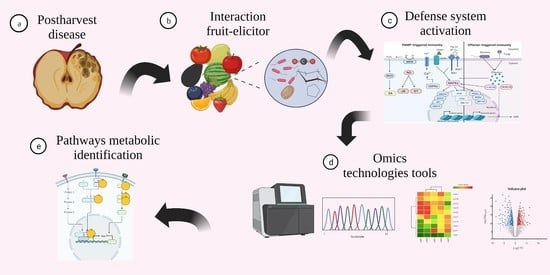
Molecular Aspects Revealed by Omics Technologies Related to the Defense System Activation in Fruits in Response to Elicitors: A Review
Abstract
1. Introduction
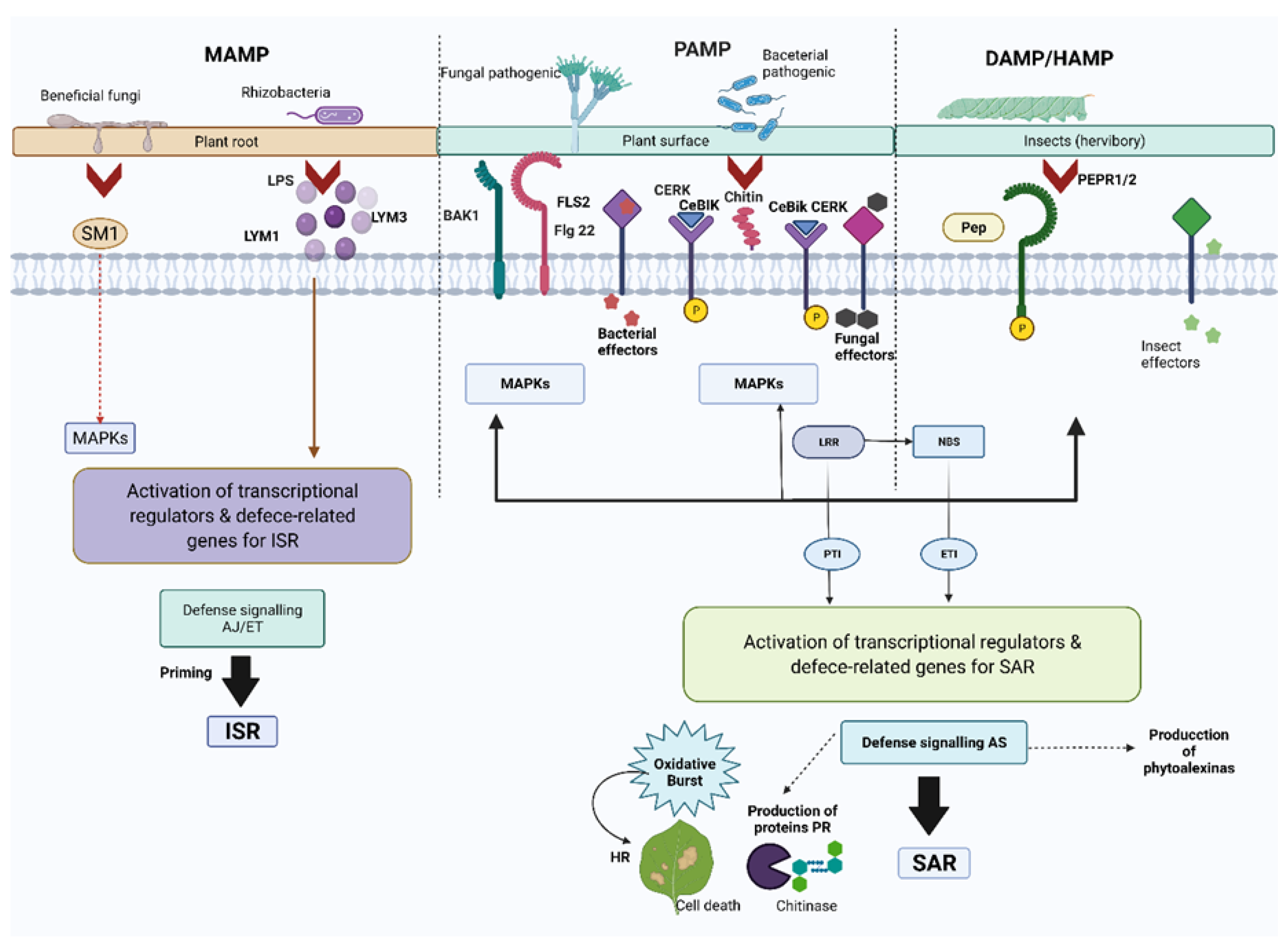
2. Plant System Immunity
3. Fruits Defense System Activation by Pathogenic Fungi
4. Classification of the Elicitors to Induced Resistance in the Postharvest Stage
5. Biocontrol Agents with Elicitor Potential
6. Physic Elicitors with Elicitor Potential
7. Chemical Elicitors: Naturals and Synthetics
8. Omics Technologies in the Study of Fruit-Elicitor Interaction
8.1. Omics Technologies in the Induction of the Defense System by Biological Elicitors
8.2. Omics Technologies in the Induction of the Defense System by Natural Chemical Elicitors
8.3. Omics Technologies in the Induction of the Defense System by Chemical Inorganic Elicitors
9. The Importance of Knowing the Information Generated by Omics Technologies in the Interaction between Fruit and Elicitor
| Elicitor | Fruit | Gene | Enzyme | Effect | Reference |
|---|---|---|---|---|---|
| Clonostachys rosea | Tomato | PAL PPO CAT ABA | Increases in indole acetic acid (IAA), salicylic acid (SA), and NO levels | [73] | |
| Meyerozyma guilliermondii | Pear | POD CAT PAL | Inhibited the blue mold decay and induced disease resistance in the pear | [74] | |
| Pseudomonas fluorescens | Grapes | PPO POD CAT PAL CHI GLU | Cell suspension of P. fluorescens inhibited spore germination of B. cinerea, and reduced the incidence of gray grape mold | [75] | |
| Pichia guilliermondii | Peach | NPR1 AtWRKY 50 PR1 GLU CHI | SOD CAT PPO GLU PAL | Biological elicitor-activated systemic acquired resistance by the SA signaling pathway | [76] |
| Wickerhamomyces anomalous | Tomato | PPO POD CAT PAL | Reduced the gray mold decay without affecting cherry tomatoes’ quality | [77] | |
| Bacillus subtilis | Blueberry | CHI PAL POD PPO | Preventive treatment was more effective than the curative one in controlling gray mold-induced decay | [78] | |
| Bacillus halotolerans | Strawberry | PPO PAL GLU CHI | The gray mold in strawberries inoculated with B. halotolerans was lower in comparison with that in the control fruit after 4 d of incubation | [79] | |
| Trichoderma asperelloides | Muskmelon | CHI GLU | CHI GLU | Reduced disease severity against gummy stem blight by overexpressed PR genes and elevated enzyme activity | [80] |
| Burkholderia contaminans | Strawberry | PAL 4CL C4H CHI | Bulkholderia contaminants reduced the incidence of postharvest disease and promoted the accumulation of lignin and total phenols. | [81] | |
| Pichia galeiformis | Citrus | PAL 4CL C4H POD CAD | PAL 4CL C4H POD PPO CAD | P. galeiformis reduced the disease incidence and lesion diameter without direct contact with the pathogen P.digitatum. | [82] |
| Elicitor | Fruit | Gene | Enzyme | Effect | Reference |
|---|---|---|---|---|---|
| Gamma irradiation | Pear | PR-1 PR-3 PR-4 | GLU PAL POD PPO | The gamma irradiation-induced resistance against P. expansum | [83] |
| Hot water rinse brushing and UV-C | Mango | POX PAL PPO | The defense-related enzymes induced resistance was an important mechanism involved in the control of stem-end rot in mango | [84] | |
| UV-C | Mangosteen | PAL POD GLU | UV-C application improves the quality of mangosteen. | [85] | |
| Light-emitting diode (LED) | Avocado | PAL LOX | LED light application can induce fruit resistance against the postharvest disease anthracnose in avocado | [86] |
