BAP (6-Benzylaminopurine) Seed-Priming Enhanced Growth, Antioxidant Accumulation and Anthocyanin Metabolism in Olive Sprouts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sprout Production
2.2. Analysis of Pigment Profile
2.3. Determination of Fatty Acid Levels
2.4. Quantification of Lipid Antioxidant Metabolites
2.5. Quantification of Water-Soluble Antioxidant Metabolites
2.6. Determination of Anthocyanin Content, Percussors and the Activity of Related Biosynthetic Enzymes
2.7. Total Antioxidant Capacity
2.8. Statistical Analyses
3. Results
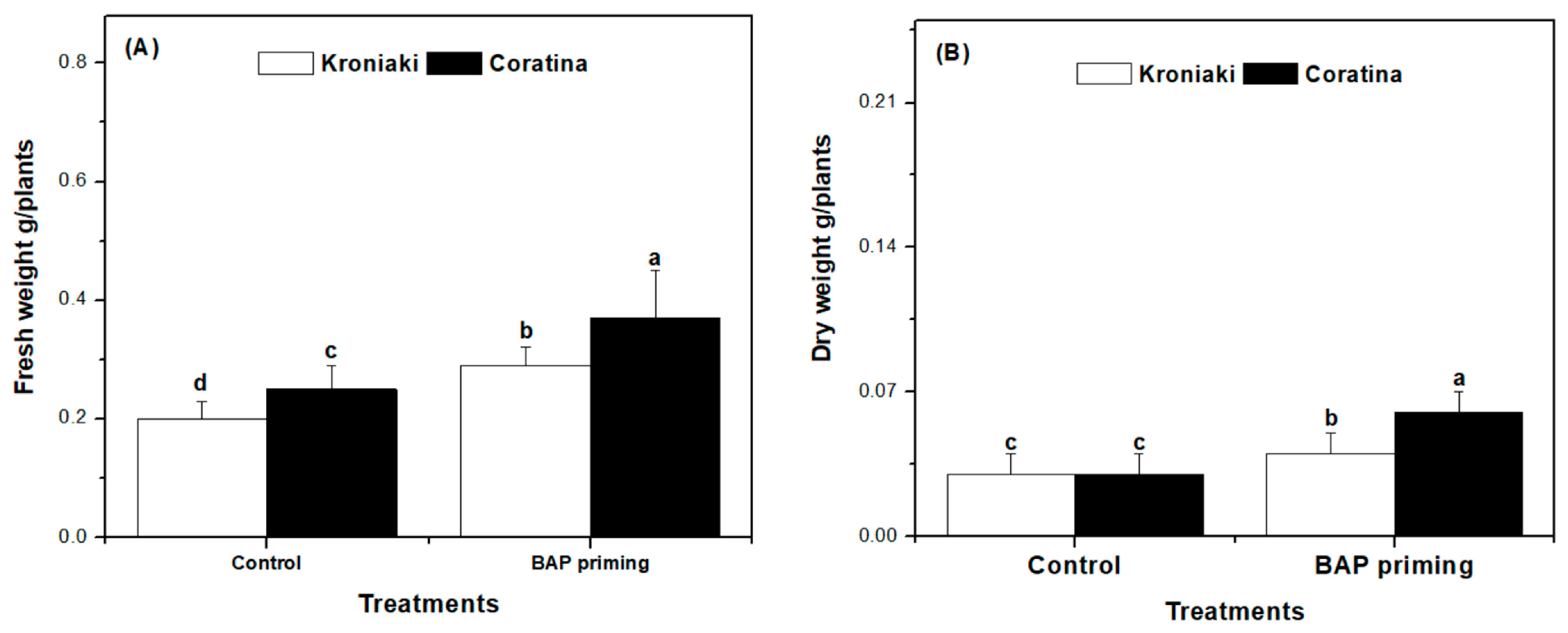
3.1. BAP Improved Biomass Accumulation in Kroniaki and Coratina Varieties
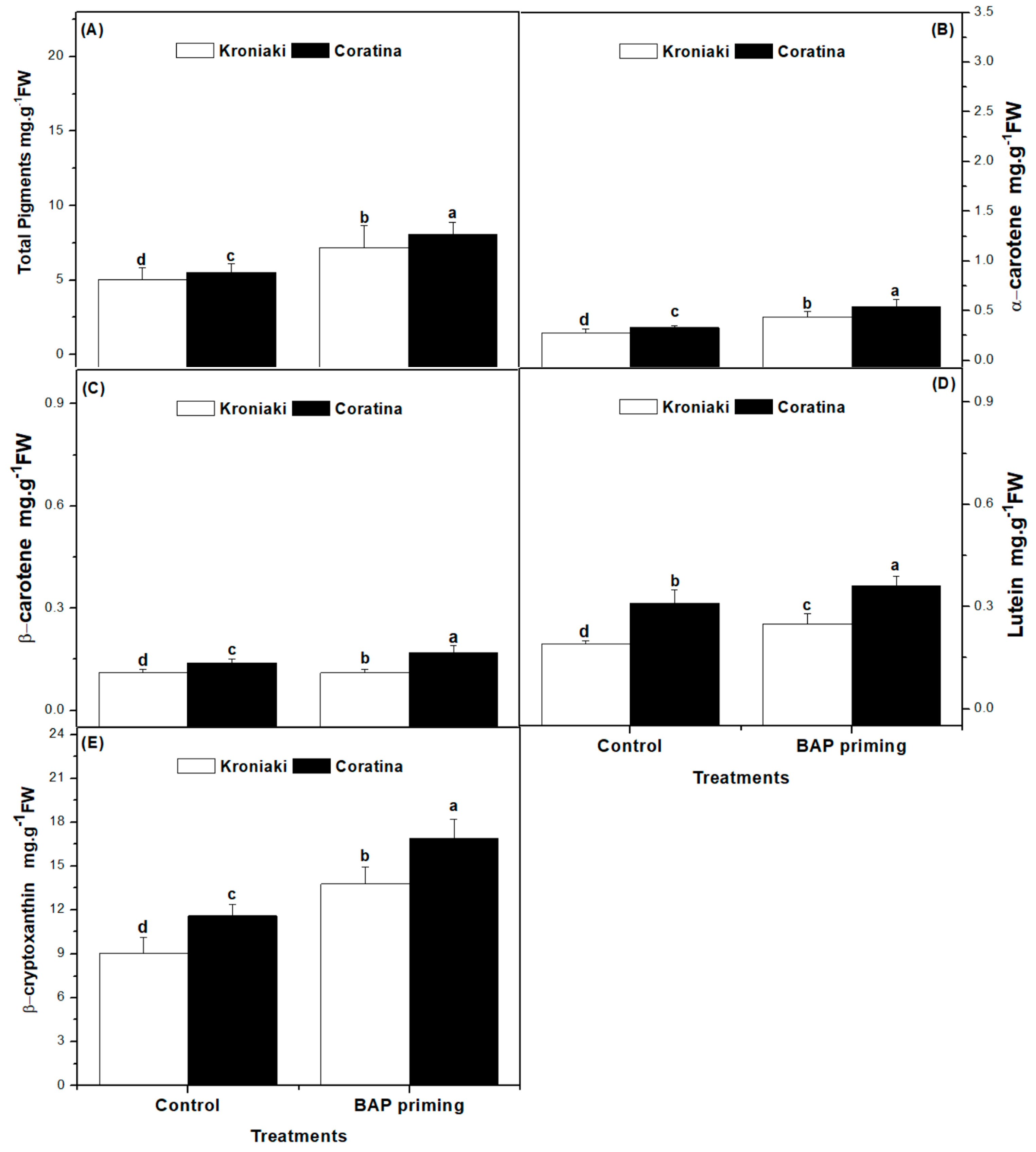
3.2. BAP Differentially Increased the Photosynthetic Pigments in Kroniaki and Coratina Varieties
3.3. BAP Increased Fatty Acids and Lipid Antioxidant Accumulation in Kroniaki and Coratina Varieties
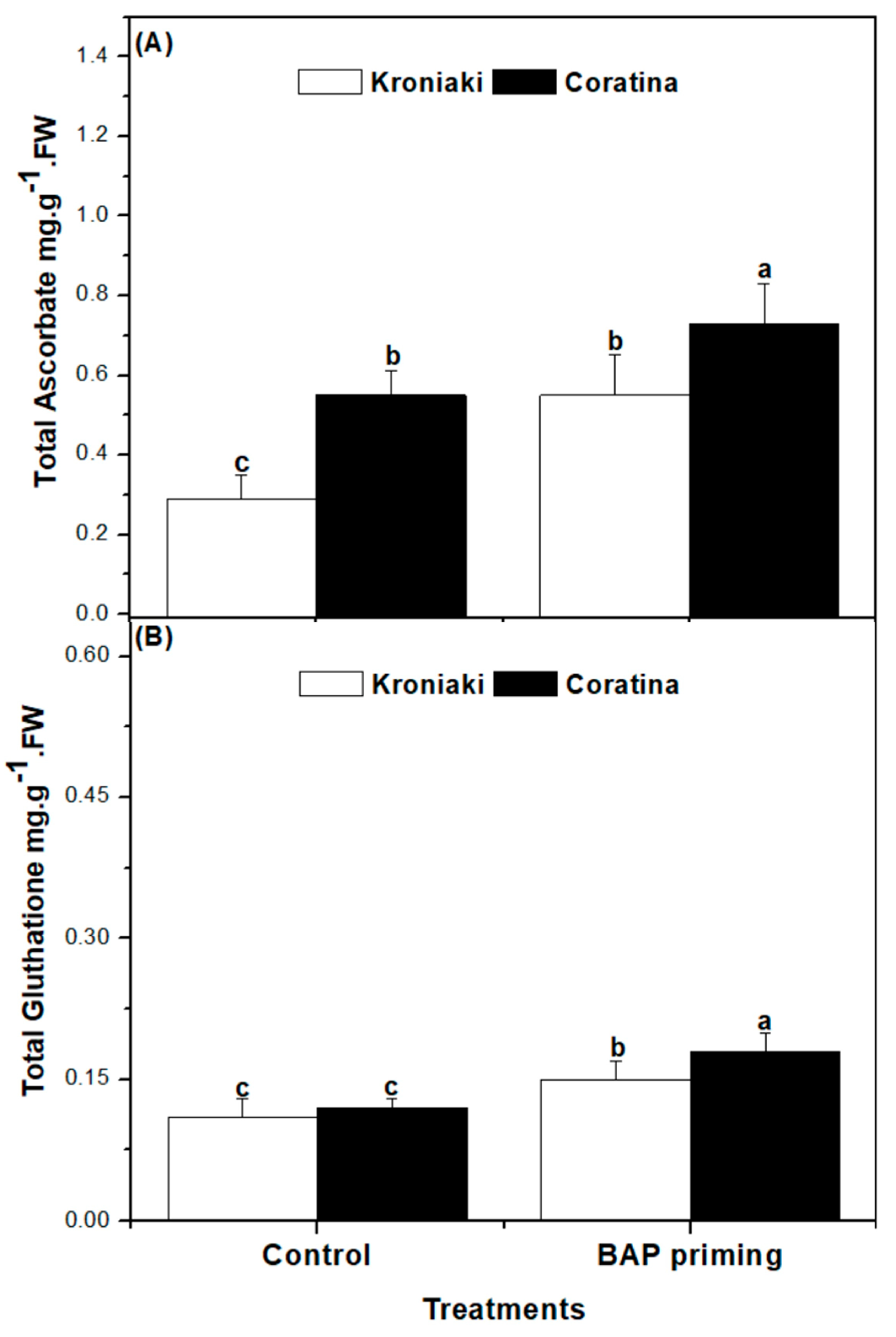
3.4. The Sprouts of BAP-Primed Olive Seeds Accumulated High Level of Water-Soluble Antioxidants
3.5. Anthocyanin Metabolism Induction in the Sprouts of BAP-Primed Olive Seeds
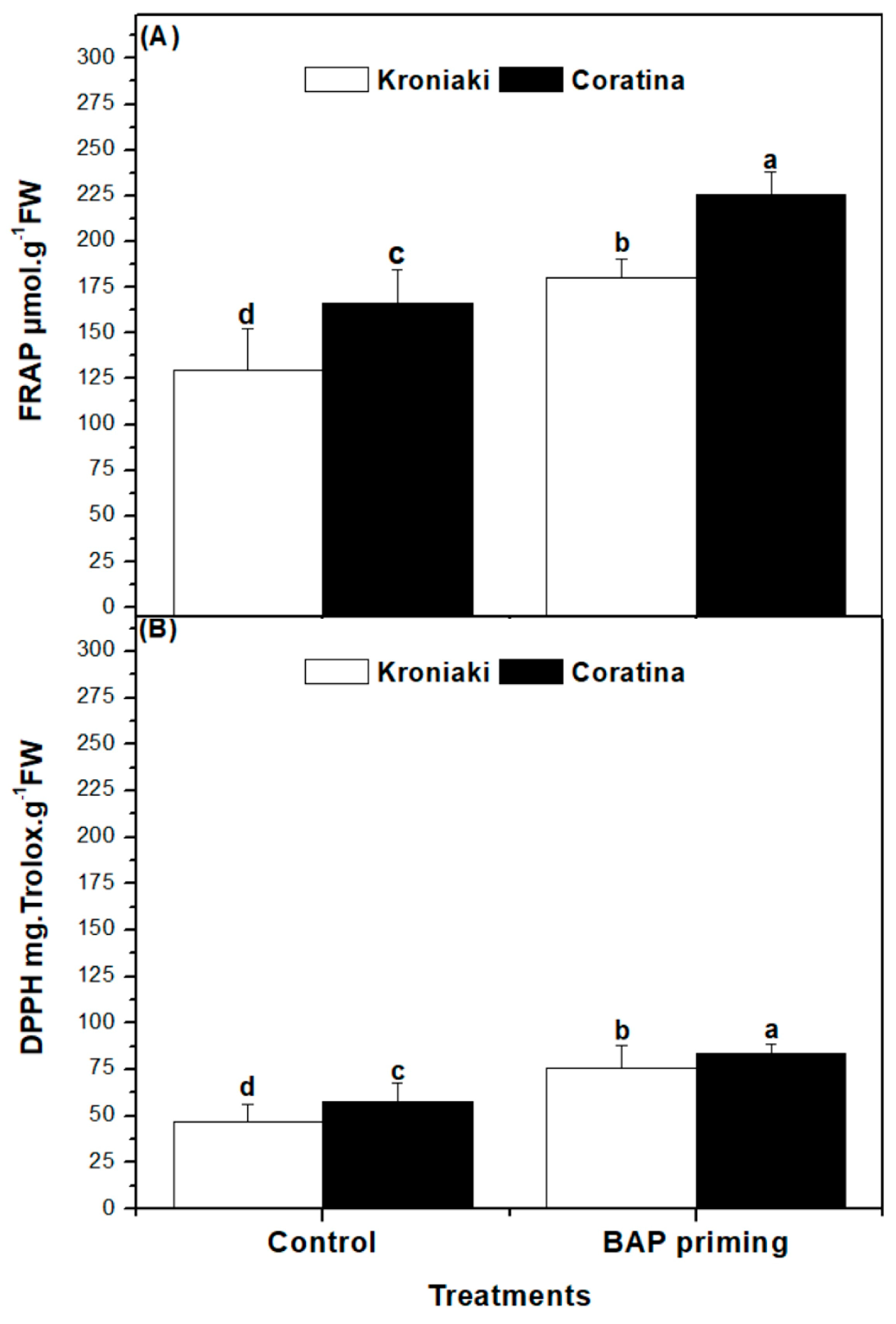
3.6. BAP Priming Effect on Antioxidant Capacity of Olive Sprouts
4. Discussion
4.1. BAP Priming Improved Sprouting Growth
4.2. BAP Priming Improved the Nutritional Values of Olive Sprouts
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Somova, L.I.; Shode, F.O.; Ramnanan, P.; Nadar, A. Antihypertensive, antiatherosclerotic and antioxidant activity of triterpenoids isolated from Olea europaea, subspecies africana leaves. J. Ethnopharmacol. 2003, 84, 299–305. [Google Scholar] [CrossRef]
- Antónia Nunes, M.; Pimentel, F.; Costa, A.S.G.; Alves, R.C.; Oliveira, M.B.P.P. Olive by-products for functional and food applications: Challenging opportunities to face environmental constraints. Innov. Food Sci. Emerg. Technol. 2016, 35, 139–148. [Google Scholar] [CrossRef]
