Phytochemical Composition and Content of Red-Fleshed Grape Accessions
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Extraction of Phenolic Compounds
2.3. Qualitative and Quantitative Analysis of Phenolic Compounds
2.4. Statistical Analyses
3. Results and Discussion
3.1. Identification of Phenolic Compounds
3.2. Total Phenolic Compounds
3.3. Anthocyanins
3.4. Flavanols and Flavonols
3.5. Hydroxycinnamic Derivatives and Hydroxybenzoic Acids
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pussa, T.; Floren, J.; Kuldepp, P.; Raal, A. Survey of grapevine Vitis vinifera stem polyphenols by liquid chromatography-diode array detection-tandem mass spectrometry. J. Agric. Food Chem. 2006, 54, 7488–7494. [Google Scholar] [CrossRef] [PubMed]
- Hammer, S.; Winterhalter, P.; Zanglein, M. Model experiments on the influence of single grape components on polyphenol input in mash-fermented white wines. Mitt. Klosterneubg. 2020, 70, 129–147. [Google Scholar]
- Ferreyra, S.; Torres-Palazzolo, C.; Bottini, R.; Camargo, A.; Fontana, A. Assessment of in-vitro bioaccessibility and antioxidant capacity of phenolic compounds extracts recovered from grapevine bunch stem and cane by-products. Food Chem. 2021, 348, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Nesbitt, W.B.; Maness, E.P.; Ballinger, W.E.; Carroll, D.E. Relationship of anthocyanins of black muscadine grapes (Vitis-Rotundifolia Michx) to wine color. Am. J. Enol. Vitic. 1974, 25, 30–32. [Google Scholar] [CrossRef]
- Bridle, P.; Timberlake, C.F. Anthocyanins as natural food colours-selected aspects. Food Chem. 1997, 58, 103–109. [Google Scholar] [CrossRef]
- Hou, D.X. Potential mechanisms of cancer chemoprevention by anthocyanins. Curr. Mol. Med. 2003, 3, 149–159. [Google Scholar] [CrossRef]
- Kelebek, H.; Canbas, A.; Selli, S. HPLC-DAD-MS analysis of anthocyanins in rose wine made from cv. okuzgiozu grapes, and effect of maceration time on anthocyanin content. Chromatographia 2007, 66, 207–212. [Google Scholar] [CrossRef]
- Willemse, C.M.; Stander, M.A.; Vestner, J.; Tredoux, A.G.J.; de Villiers, A. Comprehensive two-dimensional hydrophilic interaction chromatography (HILIC) x reversed-phase liquid chromatography coupled to high-resolution mass spectrometry (RP-LC-UV-MS) analysis of anthocyanins and derived pigments in red wine. Anal. Chem. 2015, 87, 12006–12015. [Google Scholar] [CrossRef]
- Berger, K.; Ostberg-Potthoff, J.J.; Bakuradze, T.; Winterhalter, P.; Richling, E. Carbohydrate hydrolase-inhibitory activity of juice-based phenolic extracts in correlation to their anthocyanin/copigment profile. Molecules 2020, 25, 5224. [Google Scholar] [CrossRef]
- Ge, M.Q.; Zhong, R.; Sadeghnezhad, E.; Hakeem, A.; Xiao, X.; Wang, P.P.; Fang, J.G. Genome-wide identification and expression analysis of magnesium transporter gene family in grape (Vitis vinifera). BMC Plant Biol. 2022, 22, 217. [Google Scholar] [CrossRef]
- Kielhorn, S.; Thorngate, J.H. Oral sensations associated with the flavan-3-ols (+)-catechin and (-)-epicatechin. Food Qual. Prefer. 1999, 10, 109–116. [Google Scholar] [CrossRef]
- Perez-Magarino, S.; Jose, M. Evolution of flavanols, anthocyanins, and their derivatives during the aging of red wines elaborated from grapes harvested at different stages of ripening. J. Agric. Food Chem. 2004, 52, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Aron, P.M.; Kennedy, J.A. Flavan-3-ols: Nature, occurrence and biological activity. Mol. Nutr. Food Res. 2008, 52, 79–104. [Google Scholar] [CrossRef]