| Elicitor | Fruit | Gene | Enzyme | Effect | Reference |
|---|---|---|---|---|---|
| Quercetin | Kiwi | PR1 NPR1 CHI GLU | CHI GLU PAL PPO POD | Quercetin inhibits blue mold caused by P. expansum, which may be associated with its toxic properties and induction of defense response. | [47] |
| Indole-3-acetic-acid | Pear | Endoglu9 CHI4 PR1 PR4 PAL | GLU CHI PAL | IAA induces natural resistance of pear fruit against P. expansum and suggests that the mechanisms may be closely related to the elicitation of enzymes and defense-related genes. | [44] |
| Trisodium phosphate | Apple | SOD CAT APX GR PAL POD | Enhanced disease resistance in apple fruits by TSP against A. alternata is associated with increasing antioxidative enzyme activities and accumulation of phenylpropane metabolites | [87] | |
| Chitosan | Avocado | PAL CHI LOX | SOD CAT | The control of stem-end rot and anthracnose in avocados obtained with 1.5% chitosan can be ascribed to a combination of its antifungal and eliciting properties. | [88] |
| Salicylic acid | Longan | PLD PLC Lipase LOX | SA treatment could retain the integrity of membrane structures, enhance fruit disease resistance to P. longanae, and thus suppress disease development in P. longanae-inoculated longans during storage | [89] | |
| Benzothiazole | Orange | SOD POD CAT GLU PAL CHT | BTH had promising effects on improving resistance against postharvest blue mold disease in navel orange | [43] | |
| β-aminobutyric acid (BABA) | Apple | EF-1α PR-1 PR-2 LOX Def | BABA reduced disease symptoms caused by P. expansum, in addition to the increment in the expression of the PR-1 and LOX gene and callose opposition in the cell walls that induced resistance to the pathogen. | [90] | |
| 2,6-dichloroisonicotinic acid (INA) | Citrus | GLU CHI PAL POD PPO | Treatment reduced blue and green molds and anthracnose decay in citrus | [91] | |
| Methyl jasmonate | Sweet cherry | POD PPO SOD CAT LOX AOS OPR3 MYC2 | CAT POD SOD PPO PAL CHI GLU | MeJA reduced sweet cherry fruit spoilage and is related to its induction effect rather than its fungitoxicity effect. | [92] |
| L-glutamate | Pear | PR1 GLU CHI3 CHI4 | GLU CHI PAL POD PPO | L-glutamate at 1.00 mM induced strong resistance against blue mold rot caused by P. expansum in pear fruit under either 25 °C or 4 °C conditions and reduced spore germination of P.expansum in fruit wounds and in vitro after 24 h of treatment. | [93] |
| Carboxymethyl chitosan (CMSC) and Criptococcus laurentti | Grape | POD PPO PAL | The combination of CMSC and C. laurentii treatments can maintain fruit quality and control postharvest decay more effectively than a single treatment. | [94] | |
| Chitosan and Salicylic acid | Grape | GLU POD PAL PPO | Chitosan combined with Salicylic acid reduced the lesion diameter and disease incidence, incrementing the concentration of Salicylic acid endogenous. | [95] | |
| Pectic Oligosacharides (POs) in cold-stored | Grapes | MnSOD APX CAT GR2 | POs significantly modulated the MnSOD, APX1 and CAT1 expression levels, mainly in a storage time- and temperature-dependent manner, concerning controls. By contrast, POs only significantly affected the GR2 gene expression when grapefruit were stored at non-chilling temperatures | [96] | |
| Ozone | Satsuma mandarin | SOD GLU7 Defensin-like-protein 1 | Ozone treatment effectively delayed the fruit decay, also significantly reduced fruit respiratory intensity, delayed natural fruit degreening, and prolonged shelf-life of Satsuma mandarin fruit during postharvest storage | [56] |
10. Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bourne, M.C. Food Security: Postharvest Losses. Encycl. Agric. Food Syst. 2014, 44, 338–351. [Google Scholar] [CrossRef]
- Porat, R.; Lichter, A.; Terry, L.A.; Harker, R.; Buzby, J. Postharvest Losses of Fruit and Vegetables during Retail and in Consumers’ Homes: Quantifications, Causes, and Means of Prevention. Postharvest Biol. Technol. 2018, 139, 135–149. [Google Scholar] [CrossRef]
- FAO. Global Initiative on Food Loss and Waste Reduction What IS Food; FAO: Rome, Italy, 2015. [Google Scholar]
- Chen, T.; Ji, D.; Zhang, Z.; Li, B.; Qin, G.; Tian, S. Advances and Strategies for Controlling the Quality and Safety of Postharvest Fruit. Engineering 2021, 7, 1177–1184. [Google Scholar] [CrossRef]
- Romanazzi, G.; Sanzani, S.M.; Bi, Y.; Tian, S.; Gutiérrez Martínez, P.; Alkan, N. Induced Resistance to Control Postharvest Decay of Fruit and Vegetables. Postharvest Biol. Technol. 2016, 122, 82–94. [Google Scholar] [CrossRef]
- Meena, M.; Yadav, G.; Sonigra, P.; Nagda, A.; Mehta, T.; Swapnil, P.; Harish; Marwal, A. Role of Elicitors to Initiate the Induction of Systemic Resistance in Plants to Biotic Stress. Plant Stress 2022, 5, 2–11. [Google Scholar] [CrossRef]
- Wang, B.; Bi, Y. The Role of Signal Production and Transduction in Induced Resistance of Harvested Fruits and Vegetables. Food Qual. Saf. 2021, 5, 1–8. [Google Scholar] [CrossRef]
- Petrussa, E.; Braidot, E.; Zancani, M.; Peresson, C.; Bertolini, A.; Patui, S.; Vianello, A. Plant Flavonoids—Biosynthesis, Transport and Involvement in Stress Responses. Int. J. Mol. Sci. 2013, 14, 14950–14973. [Google Scholar] [CrossRef]
- Alfieri, F. The Role of Omic Sciences in Food Security and Sustainability. In Encyclopedia of Food Security and Sustainability; Pasquale, F., Elliot, M.B., Jock, R., Eds.; Elsevier: Washington, DC, USA, 2018; Volume 1, pp. 44–49. [Google Scholar] [CrossRef]
- Xoca-Orozco, L.Á.; Cuellar-Torres, E.A.; González-Morales, S.; Gutiérrez-Martínez, P.; López-García, U.; Herrera-Estrella, L.; Vega-Arreguin, J.; Chacón-López, A. Transcriptomic Analysis of Avocado Hass (Persea Americana Mill) in the Interaction System Fruit-Chitosan-Colletotrichum. Front. Plant Sci.|Www.Front. Org 2017, 1, 1–13. [Google Scholar] [CrossRef]
- Tunsagool, P.; Wang, X.; Leelasuphakul, W.; Jutidamrongphan, W.; Phaonakrop, N.; Jaresitthikunchai, J.; Roytrakul, S.; Chen, G.; Li, L. Metabolomic Study of Stress Responses Leading to Plant Resistance in Mandarin Fruit Mediated by Preventive Applications of Bacillus Subtilis Cyclic Lipopeptides. Postharvest Biol. Technol. 2019, 156, 1–10. [Google Scholar] [CrossRef]
- Zhou, Y.; Ma, J.; Xie, J.; Deng, L.; Yao, S.; Zeng, K. Transcriptomic and Biochemical Analysis of Highlighted Induction of Phenylpropanoid Pathway Metabolism of Citrus Fruit in Response to Salicylic Acid, Pichia Membranaefaciens and Oligochitosan. Postharvest Biol. Technol. 2018, 142, 81–92. [Google Scholar] [CrossRef]
- Andersen, E.; Ali, S.; Byamukama, E.; Yen, Y.; Nepal, M. Disease Resistance Mechanisms in Plants. Genes 2018, 9, 339. [Google Scholar] [CrossRef] [PubMed]
- Saijo, Y.; Loo, E.P. Plant Immunity in Signal Integration between Biotic and Abiotic Stress Responses. New Phytol. 2020, 225, 87–104. [Google Scholar] [CrossRef] [PubMed]
