Mornet, S.; Demourgues, A.; Portier, J.; Bonnet, J.; Vekris, A.; Duguet, E. Synthesis, magnetic properties, surface modification and cytotoxicity evaluation of Y3Fe5−xAlxO12 (0 ≤ x ≤ 2) garnet submicron particles for biomedical applications. J. Magn. Magn. Mater. 2001, 234, 409–418. [Google Scholar] [CrossRef]
- Rinaldi-Montes, N.; Gorria, P.; Martínez-Blanco, D.; Amghouz, Z.; Fuertes, A.B.; Barquín, L.F.; de Pedro, I.; Olivi, L.; Blanco, J.A. Unravelling the onset of the exchange bias effect in Ni (core)@ NiO (shell) nanoparticles embedded in a mesoporous carbon matrix. J. Mater. Chem. C 2015, 3, 5674–5682. [Google Scholar] [CrossRef]
- Zhou, W.; Zheng, K.; He, L.; Wang, R.; Guo, L.; Chen, C.; Han, X.; Zhang, Z. Ni/Ni3C core–shell nanochains and its magnetic properties: One-step synthesis at low temperature. Nano Lett. 2008, 8, 1147–1152. [Google Scholar] [CrossRef] [PubMed]
- Rahal, H.; Awad, R.; Abdel-Gaber, A.; Bakeer, D. Synthesis, characterization, and magnetic properties of pure and EDTA-capped NiO nanosized particles. J. Nanomater. 2017, 2017. [Google Scholar] [CrossRef]
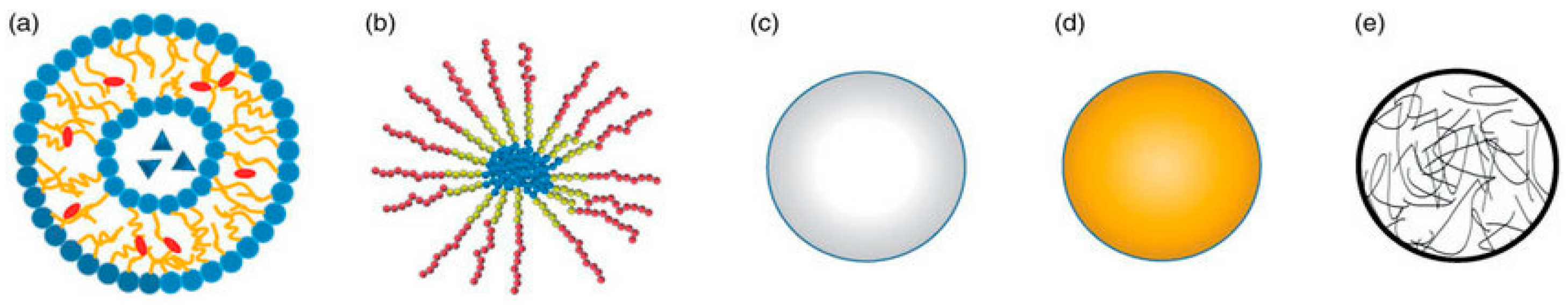
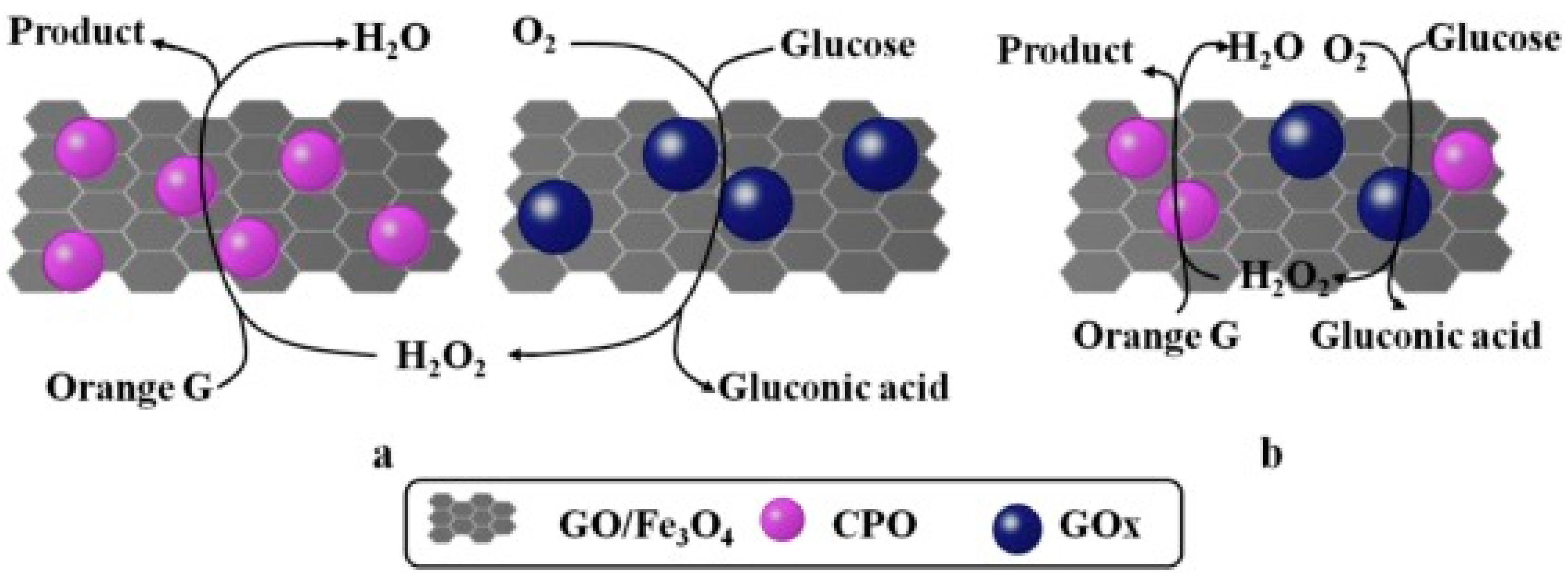
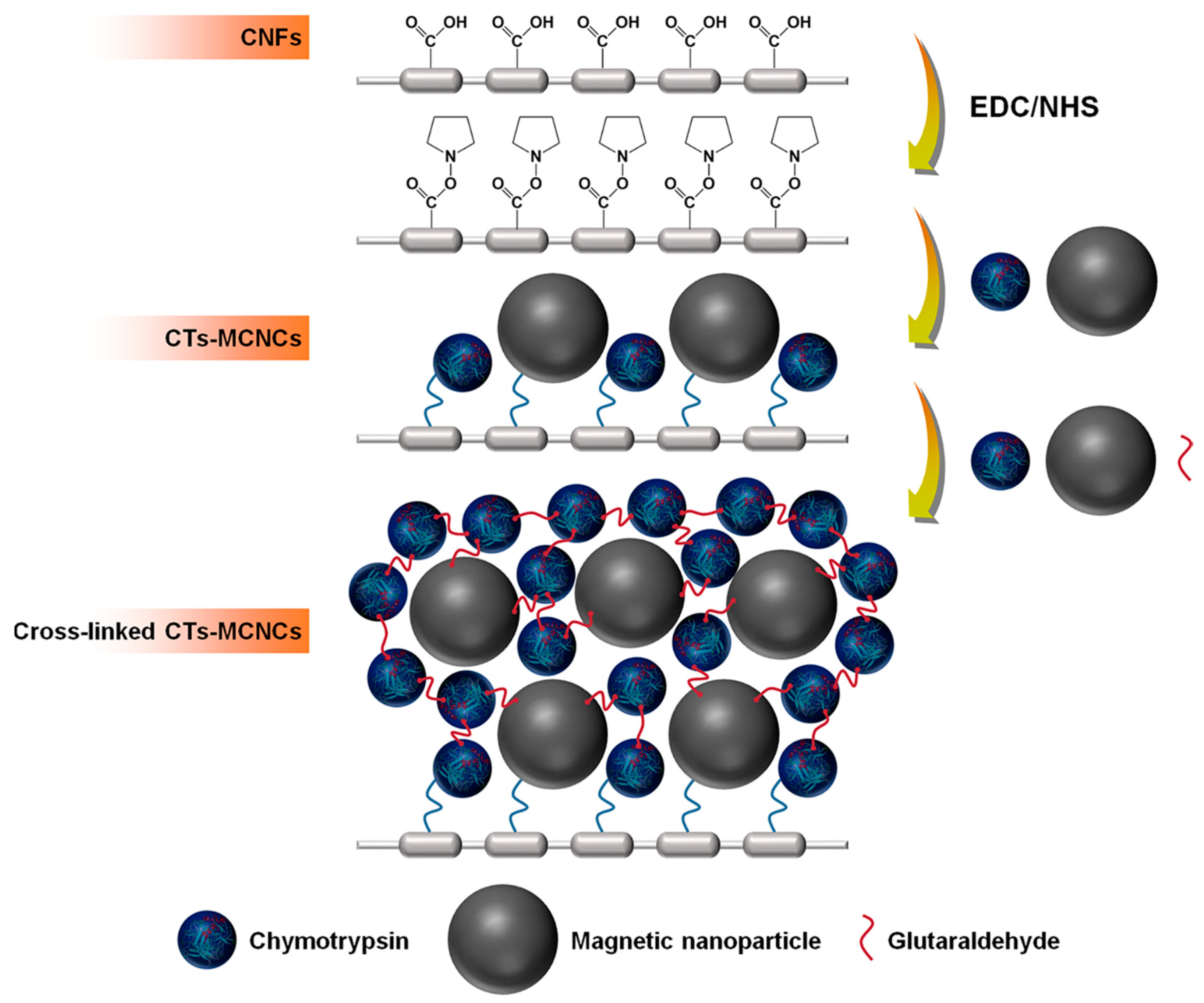
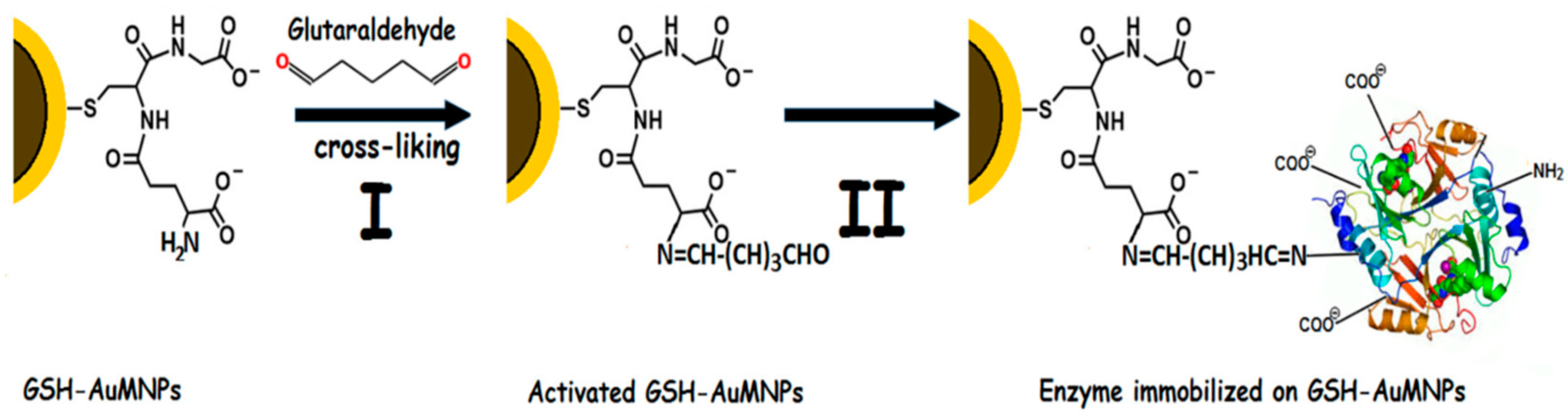
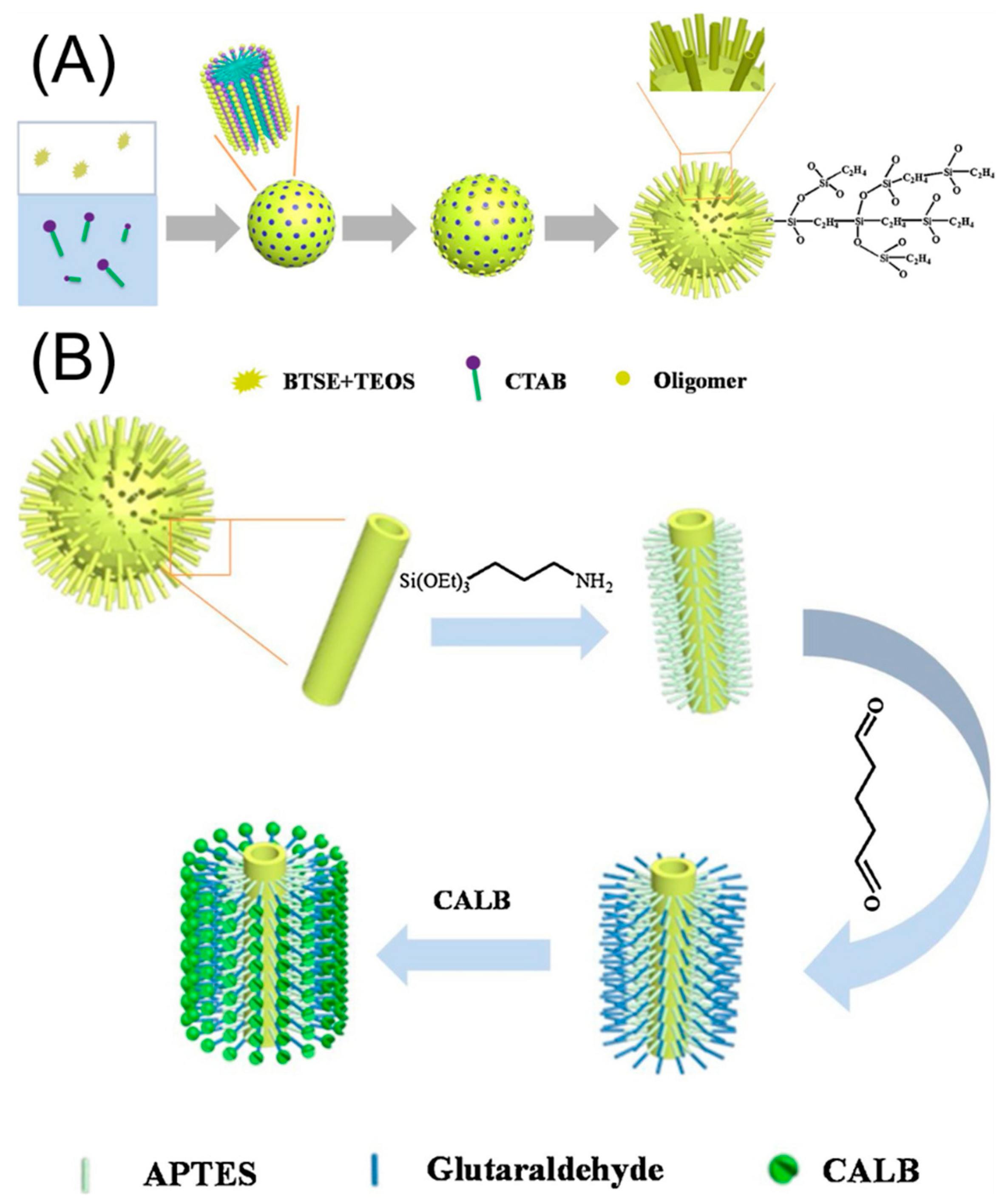
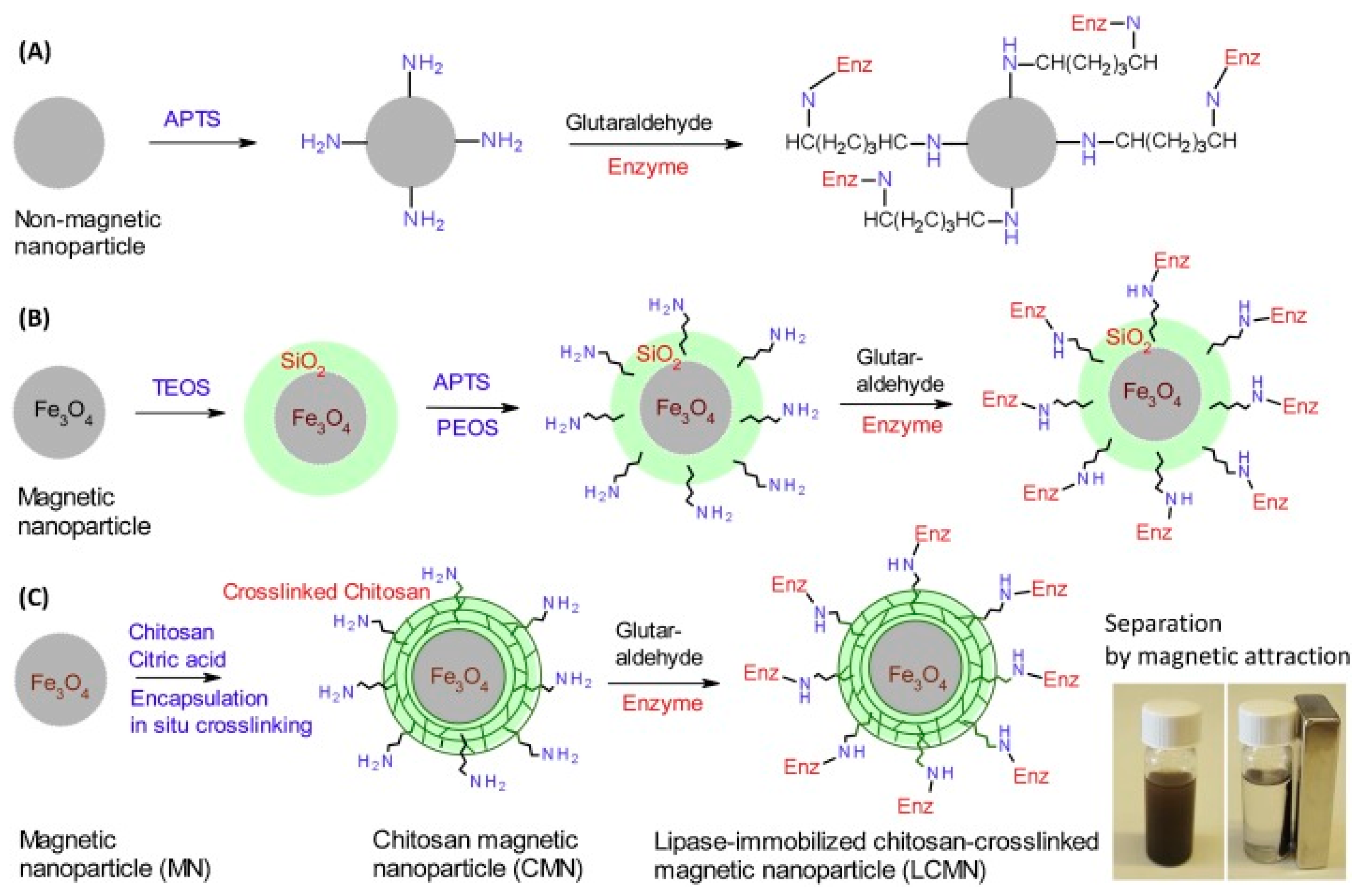
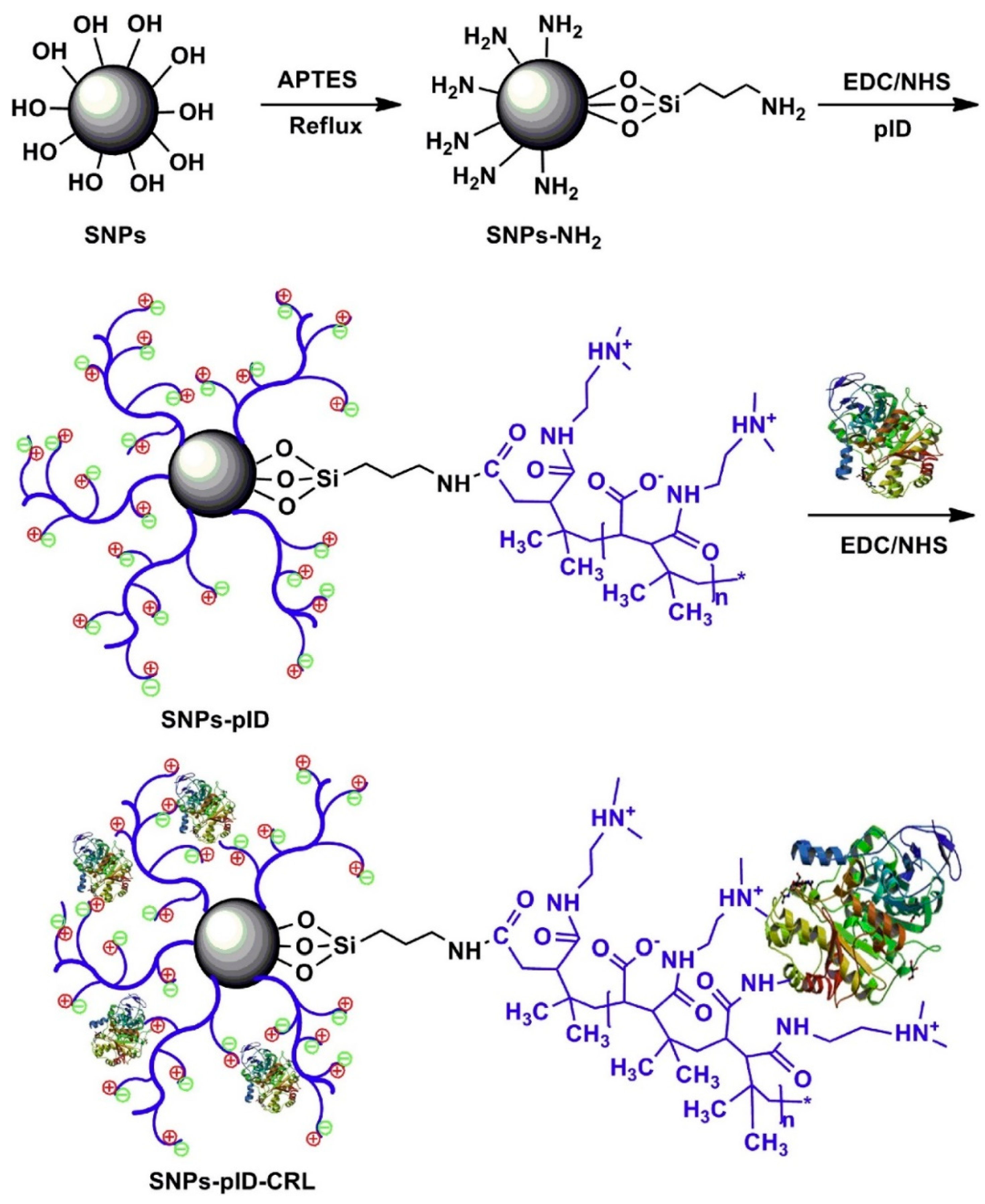
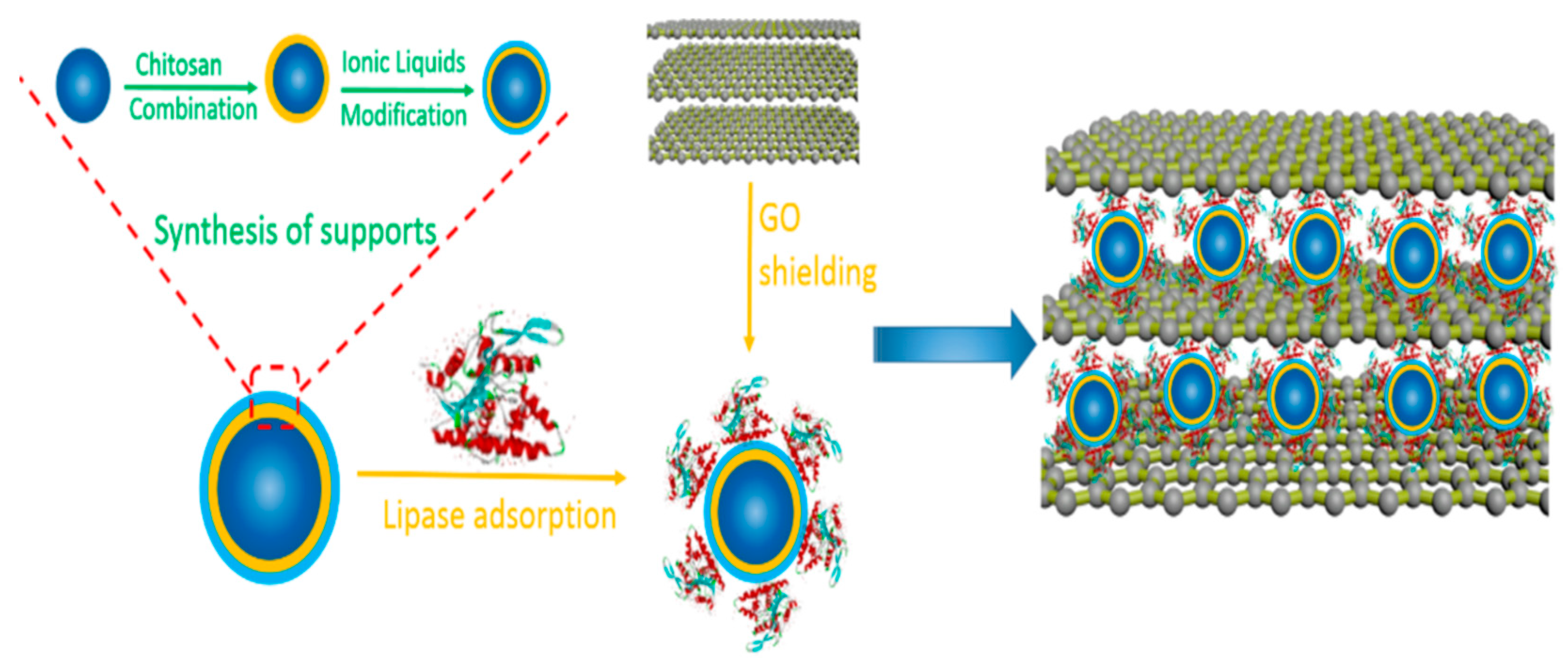
| Magnetic Carrier | Name of Enzyme | Immobilization Technique | Improved Properties and Application of Immobilized Enzymes | References |
|---|---|---|---|---|
| Magnetic graphene oxide | Chloroperoxidase/glucose oxidase | Physical adsorption | Excellent catalytic efficiency, operational durability, and