- Chen, H.; Yang, J.; Deng, X.; Lei, Y.; Xie, S.; Guo, S.; Ren, R.; Li, J.; Zhang, Z.; Xu, T. Foliar-sprayed manganese sulfate improves flavonoid content in grape berry skin of Cabernet Sauvignon (Vitis vinifera L.) growing on alkaline soil and wine chromatic characteristics. Food Chem. 2020, 314, 126182. [Google Scholar] [CrossRef] [PubMed]
- Assuncao, M.; de Freitas, V.; Paula-Barbosa, M. Grape seed flavanols, but not Port wine, prevent ethanol-induced neuronal lipofuscin formation. Brain Res. 2007, 1129, 72–80. [Google Scholar] [CrossRef]
- Es-Safi, N.E.; Cheynier, V. Flavanols and anthocyanins as potent compounds in the formation of new pigments during storage and aging of red wine. In Red Wine Color: Revealing the Mysteries; Waterhouse, A.L., Kennedy, J.A., Eds.; Acs Symposium Series; American Chemical Society: Washington, DC, USA, 2004; Volume 886, pp. 143–159. [Google Scholar]
- Budic-Leto, I.; Lovric, T. Identification of phenolic acids and changes in their content during fermentation and ageing of white wines Posip and Rukatac. Food Technol. Biotechnol. 2002, 40, 221–225. [Google Scholar]
- Smit, A.; Otero, R.R.C.; Lambrechts, M.G.; Pretorius, I.S.; Van Rensburg, P. Enhancing volatile phenol concentrations in wine by expressing various phenolic acid decarboxylase genes in Saccharomyces cerevisiae. J. Agric. Food Chem. 2003, 51, 4909–4915. [Google Scholar] [CrossRef]
- Amarowicz, R.; Carle, R.; Dongowski, G.; Durazzo, A.; Galensa, R.; Kammerer, D.; Maiani, G.; Piskula, M.K. Influence of postharvest processing and storage on the content of phenolic acids and flavonoids in foods. Mol. Nutr. Food Res. 2009, 53, S151–S183. [Google Scholar] [CrossRef]
- Coklar, H.; Akbulut, M. Changes in phenolic acids, flavonoids, anthocyanins, and antioxidant activities of Mahonia aquifolium berries during fruit development and elucidation of the phenolic biosynthetic pathway. Hortic. Environ. Biotechnol. 2021, 62, 785–794. [Google Scholar] [CrossRef]
- Falcao, A.P.; Chaves, E.S.; Kuskoski, E.M.; Fett, R.; Falcao, L.D.; Bordignon-Luiz, M.T. Total polyphenol index, total anthocyanins and antioxidant activity of a model system of grape jelly. Food Sci. Technol. 2007, 27, 637–642. [Google Scholar] [CrossRef]
- Acquaviva, R.; Russo, A.; Campisi, A.; Sorrenti, V.; Di Giacomo, C.; Barcellona, M.L.; Avitabile, M.; Vanella, A. Antioxidant activity and protective effect on DNA cleavage of resveratrol. J. Food Sci. 2002, 67, 137–141. [Google Scholar] [CrossRef]
- Azmi, A.S.; Bhat, S.H.; Hadi, S.M. Resveratrol-Cu (II) induced DNA breakage in human peripheral lymphocytes: Implications for anticancer properties. FEBS Lett. 2005, 579, 3131–3135. [Google Scholar] [CrossRef] [PubMed]
- Kasdallah-Grissa, A.; Mornagui, B.; Aouani, E.; Hammami, M.; El May, M.; Gharbi, N.; Kamoun, A.; El-Fazaa, S. Resveratrol, a red wine polyphenol, attenuates ethanol-induced oxidative stress in rat liver. Life Sci. 2007, 80, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Bechmann, L.P.; Zahn, D.; Gieseler, R.K.; Fingas, C.D.; Marquitan, G.; Jochum, C.; Gerken, G.; Friedman, S.L.; Canbay, A. Resveratrol amplifies profibrogenic effects of free fatty acids on human hepatic stellate cells. Hepatol. Res. 2009, 39, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Polyphenols and health: Update and perspectives. Arch. Biochem. Biophys. 2010, 501, 2–5. [Google Scholar] [CrossRef]