- Prusky, D.; Alkan, N.; Mengiste, T.; Fluhr, R. Quiescent and Necrotrophic Lifestyle Choice During Postharvest Disease Development. Annu. Rev. Phytopathol. 2013, 51, 155–176. [Google Scholar] [CrossRef] [PubMed]
- Alkan, N.; Fortes, A.M. Insights into Molecular and Metabolic Events Associated with Fruit Response to Post-Harvest Fungal Pathogens. Front. Plant Sci. 2015, 6, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Chen, Y.; Li, B.; Zhang, Z.; Qin, G.; Chen, T.; Tian, S. Molecular Mechanisms Underlying Multi-Level Defense Responses of Horticultural Crops to Fungal Pathogens. Hortic. Res. 2022, 9, 1–13. [Google Scholar] [CrossRef]
- Prusky, D.; Gullino, M.L. (Eds.) Post-Harvest Pathology; Springer: Dordrecht, The Netherlands, 2010; Volume 2, pp. 31–41. ISBN 978-1-4020-8929-9. [Google Scholar]
- Ramirez-Prado, J.S.; Abulfaraj, A.A.; Rayapuram, N.; Benhamed, M.; Hirt, H. Plant Immunity: From Signaling to Epigenetic Control of Defense. Trends Plant Sci. 2018, 23, 833–844. [Google Scholar] [CrossRef]
- Abdul Malik, N.A.; Kumar, I.S.; Nadarajah, K. Elicitor and Receptor Molecules: Orchestrators of Plant Defense and Immunity. Int. J. Mol. Sci. 2020, 21, 963. [Google Scholar] [CrossRef]
- Reimer-Michalski, E.-M.; Conrath, U. Innate Immune Memory in Plants. Semin. Immunol. 2016, 28, 319–327. [Google Scholar] [CrossRef]
- Trouvelot, S.; Héloir, M.-C.; Poinssot, B.; Gauthier, A.; Paris, F.; Guillier, C.; Combier, M.; Trdá, L.; Daire, X.; Adrian, M. Carbohydrates in Plant Immunity and Plant Protection: Roles and Potential Application as Foliar Sprays. Front. Plant Sci. 2014, 5, 1–14. [Google Scholar] [CrossRef]
- Vidhyasekaran, P. Plant Hormone Signaling Systems in Plant Innate Immunity; Signaling and Communication in Plants; Springer: Dordrecht, The Netherlands, 2015; Volume 2, pp. 1–26. ISBN 978-94-017-9284-4. [Google Scholar]
- Conrath, U. Molecular Aspects of Defence Priming. Trends Plant Sci. 2011, 16, 524–531. [Google Scholar] [CrossRef]
- Dolgikh, V.A.; Pukhovaya, E.M.; Zemlyanskaya, E.V. Shaping Ethylene Response: The Role of EIN3/EIL1 Transcription Factors. Front. Plant Sci. 2019, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ji, D.; Chen, T.; Li, B.; Zhang, Z.; Qin, G.; Tian, S. Production, Signaling, and Scavenging Mechanisms of Reactive Oxygen Species in Fruit–Pathogen Interactions. Int. J. Mol. Sci. 2019, 20, 2994. [Google Scholar] [CrossRef] [PubMed]
- Ku, Y.-S.; Sintaha, M.; Cheung, M.-Y.; Lam, H.-M. Plant Hormone Signaling Crosstalks between Biotic and Abiotic Stress Responses. Int. J. Mol. Sci. 2018, 19, 3206. [Google Scholar] [CrossRef]
- Aylward, J.; Steenkamp, E.T.; Dreyer, L.L.; Roets, F.; Wingfield, B.D.; Wingfield, M.J. A Plant Pathology Perspective of Fungal Genome Sequencing. IMA Fungus 2017, 8, 1–15. [Google Scholar] [CrossRef]
- Zhang, Z.-Q.; Chen, T.; Li, B.Q.; Qin, G.Z.; Tian, S.P. Molecular Basis of Pathogenesis of Postharvest Pathogenic Fungi and Control Strategy in Fruits: Progress and Prospect. Mol. Hortic. 2021, 1, 1–10. [Google Scholar] [CrossRef]
- Thera, U.; Sowmya, V.; Mounika, A.; Timsina, A.; Aravind, K. Postharvest Diseases: A Threat to the Global Food Security. In Current Research and Innovations in Plant Pathology; Kumar, S., Ed.; AkiNik Publications: New Delhi, India, 2020; Volume 8, pp. 169–189. ISBN 978-93-90846-84-9. [Google Scholar]