recyclability. Immobilized biocatalyst showed far greater thermal stability compared with the native enzyme. It presented an application in decolorization and degradation of many synthetic dyes from industrial wastewater. | [19] |
| hydrophobic virus-like organosilica nanoparticles | Candida antarctica lipase B | Covalent attachment | Improved pH and thermal resistance High tolerance to organic solvents and long-term storage stability. Efficient esterification reaction of levulinic acid retaining 75.7% of the levulinic acid transformation after 9 continuous biocatalytic cycles. | [20] |
| Chitosan-cross-linked magnetic nanoparticles | Candida antarctica lipase B | Covalent attachment | Superior separation and biocatalytic properties. Excellent storage stability and reusability. Production of bio-based photocurable oligo-esters by the ring opening esterification of polyols and itaconic anhydride. | [21] |
| Barium ferrite magnetic microparticles | Alcohol oxidase | Covalent attachment | Enhanced thermostability retaining > 65% of the original activity at 45 °C for 24 h. Good catalytic efficacy for oxidizing ethanol and methanol compared with the free enzyme. Recyclability for at three successive batches with 70% activity retention. | [22] |
| Glutathione-coated gold magnetic nanoparticles | Inulinase | Covalent binding | Enhanced storage and reusability stability. Immobilized biocatalyst preserved about 78% of its original activity after 10 repeated cycles. Improved enzyme performance at acidic pHs (3.0 and 4.0) and high temperature up to 80 °C. Complete hydrolysis of inulin to fructose and glucose. | [23] |
