Combined Use of TiO2 Nanoparticles and Biochar Produced from Moss (Leucobryum glaucum (Hedw.) Ångstr.) Biomass for Chinese Spinach (Amaranthus dubius L.) Cultivation under Saline Stress
Abstract
:1. Introduction
2. Materials and Methods
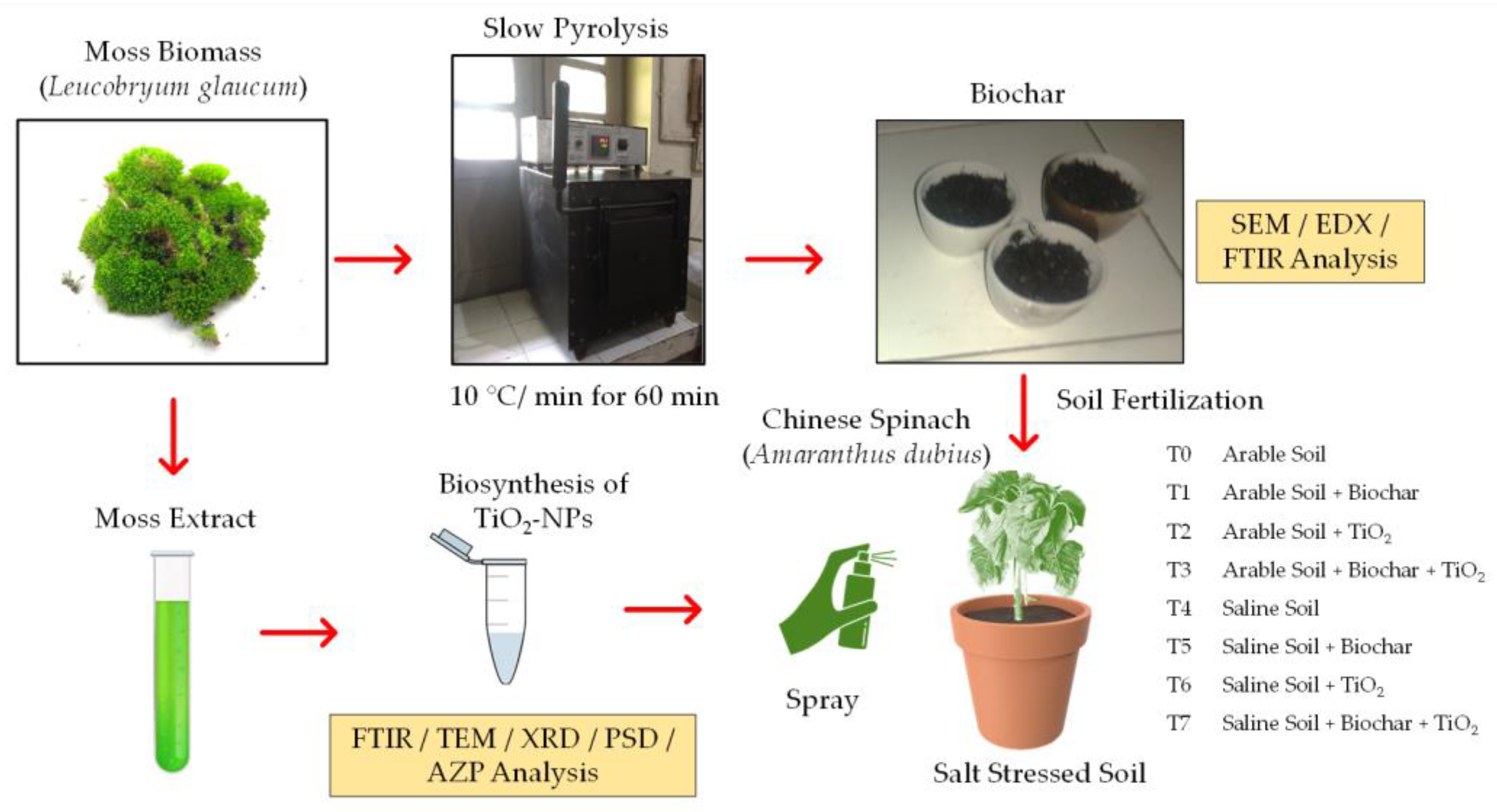
2.1. Collection of Experimental Materials
2.2. Biosynthesis of TiO2 NPs and Biochar Production
2.3. Experimental Design for Chinese Spinach Cultivation
2.4. Growth, Yield, and Biochemical Analyses
2.5. Analytical and Instrumental Methods
2.6. Data Analysis and Software
3. Results and Discussion
3.1. Characteristics of Moss Biomass, Synthesized TiO2 NPs, and Biochar
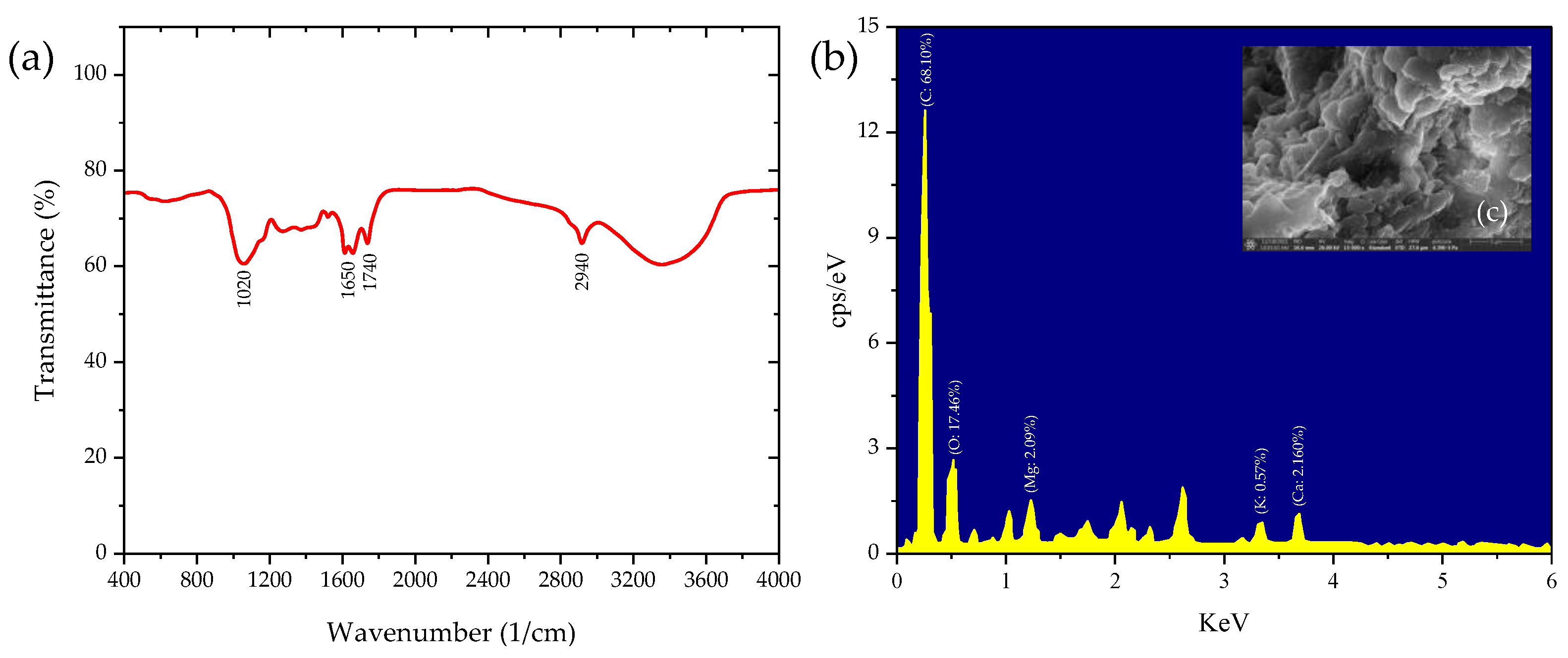
3.1.1. Properties of Moss Biomass
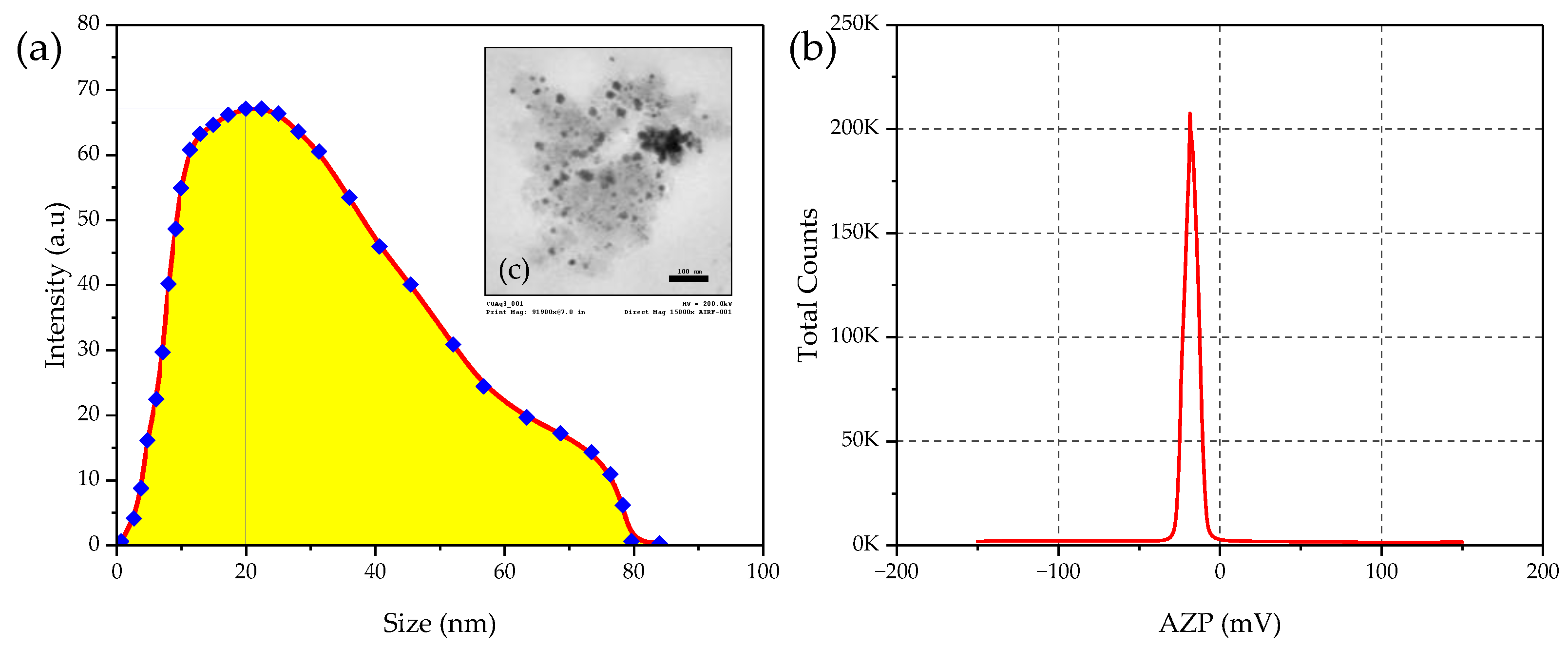
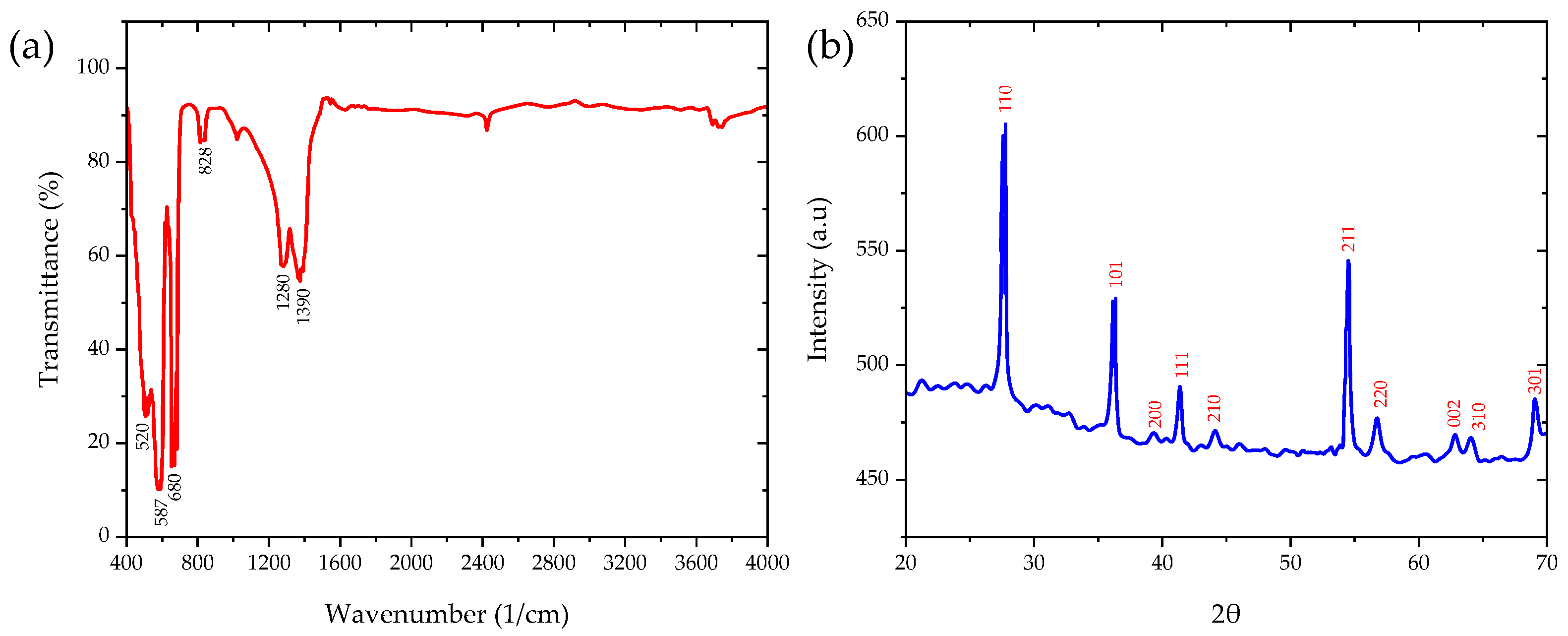
3.1.2. Properties of Synthesized TiO2 NPs
3.1.3. Properties of Produced Biochar
3.2. Effect of TiO2 NPs and Biochar on Growth and Yield of A. dubius
3.3. Effect of TiO2 NPs and Biochar on Biochemical Response of A. dubius
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grande, F.; Tucci, P. Titanium Dioxide Nanoparticles: A Risk for Human Health? Mini-Rev. Med. Chem. 2016, 16, 762–769. [Google Scholar] [CrossRef]
- Shi, H.; Magaye, R.; Castranova, V.; Zhao, J. Titanium Dioxide Nanoparticles: A Review of Current Toxicological Data. Part. Fibre Toxicol. 2013, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Rai, D.B.; Jangid, A.K.; Kulhari, H. Use of Nanotechnology in Antimicrobial Therapy. In Methods in Microbiology; Elsevier: Amsterdam, The Netherlands, 2019; Volume 46, pp. 143–172. ISBN 9780128149928. [Google Scholar]