- Singh, D.; Sharma, R.R. Postharvest Diseases of Fruits and Vegetables and Their Management. In Postharvest Desinfection of Fruits and Vegetables; Siddiqui, M., Ed.; Elsevier: Amsterdam, The Netherlands; Academic Press: Sabour, India, 2018; Volume 1, pp. 1–52. ISBN 978-0-12-812698-1. [Google Scholar]
- Ngolong Ngea, G.L.; Qian, X.; Yang, Q.; Dhanasekaran, S.; Ianiri, G.; Ballester, A.; Zhang, X.; Castoria, R.; Zhang, H. Securing Fruit Production: Opportunities from the Elucidation of the Molecular Mechanisms of Postharvest Fungal Infections. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2508–2533. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Torres, R.; Ballester, A.-R.; Li, B.; Vilanova, L.; González-Candelas, L. Molecular Aspects in Pathogen-Fruit Interactions: Virulence and Resistance. Postharvest Biol. Technol. 2016, 122, 11–21. [Google Scholar] [CrossRef]
- Ma, L.; He, J.; Liu, H.; Zhou, H. The Phenylpropanoid Pathway Affects Apple Fruit Resistance to Botrytis Cinerea. J. Phytopathol. 2018, 166, 206–215. [Google Scholar] [CrossRef]
- Liu, J.; Sui, Y.; Chen, H.; Liu, Y.; Liu, Y. Proteomic Analysis of Kiwifruit in Response to the Postharvest Pathogen, Botrytis Cinerea. Front. Plant Sci. 2018, 9, 1–18. [Google Scholar] [CrossRef]
- dos S. Costa, D.; Alviano Moreno, D.S.; Alviano, C.S.; da Silva, A.J.R. Extension of Solanaceae Food Crops Shelf Life by the Use of Elicitors and Sustainable Practices During Postharvest Phase. Food Bioprocess Technol. 2021, 15, 249–274. [Google Scholar] [CrossRef]
- Yang, B.; Yang, S.; Zheng, W.; Wang, Y. Plant Immunity Inducers: From Discovery to Agricultural Application. Stress Biol. 2022, 2, 1–13. [Google Scholar] [CrossRef]
- Köhl, J.; Kolnaar, R.; Ravensberg, W.J. Mode of Action of Microbial Biological Control Agents against Plant Diseases: Relevance beyond Efficacy. Front. Plant Sci. 2019, 10, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Jing, M.; Huang, B.; Li, W.; Zeng, J.; Shao, Y. Biocontrol of Cladosporium Cladosporioides of Mango Fruit with Bacillus Atrophaeus TE7 and Effects on Storage Quality. Curr. Microbiol. 2021, 78, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, J.; Xie, J.; Deng, L.; Yao, S.; Zeng, K. Biocontrol Efficacy Of Pichia Membranaefaciens and Kloeckera Apiculata against Monilinia Fructicola and Their Ability to Induce Phenylpropanoidpathway in Plum Fruit. Biol. Control. 2019, 129, 83–91. [Google Scholar] [CrossRef]
- Scott, G.; Rupar, M.; Fletcher, A.G.D.; Dickinson, M.; Shama, G. A Comparison of Low Intensity UV-C and High Intensity Pulsed Polychromatic Sources as Elicitors of Hormesis in Tomato Fruit. Postharvest Biol. Technol. 2017, 125, 52–58. [Google Scholar] [CrossRef]
- Pétriacq, P.; López, A.; Luna, E. Plants Fruit Decay to Diseases: Can Induced Resistance and Priming Help? Plants 2018, 7, 77. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Sun, Y.; Yang, R.; Zhang, W.; Wan, C.; Chen, J.; Kahramanoǧlu, İ.; Zhu, L. Benzothiazole (BTH) Induced Resistance of Navel Orange Fruit and Maintained Fruit Quality during Storage. J. Food Qual. 2021, 2021, 1–8. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, L.; Sun, C.; Jin, L.; Lin, M.; Huang, Y.; Zheng, X.; Yu, T. Indole-3-Acetic Acid Inhibits Blue Mold Rot by Inducing Resistance in Pear Fruit Wounds. Sci. Hortic. 2018, 231, 227–232. [Google Scholar] [CrossRef]