- Frangipane, M.T.; Cecchini, M.; Massantini, R. Extra virgin olive oil from destoned fruits to improve the quality of the oil and environmental sustainability. Foods 2022, 11, 1479. [Google Scholar] [CrossRef]
- Al-qabba, M.M.; El-mowafy, M.A.; Althwab, S.A.; Alfheeaid, H.A. Phenolic profile, antioxidant activity, and ameliorating ffficacy of Chenopodium quinoa sprouts against CCl4-induced oxidative stress in rats. Nutrients 2020, 12, 2904. [Google Scholar] [CrossRef]
- Lal, S.; Ahmed, N.; Srivastava, K.K.; Singh, D.B. Olive (Olea europaea L.) seed germination as affected by different scarification treatments. Afr. J. Agric. Res. 2015, 10, 3570–3574. [Google Scholar] [CrossRef]
- Morales-Sillero, A.; Suárez, M.P.; Jiménez, M.R.; Casanova, L.; Ordovás, J.; Rallo, P. Olive seed germination and initial seedling vigor as influenced by stratification treatment and the female parent. HortScience 2012, 47, 1672–1678. [Google Scholar] [CrossRef]
- Ghassemi-Golezani, K.; Nikpour-Rashidabad, N.; Valizadeh, M. Influence of seed pretreatments parameters of dill under salt stress on growth and yield. J. Biodivers. Environ. Sci. 2016, 9, 448–454. [Google Scholar]
- Maestri, D.; Barrionuevo, D.; Bodoira, R.; Zafra, A.; Jiménez-López, J.; Alché, J. de D. Nutritional profile and nutraceutical components of olive (Olea europaea L.) seeds. J. Food Sci. Technol. 2019, 56, 4359–4370. [Google Scholar] [CrossRef] [PubMed]
- Varmaghany, S.; Rahimi, S.; Karimi Torshizi, M.A.; Lotfollahian, H.; Hassanzadeh, M. Effect of olive leaves on ascites incidence, hematological parameters and growth performance in broilers reared under standard and cold temperature conditions. Anim. Feed. Sci. Technol. 2013, 185, 60–69. [Google Scholar] [CrossRef]
- Zrig, A.; Najar, B.; Magdy Korany, S.; Hassan, A.H.A.; Alsherif, E.A.; Ali Shah, A.; Fahad, S.; Selim, S.; AbdElgawad, H. The interaction effect of laser irradiation and 6-Benzylaminopurine improves the chemical composition and biological activities of Linseed (Linum usitatissimum) sprouts. Biology 2022, 11, 1398. [Google Scholar] [CrossRef] [PubMed]