- Aravind, M.; Amalanathan, M.; Mary, M.S.M. Synthesis of TiO2 Nanoparticles by Chemical and Green Synthesis Methods and Their Multifaceted Properties. SN Appl. Sci. 2021, 3, 409. [Google Scholar] [CrossRef]
- Gonfa, Y.H.; Tessema, F.B.; Bachheti, A.; Tadesse, M.G.; Eid, E.M.; Abou Fayssal, S.; Adelodun, B.; Choi, K.S.; Širić, I.; Kumar, P.; et al. Essential Oil Composition of Aerial Part of Pluchea ovalis (Pers.) DC., Silver Nanoparticles Synthesis, and Larvicidal Activities against Fall Armyworm. Sustainability 2022, 14, 15785. [Google Scholar] [CrossRef]
- Verma, V.; Al-Dossari, M.; Singh, J.; Rawat, M.; Kordy, M.G.M.; Shaban, M. A Review on Green Synthesis of TiO2 NPs: Photocatalysis and Antimicrobial Applications. Polymers 2022, 14, 1444. [Google Scholar] [CrossRef] [PubMed]
- Sett, A.; Gadewar, M.; Sharma, P.; Deka, M.; Bora, U. Green Synthesis of Gold Nanoparticles Using Aqueous Extract of Dillenia indica. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 025005. [Google Scholar] [CrossRef]
- Srujana, S.; Anjamma, M.; Alimuddin; Singh, B.; Dhakar, R.C.; Natarajan, S.; Hechhu, R. A Comprehensive Study on the Synthesis and Characterization of TiO2 Nanoparticles Using Aloe vera Plant Extract and Their Photocatalytic Activity against MB Dye. Adsorpt. Sci. Technol. 2022, 2022, 7244006. [Google Scholar] [CrossRef]