- Zillich, O.V.; Schweiggert-Weisz, U.; Eisner, P.; Kerscher, M. Polyphenols as active ingredients for cosmetic products. Int. J. Cosmet. Sci. 2015, 37, 455–464. [Google Scholar] [CrossRef]
- Li, Z.M.; Ren, Z.Y.; Zhao, L.; Chen, L.; Yu, Y.; Wang, D.X.; Mao, X.J.; Cao, G.T.; Zhao, Z.L.; Yang, H.S. Unique roles in health promotion of dietary flavonoids through gut microbiota regulation: Current understanding and future perspectives. Food Chem. 2023, 399, 133959. [Google Scholar] [CrossRef]
- Liang, Z.C.; Owens, C.L.; Zhong, G.Y.; Cheng, L.L. Polyphenolic profiles detected in the ripe berries of Vitis vinifera germplasm. Food Chem. 2011, 129, 940–950. [Google Scholar] [CrossRef]
- Liang, Z.C.; Yang, Y.Z.; Cheng, L.L.; Zhong, G.Y. Polyphenolic composition and content in the ripe berries of wild Vitis species. Food Chem. 2012, 132, 730–738. [Google Scholar] [CrossRef]
- Ivanova, V.; Stefova, M.; Vojnoski, B.; Dornyei, A.; Mark, L.; Dimovska, V.; Stafilov, T.; Kilar, F. Identification of polyphenolic compounds in red and white grape varieties grown in R. Macedonia and changes of their content during ripening. Food Res. Int. 2011, 44, 2851–2860. [Google Scholar] [CrossRef]
- Yue, Q.Y.; Xu, L.L.; Xiang, G.Q.; Yu, X.; Yao, Y.X. Characterization of gene expression profile, phenolic composition, and antioxidant capacity in red-fleshed grape berries and their wines. J. Agric. Food Chem. 2018, 66, 7190–7199. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.W.; Wang, F.; Zhang, X.Q.; Li, B.; Yao, Y.X. Characterization of anthocyanin and nonanthocyanidin phenolic compounds and/or their biosynthesis pathway in red-fleshed ‘Kanghong’ grape berries and their wine. Food Res. Int. 2022, 161, 111789. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.; Li, J.H.; Fan, P.G.; Chen, S.; Fang, J.B.; Li, S.H.; Wu, B.H. Anthocyanin accumulation in various organs of a teinturier cultivar (Vitis vinifera L.) during the growing season. Am. J. Enol. Vitic. 2012, 63, 177–184. [Google Scholar] [CrossRef]
- Robinson, J. (Ed.) Jancis Robinson’s Guide to Wine Grapes; Oxford University Press: Oxford, UK, 1996. [Google Scholar]
- Stafne, E.T. ‘Rubaiyat’ and Oklahoma’s wine grape legacy. J. Am. Pomol. Soc. 2006, 60, 159–163. [Google Scholar]
- Santiago, J.L.; Gonzalez, I.; Gago, P.; Alonso-Villaverde, V.; Boso, S.; Martinez, M.C. Identification of and relationships among a number of teinturier grapevines that expanded across Europe in the early 20th century. Aust. J. Grape Wine Res. 2008, 14, 223–229. [Google Scholar] [CrossRef]
- Castillo-Muñoz, N.; Fernández-González, M.; Gómez-Alonso, S.; García-Romero, E.; Hermosín-Gutiérrez, I. Red-color related phenolic composition of Garnacha Tintorera (Vitis vinifera L.) grapes and red wines. J. Agric. Food Chem. 2009, 57, 7883–7891. [Google Scholar] [CrossRef] [PubMed]
- Ageorges, A.; Fernandez, L.; Vialet, S.; Merdinoglu, D.; Terrier, N.; Romieu, C. Four specific isogenes of the anthocyanin metabolic pathway are systematically co-expressed with the red colour of grape berries. Plant Sci. 2006, 170, 372–383. [Google Scholar] [CrossRef]
- Balik, J.; Kumsta, M. Evaluation of colour content in grapes originating from South Moravia. Czech J. Food Sci. 2008, 26, S18–S24. [Google Scholar] [CrossRef]
