Abstract
The classification of grapevine cultivars into isohydric and anisohydric categories depends on their ability to close stomata under conditions of low soil water availability or high atmospheric demand. This study aimed to compare the responses of Grenache, classified as isohydric, and Cabernet Sauvignon, classified as anisohydric, both grafted onto Richter 110 rootstock, and subjected to severe drought stress. Three cycles of drought stress were applied, followed by watering, while a well-watered treatment served as the control. Stomatal conductance and stem water potential were measured at predawn and midday during the drought cycles, and primary metabolites were analyzed in leaves and roots using gas chromatography. We found that Grenache had significantly higher stomatal conductance than Cabernet Sauvignon under both well-watered and water-stressed conditions. There were no significant differences in stem water potential between the two varieties, but the control treatment maintained a higher stem water potential at predawn and midday for both varieties. Primary metabolite analysis showed that both varieties accumulated sugars and polyols in their leaves and roots under drought stress, while organic acids were more abundant in leaves than in roots. Overall, the results suggest that the hydric behavior of grapevines depends on the intensity and duration of drought stress. In this study, both varieties exhibited near-isohydric behavior by regulating stomatal closure under drought stress. The metabolites identified in this study may serve as potential biomarkers of water drought stress in Grenache and Cabernet Sauvignon grapevines under the conditions of this experiment.
1. Introduction
Several grape-growing regions in the world are facing water scarcity. Plants have developed different response mechanisms to adapt to water stress. There are plants that have the ability to control stomatal closure, modifying the delivery of water to the canopy, reducing the loss of water through transpiration, and conserving water for essential physiological processes. The accumulation of compatible solutes helps to maintain a low water potential inside the plant cells, which reduces the risk of dehydration [1,2,3]. Previous works have shown that stomatal closure adjustment is a protection mechanism used by the plant to prevent water loss and embolism formation [4,5,6,7,8,9]. Yet not all plants have this protection mechanism. Under water stress conditions, certain species are incapable of regulating stomatal closure, which can lead to the formation of xylem embolisms [7] and, as a consequence, a loss of canopy by defoliation [10,11].
Depending on the response of plants to adverse conditions, grapevine cultivars have been classified as isohydric or anisohydric [12,13,14,15]. Isohydric cultivars can maintain their midday leaf water potential constant and above the embolism formation threshold independent of soil water availability or atmospheric water demand [2] due to reduced stomatal conductance. Anisohydric cultivars keep their stomata open even with decreased leaf water potential [16]. In conditions where soil water availability decreases or atmospheric water demand increases, these cultivars may further decrease leaf water potential [2]. Some grapevines reported as isohydric in Vitis are Grenache, Trincadeira, Tempranillo, Vitis labruscana (Vitis labrusca × Vitis vinifera), and Richter 110 (Vitis berlandieri × Vitis rupestris) [17]. Some of the anisohydric cultivars reported are Chardonnay, Cabernet Sauvignon, Syrah, and Riesling [2]. These types of cultivars suffer a drop in leaf water potential during the day depending on the availability of water in the soil, something that is possible due to osmotic adjustment and changes in cell wall elasticity [18,19,20,21]. However, there are cultivars that, under water stress conditions, have the capacity to behave as iso- or anisohydric depending on the atmospheric conditions, season, and age of the plants. For example, the cultivar V. labruscana was reported as anisohydric by Liu et al. 1978 [22], but years later Naor and Wample (1994) defined it as isohydric [23]. Another variety, Pinot Noir, can behave as anisohydric when water stress is applied before veraison and in turn can behave as isohydric when the stress is applied post veraison [24]. Understanding the behavior of grapevine plants that adapt to external factors could be crucial in comprehending plant adaptation to the environment, leading to better field-level management. Additionally, it remains unclear if multiple drought cycles result in changes to the hydric behavior of the plants, potentially causing fatigue in the physiological mechanisms responsible for opening and closing stomata.
In this study, we used Grenache, reported previously as near- isohydric, and Cabernet Sauvignon, considered isohydric and anisohydric [9], with both varieties grafted onto 110R rootstock (V. berlandieri × V. rupestris) considered isohydric [17]. The objective of this study was to investigate the hydric response of these cultivars under drought conditions, including three cycles of severe water stress (<−1.5 MPa), to check for possible physiological fatigue, while maintaining a control group under well-watered conditions. In addition to analyzing the hydric behavior during drought cycles, we aimed to identify primary metabolites in foliar and root tissues, with the goal of identifying metabolite markers that can provide insights into the hydric behavior of these cultivars.