- Roopan, S.M.; Bharathi, A.; Prabhakarn, A.; Abdul Rahuman, A.; Velayutham, K.; Rajakumar, G.; Padmaja, R.D.; Lekshmi, M.; Madhumitha, G. Efficient Phyto-Synthesis and Structural Characterization of Rutile TiO2 Nanoparticles Using Annona squamosa Peel Extract. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2012, 98, 86–90. [Google Scholar] [CrossRef]
- Velayutham, K.; Rahuman, A.A.; Rajakumar, G.; Santhoshkumar, T.; Marimuthu, S.; Jayaseelan, C.; Bagavan, A.; Kirthi, A.V.; Kamaraj, C.; Zahir, A.A.; et al. Evaluation of Catharanthus Roseus Leaf Extract-Mediated Biosynthesis of Titanium Dioxide Nanoparticles against Hippobosca maculata and Bovicola ovis. Parasitol. Res. 2012, 111, 2329–2337. [Google Scholar] [CrossRef]
- Vimala, A.; Sahaya Sathish, S.; Thamizharasi, T.; Palani, R.; Vijayakanth, P.; Kavitha, R. Moss (Bryophyte) Mediated Synthesis and Characterization of Silver Nanoparticles from Campylopus flexuosus (Hedw.) Bird. J. Pharm. Sci. Res. 2017, 9, 292–297. [Google Scholar]
- Akhatova, F.; Konnova, S.; Kryuchkova, M.; Batasheva, S.; Mazurova, K.; Vikulina, A.; Volodkin, D.; Rozhina, E. Comparative Characterization of Iron and Silver Nanoparticles: Extract-Stabilized and Classical Synthesis Methods. Int. J. Mol. Sci. 2023, 24, 9274. [Google Scholar] [CrossRef]