- Difonzo, G.; Russo, A.; Trani, A.; Paradiso, V.M.; Ranieri, M.; Pasqualone, A.; Summo, C.; Tamma, G.; Silletti, R.; Caponio, F. Green extracts from Coratina olive cultivar leaves: Antioxidant characterization and biological activity. J. Funct. Foods 2017, 31, 63–70. [Google Scholar] [CrossRef]
- Anastasopoulos, E.; Kalogeropoulos, N.; Kaliora, A.C.; Kountouri, A.M.; Andrikopoulos, N.K. Quality indices, polyphenols, terpenic acids, squalene, fatty acid profile, and sterols in virgin olive oil produced by organic versus non-organic cultivation method. WIT Trans. Ecol. Environ. 2011, 152, 135–143. [Google Scholar] [CrossRef]
- Ajouri, A.; Asgedom, H.; Becker, M. Seed priming enhances germination and seedling growth of barley under conditions of P and Zn deficiency. J. Plant Nutr. Soil Sci. 2004, 167, 630–636. [Google Scholar] [CrossRef]
- Singh, K.; Gupta, N.; Dhingra, M. Effect of temperature regimes, seed priming and priming duration on germination and seedling growth on American cotton. J. Environ. Biol. 2018, 39, 83–91. [Google Scholar] [CrossRef]
- Thayer, S.S.; Bjrkman, O. Leaf Xanthophyll content and composition in sun and shade determined by HPLC. Photosynth. Res. 1990, 23, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Hassanpour, H.; Khavari-Nejad, R.A.; Niknam, V.; Razavi, K.; Najafi, F. Effect of penconazole and drought stress on the essential oil composition and gene expression of Mentha pulegium L. (Lamiaceae) at flowering stage. Acta Physiol. Plant. 2014, 36, 1167–1175. [Google Scholar] [CrossRef]
- Zinta, G.; AbdElgawad, H.; Domagalska, M.A.; Vergauwen, L.; Knapen, D.; Nijs, I.; Janssens, I.A.; Beemster, G.T.S.; Asard, H. Physiological, biochemical, and genome-wide transcriptional analysis reveals that elevated CO2 mitigates the impact of combined heat wave and drought stress in Arabidopsis thaliana at multiple organizational levels. Glob. Change Biol. 2014, 20, 3670–3685. [Google Scholar] [CrossRef]