2. Materials and Methods
2.1. Plant Materials and Growing Conditions
The experiments were performed at the Faculty of agriculture and food sciences of the Pontifícia Universidad Católica de Valparaíso, Quillota, Chile. We used 80 grafted vines (40 plants of Cabernet Sauvignon and 40 Grenache grafted on to rootstock 110 Richter). Grapevines were planted in 6 L pots filled with 75% coconut coir and 25% perlite. Growth was maintained under greenhouse conditions (approximately 16 °C to 28 °C temperature, 35% relative humidity with natural light 500–1100 µmol photons m−2 s−1 of photosynthetically active radiation (PAR), supplemented light 400–600 µmol photons m−2 s−1 PAR was provided with 600-W metal-halide bulbs (PIRANHA, Shanghai - China), and 16 h light/8 h dark cycle). Plants were irrigated with filtered water three times per week until the shoot reached 0.5 m in length. Then, plants were randomly assigned to one of two watering treatments. The well-watered plants continued to be watered every two days, whereas the drought-stressed plants were deprived of water until their stem water potential reached −2.0 Ψ MPa. After that, they were watered until the pot capacity was reached, and this cycle was repeated three times in total.
2.2. Stomatal Conductance
Two mature leaves per treatment were randomly chosen to measure stomatal conductance (predawn between 5 and 6 a.m. and midday between 12 and 13 p.m.) using a leaf Porometer model SC-1 leaf porometer (Decagon Devices). Measurements were performed every other day during the three drought cycles.
2.3. Measurements of Plant Water Status
Water status was measured immediately after stomatal conductance on the same leaf. A Scholander pressure chamber (Soil Moisture Equipment Corp 1505D, Goleta, CA, USA) was used to measure stem water potential (Ψstem) of plants. Mature leaves were placed into aluminum bags for at least 15 min, such that they were hydraulically equilibrated with Ψstem. Subsequently, leaves were excised at the base of the petiole and placed into the pressure chamber while still bagged. The chamber was pressurized, and the pressure required to force water out of the petiole base was recorded and defined as Ψstem.
2.4. Quantification of Primary Metabolites in Leaf and Root Tissues by Gas Chromatography Coupled to Mass Spectrometry [GCMS]
The extraction and derivatization of soluble sugars was performed according to [25] with minor modifications. The derivatization consisted of methoximation and trimethylsilylation reactions. Samples were analyzed using an Agilent 7890B gas chromatograph equipped with a 5977A single quadrupole MS with an electron impact ionization source, a PAL3 autosampler, and an HP-5 ms Ultra Inert (30 m × 0.25 mm × 0.25 mm) column (Agilent Technologies, Santa Clara, CA, USA). Two GC–MS methods were performed, one for more concentrated compounds, such as sugar, and the other one for less concentrated compounds, such as organic compounds and amino acids. For both methods, the injector and interface temperatures were 220 °C and 280 °C, respectively, and 1 mL of sample was injected. Helium (Indura, Santiago, Chile) was used as a carrier gas with a constant flow of 1 mL min−1. Mass spectra in the 50–600 m/z range were recorded at a scanning speed of 2.66 scan cycles per second. The MS ion source and quadrupole temperatures were 230 °C and 150 °C, respectively. The method for more abundant compounds included an injection with a split ratio of 1:150, and the oven temperature was programmed to start at 120 °C (for 1 min), increase to 300 °C at 10 °C min−1, and then hold for 6 min.
2.5. Statistical Analysis
T-tests were performed using R v4.2.1 statistical computing environment with aid from the car software package. When appropriate, the Shapiro–Wilk test and Levene’s test were used to test the assumptions of normality of residuals and homogeneity of variances, respectively. Data were transformed as necessary when assumptions were not met.