- Hernandez-Soriano, M.C.; Kerré, B.; Kopittke, P.M.; Horemans, B.; Smolders, E. Biochar Affects Carbon Composition and Stability in Soil: A Combined Spectroscopy-Microscopy Study. Sci. Rep. 2016, 6, 25127. [Google Scholar] [CrossRef] [PubMed]
- Širić, I.; Eid, E.M.; Taher, M.A.; El-Morsy, M.H.E.; Osman, H.E.M.; Kumar, P.; Adelodun, B.; Abou Fayssal, S.; Mioč, B.; Andabaka, Ž.; et al. Combined Use of Spent Mushroom Substrate Biochar and PGPR Improves Growth, Yield, and Biochemical Response of Cauliflower (Brassica oleracea var. botrytis): A Preliminary Study on Greenhouse Cultivation. Horticulturae 2022, 8, 830. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar Physicochemical Properties: Pyrolysis Temperature and Feedstock Kind Effects. Rev. Environ. Sci. Biotechnol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Shaaban, A.; Se, S.-M.; Dimin, M.F.; Juoi, J.M.; Mohd Husin, M.H.; Mitan, N.M.M. Influence of Heating Temperature and Holding Time on Biochars Derived from Rubber Wood Sawdust via Slow Pyrolysis. J. Anal. Appl. Pyrolysis 2014, 107, 31–39. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Liu, R. Effects of Pyrolysis Temperature and Heating Time on Biochar Obtained from the Pyrolysis of Straw and Lignosulfonate. Bioresour. Technol. 2015, 176, 288–291. [Google Scholar] [CrossRef]
- Maximize Market Research Amaranth Market: Global Industry Analysis and Forecast (2022–2029). Available online: https://www.maximizemarketresearch.com/market-report/global-amaranth-market/81299/ (accessed on 13 August 2023).
