Ectopic Expression of AGAMOUS-like 18 from Litchi (Litchi chinensis Sonn.) Delayed the Floral Organ Abscission in Arabidopsis
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatments
2.2. Isolation of Genes, Sequence Alignment, and qRT-PCR
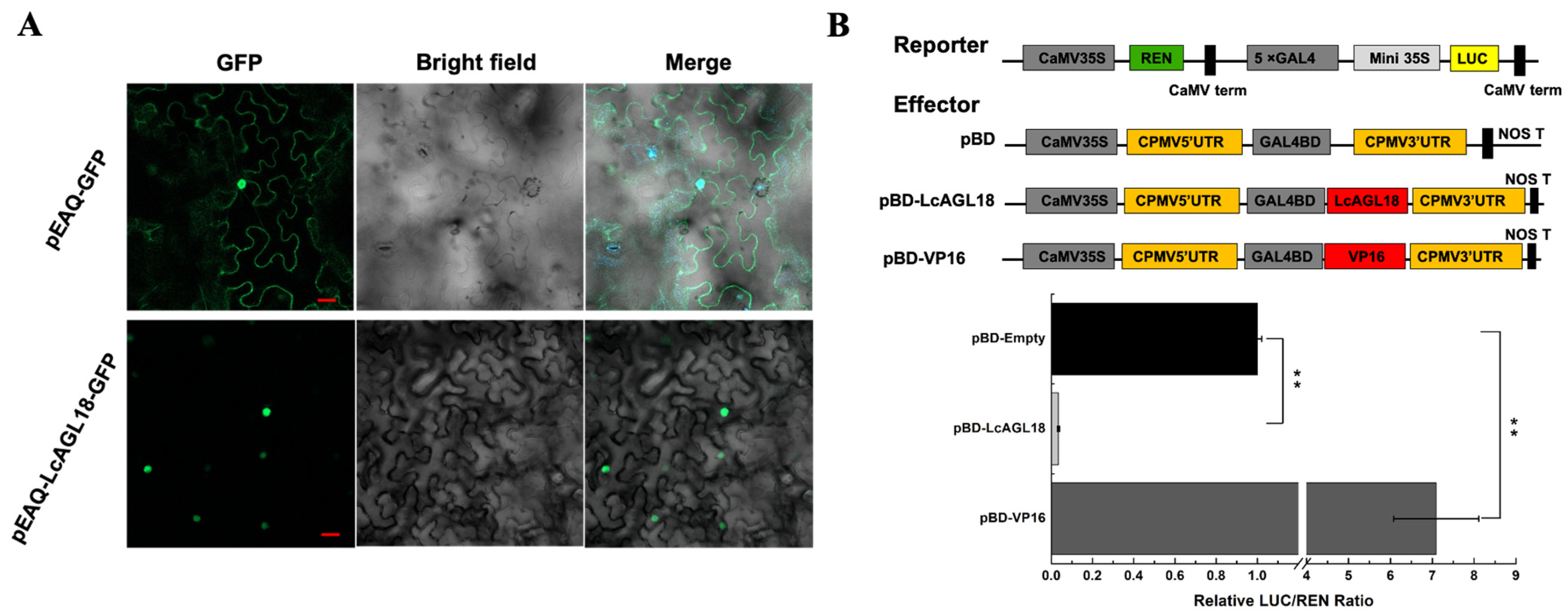
2.3. Subcellular Compartmentation of LcAGL18
2.4. BCECF Fluorescence Assay
2.5. Histochemical GUS Assays
2.6. Scanning Electron Microscopy
2.7. Transcriptional Activity Assays
2.8. Data Analysis
2.9. Primers
3. Results
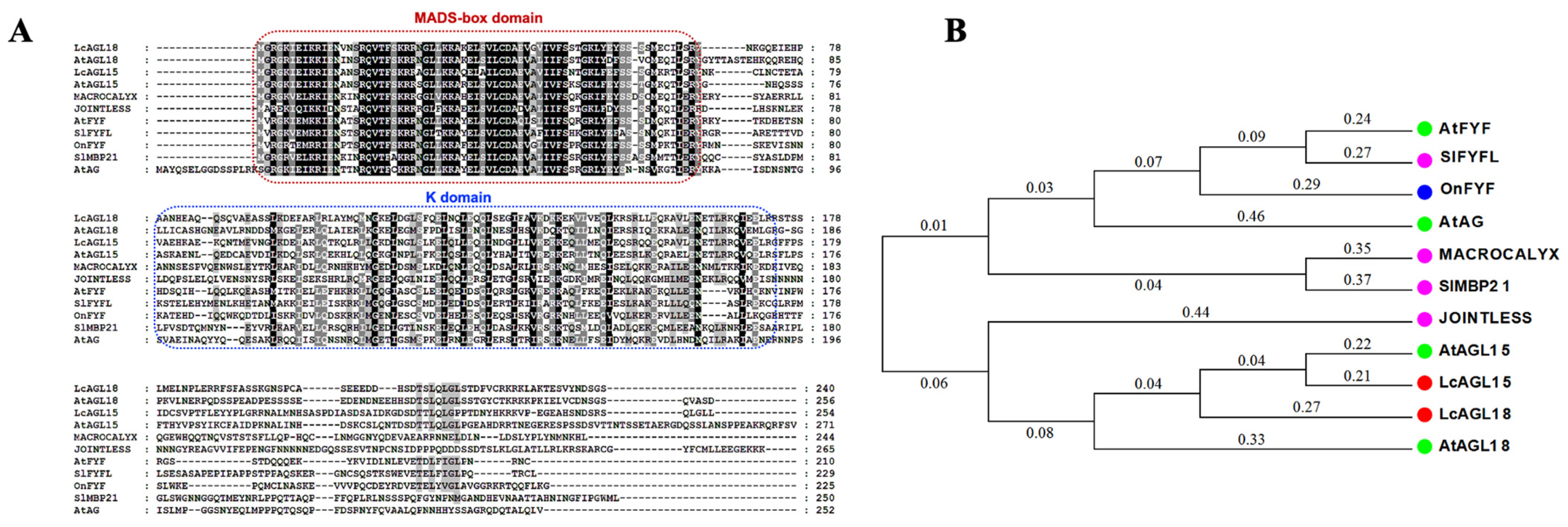
3.1. Identification of the Closest Homologs of AGL15/18 in Litchi
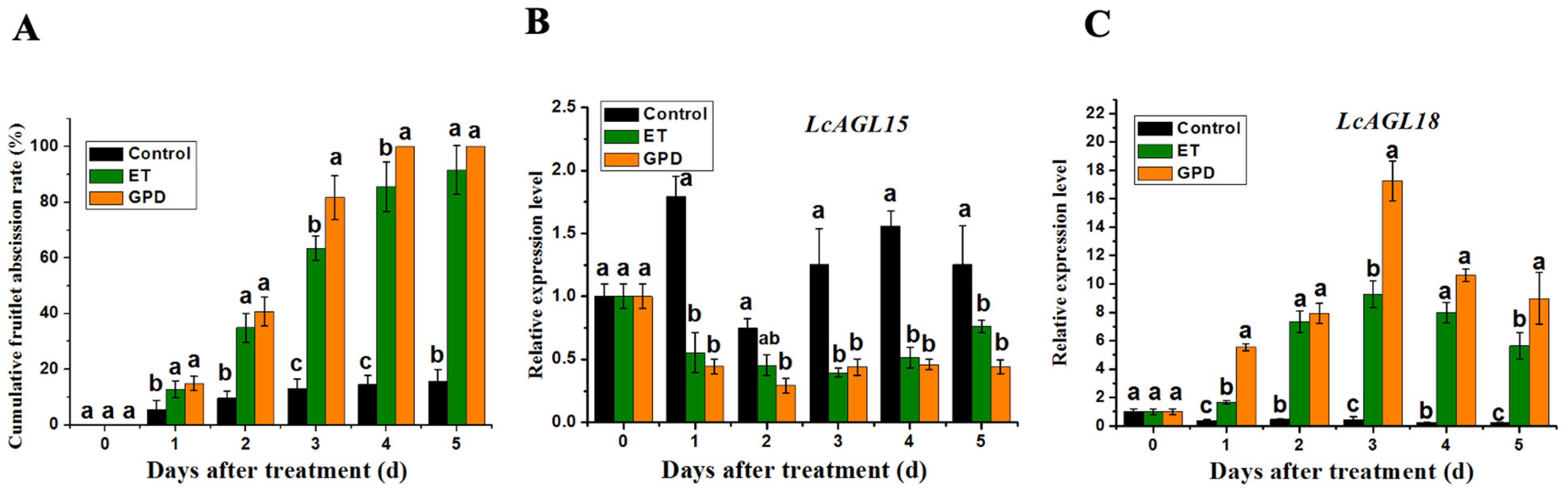
3.2. Expression Patterns of LcAGL15 and LcAGL18 during the Litchi Fruitlet Abscission
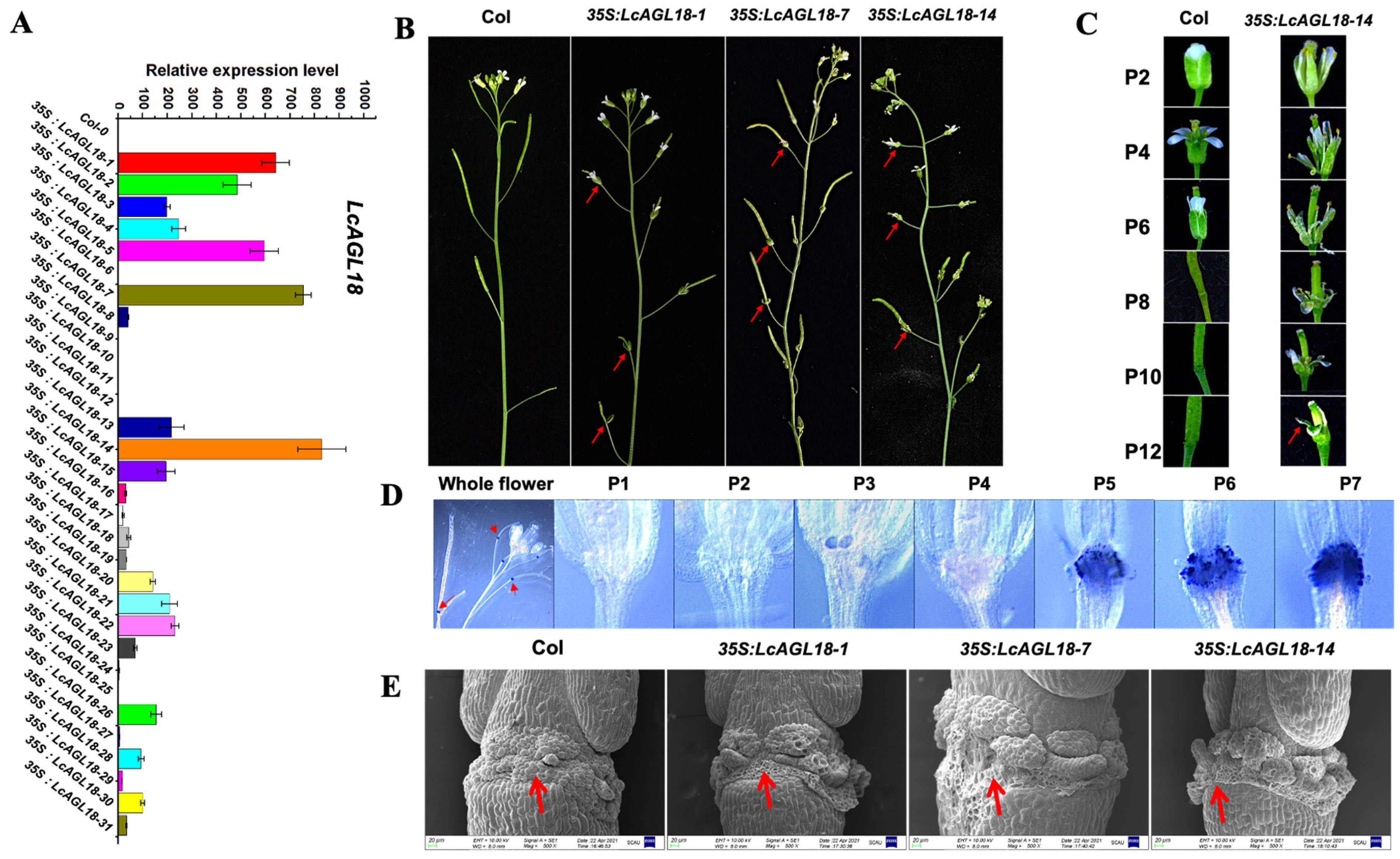
3.3. High Expression of LcAGL18 in Arabidopsis Leads to the Delay of Floral Organ Abscission
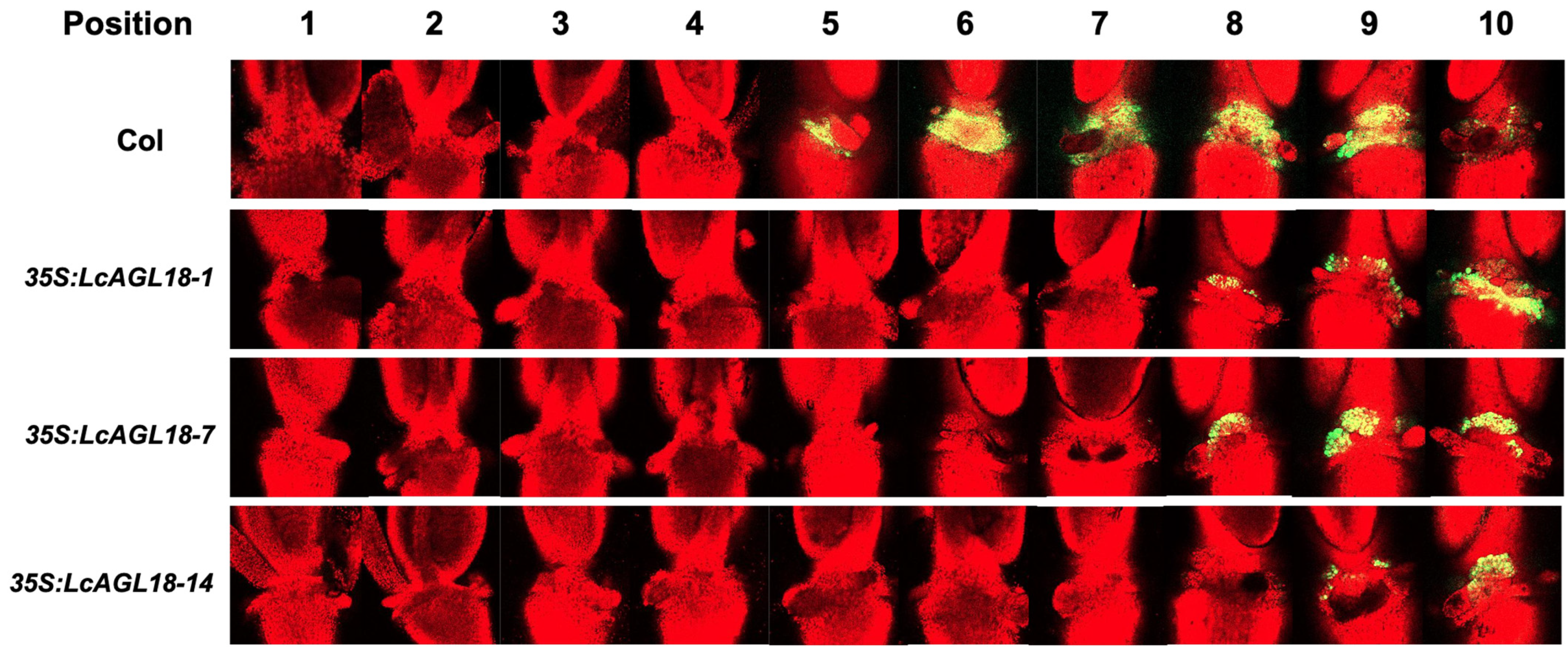
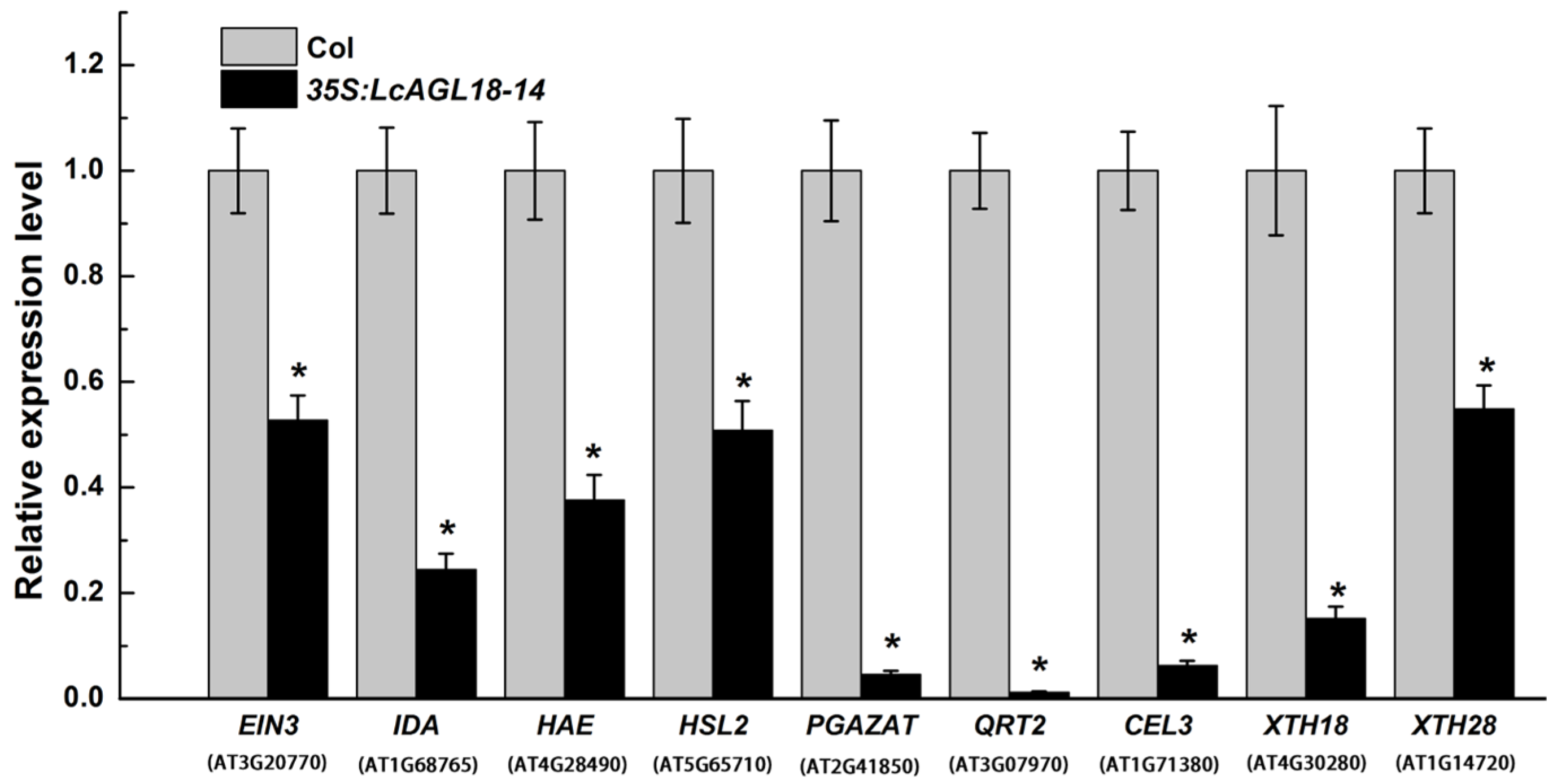
3.4. LcAGL18 Inhibits Abscission-Related Gene Expression in Arabidopsis
3.5. LcAGL18 Is a Transcriptional Repressor
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Estornell, L.H.; Agusti, J.; Merelo, P.; Talon, M.; Tadeo, F.R. Elucidating mechanisms underlying organ abscission. Plant Sci. 2013, 199, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Patterson, S.E. Cutting loose. Abscission and dehiscence in Arabidopsis. Plant Physiol. 2001, 126, 494–500. [Google Scholar] [CrossRef]
- Jinn, T.L.; Stone, J.M.; Walker, J.C. HAESA, an Arabidopsis leucine-rich repeat receptor kinase, controls floral organ abscission. Genes Dev. 2000, 14, 108–117. [Google Scholar] [CrossRef]
- Cho, S.K.; Larue, C.T.; Chevalier, D.; Wang, H.; Jinn, T.L.; Zhang, S.; Walker, J.C. Regulation of floral organ abscission in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2008, 105, 15629–15634. [Google Scholar] [CrossRef] [PubMed]