- Potters, G.; Horemans, N.; Bellone, S.; Caubergs, R.J.; Trost, P.; Guisez, Y.; Asard, H. Dehydroascorbate influences the plant cell cycle through a glutathione-independent reduction mechanism. Plant Physiol. 2004, 134, 1479–1487. [Google Scholar] [CrossRef]
- Selim, S.; Abuelsoud, W.; Al-Sanea, M.M.; AbdElgawad, H. Elevated CO2 differently suppresses the arsenic oxide nanoparticles-induced stress in C3 (Hordeum vulgare) and C4 (Zea maize) plants via altered homeostasis in metabolites specifically proline and anthocyanin metabolism. Plant Physiol. Biochem. 2021, 166, 235–245. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP Assay. Anal. Biochem. 1996, 76, 70–76. [Google Scholar] [CrossRef]
- Zrig, A.; Saleh, A.; Sheteiwy, M.S.; Hamouda, F.; Selim, S.; Abdel-Mawgoud, M.; Almuhayawi, M.S.; Okla, M.K.; Abbas, Z.K.; Al-Qahtani, W.H.; et al. Melatonin priming as a promising approach to improve biomass accumulation and the nutritional values of Chenopodium quinoa sprouts: A genotype-based study. Sci. Hort. 2022, 301, 111088. [Google Scholar] [CrossRef]
- Zrig, A.; Saleh, A.; Hamouda, F.; Okla, M.K.; Al-Qahtani, W.H.; Alwasel, Y.A.; Al-Hashimi, A.; Hegab, M.Y.; Hassan, A.H.; AbdElgawad, H. Impact of sprouting under potassium nitrate priming on nitrogen assimilation and bioactivity of three Medicago species. Plants 2021, 11, 71. [Google Scholar] [CrossRef]
- Buta, S.D. Seed priming with potassium nitrate and gibberellic acid enhances the performance of dry direct seeded rice. Agronomy 2021, 11, 849. [Google Scholar]
- Iqbal, M.; Ashraf, M. Presowing seed treatment with cytokinins and its effect on growth, photosynthetic rate, ionic levels and yield of two wheat cultivars differing in salt tolerance. J. Integr. Plant Biol. 2005, 47, 1315–1325. [Google Scholar] [CrossRef]
- Nambiar, N.; Siang, T.C.; Mahmood, M. Effect of 6-Benzylaminopurine on flowering of a Dendrobium orchid. Aust. J. Crop Sci. 2012, 6, 225–231. [Google Scholar]