- Raiger, H.L.; Phogat, B.S.; Dua, R.P.; Sharma, S.K. Improved Varieties and Cultivation Practices of Grain Amaranth; Indian Council of Agricultural Research, Krishi Bhavan: New Delhi, India, 2009. [Google Scholar]
- Sarker, U.; Islam, M.T.; Oba, S. Salinity Stress Accelerates Nutrients, Dietary Fiber, Minerals, Phytochemicals and Antioxidant Activity in Amaranthus tricolor Leaves. PLoS ONE 2018, 13, e0206388. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Protein, Dietary Fiber, Minerals, Antioxidant Pigments and Phytochemicals, and Antioxidant Activity in Selected Red Morph Amaranthus Leafy Vegetable. PLoS ONE 2019, 14, e0222517. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Salinity Stress Enhances Color Parameters, Bioactive Leaf Pigments, Vitamins, Polyphenols, Flavonoids and Antioxidant Activity in Selected Amaranthus Leafy Vegetables. J. Sci. Food Agric. 2019, 99, 2275–2284. [Google Scholar] [CrossRef]
- Estrada, Y.; Fernández-Ojeda, A.; Morales, B.; Egea-Fernández, J.M.; Flores, F.B.; Bolarín, M.C.; Egea, I. Unraveling the Strategies Used by the Underexploited Amaranth Species to Confront Salt Stress: Similarities and Differences with Quinoa Species. Front. Plant Sci. 2021, 12, 604481. [Google Scholar] [CrossRef]
- Bellache, M.; Allal Benfekih, L.; Torres-Pagan, N.; Mir, R.; Verdeguer, M.; Vicente, O.; Boscaiu, M. Effects of Four-Week Exposure to Salt Treatments on Germination and Growth of Two Amaranthus Species. Soil. Syst. 2022, 6, 57. [Google Scholar] [CrossRef]
- Hoang, L.H.; De Guzman, C.C.; Cadiz, N.M.; Tran, D.H. Physiological and Phytochemical Responses of Red Amaranth (Amaranthus tricolor L.) and Green Amaranth (Amaranthus dubius L.) to Different Salinity Levels. Legume Res. An. Int. J. 2019, 43, 206–211. [Google Scholar] [CrossRef]
- Sardar, H.; Khalid, Z.; Ahsan, M.; Naz, S.; Nawaz, A.; Ahmad, R.; Razzaq, K.; Wabaidur, S.M.; Jacquard, C.; Širić, I.; et al. Enhancement of Salinity Stress Tolerance in Lettuce (Lactuca sativa L.) via Foliar Application of Nitric Oxide. Plants 2023, 12, 1115. [Google Scholar] [CrossRef] [PubMed]
- Gour, T.; Sharma, A.; Lal, R.; Heikrujam, M.; Gupta, A.; Agarwal, L.K.; Chetri, S.P.K.; Kumar, R.; Sharma, K. Amelioration of the Physio-Biochemical Responses to Salinity Stress and Computing the Primary Germination Index Components in Cauliflower on Seed Priming. SSRN Electron. J. 2022, 9, e14403. [Google Scholar] [CrossRef]
- El-Shal, R.M.; El-Naggar, A.H.; El-Beshbeshy, T.R.; Mahmoud, E.K.; El-Kader, N.I.A.; Missaui, A.M.; Du, D.; Ghoneim, A.M.; El-Sharkawy, M.S. Effect of Nano-Fertilizers on Alfalfa Plants Grown under Different Salt Stresses in Hydroponic System. Agriculture 2022, 12, 1113. [Google Scholar] [CrossRef]
- Zhang, S. Mechanism of Migration and Transformation of Nano Selenium and Mitigates Cadmium Stress in Plants. Master’s Thesis, Shandong University, Jinan, China, 2019. [Google Scholar]
- Francis, D.V.; Sood, N.; Gokhale, T. Biogenic CuO and ZnO Nanoparticles as Nanofertilizers for Sustainable Growth of Amaranthus hybridus. Plants 2022, 11, 2776. [Google Scholar] [CrossRef]
- Cai, Y.; Yuan, B.; Ma, X.; Fang, G.; Zhou, D.; Gao, J. Foliar Application of SiO2 and ZnO Nanoparticles Affected Polycyclic Aromatic Hydrocarbons Uptake of Amaranth (Amaranthus tricolor L.): A Metabolomics and Typical Statistical Analysis. SSRN Electron. J. 2022, 833, 155258. [Google Scholar] [CrossRef]
- Situmeang, Y.P.; Suarta, M.; Irianto, I.K.; Andriani, A.A.S.P.R. Biochar Bamboo Application on Growth and Yield of Red Amaranth (Amaranthus tricolor L). IOP Conf. Ser. Mater. Sci. Eng. 2018, 434, 012231. [Google Scholar] [CrossRef]
- Jiang, S.; Dai, G.; Zhou, J.; Zhong, J.; Liu, J.; Shu, Y. An Assessment of Integrated Amendments of Biochar and Soil Replacement on the Phytotoxicity of Metal(Loid)s in Rotated Radish-Soya Bean-Amaranth in a Mining Acidy Soil. Chemosphere 2022, 287, 132082. [Google Scholar] [CrossRef]
- Daryabeigi Zand, A.; Tabrizi, A.M.; Heir, A.V. Co-Application of Biochar and Titanium Dioxide Nanoparticles to Promote Remediation of Antimony from Soil by Sorghum bicolor: Metal Uptake and Plant Response. Heliyon 2020, 6, e04669. [Google Scholar] [CrossRef]
- González, L.; González-Vilar, M. Determination of Relative Water Content. In Handbook of Plant Ecophysiology Techniques; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2006; pp. 207–212. [Google Scholar]