- Stenvik, G.E.; Tandstad, N.M.; Guo, Y.; Shi, C.L.; Kristiansen, W.; Holmgren, A.; Clark, S.E.; Aalen, R.B.; Butenko, M.A. The EPIP peptide of Inflorescence Deficient in Abscission is sufficient to induce abscission in arabidopsis through the receptor-like kinases HAESA and HAESA-LIKE2. Plant Cell 2008, 20, 1805–1817. [Google Scholar] [CrossRef] [PubMed]
- Liljegren, S.J.; Leslie, M.E.; Darnielle, L.; Lewis, M.W.; Taylor, S.M.; Luo, R.; Geldner, N.; Chory, J.; Randazzo, P.A.; Yanofsky, M.F.; et al. Regulation of membrane trafficking and organ separation by the NEVERSHED ARF-GAP protein. Development 2009, 136, 1909–1918. [Google Scholar] [CrossRef]
- Santiago, J.; Brandt, B.; Wildhagen, M.; Hohmann, U.; Hothorn, L.A.; Butenko, M.A.; Hothorn, M. Mechanistic insight into a peptide hormone signaling complex mediating floral organ abscission. eLife 2016, 5, e15075. [Google Scholar] [CrossRef]
- Meng, X.; Zhou, J.; Tang, J.; Li, B.; de Oliveira, M.; Chai, J.; He, P.; Shan, L. Ligand-induced receptor-like kinase complex regulates floral organ abscission in Arabidopsis. Cell Rep. 2016, 14, 1330–1338. [Google Scholar] [CrossRef]
- Meir, S.; Philosoph-Hadas, S.; Sundaresan, S.; Selvaraj, K.S.; Burd, S.; Ophir, R.; Kochanek, B.; Reid, M.S.; Jiang, C.Z.; Lers, A. Microarray analysis of the abscission-related transcriptome in the tomato flower abscission zone in response to auxin depletion. Plant Physiol. 2010, 154, 1929–1956. [Google Scholar] [CrossRef]
- Ma, C.; Meir, S.; Xiao, L.; Tong, J.; Liu, Q.; Reid, M.S.; Jiang, C.Z. A KNOTTED1-LIKE HOMEOBOX protein regulates abscission in tomato by modulating the auxin pathway. Plant Physiol. 2015, 167, 844–853. [Google Scholar] [CrossRef]