- Bewley, J.D. Seed germination and dormancy. Plant Cell 1997, 9, 1055–1066. [Google Scholar] [CrossRef] [PubMed]
- Logan, D.C.; Millar, A.H.; Sweetlove, L.J.; Hill, S.A.; Leaver, C.J. Mitochondrial biogenesis during germination in maize embryos. Plant Physiol. 2001, 125, 662–672. [Google Scholar] [CrossRef]
- León-López, L.; Escobar-Zúñiga, Y.; Salazar-Salas, N.Y.; Mora Rochín, S.; Cuevas-Rodríguez, E.O.; Reyes-Moreno, C.; Milán-Carrillo, J. Improving polyphenolic compounds: Antioxidant activity in chickpea sprouts through elicitation with hydrogen peroxide. Foods 2020, 9, 1791. [Google Scholar] [CrossRef]
- León, L.; Uceda, M.; Jiménez, A.; Martín, L.M.; Rallo, L. Variability of fatty acid composition in olive (Olea europaea L.) progenies. Span. J. Agric. Res. 2004, 2, 353. [Google Scholar] [CrossRef]
- Vollár, M.; Feigl, G.; Oláh, D.; Horváth, A.; Molnár, Á.; Kúsz, N.; Ördög, A.; Csupor, D.; Kolbert, Z. Nitro-oleic acid in seeds and differently developed. Plants 2020, 9, 406. [Google Scholar] [CrossRef]
- Polari, J.J.; Crawford, L.M.; Wang, S.C. Cultivar determines fatty acids and phenolics dynamics for olive fruit and oil in super-high-density orchards. Agronomy 2021, 11, 313. [Google Scholar] [CrossRef]
- Contreras, C.; Pierantozzi, P.; Maestri, D.; Tivani, M.; Searles, P.; Brizuela, M.; Fernández, F.; Toro, A.; Puertas, C.; Trentacoste, E.R. How temperatures may affect the synthesis of fatty acids during olive fruit ripening: Genes at work in the field. Plants 2023, 12, 54. [Google Scholar] [CrossRef] [PubMed]
- Gucci, R.; Lombardini, L.; Tattini, M. Analysis of leaf water relations in leaves of two olive (Olea europaea) cultivars differing in tolerance to salinity. Tree Physiol. 