- Uma, S.; Karthic, R.; Kalpana, S.; Backiyarani, S. A Comparative Assessment of Photosynthetic Pigments and Defense Enzymes in Ex Vitro and In Vitro Propagated Plants of Banana (Musa spp.). Biocatal. Agric. Biotechnol. 2023, 51, 102799. [Google Scholar] [CrossRef]
- Upadhyay, P.; Tewari, A.K.; Punetha, H. Biochemical Estimation of Polyphenol and Peroxidases in Resistant Sources against White Rust Disease of Rapeseed Mustard In-Cited by Albugo candida. Pharma Innov. J. 2023, 12, 4964–4974. [Google Scholar]
- Toor, S.S.; Reddy, H.; Deng, S.; Hoffmann, J.; Spangsmark, D.; Madsen, L.B.; Holm-Nielsen, J.B.; Rosendahl, L.A. Hydrothermal Liquefaction of Spirulina and Nannochloropsis salina under Subcritical and Supercritical Water Conditions. Bioresour. Technol. 2013, 131, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Alpert, P. The Limits and Frontiers of Desiccation-Tolerant Life. Integr. Comp. Biol. 2005, 45, 685–695. [Google Scholar] [CrossRef]
- Hoekstra, F.A.; Golovina, E.A.; Buitink, J. Mechanisms of Plant Desiccation. Trends Plant Sci. 2001, 6, 431–438. [Google Scholar] [CrossRef]
- Ihl, C.; Barboza, P.S. Nutritional Value of Moss for Arctic Ruminants: A Test with Muskoxen. J. Wildl. Manag. 2007, 71, 752–758. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper Enzymes in Isolated Chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Orlov, D.S.; Sadovnikova, L.K. Soil Organic Matter and Protective Functions of Humic Substances in the Bioshere. In Use of Humic Substances to Remediate Polluted Environments: From Theory to Practice; Springer: Dordrecht, The Netherlands, 2005; ISBN 978-1-4020-3252-3. [Google Scholar]
- Ryde, I.; Davie-Martin, C.L.; Li, T.; Naursgaard, M.P.; Rinnan, R. Volatile Organic Compound Emissions from Subarctic Mosses and Lichens. Atmos. Environ. 2022, 290, 119357. [Google Scholar] [CrossRef]
- McCuaig, B.; Dufour, S.C.; Raguso, R.A.; Bhatt, A.P.; Marino, P. Structural Changes in Plastids of Developing Splachnum ampullaceum Sporophytes and Relationship to Odour Production. Plant Biol. 2015, 17, 466–473. [Google Scholar] [CrossRef]
- Resemann, H.C.; Lewandowska, M.; G�mann, J.; Feussner, I. Membrane Lipids, Waxes and Oxylipins in the Moss Model Organism Physcomitrella patens. Plant Cell Physiol. 2019, 60, 1166–1175. [Google Scholar] [CrossRef]
- Zaccone, C.; Miano, T.M.; Shotyk, W. Qualitative Comparison between Raw Peat and Related Humic Acids in an Ombrotrophic Bog Profile. Org. Geochem. 2007, 38, 151–160. [Google Scholar] [CrossRef]
- Klavina, L. Composition of Mosses, Their Metabolites and Environmental Stress Impacts. Ph.D. Thesis, University of Latvia, Riga, Latvia, 2018. [Google Scholar]
- Serk, H.; Nilsson, M.B.; Bohlin, E.; Ehlers, I.; Wieloch, T.; Olid, C.; Grover, S.; Kalbitz, K.; Limpens, J.; Moore, T.; et al. Global CO2 Fertilization of Sphagnum Peat Mosses via Suppression of Photorespiration during the Twentieth Century. Sci. Rep. 2021, 11, 24517. [Google Scholar] [CrossRef] [PubMed]
- Ramdath, S.; Mellem, J.; Mbatha, L.S. Anticancer and Antimicrobial Activity Evaluation of Cowpea-Porous-Starch-Formulated Silver Nanoparticles. J. Nanotechnol. 2021, 2021, 5525690. [Google Scholar] [CrossRef]
- Larue, C.; Castillo-Michel, H.; Sobanska, S.; Trcera, N.; Sorieul, S.; Cécillon, L.; Ouerdane, L.; Legros, S.; Sarret, G. Fate of Pristine TiO2 Nanoparticles and Aged Paint-Containing TiO2 Nanoparticles in Lettuce Crop after Foliar Exposure. J. Hazard. Mater. 2014, 273, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Palocci, C.; Valletta, A.; Chronopoulou, L.; Donati, L.; Bramosanti, M.; Brasili, E.; Baldan, B.; Pasqua, G. Endocytic Pathways Involved in PLGA Nanoparticle Uptake by Grapevine Cells and Role of Cell Wall and Membrane in Size Selection. Plant Cell Rep. 2017, 36, 1917–1928. [Google Scholar] [CrossRef]
- Cai, L.; Cai, L.; Jia, H.; Liu, C.; Wang, D.; Sun, X. Foliar Exposure of Fe3O4 Nanoparticles on Nicotiana benthamiana: Evidence for Nanoparticles Uptake, Plant Growth Promoter and Defense Response Elicitor against Plant Virus. J. Hazard. Mater. 2020, 393, 122415. [Google Scholar] [CrossRef] [PubMed]