- He, F.; Mu, L.; Yan, G.L.; Liang, N.N.; Pan, Q.H.; Wang, J.; Reeves, M.J.; Duan, C.Q. Biosynthesis of anthocyanins and their regulation in colored grapes. Molecules 2010, 15, 9057–9091. [Google Scholar] [CrossRef]
- Xie, S.; Song, C.Z.; Wang, X.J.; Liu, M.Y.; Zhang, Z.W.; Xi, Z.M. Tissue-specific expression analysis of anthocyanin biosynthetic genes in white- and red-Fleshed grape cultivars. Molecules 2015, 20, 22767–22780. [Google Scholar] [CrossRef]
- Tian, M.B.; Yuan, L.; Zheng, M.Y.; Xi, Z.M. Differences in anthocyanin accumulation profiles between teinturier and non-teinturier cultivars during ripening. Foods 2021, 10, 1073. [Google Scholar] [CrossRef] [PubMed]
- Korosi, L.; Molnar, S.; Teszlak, P.; Dornyei, A.; Maul, E.; Topfer, R.; Marosvolgyi, T.; Szabo, E.; Rockel, F. Comparative study on grape berry anthocyanins of various teinturier varieties. Foods 2022, 11, 3668. [Google Scholar] [CrossRef] [PubMed]
- He, J.J.; Liu, Y.X.; Pan, Q.H.; Cui, X.Y.; Duan, C.Q. Different anthocyanin profiles of the skin and the pulp of Yan73 (Muscat Hamburg x Alicante Bouschet) grape berries. Molecules 2010, 15, 1141–1153. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.K.; Wang, Y.; Gao, X.T.; Yang, X.H.; He, F.; Duan, C.Q.; Wang, J. Flavonoid and aromatic profiles of two Vitis vinifera L. teinturier grape cultivars. Aust. J. Grape Wine Res. 2018, 24, 379–389. [Google Scholar] [CrossRef]
- Schwarz, M.; Picazo-Bacete, J.J.; Winterhalter, P.; Hermosin-Gutierrez, I. Effect of copigments and grape cultivar on the color of red wines fermented after the addition of copigments. J. Agric. Food Chem. 2005, 53, 8372–8381. [Google Scholar] [CrossRef]
- Rockel, F.; Moock, C.; Braun, U.; Schwander, F.; Cousins, P.; Maul, E.; Topfer, R.; Hausmann, L. Color intensity of the red-fleshed berry phenotype of Vitis vinifera teinturier grapes varies due to a 408bp duplication in the promoter of VvmybA1. Genes 2020, 11, 891. [Google Scholar] [CrossRef]
- Sikuten, I.; Stambuk, P.; Andabaka, Z.; Tomaz, I.; Markovic, Z.; Stupic, D.; Maletic, E.; Kontic, J.K.; Preiner, D. Grapevine as a rich source of polyphenolic compounds. Molecules 2020, 25, 5604. [Google Scholar] [CrossRef]
- Kong, J.H.; Wu, J.; Guan, L.; Hilbert, G.; Delrot, S.; Fan, P.G.; Liang, Z.C.; Wu, B.H.; Matus, J.T.; Gomes, E.; et al. Metabolite analysis reveals distinct spatio-temporal accumulation of anthocyanins in two teinturier variants of cv. ‘Gamay’ grapevines (Vitis vinifera L.). Planta 2021, 253, 84. [Google Scholar] [CrossRef]
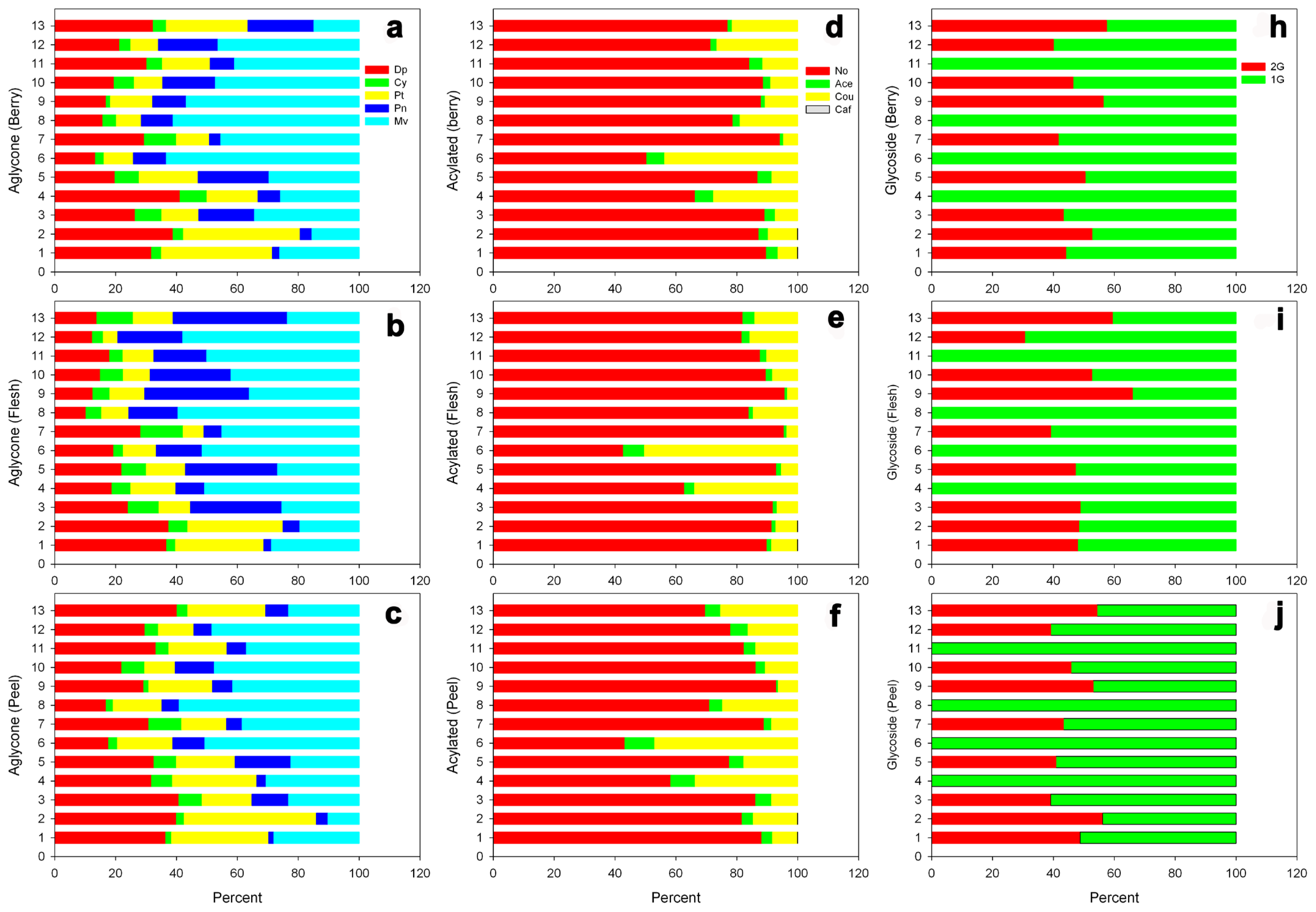
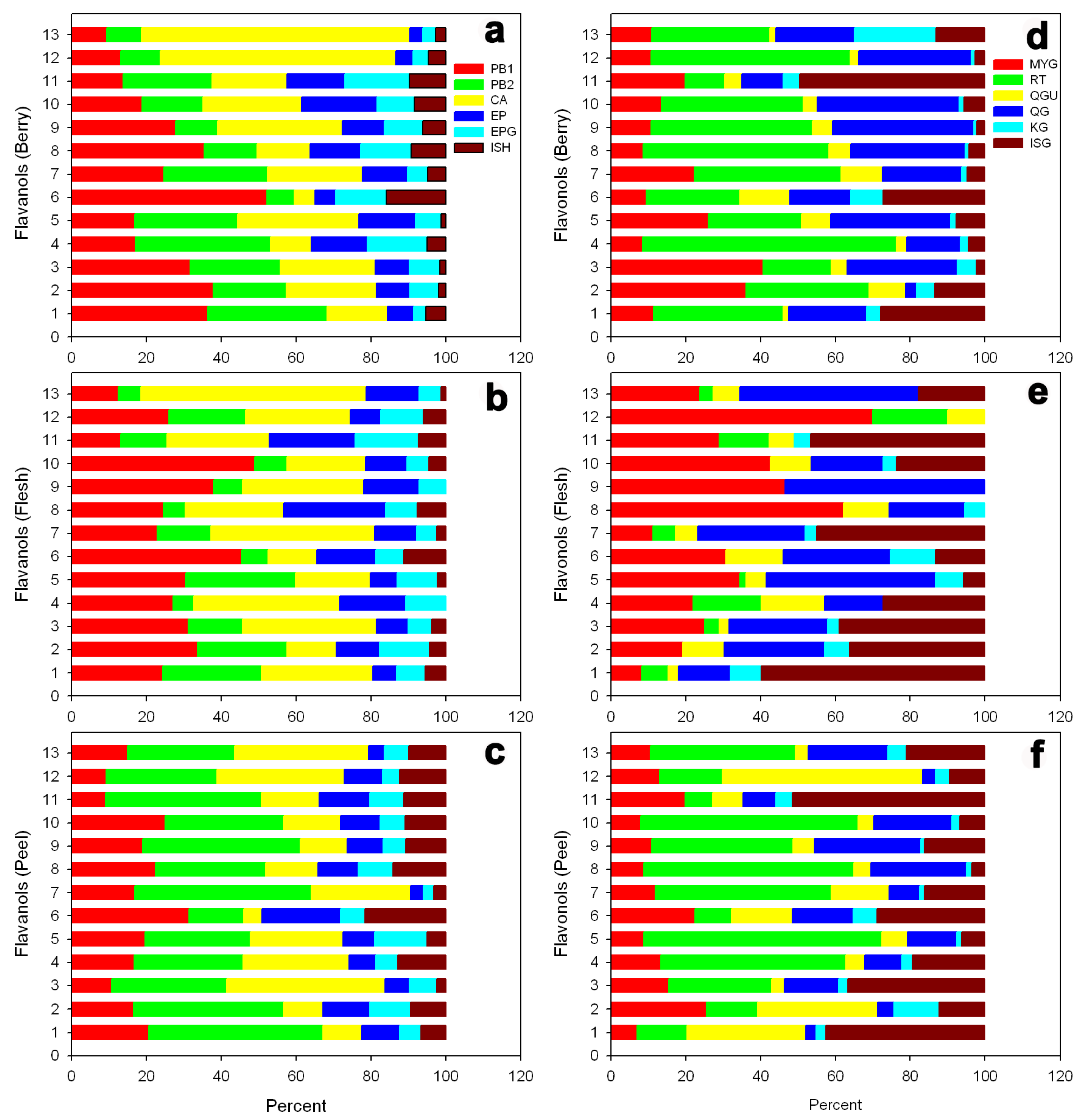
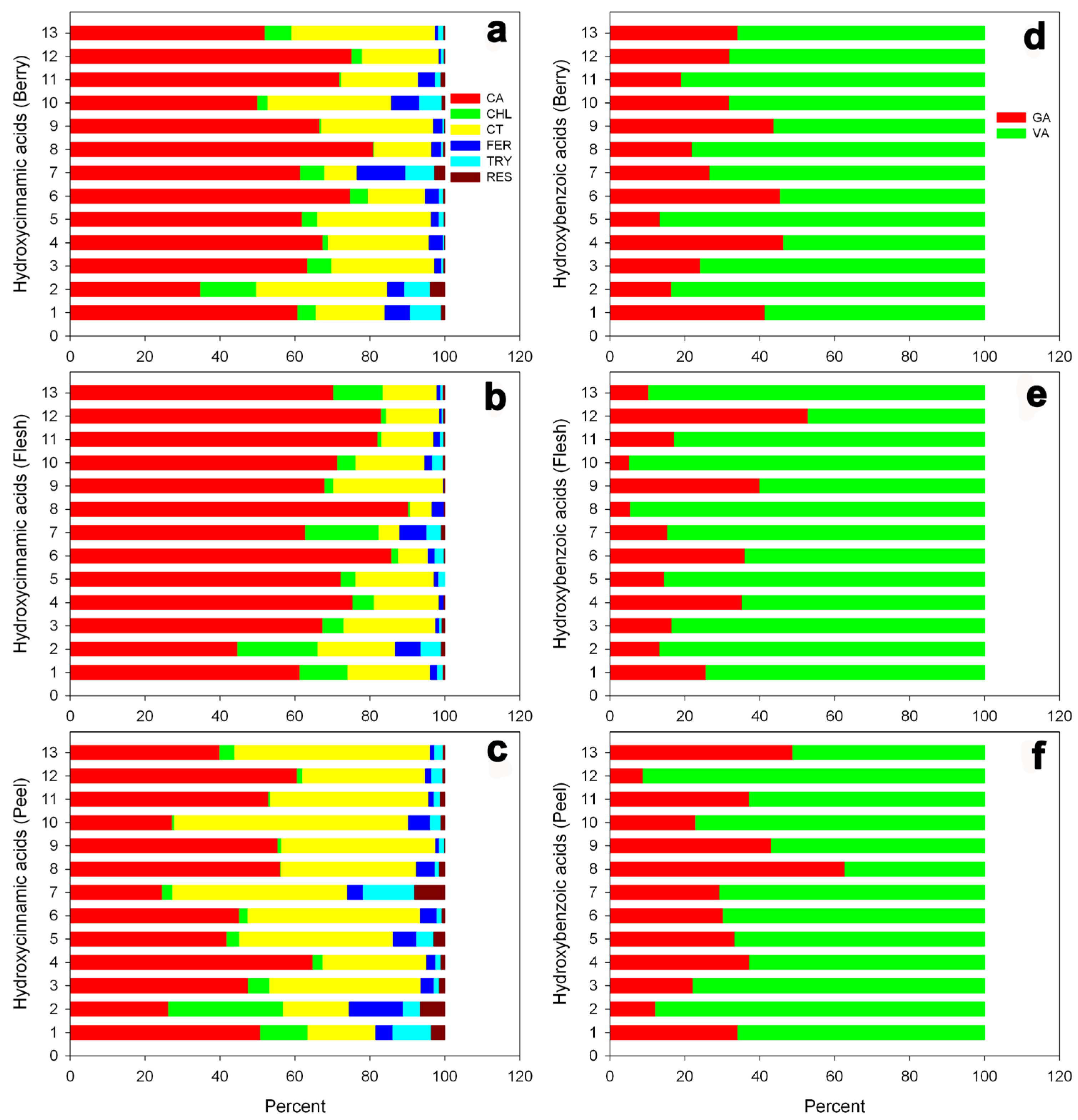
| No | Cultivar Name | Accession ID (PI No.) | Pedigree | Origin |
|---|---|---|---|---|
| 1 | Sori | 588103 a | V. acerifolia x V. riparia | Unknown |
| 2 | Pulliat | 588190 | Herbemont seedling, bourq., vinifera (all according to Loomis card), note Loomis does not show riparia for Pulliat | United States |
| 3 | Bailey Alicante | 588361 | Bailey Alicante | Unknown |
| 4 | NY 65.548.3 | 588521 | Ill 791-1 (Jaeger 70 x Victoria’s Choice) x Ill 820-1 (Ill 271-1 x Black Monukka) | United States |
| 5 | Bailey Alicante A | 588579 | Bailey x Alicante Bouschet | Japan |
| 6 | Agria | 588670 | Unknown | Hungary |
| 7 | GVIT 1392 | 588681 | V. rupestris x V. vulpina | United States |