- Sundaresan, S.; Philosoph-Hadas, S.; Riov, J.; Belausov, E.; Kochanek, B.; Tucker, M.L.; Meir, S. Abscission of flowers and floral organs is closely associated with alkalization of the cytosol in abscission zone cells. J. Exp. Bot. 2015, 66, 1355–1368. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Li, R.; Wang, X.; Ge, S.; Wang, S.; Liu, X.; He, J.; Jiang, C.Z.; Qi, M.; Xu, T.; et al. A SlCLV3-SlWUS module regulates auxin and ethylene homeostasis in low light-induced tomato flower abscission. Plant Cell 2022, 34, 4388–4408. [Google Scholar] [CrossRef] [PubMed]
- Reichardt, S.; Piepho, H.P.; Stintzi, A.; Schaller, A. Peptide signaling for drought-induced tomato flower drop. Science 2020, 367, 1482–1485. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Shi, C.L.; Wang, X.; Meng, Y.; Cheng, L.; Jiang, C.Z.; Qi, M.; Xu, T.; Li, T. Inflorescence abscission protein SlIDL6 promotes low light intensity-induced tomato flower abscission. Plant Physiol. 2021, 186, 1288–1301. [Google Scholar] [CrossRef]
- Nakano, T.; Fujisawa, M.; Shima, Y.; Ito, Y. The AP2/ERF transcription factor SlERF52 functions in flower pedicel abscission in tomato. J. Exp. Bot. 2014, 65, 3111–3119. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, L.; Li, R.; Cai, Y.; Wang, X.; Fu, X.; Dong, X.; Qi, M.; Jiang, C.Z.; Xu, T.; et al. The HD-Zip transcription factor SlHB15A regulates abscission by modulating jasmonoyl-isoleucine biosynthesis. Plant Physiol. 2022, 189, 2396–2412. [Google Scholar] [CrossRef]
- Xie, Q.; Hu, Z.; Zhu, Z.; Dong, T.; Zhao, Z.; Cui, B.; Chen, G. Overexpression of a novel MADS-box gene SlFYFL delays senescence, fruit ripening and abscission in tomato. Sci. Rep. 2014, 4, 4367. [Google Scholar] [CrossRef]
- Mao, L.; Begum, D.; Chuang, H.W.; Budiman, M.A.; Szymkowiak, E.J.; Irish, E.E.; Wing, R.A. JOINTLESS is a MADS-box gene controlling tomato flower abscission zone development. Nature 2000, 406, 910–913. [Google Scholar] [CrossRef]