- Movasaghi, Z.; Rehman, S.; Ur Rehman, I. Fourier Transform Infrared (FTIR) Spectroscopy of Biological Tissues. Appl. Spectrosc. Rev. 2008, 43, 134–179. [Google Scholar] [CrossRef]
- El-Desoky, M.M.; Morad, I.; Wasfy, M.H.; Mansour, A.F. Synthesis, Structural and Electrical Properties of PVA/TiO2 Nanocomposite Films with Different TiO2 Phases Prepared by Sol–Gel Technique. J. Mater. Sci. Mater. Electron. 2020, 31, 17574–17584. [Google Scholar] [CrossRef]
- Usgodaarachchi, L.; Thambiliyagodage, C.; Wijesekera, R.; Vigneswaran, S.; Kandanapitiye, M. Fabrication of TiO2 Spheres and a Visible Light Active α-Fe2O3/TiO2 -Rutile/TiO2 -Anatase Heterogeneous Photocatalyst from Natural Ilmenite. ACS Omega 2022, 7, 27617–27637. [Google Scholar] [CrossRef]
- Sun, Z.; Dai, L.; Lai, P.; Shen, F.; Shen, F.; Zhu, W. Air Oxidation in Surface Engineering of Biochar-Based Materials: A Critical Review. Carbon Res. 2022, 1, 32. [Google Scholar] [CrossRef]
- Bakshi, S.; Banik, C.; Laird, D.A. Estimating the Organic Oxygen Content of Biochar. Sci. Rep. 2020, 10, 13082. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar Effects on Soil Biota—A Review. Soil. Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar]
- Tomar, R.S.; Kataria, S.; Jajoo, A. Behind the Scene: Critical Role of Reactive Oxygen Species and Reactive Nitrogen Species in Salt Stress Tolerance. J. Agron. Crop Sci. 2021, 207, 577–588. [Google Scholar] [CrossRef]
- Shaheen, S.M.; Mosa, A.; Natasha; Arockiam Jeyasundar, P.G.S.; Hassan, N.E.E.; Yang, X.; Antoniadis, V.; Li, R.; Wang, J.; Zhang, T.; et al. Pros and Cons of Biochar to Soil Potentially Toxic Element Mobilization and Phytoavailability: Environmental Implications. Earth Syst. Environ. 2023, 7, 321–345. [Google Scholar] [CrossRef]
- Gohari, G.; Mohammadi, A.; Akbari, A.; Panahirad, S.; Dadpour, M.R.; Fotopoulos, V.; Kimura, S. Titanium Dioxide Nanoparticles (TiO2 NPs) Promote Growth and Ameliorate Salinity Stress Effects on Essential Oil Profile and Biochemical Attributes of Dracocephalum moldavica. Sci. Rep. 2020, 10, 912. [Google Scholar] [CrossRef]
- Odjegba, V.J.; Chukwunwike, I.C. Physiological Responses of Amaranthus hybridus L. Under Salinity Stress. Niger. J. Life Sci. 2022, 5, 242–252. [Google Scholar] [CrossRef]
- Amukali, O.; Obadoni, B.O.; Mensah, J.K. Effects of Different NaCl Concentrations on Germination and Seedling Growth of Amaranthus hybridus and Celosia argentea. Afr. J. Environ. Sci. Tech. 2015, 9, 301–306. [Google Scholar] [CrossRef]
- Menezes, R.V.; De Azevedo Neto, A.D.; Ribeiro, M.d.O.; Cova, A.M.W. Growth and Contents of Organic and Inorganic Solutes in Amaranth under Salt Stress. Pesqui. Agropecu. Trop. 2017, 47, 22–30. [Google Scholar] [CrossRef]
- Ferreira, J.; Sandhu, D.; Liu, X.; Halvorson, J. Spinach (Spinacea oleracea L.) Response to Salinity: Nutritional Value, Physiological Parameters, Antioxidant Capacity, and Gene Expression. Agriculture 2018, 8, 163. [Google Scholar] [CrossRef]
- Seymen, M. Comparative Analysis of the Relationship between Morphological, Physiological, and Biochemical Properties in Spinach (Spinacea oleracea L.) under Deficit Irrigation Conditions. Turk. J. Agric. For. 2021, 45, 55–67. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive Oxygen Species (ROS) and Response of Antioxidants as ROS-Scavengers during Environmental Stress in Plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Farhangi-Abriz, S.; Torabian, S. Effect of Biochar on Growth and Ion Contents of Bean Plant under Saline Condition. Environ. Sci. Pollut. Res. 2018, 25, 11556–11564. [Google Scholar] [CrossRef] [PubMed]
- Naz, T.; Mazhar Iqbal, M.; Tahir, M.; Hassan, M.M.; Rehmani, M.I.A.; Zafar, M.I.; Ghafoor, U.; Qazi, M.A.; EL Sabagh, A.; Sakran, M.I. Foliar Application of Potassium Mitigates Salinity Stress Conditions in Spinach (Spinacia oleracea L.) through Reducing NaCl Toxicity and Enhancing the Activity of Antioxidant Enzymes. Horticulturae 2021, 7, 566. [Google Scholar] [CrossRef]
- González-García, Y.; Cárdenas-Álvarez, C.; Cadenas-Pliego, G.; Benavides-Mendoza, A.; Cabrera-de-la-Fuente, M.; Sandoval-Rangel, A.; Valdés-Reyna, J.; Juárez-Maldonado, A. Effect of Three Nanoparticles (Se, Si and Cu) on the Bioactive Compounds of Bell Pepper Fruits under Saline Stress. Plants 2021, 10, 217. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Value |
|---|---|