| 8 | Landot 234 | 597158 | Seibel 5455 x Gamay Mourot | Unknown |
| 9 | Seibel 6339 | 597175 | Seibel 867 x Seibel 2524–V. cinerea, V. labrusca, V. lincecumii, V. riparia, V. rupestris, V. vinifera | France |
| 10 | Seibel 4646 | 597193 | Seibel 2508 x Seibel 880–V. aestivalis, V. cinerea, V. rupestris, V. vinifera | France |
| 11 | HN 12 | 597259 | Seibel 10878 x Couderc 299-35 | Unknown |
| 12 | Rubaiyat | 597289 | Seibel 5437 x Bailey | United States |
| 13 | Seibel 5437 | GVIT1616 | Seibel 867 x Seibel 2512 | France |
| No | RT (min) | Molecular Ion M (m/z) | Fragment Ions M (m/z) | Absorbance Maxima (nm) | Identity | Berry (mg g−1 FW) | Flesh (mg g−1 FW) | Peel (mg g−1 FW) |
|---|---|---|---|---|---|---|---|---|
| 1 | 4.13 | 170 | 170 | 280 | Gallic acid | 0.006 ± 0.001 b * | 0.003 ± 0.001 c | 0.011 ± 0.002 a |
| 2 | 15.94 | 168 | 168 | 280 | Vanillic acid | 0.013 ± 0.002 b | 0.015 ± 0.003 b | 0.033 ± 0.008 a |
| 3 | 6.95 | 578 | 577 | 280 | Procyanidin B1 | 0.111 ± 0.013 b | 0.061 ± 0.006 c | 0.147 ± 0.016 a |
| 4 | 9.63 | 290 | 290 | 276 | Catechin | 0.121 ± 0.016 b | 0.068 ± 0.010 c | 0.207 ± 0.026 a |
| 5 | 16.34 | 290 | 289 | 280 | Epicatechin | 0.044 ± 0.005 b | 0.026 ± 0.004 c | 0.084 ± 0.011 a |
| 6 | 23.3 | 442 | 290 | 280 | Epicatechin gallate | 0.036 ± 0.004 b | 0.018 ± 0.002 c | 0.067 ± 0.011 a |
| 7 | 35.96 | 316 | 315 | 280 | Isorhamnetin | 0.020 ± 0.001 b | 0.008 ± 0.001 c | 0.08 ± 0.012 a |
| 8 | 39.59 | 161 | 161 | 280 | Tryptophol | 0.010 ± 0.001 b | 0.004 ± 0.000 c | 0.025 ± 0.001 a |
| 9 | 10.34 | 578 | 577 | 280 | Procyanidin B2 | 0.092 ± 0.008 b | 0.033 ± 0.006 c | 0.307 ± 0.036 a |
| 10 | 7.9 | 312 | 180 | 318 | Caftaric acid | 0.461 ± 0.025 a | 0.254 ± 0.020 b | 0.518 ± 0.073 a |
| 11 | 11.56 | 296 | 164 | 331 | Coutaric acid | 0.172 ± 0.011 b | 0.057 ± 0.012 c | 0.418 ± 0.068 a |
| 12 | 11.83 | 354 | 354 | 320 | Chlorogenic acid | 0.020 ± 0.003 a | 0.017 ± 0.002 a | 0.029 ± 0.003 a |
| 13 | 26.98 | 194 | 194 | 320 | Ferulic acid | 0.023 ± 0.001 b | 0.006 ± 0.001 c | 0.032 ± 0.004 a |
| 14 | 41.76 | 228 | 228 | 318 | Resveratrol | 0.003 ± 0.000 b | 0.001 ± 0.000 c | 0.013 ± 0.002 a |
| 15 | 38.34 | 449 | 287 | 320 | Kaempferol 3-O-glucoside | 0.005 ± 0.004 b | 0.002 ± 0.001 c | 0.011 ± 0.007 a |
| 16 | 23.6 | 480 | 318 | 365 | Myricetin 3-O-glucoside | 0.025 ± 0.001 b | 0.009 ± 0.000 c | 0.048 ± 0.002 a |
| 17 | 29.42 | 610 | 609 | 365 | Rutin | 0.062 ± 0.002 b | 0.002 ± 0.000 c | 0.134 ± 0.029 a |
| 18 | 30.65 | 478 | 302 | 356 | Quercetin 3-O-glucuronide | 0.008 ± 0.001 b | 0.002 ± 0.000 c | 0.051 ± 0.006 a |
| 19 | 31.78 | 464 | 302 | 355 | Quercetin 3-O-glucoside | 0.032 ± 0.003 b | 0.009 ± 0.001 c | 0.049 ± 0.007 a |
| 20 | 39.95 | 479 | 317 | 350 | Isorhamnetin 3-O-glucoside | 0.017 ± 0.001 b | 0.010 ± 0.001 c | 0.075 ± 0.009 a |
| 21 | 9.51 | 627 | 303, 465 | 282, 520 | Delphinidin 3-O-glucoside-5-O-glucoside | 0.730 ± 0.031 b | 0.284 ± 0.045 c | 2.911 ± 0.325 a |
| 22 | 12.64 | 611 | 287, 449 | 282, 516 | Cyanidin 3-O-glucoside-5-O-glucoside | 0.15 ± 0.009 b | 0.083 ± 0.011 c | 0.492 ± 0.081 a |
| 23 | 14.22 | 465 | 303 | 280, 523 | Delphinidin 3-O-glucoside | 2.299 ± 0.098 b | 0.876 ± 0.072 c | 7.26 ± 0.451 a |
| 24 | 14.86 | 641 | 317, 479 | 274, 523 | Petunidin 3-O-glucoside-5-O-glucoside | 0.781 ± 0.036 b | 0.286 ± 0.030 c | 2.29 ± 0.201 a |