1997, 17, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Li, X.; Chen, P.X.; Zhang, B.; Liu, R.; Hernandez, M.; Draves, J.; Marcone, M.F.; Tsao, R. Assessing the fatty acid, carotenoid, and tocopherol compositions of amaranth and quinoa seeds grown in Ontario and their overall contribution to nutritional quality. J. Agric. Food Chem. 2016, 64, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Zeb, A.; Murkovic, M. Olive (Olea europaea L.) Seeds, From chemistry to health benefits. In Nuts and Seeds in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2011; pp. 847–853. [Google Scholar] [CrossRef]
- Shah, T.; Latif, S.; Khan, H.; Munsif, F.; Nie, L. Ascorbic acid priming enhances seed germination and seedling growth of winter wheat under low temperature due to late sowing in Pakistan. Agronomy 2019, 9, 757. [Google Scholar] [CrossRef]
- Saldeen, K.; Saldeen, T. Importance of tocopherols beyond α-tocopherol: Evidence from animal and human studies. Nutr. Res. 2005, 25, 877–889. [Google Scholar] [CrossRef]
- Ergönül, P.G.; Köseoǧlu, O. Changes in α-, β-, γ- And δ-tocopherol contents of mostly consumed vegetable oils during refining process. CYTA-J. Food 2014, 12, 199–202. [Google Scholar] [CrossRef]
- Okla, M.K.; Rubnawaz, S.; Dawoud, T.M.; Al-Amri, S.; El-Tayeb, M.A.; Abdel-Maksoud, M.A.; Akhtar, N.; Zrig, A.; AbdElgayed, G.; AbdElgawad, H. Laser light treatment improves the mineral composition, essential oil production and antimicrobial activity of mycorrhizal treated Pelargonium graveolens. Molecules 2022, 27, 1752. [Google Scholar] [CrossRef]
- Okla, M.K.; El-tayeb, M.A.; Qahtan, A.A.; Abdel-maksoud, M.A.; Elbadawi, Y.B.; Alaskary, M.K.; Balkhyour, M.A.; Hassan, A.H.A. Laser light treatment of seeds for improving the biomass photosynthesis, chemical composition and biological activities of lemongrass sprouts. Agronomy 2021, 11, 478. [Google Scholar] [CrossRef]

| Varieties | Kroniaki | Coratina | ||
|---|---|---|---|---|
| mg.g−1 FW | Control | BAP | Control | BAP |
| Myristic (C14:0) | 1.08 ± 0.07 c | 1.64 ± 0.04 b | 1.69 ± 0.22 b | 2.06 ± 0.04 a |
| Palmitic (C16:0) | 15.13 ± 5.11 b | 27.23 ± 5.02 a | 17.28 ± 8.26 b | 25.92 ± 4.98 a |