- Liu, D.; Wang, D.; Qin, Z.; Zhang, D.; Yin, L.; Wu, L.; Colasanti, J.; Li, A.; Mao, L. The SEPALLATA MADS-box protein SLMBP21 forms protein complexes with JOINTLESS and MACROCALYX as a transcription activator for development of the tomato flower abscission zone. Plant J. 2014, 77, 284–296. [Google Scholar] [CrossRef]
- Adamczyk, B.J.; Lehti-Shiu, M.D.; Fernandez, D.E. The MADS domain factors AGL15 and AGL18 act redundantly as repressors of the floral transition in Arabidopsis. Plant J. 2007, 50, 1007–1019. [Google Scholar] [CrossRef]
- Verelst, W.; Twell, D.; de Folter, S.; Immink, R.; Saedler, H.; Munster, T. MADS-complexes regulate transcriptome dynamics during pollen maturation. Genome Biol. 2007, 8, R249. [Google Scholar] [CrossRef] [PubMed]
- Paul, P.; Joshi, S.; Tian, R.; Diogo, J.R.; Chakrabarti, M.; Perry, S.E. The MADS-domain factor AGAMOUS-Like18 promotes somatic embryogenesis. Plant Physiol. 2022, 188, 1617–1631. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Perry, S.E. Alterations in the transcriptome of soybean in response to enhanced somatic embryogenesis promoted by orthologs of Agamous-like15 and Agamous-like18. Plant Physiol. 2014, 164, 1365–1377. [Google Scholar] [CrossRef] [PubMed]
- Patharkar, O.R.; Walker, J.C. Floral organ abscission is regulated by a positive feedback loop. Proc. Natl. Acad. Sci. USA 2015, 112, 2906–2911. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.K.; Lee, P.F.; Yang, C.H. Delay of flower senescence and abscission in Arabidopsis transformed with a Forever Young Flower homolog from Oncidium orchid. Plant Signal. Behav. 2011, 6, 1841–1843. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.H.; Lee, Y.I.; Yang, C.H. Ectopic expression of two FOREVER YOUNG FLOWER orthologues from Cattleya Orchid suppresses ethylene signaling and DELLA results in delayed flower senescence/abscission and reduced flower organ elongation in Arabidopsis. Plant Mol. Biol. Rep. 2018, 36, 710–724. [Google Scholar] [CrossRef]
- Chen, W.H.; Jiang, Z.Y.; Hsu, H.F.; Yang, C.H. Silencing of FOREVER YOUNG FLOWER-Like Genes from Phalaenopsis Orchids Promotes Flower Senescence and Abscission. Plant Cell Physiol. 2021, 62, 111–124. [Google Scholar] [CrossRef]
- Hu, G.; Feng, J.; Xiang, X.; Wang, J.; Salojärvi, J.; Liu, C.; Wu, Z.; Zhang, J.; Liang, X.; Jiang, Z.; et al. Two divergent haplotypes from a highly heterozygous lychee genome suggest independent domestication events for early and late-maturing cultivars. Nat. Genet. 2022, 54, 73–83. [Google Scholar] [CrossRef]
- Zhao, M.; Li, J. Molecular Events Involved in Fruitlet Abscission in Litchi. Plants 2020, 9, 151. [Google Scholar] [CrossRef]
- Stern, R.A.; Kigel, J.; Tomer, E.; Gazit, S. ‘Mauritius’ lychee fruit development and reduced abscission after treatment with the auxin 2,4,5-TP. Am. Soc. Hortic. Sci. 1995, 120, 65–70. [Google Scholar] [CrossRef]
- Mitra, S.K.; Pereira, L.S.; Pathak, P.K.; Majumdar, D. Fruit abscission pattern of lychee cultivars. In II International Symposium on Lychee, Longan, Rambutan and Other Sapindaceae Plants; ISHS Acta Horticulturae: Chiang Mai, Thailand, 2005; Volume 665, pp. 215–218. [Google Scholar]
- Li, C.Q.; Wang, Y.; Ying, P.Y.; Ma, W.Q.; Li, J.G. An improved fruit transcriptome and the identification of the candidate genes involved in fruit abscission induced by carbohydrate stress in litchi. Front. Plant Sci. 2015, 6, 439. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.L.; Li, C.Q.; Ma, X.S.; Xia, R.; Chen, J.Y.; Liu, X.C.; Ying, P.Y.; Peng, M.J.; Wang, J.; Shi, C.L.; et al. KNOX protein KNAT1 regulates fruitlet abscission in litchi by repressing ethylene biosynthetic genes. J. Exp. Bot. 2020, 71, 4069–4082. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.S.; Yuan, Y.; Wu, Q.; He, Z.D.; Li, J.G.; Zhao, M.L. Brassinosteroids suppress ethylene-induced fruitlet abscission through LcBZR1/2-mediated transcriptional repression of LcACS1/4 and LcACO2/3 in litchi. Hortic. Res. 2021, 8, 105. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Yuan, Y.; Wu, Q.; Wang, J.; Li, J.; Zhao, M. LcEIL2/3 are involved in fruitlet abscission via activating genes related to ethylene biosynthesis and cell wall remodeling in litchi. Plant J. 2020, 103, 1338–1350. [Google Scholar] [CrossRef]
- Kuang, J.F.; Wu, J.Y.; Zhong, H.Y.; Li, C.Q.; Chen, J.Y.; Lu, W.J.; Li, J.G. Carbohydrate stress affecting fruitlet abscission and expression of genes related to auxin signal transduction pathway in litchi. Int. J. Mol. Sci. 2012, 13, 16084–16103. [Google Scholar] [CrossRef]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 2010, 16, 735–743. [Google Scholar] [CrossRef]
- Zhong, H.Y.; Chen, J.W.; Li, C.Q.; Chen, L.; Wu, J.Y.; Chen, J.Y.; Lu, W.J.; Li, J.G. Selection of reliable reference genes for expression studies by reverse transcription quantitative real-time PCR in litchi under different experimental conditions. Plant Cell Rep. 2011, 30, 641–653. [Google Scholar] [CrossRef]
- Ying, P.; Li, C.; Liu, X.; Xia, R.; Zhao, M.; Li, J. Identification and molecular characterization of an IDA-like gene from litchi, LcIDL1, whose ectopic expression promotes floral organ abscission in Arabidopsis. Sci. Rep. 2016, 6, 37135. [Google Scholar] [CrossRef]