| Moisture content (%) | 63.30 ± 2.04 |
| Dry weight (%) | 36.70 ± 0.31 |
| Crude protein (%) | 7.25 ± 0.07 |
| Volatile matter (%) | 38.15 ± 1.78 |
| Crude lipid (%) | 2.41 ± 0.04 |
| Total ash (%) | 2.97 ± 0.02 |
| Carbon (%) | 49.28 ± 2.60 |
| Nitrogen (%) | 3.10 ± 0.04 |
| Oxygen (%) | 28.05 ± 1.52 |
| Parameters | Treatments | |||||||
|---|---|---|---|---|---|---|---|---|
| T0 | T1 | T2 | T3 | T4 | T5 | T6 | T7 | |
| Fresh weight (g/plant) | 31.10 ± 1.40 b | 34.05 ± 1.07 a | 32.51 ± 1.16 ab | 36.34 ± 2.07 a | 20.52 ± 2.25 d | 22.29 ± 1.09 cd | 24.09 ± 0.64 c | 30.81 ± 0.72 b |
| Dry weight (g/plant) | 5.09 ± 0.15 bc | 5.23 ± 0.09 b | 5.16 ± 0.08 bc | 5.30 ± 0.06 a | 3.42 ± 0.10 e | 3.45 ± 0.03 de | 3.53 ± 0.07 d | 4.90 ± 0.14 c |
| Shoot length (cm) | 30.57 ± 0.90 b | 33.42 ± 1.08 a | 30.90 ± 0.41 b | 33.04 ± 0.57 ab | 17.20 ± 1.01 f | 19.16 ± 0.35 e | 24.75 ± 0.81 d | 28.64 ± 1.30 c |
| Root length (cm) | 14.10 ± 0.41 c | 15.36 ± 0.14 b | 14.28 ± 0.20 c | 16.09 ± 0.24 a | 7.53 ± 0.12 g | 8.10 ± 0.04 f | 11.03 ± 0.09 e | 12.54 ± 0.17 d |
| Leaf number | 19.00 ± 0.56 c | 20.50 ± 0.42 b | 19.70 ± 0.28 c | 22.20 ± 0.35 a | 9.80 ± 0.49 g | 10.20 ± 0.50 fg | 15.40 ± 0.18 e | 17.50 ± 0.44 d |
| Leaf area (cm2/plant) | 13.80 ± 0.35 c | 14.02 ± 0.12 b | 14.07 ± 0.15 b | 17.08 ± 0.07 a | 8.10 ± 0.14 g | 8.95 ± 0.26 f | 10.62 ± 0.31 e | 12.50 ± 0.20 d |
| Parameters | Treatments | |||||||
|---|---|---|---|---|---|---|---|---|
| T0 | T1 | T2 | T3 | T4 | T5 | T6 | T7 | |
| Chlorophyll content (mg/g) | 2.49 ± 0.05 d | 2.53 ± 0.03 c | 2.94 ± 0.05 b | 3.25 ± 0.07 a | 1.13 ± 0.03 h | 1.39 ± 0.04 g | 1.87 ± 0.06 f | 2.36 ± 0.05 e |
| Carotenoids (mg/g) | 3.36 ± 0.07 a | 3.42 ± 0.05 a | 3.40 ± 0.06 a | 3.49 ± 0.03 a | 2.02 ± 0.03 e | 2.23 ± 0.04 d | 2.68 ± 0.03 c | 2.85 ± 0.02 b |
| Relative water content (%) | 87.01 ± 1.14 b | 88.69 ± 0.64 b | 89.05 ± 0.21 ab | 91.40 ± 0.70 a | 72.50 ± 1.63 e | 73.14 ± 0.19 e | 78.32 ± 0.24 d | 82.10 ± 0.86 c |
| SOD (U/mg P) | 2.20 ± 0.03 e | 2.16 ± 0.02 ef | 2.12 ± 0.03 f | 2.04 ± 0.01 g | 4.05 ± 0.04 a | 3.50 ± 0.07 b | 2.98 ± 0.03 c | 2.47 ± 0.05 d |
| POD (µmol/min/mg P) | 38.29 ± 0.58 e | 37.65 ± 0.19 f | 37.08 ± 0.35 fg | 36.59 ± 0.52 g | 58.72 ± 1.29 a | 51.84 ± 0.91 b | 48.01 ± 1.46 c | 40.03 ± 2.49 d |
| CAT (µmol/min/mg P) | 1.90 ± 0.03 e | 1.82 ± 0.02 f | 1.84 ± 0.04 ef | 1.85 ± 0.02 ef | 5.84 ± 0.07 a | 5.10 ± 0.04 b | 4.26 ± 0.10 c | 2.93 ± 0.12 d |
| APX (µmol/min/mg P) | 2.71 ± 0.05 e | 2.66 ± 0.07 ef | 2.64 ± 0.03 f | 2.56 ± 0.04 g | 7.45 ± 0.10 a | 6.57 ± 0.09 b | 4.74 ± 0.04 c | 3.10 ± 0.05 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Širić, I.; Alhag, S.K.; Al-Shuraym, L.A.; Mioč, B.; Držaić, V.; Abou Fayssal, S.; Kumar, V.; Singh, J.; Kumar, P.; Singh, R.; et al. Combined Use of TiO2 Nanoparticles and Biochar Produced from Moss (Leucobryum glaucum (Hedw.) Ångstr.) Biomass for Chinese Spinach (Amaranthus dubius L.) Cultivation under Saline Stress. Horticulturae 2023, 9, 1056. https://doi.org/10.3390/horticulturae9091056
Širić I, Alhag SK, Al-Shuraym LA, Mioč B, Držaić V, Abou Fayssal S, Kumar V, Singh J, Kumar P, Singh R, et al. Combined Use of TiO2 Nanoparticles and Biochar Produced from Moss (Leucobryum glaucum (Hedw.) Ångstr.) Biomass for Chinese Spinach (Amaranthus dubius L.) Cultivation under Saline Stress. Horticulturae. 2023; 9(9):1056. https://doi.org/10.3390/horticulturae9091056
Chicago/Turabian StyleŠirić, Ivan, Sadeq K. Alhag, Laila A. Al-Shuraym, Boro Mioč, Valentino Držaić, Sami Abou Fayssal, Vinod Kumar, Jogendra Singh, Piyush Kumar, Rattan Singh, and et al. 2023. "Combined Use of TiO2 Nanoparticles and Biochar Produced from Moss (Leucobryum glaucum (Hedw.) Ångstr.) Biomass for Chinese Spinach (Amaranthus dubius L.) Cultivation under Saline Stress" Horticulturae 9, no. 9: 1056. https://doi.org/10.3390/horticulturae9091056
APA StyleŠirić, I., Alhag, S. K., Al-Shuraym, L. A., Mioč, B., Držaić, V., Abou Fayssal, S., Kumar, V., Singh, J., Kumar, P., Singh, R., Bachheti, R. K., Goala, M., Kumar, P., & Eid, E. M. (2023). Combined Use of TiO2 Nanoparticles and Biochar Produced from Moss (Leucobryum glaucum (Hedw.) Ångstr.) Biomass for Chinese Spinach (Amaranthus dubius L.) Cultivation under Saline Stress. Horticulturae, 9(9), 1056. https://doi.org/10.3390/horticulturae9091056