3. Results
3.1. Stomatal Conductance
During the three drought cycles, we assessed stomatal conductance at predawn and midday. Our findings at predawn (as shown in Figure 1a) revealed significant differences between Cabernet Sauvignon and Grenache varieties under well-watered conditions (p = 0.001), with Cabernet Sauvignon exhibiting a mean of 66.2 mmol s−1 m−2 and Grenache showing a mean of 80.4 mmol s−1 m−2. Similarly, under drought stress treatment, there were differences observed between the two varieties at predawn (p = 0.0008), with Cabernet Sauvignon displaying a mean of 37.1 mmol s−1 m−2 and Grenache showing a mean of 50.3 mmol s−1 m−2. At midday (Figure 1b), the well-watered plants also exhibited significant differences between Cabernet Sauvignon and Grenache varieties (p = 0.0002), with Cabernet Sauvignon showing a mean of 239 mmol s−1 m−2 and Grenache showing a mean of 288 mmol s−1 m−2. Furthermore, the drought treatment measured at midday showed differences between the two varieties (p = 0.005), with Cabernet Sauvignon displaying a mean of 36 mmol s−1 m−2 and Grenache showing a mean of 91.8 mmol s−1 m−2.
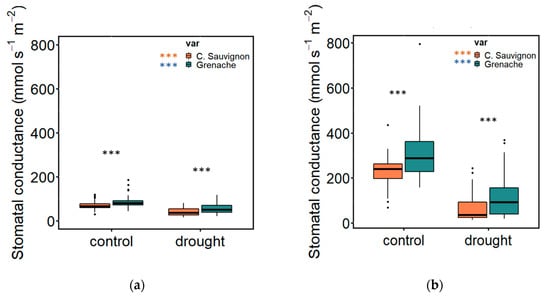
Figure 1.
This figure shows the stomatal conductance (mmol s−1 m−2) of Cabernet Sauvignon and Grenache varieties under well-watered and drought conditions. (a) shows the stomatal conductance at predawn, and (b) shows the stomatal conductance at midday. The asterisks indicate significant differences between the two varieties and the treatments at each stress level as determined by t-test. The significant differences between the varieties and treatment at each stress level are represented by *** (p = 0.001).
3.2. Plant Water Status
We measured stem water potential at predawn and midday during the three drought cycles (Figure 2). During predawn, the mean water potentials for well-watered Cabernet Sauvignon and Grenache were −0.39 MPa and −0.42 MPa, respectively, while under drought stress, Cabernet Sauvignon had a mean of −0.95 MPa and Grenache had a mean of −0.81 MPa. Significant differences were found across treatments at predawn for Cabernet Sauvignon (p = 3.184 × 10−14), while no differences were observed in Granache. At midday, well-watered Cabernet Sauvignon had a mean of −0.62 MPa and Grenache −0.57 MPa. The Cabernet Sauvignon under drought stress showed a mean of −1.15 MPa and Grenache −1.12 MPa. Significant differences were observed across treatments for Cabernet Sauvignon (p = 8.8 × 10−15) and Grenache (p = 2.2 × 10−16).
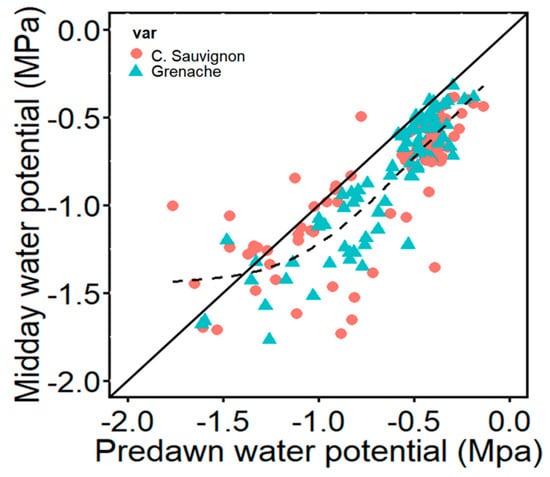
Figure 2.
Relationship between predawn and midday stem water potential (MPa) observed in Cabernet Sauvignon and Grenache plants during drying cycles. The solid lines represent 1:1 relation. The dashed line is a smoothed line that best followed the pattern of data points.