- Zheng, F.; Chen, L.; Zhang, P.; Zhou, J.; Lu, X.; Tian, W. Carbohydrate Polymers Exhibit Great Potential as Effective Elicitors in Organic Agriculture: A Review. Carbohydr. Polym. 2020, 230, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Cuéllar-Torres, E.A.; Aguilera-Aguirre, S.; Bañuelos-Gonzáles, M.D.C.; Xoca-Orozco, L.Á.; Ortiz-Basurto, R.I.; Efigenia, M.-G.; Vega-Arreguín, J.; Chacón-López, M.A. Postharvest Application Effect of Agave Fructans on Anthracnose Disease, Defense—Related Enzyme Activities, and Quality Attributes in Avocado Fruit. Food Sci. Biotechnol. 2022, 31, 1411–1421. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xu, L.; Zhang, L.; Guo, Y.; Qi, X.; He, L. Effects of Quercetin on Postharvest Blue Mold Control in Kiwifruit. Sci. Hortic. 2018, 228, 18–25. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, H.; Yue, Z.; Wang, X.; Zhou, H. The Effects of Caffeic Acid and Epicatechin Treatment on Gray Mold Resistance and Antioxidant Metabolism in Apples. J. Plant Pathol. 2022, 104, 661–670. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Y.; Jiang, H.; Song, R.; Liu, Y.; Guo, H.; Meng, D. Antimicrobial Peptide CB-M Exhibits Direct Antifungal Activity against Botrytis Cinerea and Induces Disease Resistance to Gray Mold in Cherry Tomato Fruit. Postharvest Biol. Technol. 2023, 196, 1–10. [Google Scholar] [CrossRef]
- Dou, Y.; Routledge, M.N.; Gong, Y.; Godana, E.A.; Dhanasekaran, S.; Yang, Q.; Zhang, X.; Zhang, H. Efficacy of Epsilon-Poly-L-Lysine Inhibition of Postharvest Blue Mold in Apples and Potential Mechanisms. Postharvest Biol. Technol. 2020, 171, 1–10. [Google Scholar] [CrossRef]
- Scariotto, S.; Tomazeli, V.N.; Paladini, M.V.; de Oliveira Bolina, C.; Sobrinho, R.L.; da Silva, E.P.; Dallacorte, L.V.; de Cássia Oliveira, M.; dos Santos, I.; Marchese, J.A. Plant Innate Immunity in Strawberry Induced by Pathogen-Associated Molecular Pattern Harpin and Acibenzolar-S-Methyl. Theor. Exp. Plant Physiol. 2021, 33, 357–367. [Google Scholar] [CrossRef]
- Tesfay, S.Z.; Bertling, I.; Bower, J.P. Effects of Postharvest Potassium Silicate Application on Phenolics and Other Anti-Oxidant Systems Aligned to Avocado Fruit Quality. Postharvest Biol. Technol. 2011, 60, 92–99. [Google Scholar] [CrossRef]
- Youssef, K.; Sanzani, S.M.; Ligorio, A.; Ippolito, A.; Terry, L.A. Sodium Carbonate and Bicarbonate Treatments Induce Resistance to Postharvest Green Mould on Citrus Fruit. Postharvest Biol. Technol. 2014, 87, 61–69. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, S.; Zeng, K. Exogenous Nitric Oxide-Induced Postharvest Disease Resistance in Citrus Fruit to Colletotrichum Gloeosporioides. J. Sci. Food Agric. 2016, 96, 505–512. [Google Scholar] [CrossRef]
- Wu, Q.; Zhu, X.; Gao, H.; Zhang, Z.; Zhu, H.; Duan, X.; Qu, H.; Yun, Z.; Jiang, Y. Comparative Profiling of Primary Metabolites and Volatile Compounds in Satsuma Mandarin Peel after Ozone Treatment. Postharvest Biol. Technol. 2019, 153, 1–12. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Z.; Li, X.; Wei, J.; Wu, B. Effect of Nitrous Oxide against Botrytis Cinerea and Phenylpropanoid Pathway Metabolism in Table Grapes. Sci. Hortic. 2019, 254, 99–105. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Q.; Guo, Y.; Kang, L.; Yu, Y. Carbon Monoxide Enhances the Resistance of Jujube Fruit against Postharvest Alternaria Rot. Postharvest Biol. Technol. 2020, 168, 1–6. [Google Scholar] [CrossRef]