| 25 | 17.85 | 449 | 287 | 279, 515 | Cyanidin 3-O-glucoside | 0.325 ± 0.025 b | 0.218 ± 0.023 b | 0.655 ± 0.064 a |
| 26 | 18.53 | 625 | 301, 463 | 278, 513 | Peonidin 3-O-glucoside-5-O-glucoside | 0.58 ± 0.025 b | 0.471 ± 0.068 b | 1.292 ± 0.159 a |
| 27 | 20.31 | 479 | 317 | 277, 526 | Petunidin 3-O-glucoside | 1.614 ± 0.060 b | 0.849 ± 0.048 c | 4.216 ± 0.341 a |
| 28 | 20.53 | 655 | 331, 493 | 275, 524 | Malvidin 3-O-glucoside-5-O-glucoside | 1.474 ± 0.069 b | 0.452 ± 0.052 c | 4.358 ± 0.257 a |
| 29 | 23.82 | 653 | 287, 449, 611 | 280, 516 | Cyanidin 3-O-(6-O-acetyl)-glucoside-5-O-glucoside | 0.003 ± 0.000 a | 0 ± 0.000 b | 0.005 ± 0.000 a |
| 30 | 24.58 | 463 | 301 | 279, 515 | Peonidin 3-O-glucoside | 0.445 ± 0.027 b | 0.331 ± 0.038 b | 0.859 ± 0.084 a |
| 31 | 25.83 | 683 | 317, 479, 641 | 280, 530 | Petunidin 3-O-(6-O-acetyl)-glucoside-5-O-glucoside | 0.013 ± 0.001 b | 0.004 ± 0.001 c | 0.038 ± 0.007 a |
| 32 | 27.05 | 493 | 331 | 278, 530 | Malvidin 3-O-glucoside | 1.571 ± 0.055 b | 0.65 ± 0.057 c | 4.564 ± 0.253 a |
| 33 | 27.79 | 773 | 287, 611 | 281, 525 | Cyanidin 3-O-(6-O-caffeoyl)-glucoside-5-O-glucoside | 0.002 ± 0.000 b | 0.001 ± 0.000 b | 0.006 ± 0.001 a |
| 34 | 29.75 | 507 | 303, 465 | 280, 521 | Delphinidin 3-O-(6-O-acetyl)-glucoside | 0.078 ± 0.003 b | 0.018 ± 0.002 c | 0.263 ± 0.034 a |
| 35 | 33.42 | 491 | 287 | 279, 514 | Cyanidin 3-O-(6-O-acetyl)-glucoside | 0.014 ± 0.001 b | 0.007 ± 0.002 c | 0.084 ± 0.011 a |
| 36 | 34.37 | 773 | 303, 465, 627 | 279, 530 | Delphinidin 3-O-(6-O-coumaryl)-glucoside-5-O-glucoside | 0.133 ± 0.009 b | 0.049 ± 0.009 b | 0.565 ± 0.027 a |
| 37 | 36.5 | 521 | 317, 479 | 280, 530 | Petunidin 3-O-(6-O-acetyl)-glucoside | 0.045 ± 0.002 b | 0.007 ± 0.001 c | 0.229 ± 0.017 a |
| 38 | 38.2 | 757 | 287, 449, 611 | 280, 524 | Cyanidin 3-O-(6-O-coumaryl)-glucoside-5-O-glucoside | 0.013 ± 0.001 b | 0.008 ± 0.001 c | 0.083 ± 0.010 a |
| 39 | 39.74 | 505 | 301 | 280, 518 | Peonidin 3-O-(6-O-acetyl)-glucoside | 0.062 ± 0.003 b | 0.014 ± 0.002 c | 0.205 ± 0.025 a |
| 40 | 41.12 | 773 | 303, 465, 627 | 282, 530 | Delphinidin 3-O-(6-O-coumaryl)-glucoside | 0.305 ± 0.025 b | 0.065 ± 0.007 c | 1.005 ± 0.084 a |
| 41 | 42.4 | 535 | 331, 493 | 280, 521 | Malvidin 3-O-(6-O-acetyl)-glucoside | 0.203 ± 0.016 b | 0.044 ± 0.007 c | 0.894 ± 0.067 a |
| 42 | 43.38 | 783 | 317, 479, 641 | 280, 530 | Petunidin 3-O-(6-O-coumaryl)-glucoside-5-O-glucoside | 0.039 ± 0.004 b | 0.016 ± 0.003 c | 0.143 ± 0.021 a |
| 43 | 43.95 | 771 | 301, 463, 625 | 279, 520 | Peonidin 3-O-(6-O-coumaryl)-glucoside-5-O-glucoside | 0.061 ± 0.002 b | 0.02 ± 0.004 c | 0.232 ± 0.046 a |
| 44 | 45.23 | 801 | 331, 493,655 | 280, 530 | Malvidin 3-O-(6-O-coumaryl)-glucoside-5-O-glucoside | 0.132 ± 0.008 b | 0.052 ± 0.008 c | 0.654 ± 0.088 a |
| 45 | 46.98 | 595 | 287, 449 | 283, 522 | Cyanidin 3-O-(6-O-coumaryl)-glucoside | 0.194 ± 0.012 b | 0.047 ± 0.004 c | 0.553 ± 0.063 a |
| 46 | 48.78 | 625 | 317, 479 | 280, 531 | Petunidin 3-O-(6-O-coumaryl)-glucoside | 0.203 ± 0.012 b | 0.074 ± 0.007 c | 1.140 ± 0.131 a |
| 47 | 51.35 | 609 | 301, 463 | 279, 523 | Peonidin 3-O-(6-O-coumaryl)-glucoside | 0.078 ± 0.008 b | 0.031 ± 0.002 c | 0.224 ± 0.026 a |
| 48 | 52.59 | 639 | 331, 493 | 280, 521 | Malvidin 3-O-(6-O-coumaryl)-glucoside | 0.510 ± 0.032 b | 0.148 ± 0.014 c | 1.705 ± 0.156 a |