3.3. Grapevine Primary Metabolites Determined in Leaf and Root Tissues by GS-MS
In both leaves and roots, we detected eighteen primary metabolites. Under drought conditions, Cabernet Sauvignon leaves showed a higher relative abundance of fructose, methylfructoside, citric acid, tartaric acid, and scyllo inositol, while the control treatment had a higher relative abundance of erythrono-1,4-lactone and epigallocatechin. Similarly, in Grenache leaves under drought, we observed a greater relative abundance of fructose, methylfructoside, citric acid, malic acid, and scyllo inositol compared to the control treatment, while the control treatment had a higher relative abundance of glucose and catechine.
In roots of Cabernet Sauvignon under drought, we observed a higher relative abundance of fructose, methylfructoside, galactinol, scyllo inositol, and β-sitosterol compared to the control treatment. The control treatment had a higher relative abundance of citric acid, tartaric acid, shikimic acid, malic acid, gluconic acid, threonic acid, catechin, epigallocatechin, and erythrono-1,4-lactone compared to the drought treatment.
In Grenache roots under drought, we observed a greater abundance of glucose, fructose, sucrose, 3-α-mannobiose, methylfructoside, and galactinol compared to the control treatment. The control treatment had a higher relative abundance of citric acid, tartaric acid, shikimic acid, malic acid, gluconic acid, threonic acid, epigallocatechin, and erythrono-1,4-lactone compared to the drought treatment (as shown in Figure 3).
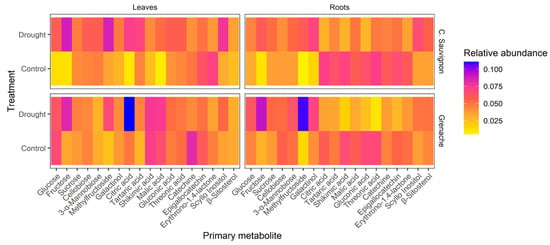
Figure 3.
Heatmap of primary metabolites detected in leaves of Cabernet Sauvignon and Grenache variety and roots of Richter 110 rootstock grown under well-watered and drought conditions.
4. Discussion
In this study, we provide a depiction of the relation between the hydric behavior of two supposedly contrasting grapevine varieties during several drought cycles and the primary metabolism of the plants. Our data suggest that the hydric behavior of grapevines deeply depends on the type of drought stress, highlighting that multiple drought cycles generate a fatigue of the physiological mechanisms that open and close stomata. According to the literature, the Cabernet Sauvignon variety should have a higher stomatal conductance according to the anisohydric classification and definition, since it stands out for keeping its stomata open at the low water potential of −1.5 MPa [2], but according to our reported data, the Grenache variety maintained a greater stomatal conductance both at predawn and midday, presenting an even greater gap at midday. In this experiment, both varieties behaved in a near-isohydric region, regulating stomata closure as a response to drought stress. Previous research has shown that grapevines adjust osmotically in response to drought conditions [26,27]. This process reduces osmotic potential in leaf cells, allowing them to maintain a positive cell turgor despite the decrease in water potential [28,29]. However, it is important to mention that different species accumulate different osmolytes [19,30] and there is little information on the compounds involved in grapevine osmoregulation. In our study, we observed that the greatest accumulation of solutes occurred in Grenache in leaves, something that might explain the near-isohydric behavior, yet still maintaining a greater stomatal conductance compared to Cabernet Sauvignon.
In both varieties, our results showed a higher content of sugars and polyols in leaves and roots under drought treatment. The water deficit in Cabernet Sauvignon plants grafted onto 101-14 Mg and Milano-4 (M4) rootstocks have been shown to elicit a total soluble sugars accumulation in roots and leaves [31]. Moreover, roots of the Grenache variety showed a higher content of sucrose under drought conditions [32]. Soluble sugars integrate energetic and biosynthetic metabolism, while also having an osmoprotective role that re-establishes the osmotic balance and maintains cell turgor [33,34]. Galactinol, myo-inositol, and sucrose act as precursors of the raffinose pathway that can provide osmolytes; however, studies have suggested that they can also act as osmoprotective compounds [35,36,37]. Drought stress induces the breakdown of storage sugars into soluble sugars such as sucrose, glucose, and fructose, and an accumulation of sugars triggers the expression of sugar transporters for the source-to-sink distribution of carbohydrates [34].