- Waewthongrak, W.; Leelasuphakul, W.; McCollum, G. Cyclic Lipopeptides from Bacillus Subtilis ABS–S14 Elicit Defense-Related Gene Expression in Citrus Fruit. PLoS ONE 2014, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tunsagool, P.; Leelasuphakul, W.; Jaresitthikunchai, J.; Phaonakrop, N.; Roytrakul, S.; Jutidamrongphan, W. Targeted Transcriptional and Proteomic Studies Explicate Specific Roles of Bacillus Subtilis Iturin A, Fengycin, and Surfactin on Elicitation of Defensive Systems in Mandarin Fruit during Stress. PLoS ONE 2019, 14, 1–21. [Google Scholar] [CrossRef]
- Tunsagool, P.; Jutidamrongphan, W.; Phaonakrop, N.; Jaresitthikunchai, J.; Roytrakul, S.; Leelasuphakul, W. Insights into Stress Responses in Mandarins Triggered by Bacillus Subtilis Cyclic Lipopeptides and Exogenous Plant Hormones upon Penicillium Digitatum Infection. Plant Cell Rep. 2019, 38, 559–575. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.-Y.; Dou, G.-X.; Sun, X.-X.; Chen, L.; Zheng, Y.; Xiao, H.-M.; Wang, Y.-P.; Li, H.-Y.; Guo, J.-H.; Jiang, C.-H. Transcriptome and Biochemical Analysis Jointly Reveal the Effects of Bacillus Cereus AR156 on Postharvest Strawberry Gray Mold and Fruit Quality. Front. Plant Sci. 2021, 12, 1–14. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, R.; Tang, Y.; Cheng, Z.; Qian, M.; Li, W.; Shao, Y. Transcriptome Analysis Reveals the Inducing Effect of Bacillus Siamensis on Disease Resistance in Postharvest Mango Fruit. Foods 2022, 11, 107. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, L.; Sun, Y.; Zhao, L.; Zheng, X.; Yang, Q.; Zhang, X. Investigating Proteome and Transcriptome Defense Response of Apples Induced by Yarrowia Lipolytica. Mol Plant Microbe In 2017, 30, 301–311. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, F.; Gu, N.; Yan, X.; Wang, K.; Dhanasekaran, S.; Gu, X.; Zhao, L.; Zhang, H. Postharvest Biological Control of Rhizopus Rot and the Mechanisms Involved in Induced Disease Resistance of Peaches by Pichia Membranefaciens. Postharvest Biol. Technol. 2020, 163, 1–14. [Google Scholar] [CrossRef]
- Zhang, H.; Li, R.; Liu, W. Effects of Chitin and Its Derivative Chitosan on Postharvest Decay of Fruits: A Review. Int. J. Mol. Sci. 2011, 12, 917–934. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, P.; Zhang, P.; Su, L.; Jia, H.; Wei, X.; Fang, J.; Jia, H. Integrative Transcriptomics and Metabolomics Data Exploring the Effect of Chitosan on Postharvest Grape Resistance to Botrytis Cinerea. Postharvest Biol. Technol. 2020, 167, 1–14. [Google Scholar] [CrossRef]
- Lu, L.; Ji, L.; Shi, R.; Li, S.; Zhang, X.; Guo, Q.; Wang, C.; Qiao, L. Dextran as an Elicitor of Phenylpropanoid and Flavonoid Biosynthesis in Tomato Fruit against Gray Mold Infection. Carbohydr. Polym. 2019, 225, 1–9. [Google Scholar] [CrossRef]
- Zhao, L.; Han, J.; Li, B.; Zhang, X.; Gu, X.; Yang, Q.; Wang, K.; Zhang, H. Transcriptome Analysis of the Disease Resistance in Postharvest Pears Induced by Meyerozyma Guilliermondii Combined with Alginate Oligosaccharide. Biol. Control. 2022, 170, 1–13. [Google Scholar] [CrossRef]
- Lyu, L.; Bi, Y.; Li, S.; Xue, H.; Li, Y.; Prusky, D.B. Sodium Silicate Prime Defense Responses in Harvested Muskmelon by Regulating Mitochondrial Energy Metabolism and Reactive Oxygen Species Production. Food Chem. 2019, 289, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Khan, A.S.; Malik, A.U.; Nawaz, A.; Shahid, M. Postharvest Application of Antibrowning Chemicals Modulates Oxidative Stress and Delays Pericarp Browning of Controlled Atmosphere Stored Litchi Fruit. J. Food Biochem. 2019, 43, 1–14. [Google Scholar] [CrossRef]