| Accession ID | Tissue | Total Phenolic Compounds | Anthocyanins | Flavanols | Flavonols | Hydroxycinnamic Derivatives | Hydroxybenzoic Acids |
|---|---|---|---|---|---|---|---|
| 588103 | Berry | 17.893 ± 0.093 c * | 17.157 ± 0.120 c | 0.479 ± 0.015 bc | 0.067 ± 0.003 e | 0.177 + 0.009 f | 0.015 ± 0.001 cd |
| 588190 | Berry | 29.875 ± 0.882 a | 28.290 ± 0.739 a | 1.108 ± 0.136 a | 0.221 ± 0.027 b | 0.221 ± 0.027 ef | 0.037 ± 0.009 b |
| 588361 | Berry | 14.571 ± 0.279 d | 13.390 ± 0.240 d | 0.482 ± 0.011 bc | 0.085 ± 0.001 de | 0.606 ± 0.031 c | 0.007 ± 0.000 d |
| 588521 | Berry | 23.377 ± 0.072 b | 19.612 ± 0.047 b | 0.549 ± 0.033 b | 0.431 ± 0.020 a | 2.723 ± 0.073 a | 0.063 ± 0.006 a |
| 588579 | Berry | 15.235 ± 0.426 d | 14.055 ± 0.422 d | 0.437 ± 0.019 bc | 0.134 ± 0.010 cd | 0.596 ± 0.024 c | 0.013 ± 0.001 d |
| 588670 | Berry | 8.882 ± 0.176 f | 7.603 ± 0.115 f | 0.240 ± 0.004 cd | 0.150 ± 0.008 c | 0.874 ± 0.068 b | 0.017 ± 0.002 cd |
| 588681 | Berry | 14.997 ± 0.626 d | 14.297 ± 0.614 d | 0.497 ± 0.013 bc | 0.064 ± 0.003 e | 0.109 ± 0.006 f | 0.030 ± 0.004 bc |
| 597158 | Berry | 6.074 ± 0.033 g | 4.804 ± 0.001 g | 0.162 ± 0.007 d | 0.206 ± 0.005 b | 0.889 ± 0.043 b | 0.013 ± 0.000 d |
| 597175 | Berry | 9.101 ± 0.035 f | 8.021 ± 0.025 f | 0.155 ± 0.003 d | 0.122 ± 0.002 cd | 0.800 ± 0.016 b | 0.004 ± 0.000 d |
| 597193 | Berry | 8.473 ± 0.059 f | 7.591 ± 0.086 f | 0.282 ± 0.010 bcd | 0.143 ± 0.004 c | 0.445 ± 0.014 cd | 0.012 ± 0.001 d |
| 597259 | Berry | 11.868 ± 0.709 e | 10.853 ± 0.697 e | 0.332 ± 0.004 bcd | 0.141 ± 0.010 c | 0.526 ± 0.023 cd | 0.017 ± 0.000 cd |
| 597289 | Berry | 4.795 ± 0.218 g | 3.790 ± 0.217 g | 0.290 ± 0.075 bcd | 0.110 ± 0.001 cde | 0.599 ± 0.048 c | 0.006 ± 0.001 d |
| GVIT1616 | Berry | 8.192 ± 0.480 f | 7.235 ± 0.405 f | 0.509 ± 0.115 bc | 0.060 ± 0.006 e | 0.378 ± 0.042 de | 0.010 ± 0.002 d |
| 588103 | Flesh | 12.593 ± 0.352 a | 12.030 ± 0.356 a | 0.313 ± 0.016 abc | 0.036 ± 0.004 cd | 0.206 ± 0.019 bcd | 0.009 ± 0.001 b |
| 588190 | Flesh | 9.725 ± 0.521 b | 9.266 ± 0.502 b | 0.279 ± 0.008 abcd | 0.030 ± 0.004 cde | 0.130 ± 0.009 d | 0.021 ± 0.002 ab |
| 588361 | Flesh | 8.042 ± 0.550 b | 7.248 ± 0.558 c | 0.342 ± 0.018 ab | 0.086 ± 0.003 a | 0.339 ± 0.006 abcd | 0.027 ± 0.015 ab |
| 588521 | Flesh | 1.960 ± 0.105 d | 1.388 ± 0.090 e | 0.158 ± 0.018 def | 0.019 ± 0.001 ef | 0.387 ± 0.035 abc | 0.009 ± 0.002 b |
| 588579 | Flesh | 8.709 ± 0.776 b | 8.048 ± 0.733 bc | 0.227 ± 0.017 bcdef | 0.061 ± 0.006 b | 0.361 ± 0.029 abcd | 0.012 ± 0.002 b |
| 588670 | Flesh | 3.137 ± 0.234 cd | 2.514 ± 0.202 de | 0.108 ± 0.002 f | 0.023 ± 0.002 def | 0.479 ± 0.030 a | 0.013 ± 0.001 b |
| 588681 | Flesh | 8.015 ± 0.119 b | 7.364 ± 0.019 c | 0.395 ± 0.087 a | 0.058 ± 0.004 b | 0.172 ± 0.015 cd | 0.026 ± 0.000 ab |
| 597158 | Flesh | 2.690 ± 0.007 cd | 2.114 ± 0.017 de | 0.110 ± 0.011 ef | 0.019 ± 0.001 ef | 0.420 ± 0.018 ab | 0.028 ± 0.006 ab |
| 597175 | Flesh | 3.502 ± 0.439 cd | 2.729 ± 0.341 de | 0.177 ± 0.018 cdef | 0.015 ± 0.003 ef | 0.574 ± 0.154 a | 0.005 ± 0.001 b |
| 597193 | Flesh | 4.218 ± 0.186 c | 3.771 ± 0.195 d | 0.192 ± 0.008 cdef | 0.028 ± 0.001 cde | 0.210 ± 0.023 bcd | 0.019 ± 0.005 ab |
| 597259 | Flesh | 3.379 ± 0.430 cd | 2.838 ± 0.418 de | 0.127 ± 0.006 ef | 0.041 ± 0.003 c | 0.358 ± 0.030 abcd | 0.016 ± 0.002 b |