In this study, organic acids of grapevines exposed to drought were more abundant in leaves than in roots. Cabernet Sauvignon leaves under water deficit displayed an increase to a peak in citrate levels before they decreased, while malate showed a similar trend [26]. Citric and malic acids participate as intermediaries of the tricarboxylic acid (TCA) cycle, which is crucial for energy metabolism and the supply of carbon skeletons [38,39]. However, organic acid accumulation can also be attributed to vacuolar storage [40]. The roots of the drought-tolerant M4 rootstock under water deficit induce a fall in intermediaries of the TCA cycle and, at the same time, an overexpression of enzymes of this cycle was observed [39]. Our results suggest that water deficit could induce an imbalance in energy metabolism and/or vacuolar storage, which can impact turgor pressure.
The shikimate pathway directs bulk carbon towards the biosynthesis of aromatic amino acids, and various metabolic pathways are derived from these precursors, for example, the phytohormone biosynthesis and phenylpropanoid pathways [41]. The secondary metabolites (shikimic acid, epigallocatechin, and catechin) found in this study were downregulated in both tissues under water-deficit treatment. Drought stress triggers an increase in enzymes involved in the flavonoid and stilbene biosynthesis in the roots of grapevine rootstock [39]. Cabernet Sauvignon leaves presented a low content of shikimic acid and flavanols, such as catechin, epicatechin, and epigallocatechin, in response to water-deficit stress [26]. Drought stress induces an increase in reactive oxygen species (ROS) and also an increase of secondary metabolites that can act as scavenge ROS to protect plant cells from lipid peroxidation and other oxidative damage [42,43]. Our results suggest a decrease in secondary metabolic flux independent of tissue and variety; however, further omic approaches are needed.
In different plant species, other compounds, such as mannobiose, β-sitosterol, gluconic acid, and threonic acid, have been reported as drought-stress-response metabolites [43,44,45,46]. Regarding β-sitosterol, this was upregulated in the roots of Cabernet Sauvignon under drought treatment. Phytosterols are integral components of cell membranes and regulators of membrane fluidity, and they improve the resistance of plants to abiotic stress by reinforcing the cohesion of cell membranes [45,47]. The metabolites found in this study could potentially be biomarkers of water drought stress in grapevines varieties; however, the metabolic responses differ among plant tissues and are reliant on the duration and severity of drought treatment [38]. In further studies, we think there is still a need to describe how other parameters such as the activity of defense enzymes, the content of pigments such as carotenoids, and the content of total proteins are modulated in relation to isohydric and anisohydric behavior.
5. Conclusions
This study provides valuable insights into the hydric behavior and physiological mechanisms of grapevines under drought stress, with a particular focus on two grape varieties: Cabernet Sauvignon and Grenache. The findings suggest that the type of drought stress has a significant impact on grapevines, with multiple drought cycles leading to a fatigue of the physiological mechanisms that regulate stomatal opening and closing. Both varieties displayed a near-isohydric behavior in response to drought stress, with the Grenache variety showing a greater stomatal conductance and solute accumulation than Cabernet Sauvignon. The accumulation of sugars and organic acids as well as the downregulation of secondary metabolites were observed in both varieties under drought treatment, highlighting their role in osmoregulation and energy metabolism. The metabolites identified in this study, such as β-sitosterol, could potentially serve as biomarkers of water drought stress in grapevines, but further studies are needed to fully understand the metabolic response to drought and its impact on other parameters.
Author Contributions
Conceptualization, M.T. and I.F.C.; formal analysis, M.T. and I.F.C.; investigation, M.T., L.S., E.P.G. and P.S.; resources, R.P., A.C.-M. and I.F.C.; data curation, I.F.C. and M.T.; writing—original draft preparation, M.T.; writing—review and editing, I.F.C.; visualization, I.F.C., J.E.A., R.P. and A.C.-M.; project administration, I.F.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Agencia Nacional de Investigación y Desarrollo (ANID), within the Fondecyt Regular grant No 1220235.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors thank Viveros Guillaume for their collaboration with the plant material.