- Fan, Z.Q.; Tan, X.L.; Chen, J.W.; Liu, Z.L.; Kuang, J.F.; Lu, W.J.; Shan, W.; Chen, J.Y. Characterization of a Transcriptional Regulator BrWRKY6 that Associates with Gibberellin-Suppressed Leaf Senescence of Chinese Flowering Cabbage. J. Agric. Food Chem. 2018, 66, 7b06085. [Google Scholar] [CrossRef]
- Immink, R.G.; Gadella, T.J.; Ferrario, S.; Busscher, M.; Angenent, G.C. Analysis of MADS box protein-protein interactions in living plant cells. Proc. Natl. Acad. Sci. USA 2002, 99, 2416–2421. [Google Scholar] [CrossRef]
- Ogawa, M.; Kay, P.; Wilson, S.; Swain, S.M. Arabidopsis Dehiscence Zone Polygalacturonase1 (ADPG1), ADPG2, and QUARTET2 are Polygalacturonases required for cell separation during reproductive development in Arabidopsis. Plant Cell 2009, 21, 216–233. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Yoon, T.H.; Lee, J.; Jeon, S.Y.; Lee, J.H.; Lee, M.K.; Chen, H.; Yun, J.; Oh, S.Y.; Wen, X.; et al. A Lignin Molecular Brace Controls Precision Processing of Cell Walls Critical for Surface Integrity in Arabidopsis. Cell 2018, 173, 1468. [Google Scholar] [CrossRef] [PubMed]
- Crick, J.; Corrigan, L.; Belcram, K.; Khan, M.; Dawson, J.W.; Adroher, B.; Li, S.; Hepworth, S.R.; Pautot, V. Floral organ abscission in Arabidopsis requires the combined activities of three TALE homeodomain transcription factors. J. Exp. Bot. 2022, 73, 6150–6169. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Jiang, C.Z.; Gao, J. Regulatory Mechanisms Underlying Activation of Organ Abscission. Annu. Plant Rev. 2021, 4, 27–55. [Google Scholar]
- Chen, W.H.; Li, P.F.; Chen, M.K.; Lee, Y.I.; Yang, C.H. Forever Young Flower Negatively Regulates Ethylene Response DNA-Binding Factors by Activating an Ethylene-Responsive Factor to Control Arabidopsis Floral Organ Senescence and Abscission. Plant Physiol. 2015, 168, 1666–1683. [Google Scholar] [CrossRef]