| Ionic liquid-modified magnetic chitosan composites | Lipase | Adsorption | Elevated catalytic activity (6.72-fold) as compared to the free enzyme Enhanced thermal stability and reusability retaining 92.1% of residual activity after 10 cycles of reuse. | [24] |
| Fe3O4 magnetic nanoparticles functionalized with wheat gluten hydrolysates | Inulinase | Covalent binding | High activity over a broader pH and temperature ranges, and also exhibited pronounced storage and thermal stability. The inulinase showed 12.3 folds rise in enzyme half-life value at 75 °C Potential recyclability retaining 70% of its preliminary catalytic activity after 12 continuous inulin hydrolysis cycles. | [25] |
| FunctionalizedAPTMS-magnetite nanoparticles | Cellulase and pectinase | Covalent immobilization | Improved characteristics such as high activities recovery, enhanced temperature stability (2.39-times greater than that to the free enzyme), and reusability for up to 8 continuous cycles in grape juice clarification. | [26] |
| Chitosan-montmorillonite nanocomposite beads | α-amylase | Cross-linking | High enzyme activity and stability at varying pH and temperature conditions than the free enzyme. Retention of about 53% enzyme relative activity after recycling 5 times | [27] |
| Chitosan magnetic nanoparticles | Pectinase | Cross-linking | Superior thermal stability than the soluble form of the enzyme. High stabilization retaining 87% of original activity after seven repeated cycles. Excellent durability. Potential apple juice clarification with up to 74% turbidity reduction after 2.5 h of treatment. | [28] |
| Amino-functionalized magnetic nanoparticle | α-amylase, cellulose, and pectinase | Cross-linking | Increased pH and thermal stability Encouraging enzyme reusability preserving up to 75% of activity after 8 reuse cycles. Clarification of fruit juices. Significant decrease in turbidity. | [29] |