Conflicts of Interest
The authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Skirycz, A.; Inze, D. More from less: Plant growth under limited water. Curr. Opin. Biotechnol. 2010, 21, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Lovisolo, C.; Perrone, I.; Carra, A.; Ferrandino, A.; Flexas, J.; Medrano, H.; Schubert, A. Drought-induced changes in development and function of grapevine (Vitis spp.) organs and in their hydraulic and non-hydraulic interactions at the whole-plant level: A physiological and molecular update. Funct. Plant Biol. 2010, 37, 98–116. [Google Scholar] [CrossRef]
- Chaves, M.; Pereira, J.S.; Maroco, J. Underestanding plant response to drought-from genes to the whole plant. Funct. Plant Biol. 2003, 30, 239–264. [Google Scholar] [CrossRef] [PubMed]
- Jones, H.G.; Southerland, R.A. Stomatal control of xylem embolism. Plant Cell Environ. 1991, 14, 607–612. [Google Scholar] [CrossRef]
- Cochard, H.; Coll, L.; Le Roux, X.; Améglio, T. Unraveling the effects of plant hydraulics on stomatal closure during water stress in Walnut. Plant Physiol. 2002, 128, 282–290. [Google Scholar] [CrossRef]
- Buckley, T.N.; Mott, K.A.; Farquhar, G.D. A hydromechanical and biochemical model of stomatal conductance. Plant Cell Environ. 2003, 26, 1767–1785. [Google Scholar] [CrossRef]
- Vilagrosa, A.; Bellot, J.; Vallejo, V.R.; Gil-Pelegrin, E. Cavitation, stomatal conductance, and leaf dieback in seedlings of two co-ocurring Mediterranean schrubs during an intense drought. J. Exp. Bot. 2003, 54, 2015–2024. [Google Scholar]
- Cruiziat, P.; Cochard, H.; Améglio, T. Hydraulic architecture of trees: Main concepts and results. Ann. For. Sci. 2002, 59, 723–752. [Google Scholar] [CrossRef]
- Chaves, M.; Zarrouk, O.; Francisco, R.; Costa, J.M.; Santos, T.; Regalado, A.P.; Rodrigues, M.L.; Lopes, C.M. Grapevine under deficit irrigation: Hints from physiological and molecular data. Ann. Bot. 2010, 105, 661–676. [Google Scholar]
- Tyree, M.T.; Cochard, H.; Cruiziat, P.; Sinclair, B.; Ameglio, T. Drought-induced leaf shedding in walnut: Evidence for vulnerability segmentation. Plant Cell Environ. 1993, 16, 879–882. [Google Scholar] [CrossRef]
- Sperry, J.; Saliendra, N.Z. Intra- and inter-plant variation in xylem cavitation in Betula occidentalis. Plant Cell Environ. 1994, 17, 1233–1241. [Google Scholar] [CrossRef]
- Tardieu, F.; Simonneau, T. Variability among species of stomatal control under fluctuating soil water status and evaporative demand: Modelling isohydric and anisohydric behaviors. J. Exp. Bot. 1998, 49, 419–432. [Google Scholar] [CrossRef]
- Schultz, H.R. Differences in hydraulic architecture account for near-isohydric and anisohydric behaviour of two field-grown Vitis vinifera L. cultivars during drought. Plant Cell Environ. 2003, 26, 1393–1405. [Google Scholar] [CrossRef]
- Franks, P.; Drake, P.L.; Froend, R.H. Anisohydric but isohydrodinamic: Seasonally constant plant water potential gradient explained by a stomatal control mechanism incorporating variable plant hydraulic conductance. Plant Cell Environ. 2007, 30, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Vandeleur, R.K.; Mayo, G.; Shelden, M.C.; Gilliham, M.; Kaiser, B.N.; Tyerman, S.D. The role of plasma membrane intrinsic protein aquaporins in water transport through roots: Diurnal and drought stress responses reveal different strategies between isohydric and anisohydric cultivars of grapevine. Plant Physiol. 2009, 149, 445–460. [Google Scholar] [CrossRef]