| Heptadecanoic (C17:0) | 0.07 ± 0.01 b | 0.07 ± 0.01 b | 0.14 ± 0.01 a | 0.14 ± 0.01 a |
| Stearic (C18:0) | 2.97 ± 0.05 b | 4.3 ± 0.32 a | 3.59 ± 0.52 a | 6.00 ± 0.83 a |
| Arachidic (C20:0) | 1.56 ± 0.29 b | 1.71 ± 0.12 ab | 20.4 ± 0.3 ab | 2.24 ± 0.13 a |
| Docosanoic (C22:0) | 0.86 ± 0.06 b | 1.15 ± 0.08 a | 0.82 ± 0.03 b | 1.47 ± 0.19a |
| Tricosanoic (C23:0) | 0.06 ± 0.02 b | 0.04 ± 0.01 b | 0.12 ± 0.01 a | 0.04 ± 0.01 b |
| Pentacosanoic (C25:0) | 0.01 ± 0.00 a | 0.00 ± 0.00 b | 0.01 ± 0.00 a | 0.01 ± 0.00 a |
| Palmitoleic (C16:1) | 0.13 ± 0.01 b | 0.12 ± 0.03 b | 0.26 ± 0.07 a | 0.26 ± 0.07 a |
| Heptadecenoic (C17:1) | 0.34 ± 0.12 b | 0.44 ± 0.10 a | 0.29 ± 0.03 b | 0.48 ± 0.19 a |
| Oleic (C18:1) | 68.2 ± 2 c | 101.7 ± 4 b | 82.8 ± 4 b | 128.2 ± 19 a |
| Linoleic (C18:2) | 11.7 ± 1.38 b | 19.04 ± 0.51 ab | 11.33 ± 1.15 b | 19.18 ± 1.24 a |
| Linolenic (C18:3 ω−3) | 1.13 ± 0.13 a | 0.05 ± 0.02 c | 1.17 ± 0.26 a | 0.77 ± 0.09 b |
| Arachidonic (C20:4) | ND | ND | 1.11 ± 0.44 b | 2.01 ± 0.32 a |
| Eicosenoic (C20:1) | 0.51 ± 0.05 a | 0.22 ± 0.05 b | ND | ND |
| Varieties | Kroniaki | Coratina | ||
|---|---|---|---|---|
| mg.g−1 FW | Control | BAP | Control | BAP |
| α-tocopherol | 1.93 ± 0.44 b | 2.87 ± 0.21 a | 2.32 ± 0.15 b | 3.39 ± 0.41 a |
| β-tocopherol | 0.29 ± 0.02 b | 0.49 ± 0.04 b | 0.37 ± 0.02 b | 0.60 ± 0.04 a |
| γ-tocopherol | 0.09 ± 0.02 c | 0.08 ± 0.01 c | 0.10 ± 0.00 b | 0.15 ± 0.04 a |
| Total tocopherols | 1.89 ± 0.20 c | 3.42 ± 0.25 a | 2.47 ± 0.27 c | 3.68 ± 0.38 a |
| Varieties | Kroniaki | Coratina | ||
|---|---|---|---|---|
| Control | BAP | Control | BAP | |
| Metabolite level (mg. g−1 FW) | ||||
| Anthocyanin | 1.04 ± 0.39 c | 2.50 ± 0.28 c | 3.29 ± 1.23 b | 6.19 ± 0.13 a |
| Phenylalanine | 1.04 ± 0.12 c | 1.68 ± 0.16 a | 1.30 ± 0.17 b | 1.96 ± 0.24 a |
| Cinnamic acid | 6.04 ± 0.51 b | 9.59 ± 1.23 a | 8.12 ± 1.05 b | 11.44 ± 0.59 a |
| Coumaric acid | 1.89 ± 0.23 b | 1.71 ± 0.17 b | 3.74 ± 0.84 a | 3.63 ± 0.47 a |