- Chen, W.H.; Lin, P.T.; Hsu, W.H.; Hsu, H.F.; Li, Y.C.; Tsao, C.W.; Hsu, M.C.; Mao, W.T.; Yang, C.H. Regulatory network for Forever Young Flower-like genes in regulating Arabidopsis flower senescence and abscission. Commun. Biol. 2022, 5, 662. [Google Scholar] [CrossRef]
- Chen, M.K.; Hsu, W.H.; Lee, P.F.; Thiruvengadam, M.; Chen, H.I.; Yang, C.H. The MADS box gene, FOREVER YOUNG FLOWER, acts as a repressor controlling floral organ senescence and abscission in Arabidopsis. Plant J. 2011, 68, 168–185. [Google Scholar] [CrossRef]
- Wang, F.; Zheng, Z.; Yuan, Y.; Li, J.; Zhao, M. Identification and Characterization of HAESA-Like Genes Involved in the Fruitlet Abscission in Litchi. Int. J. Mol. Sci. 2019, 20, 5945. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Liang, Z.; He, Z.; Ma, X.; Li, J.; Zhao, M. Ectopic Expression of AGAMOUS-like 18 from Litchi (Litchi chinensis Sonn.) Delayed the Floral Organ Abscission in Arabidopsis. Horticulturae 2023, 9, 578. https://doi.org/10.3390/horticulturae9050578
Wang F, Liang Z, He Z, Ma X, Li J, Zhao M. Ectopic Expression of AGAMOUS-like 18 from Litchi (Litchi chinensis Sonn.) Delayed the Floral Organ Abscission in Arabidopsis. Horticulturae. 2023; 9(5):578. https://doi.org/10.3390/horticulturae9050578
Chicago/Turabian StyleWang, Fei, Zhijian Liang, Zidi He, Xingshuai Ma, Jianguo Li, and Minglei Zhao. 2023. "Ectopic Expression of AGAMOUS-like 18 from Litchi (Litchi chinensis Sonn.) Delayed the Floral Organ Abscission in Arabidopsis" Horticulturae 9, no. 5: 578. https://doi.org/10.3390/horticulturae9050578
APA StyleWang, F., Liang, Z., He, Z., Ma, X., Li, J., & Zhao, M. (2023). Ectopic Expression of AGAMOUS-like 18 from Litchi (Litchi chinensis Sonn.) Delayed the Floral Organ Abscission in Arabidopsis. Horticulturae, 9(5), 578. https://doi.org/10.3390/horticulturae9050578