- Sade, N.; Gebremedhin, A.; Moshelion, M. Risk-taking plants: Anisohydric behavior as a stress-resistance trait. Plant Signal. Behav. 2012, 7, 767–770. [Google Scholar] [CrossRef]
- Lovisolo, C.; Perrone, I.; Hartung, W.; Schubert, A. An abscisic acidrelated reduced transpiration promotes gradual embolism repair when grapevines are rehydrated after drought. New Phytol. 2008, 180, 642–651. [Google Scholar] [CrossRef]
- Downton, W.J. Osmotic adjustment during water stress protects the photosynthetic apparatus against photoinhibition. Plant Sci. Lett. 1983, 30, 137–143. [Google Scholar] [CrossRef]
- Düring, H. Evidence for osmotic adjustment to drought in grapevines (Vitis vinifera L.). Vitis 1984, 23, 1–10. [Google Scholar]
- Schultz, H.R.; Matthews, M.A. Growth, osmotic adjustment, and cellwall mechanics of expanding grape leaves during water deficits. Crop Sci. 1993, 33, 287–294. [Google Scholar] [CrossRef]
- Patakas, A.; Noitsakis, B. Cell wall elasticity as a mechanism to maintain favourable water relations during leaf ontogeny in grapevines. Am. J. Enol. Vitic. 1997, 48, 352–356. [Google Scholar] [CrossRef]
- Liu, W.T.; Pool, R.; Wenkert, W.; Kriedemann, P.E. Changes in photosynthesis, stomatal resistance and abscisic acid of Vitis labruscana through drought and irrigation cycles. Am. J. Enol. Vitic. 1978, 29, 239–246. [Google Scholar] [CrossRef]
- Naor, A.; Wample, R.L. Gas-exchange and water relations of fieldgrown Concord (Vitis labruscana Bailey) grapevines. Am. J. Enol. Vitic. 1994, 45, 333–337. [Google Scholar] [CrossRef]
- Poni, S.; Lakso, A.N.; Turner, J.R.; Melious, R.E. The effects of pre- and post-veraison water stress on growth and physiology of potted Pinot Noir grapevines at varying crop levels. Vitis 1993, 32, 207–214. [Google Scholar]
- Fuentealba, C.; Hernández, I.; Saa, S.; Toledo, L.; Burdiles, P.; Chirinos, R.; Pedreschi, R. Colour and in vitro quality attributes of walnuts from different growing conditions correlate with key precursors of primary and secondary metabolism. Food Chem. 2017, 232, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Hochberg, U.; Degu, A.; Toubiana, D.; Gendler, T.; Nikoloski, Z.; Rachmilevitch, S.; Fait, A. Metabolite profiling and network analysis reveal coordinated changes in grapevine water stress response. BMC Plant Biol. 2013, 13, 184. [Google Scholar] [CrossRef] [PubMed]
- Martorell, S.; Medrano, H.; Tomas, M.; Escalona, J.M.; Flexas, J.; Diaz-Espejo, A. Plasticity of vulnerability to leaf hydraulic dysfunctionduring acclimation to drought in grapevines: An osmotic-mediated process. Physiol. Plant. 2015, 153, 381–391. [Google Scholar] [CrossRef]
- Bartlett, M.K.; Zhang, Y.; Kreidler, N.; Sun, S.; Ardy, R.; Cao, K.; Sack, L. Global analysis of plasticity in turgor loss point, a key drought tolerance trait. Ecol. Lett. 2014, 17, 1580–1590. [Google Scholar] [CrossRef]
- Maréchaux, I.; Bartlett, M.K.; Iribar, A.; Sack, L.; Chave, J. Stronger seasonal adjustment in leaf turgor loss point in lianas than trees in an Amazonian forest. Biol. Lett. 2017, 13, 20160819. [Google Scholar] [CrossRef]
- Turner, N.C. Turgor maintenance by osmotic adjustment: 40 years of progress. J. Exp. Bot. 2018, 69, 3223–3233. [Google Scholar] [CrossRef]
- Prinsi, B.; Simeoni, F.; Galbiati, M.; Meggio, F.; Tonelli, C.; Scienza, A.; Espen, L. Grapevine Rootstocks Differently Affect Physiological and Molecular Responses of the Scion under Water Deficit Condition. Agronomy 2021, 11, 289. [Google Scholar] [CrossRef]
- Rogiers, S.; Holzapfel, B.; Smith, J. Sugar accumulation in roots of two grape varieties with contrasting response to water stress. Ann. Appl. Biol. 2011, 159, 399–413. [Google Scholar] [CrossRef]