| Magnetic cornstarch microspheres | Pectinase | Adsorption | Improved pH and thermal stability. Good reusability and operability of the immobilized biocatalyst preserving 60% of its initial activity after 8 reuses in apple juice processing. | [30] |
| Magnetic Fe3O4@chitosan nanoparticles | Lipase | Covalent immobilization | Immobilized biocatalyst presented more than 50% and 75% residual activity in the pH range 7.0–11.0, and 70 °C. Satisfactory reusability preserving 70% of its original activity after 10 repeated cycles. More than 50% conversion of ascorbic acid was achieved when used for ascorbyl palmitate synthesis in tert-butanol at 50 °C. | [31] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bilal, M.; Mehmood, S.; Rasheed, T.; Iqbal, H.M.N. Bio-Catalysis and Biomedical Perspectives of Magnetic Nanoparticles as Versatile Carriers. Magnetochemistry 2019, 5, 42. https://doi.org/10.3390/magnetochemistry5030042
Bilal M, Mehmood S, Rasheed T, Iqbal HMN. Bio-Catalysis and Biomedical Perspectives of Magnetic Nanoparticles as Versatile Carriers. Magnetochemistry. 2019; 5(3):42. https://doi.org/10.3390/magnetochemistry5030042
Chicago/Turabian StyleBilal, Muhammad, Shahid Mehmood, Tahir Rasheed, and Hafiz M. N. Iqbal. 2019. "Bio-Catalysis and Biomedical Perspectives of Magnetic Nanoparticles as Versatile Carriers" Magnetochemistry 5, no. 3: 42. https://doi.org/10.3390/magnetochemistry5030042
APA StyleBilal, M., Mehmood, S., Rasheed, T., & Iqbal, H. M. N. (2019). Bio-Catalysis and Biomedical Perspectives of Magnetic Nanoparticles as Versatile Carriers. Magnetochemistry, 5(3), 42. https://doi.org/10.3390/magnetochemistry5030042