| Naringenin | 6.41 ± 0.86 c | 5.38 ± 0.8 b | 9.9 ± 0.54 ab | 9.32 ± 0.41 a |
| Enzyme activity (µmol. mg−1 protein. g−1 FW) | ||||
| Phenylalanine ammonia lyase | 2.81 ± 0.22 b | 1.97 ± 0.5 b | 2.70 ± 0.41 a | 3.22 ± 0.74 a |
| Chalcone synthase | 0.83 ± 0.14 c | 1.09 ± 0.14 a | 1.04 ± 0.14 b | 2.01 ± 0.22 c |
| Cinnamate 4-hydroxylase | 2.75 ± 0.37 b | 4.32 ± 0.25 b | 2.10 ± 0.11 b | 1.23 ± 0.31 a |
| Coenzyme A ligase | 0.40 ± 0.07 b | 0.37 ± 0.08 a | 1.06 ± 0.06 a | 1.48 ± 0.19 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selim, S.; Zrig, A.; Albqmi, M.; M. Al-Sanea, M.; Alnusaire, T.S.; Almuhayawi, M.S.; Jaouni, S.K.A.; Hussein, S.; Warrad, M.; AbdElgawad, H. BAP (6-Benzylaminopurine) Seed-Priming Enhanced Growth, Antioxidant Accumulation and Anthocyanin Metabolism in Olive Sprouts. Horticulturae 2023, 9, 1055. https://doi.org/10.3390/horticulturae9091055
Selim S, Zrig A, Albqmi M, M. Al-Sanea M, Alnusaire TS, Almuhayawi MS, Jaouni SKA, Hussein S, Warrad M, AbdElgawad H. BAP (6-Benzylaminopurine) Seed-Priming Enhanced Growth, Antioxidant Accumulation and Anthocyanin Metabolism in Olive Sprouts. Horticulturae. 2023; 9(9):1055. https://doi.org/10.3390/horticulturae9091055
Chicago/Turabian StyleSelim, Samy, Ahlem Zrig, Mha Albqmi, Mohammad M. Al-Sanea, Taghreed S. Alnusaire, Mohammed S. Almuhayawi, Soad K. Al Jaouni, Shaimaa Hussein, Mona Warrad, and Hamada AbdElgawad. 2023. "BAP (6-Benzylaminopurine) Seed-Priming Enhanced Growth, Antioxidant Accumulation and Anthocyanin Metabolism in Olive Sprouts" Horticulturae 9, no. 9: 1055. https://doi.org/10.3390/horticulturae9091055
APA StyleSelim, S., Zrig, A., Albqmi, M., M. Al-Sanea, M., Alnusaire, T. S., Almuhayawi, M. S., Jaouni, S. K. A., Hussein, S., Warrad, M., & AbdElgawad, H. (2023). BAP (6-Benzylaminopurine) Seed-Priming Enhanced Growth, Antioxidant Accumulation and Anthocyanin Metabolism in Olive Sprouts. Horticulturae, 9(9), 1055. https://doi.org/10.3390/horticulturae9091055