| 597289 | Flesh | 3.941 ± 0.150 c | 3.286 ± 0.184 d | 0.096 ± 0.013 f | 0.010 ± 0.000 f | 0.546 ± 0.022 a | 0.004 ± 0.000 b |
| GVIT1616 | Flesh | 4.321 ± 0.420 c | 3.796 ± 0.401 d | 0.246 ± 0.021 bcde | 0.021 ± 0.002 def | 0.213 ± 0.007 bcd | 0.044 ± 0.004 a |
| 588103 | Peel | 51.018 ± 3.111 abc | 49.412 ± 3.029 abc | 0.905 ± 0.055 bcde | 0.239 ± 0.013 bc | 0.435 ± 0.055 de | 0.030 ± 0.001 b |
| 588190 | Peel | 52.996 ± 3.657 ab | 51.803 ± 3.606 ab | 0.719 ± 0.024 cdef | 0.220 ± 0.026 c | 0.209 ± 0.002 e | 0.045 ± 0.008 b |
| 588361 | Peel | 43.618 ± 3.516 bc | 40.646 ± 3.227 bcd | 1.390 ± 0.130 a | 0.449 ± 0.055 abc | 1.104 ± 0.204 bcd | 0.029 ± 0.005 b |
| 588521 | Peel | 41.124 ± 0.683 bcd | 38.102 ± 0.758 cde | 1.092 ± 0.101 abc | 0.413 ± 0.066 abc | 1.470 ± 0.057 abc | 0.047 ± 0.010 b |
| 588579 | Peel | 59.293 ± 2.954 a | 56.561 ± 2.723 a | 1.304 ± 0.074 ab | 0.540 ± 0.111 a | 0.857 ± 0.065 cde | 0.031 ± 0.005 b |
| 588670 | Peel | 28.100 ± 2.014 ef | 25.970 ± 1.892 fgh | 0.604 ± 0.031 def | 0.218 ± 0.025 c | 1.249 ± 0.145 abcd | 0.058 ± 0.010 b |
| 588681 | Peel | 42.104 ± 2.504 bc | 40.252 ± 2.371 cd | 1.137 ± 0.088 abc | 0.460 ± 0.067 abc | 0.202 ± 0.007 e | 0.052 ± 0.006 b |
| 597158 | Peel | 17.067 ± 0.377 f | 15.138 ± 2.371 h | 0.492 ± 0.015 ef | 0.428 ± 0.023 abc | 0.992 ± 0.082 bcde | 0.017 ± 0.003 b |
| 597175 | Peel | 25.213 ± 2.363 f | 22.426 ± 2.136 gh | 0.438 ± 0.039 f | 0.258 ± 0.040 bc | 2.080 ± 0.234 a | 0.013 ± 0.002 b |
| 597193 | Peel | 21.269 ± 1.060 f | 19.468 ± 0.875 gh | 0.513 ± 0.073 ef | 0.398 ± 0.065 abc | 0.837 ± 0.100 cde | 0.052 ± 0.004 b |
| 597259 | Peel | 39.093 ± 2.258 cde | 35.337 ± 1.694 def | 1.405 ± 0.153 a | 0.497 ± 0.051 ab | 1.800 ± 0.430 ab | 0.055 ± 0.016 b |
| 597289 | Peel | 60.182 ± 3.097 a | 57.515 ± 3.081 a | 0.958 ± 0.013 bcd | 0.450 ± 0.039 abc | 1.122 ± 0.086 bcd | 0.135 ± 0.050 a |
| GVIT1616 | Peel | 29.343 ± 1.657 def | 27.382 ± 1.385 efg | 0.630 ± 0.127 def | 0.220 ± 0.030 c | 1.101 ± 0.195 bcd | 0.011 ± 0.001 b |
| Berry | Flesh | Peel | |
|---|---|---|---|
| Total phenolic compounds | 13.333 ± 1.992 b * | 5.710 ± 0.915 c | 39.263 ± 3.949 a |
| Anthocyanins | 12.054 ± 1.893 b | 5.107 ± 0.915 b | 36.924 ± 3.936 a |
| Flavanols | 0.425 ± 0.068 b | 0.213 ± 0.027 c | 0.891 ± 0.097 a |
| Flavonols | 0.149 ± 0.027 b | 0.034 ± 0.006 c | 0.368 ± 0.033 a |
| Hydroxycinnamic derivatives | 0.688 ± 0.183 ab | 0.338 ± 0.040 b | 1.035 ± 0.155 a |
| Hydroxybenzoic acids | 0.019 ± 0.004 b | 0.018 ± 0.003 b | 0.044 ± 0.009 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, L.; Yang, Y.; Zhong, G.-Y.; Liang, Z.; Cheng, L. Phytochemical Composition and Content of Red-Fleshed Grape Accessions. Horticulturae 2023, 9, 579. https://doi.org/10.3390/horticulturae9050579
Lu L, Yang Y, Zhong G-Y, Liang Z, Cheng L. Phytochemical Composition and Content of Red-Fleshed Grape Accessions. Horticulturae. 2023; 9(5):579. https://doi.org/10.3390/horticulturae9050579
Chicago/Turabian StyleLu, Lizhen, Yingzhen Yang, Gan-Yuan Zhong, Zhenchang Liang, and Lailiang Cheng. 2023. "Phytochemical Composition and Content of Red-Fleshed Grape Accessions" Horticulturae 9, no. 5: 579. https://doi.org/10.3390/horticulturae9050579
APA StyleLu, L., Yang, Y., Zhong, G.-Y., Liang, Z., & Cheng, L. (2023). Phytochemical Composition and Content of Red-Fleshed Grape Accessions. Horticulturae, 9(5), 579. https://doi.org/10.3390/horticulturae9050579