- Gurrieri, L.; Merico, M.; Trost, P.; Forlani, G.; Sparla, F. Impact of Drought on Soluble Sugars and Free Proline Content in Selected Arabidopsis Mutants. Biology 2020, 9, 367. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Manna, M.; Thakur, T.; Gautam, V.; Salvi, P. Imperative role of sugar signaling and transport during drought stress responses in plants. Physiol. Plant 2021, 171, 833–848. [Google Scholar] [CrossRef] [PubMed]
- Griesser, M.; Weingart, G.; Schoedl-Hummel, K.; Neumann, N.; Becker, M.; Varmuza, K.; Liebner, F.; Schuhmacher, R.; Forneck, A. Severe drought stress is affecting selected primary metabolites, polyphenols, and volatile metabolites in grapevine leaves (Vitis vinifera cv. Pinot noir). Plant Physiol. Biochem. 2015, 88, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, A.; Yabuta, Y.; Shigeoka, S. Galactinol and raffinose constitute a novel function to protect plants from oxidative damage. Plant Physiol. 2008, 147, 1251–1263. [Google Scholar] [CrossRef] [PubMed]
- Rabara, R.C.; Tripathi, P.; Reese, R.N.; Rushton, D.L.; Alexander, D.; Timko, M.P.; Shen, Q.J.; Rushton, P.J. Tobacco drought stress responses reveal new targets for Solanaceae crop improvement. BMC Genom. 2015, 16, 484. [Google Scholar] [CrossRef]
- Fàbregas, N.; Fernie, A.R. The metabolic response to drought. J. Exp. Bot. 2019, 70, 1077–1085. [Google Scholar] [CrossRef]
- Prinsi, B.; Negri, A.S.; Failla, O.; Scienza, A.; Espen, L. Root proteomic and metabolic analyses reveal specific responses to drought stress in differently tolerant grapevine rootstocks. BMC Plant Biol. 2018, 18, 126. [Google Scholar] [CrossRef]
- Huang, X.Y.; Wang, C.K.; Zhao, Y.W.; Sun, C.H.; Hu, D.G. Mechanisms and regulation of organic acid accumulation in plant vacuoles. Hortic. Res. 2021, 8, 227. [Google Scholar] [CrossRef]
- Yokoyama, R.; De Oliveira, M.V.; Kleven, B.; Maeda, H.A. The entry reaction of the plant shikimate pathway is subjected to highly complex metabolite-mediated regulation. Plant Cell 2021, 33, 671–696. [Google Scholar] [CrossRef]
- Yadav, B.; Jogawat, A.; Rahman, M.S.; Narayan, O.P. Secondary metabolites in the drought stress tolerance of crop plants: A review. Gene Rep. 2021, 23, 101040. [Google Scholar] [CrossRef]
- Kumar, M.; Kumar, P.M.; Kumar, N.; Bajpai, A.B.; Siddique, K.H. Metabolomics and Molecular Approaches Reveal Drought Stress Tolerance in Plants. Int. J. Mol. Sci. 2021, 22, 9108. [Google Scholar] [CrossRef] [PubMed]
- Degenkolbe, T.; Do, P.T.; Kopka, J.; Zuther, E.; Hincha, D.K.; Köhl, K.I. Identification of drought tolerance markers in a diverse population of rice cultivars by expression and metabolite profiling. PLoS ONE 2013, 8, e63637. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.S.; Ali, K.; Dahuja, A.; Tyagi, A. Role of phytosterols in drought stress tolerance in rice. Plant Physiol. Biochem. 2015, 96, 83–89. [Google Scholar] [CrossRef]
- Nisa, Z.U.; Arif, A.; Waheed, M.Q.; Shah, T.M.; Iqbal, A.; Siddiqui, A.J.; Choudhary, M.I.; El-Seedi, H.R.; Musharraf, S.G. A comparative metabolomic study on desi and kabuli chickpea (Cicer arietinum L.) genotypes under rainfed and irrigated field conditions. Sci. Rep. 2020, 10, 13919. [Google Scholar] [CrossRef]
- Du, Y.; Fu, X.; Chu, Y.; Wu, P.; Liu, Y.; Ma, L.; Tian, H.; Zhu, B. Biosynthesis and the Roles of Plant Sterols in Development and Stress Responses. Int. J. Mol. Sci. 2022, 23, 2332. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).