- Bang, J.; Lim, S.; Yi, G.; Lee, J.G.; Lee, E.J. Integrated Transcriptomic-Metabolomic Analysis Reveals Cellular Responses of Harvested Strawberry Fruit Subjected to Short-Term Exposure to High Levels of Carbon Dioxide. Postharvest Biol. Technol. 2019, 148, 120–131. [Google Scholar] [CrossRef]
- Xoca-Orozco, L.-Á.; Aguilera-Aguirre, S.; Vega-Arreguín, J.; Acevedo-Hernández, G.; Tovar-Pérez, E.; Stoll, A.; Herrera-Estrella, L.; Chacón-López, A. Activation of the Phenylpropanoid Biosynthesis Pathway Reveals a Novel Action Mechanism of the Elicitor Effect of Chitosan on Avocado Fruit Epicarp. Food Res. Int. 2019, 121, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Liu, Y.; Liu, S.-Y.; Cheng, M.-Z.; Zhang, Y.; Wang, R.-H.; Chen, H.-Y.; Li, J.-F.; Chen, X.-L.; Wang, A.-X. Analysis of Clonostachys Rosea-Induced Resistance to Grey Mould Disease and Identification of the Key Proteins Induced in Tomato Fruit. Postharvest Biol. Technol. 2017, 123, 83–93. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, X.; Zheng, X.; Apaliya, M.T.; Yang, Q.; Zhao, L.; Gu, X.; Zhang, H. Control of Postharvest Blue Mold Decay in Pears by Meyerozyma Guilliermondii and It’s Effects on the Protein Expression Profile of Pears. Postharvest Biol. Technol. 2018, 136, 124–131. [Google Scholar] [CrossRef]
- Jiang, M.Y.; Wang, Z.R.; Chen, K.W.; Kan, J.Q.; Wang, K.T.; Zalán, Z.S.; Hegyi, F.; Takács, K.; Du, M.Y. Inhibition of Postharvest Gray Mould Decay and Induction of Disease Resistance by Pseudomonas Fluorescens in Grapes. Acta Aliment. 2019, 48, 288–296. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Y.; Zhang, B. Induced Resistance in Peach Fruit as Treated by Pichia Guilliermondii and Their Possible Mechanism. Int. J. Food Prop. 2020, 23, 34–51. [Google Scholar] [CrossRef]
- Raynaldo, F.A.; Dhanasekaran, S.; Ngea, G.L.N.; Yang, Q.; Zhang, X.; Zhang, H. Investigating the Biocontrol Potentiality of Wickerhamomyces Anomalus against Postharvest Gray Mold Decay in Cherry Tomatoes. Sci. Hortic. 2021, 285, 1–6. [Google Scholar] [CrossRef]
- Lu, Y.; Ma, D.; He, X.; Wang, F.; Wu, J.; Liu, Y.; Jiao, J.; Deng, J. Bacillus Subtilis KLBC BS6 Induces Resistance and Defence-Related Response against Botrytis Cinerea in Blueberry Fruit. Physiol. Mol. Plant Pathol. 2020, 114, 1–9. [Google Scholar] [CrossRef]
- Wang, F.; Xiao, J.; Zhang, Y.; Li, R.; Liu, L.; Deng, J. Biocontrol Ability and Action Mechanism of Bacillus Halotolerans against Botrytis Cinerea Causing Grey Mould in Postharvest Strawberry Fruit. Postharvest Biol. Technol. 2021, 174, 1–9. [Google Scholar] [CrossRef]
- Intana, W.; Wonglom, P.; Suwannarach, N.; Sunpapao, A. Trichoderma Asperelloides PSU-P1 Induced Expression of Pathogenesis-Related Protein Genes against Gummy Stem Blight of Muskmelon (Cucumis Melo) in Field Evaluation. J. Fungi 2022, 8, 156. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shi, J.; Wang, R. Effect of Burkholderia Contaminans on Postharvest Diseases and Induced Resistance of Strawberry Fruits. Plant Pathol. J. 2018, 34, 403–411. [Google Scholar] [CrossRef]
- Chen, O.; Deng, L.; Ruan, C.; Yi, L.; Zeng, K. Pichia Galeiformis Induces Resistance in Postharvest Citrus by Activating the Phenylpropanoid Biosynthesis Pathway. J. Agric. Food Chem. 2021, 69, 2619–2631. [Google Scholar] [CrossRef]
- Jeong, M.-A.; Jeong, R.-D. Applications of Ionizing Radiation for the Control of Postharvest Diseases in Fresh Produce: Recent Advances. Plant Pathol. 2018, 67, 18–29. [Google Scholar] [CrossRef]
- Terao, D.; de Lima Nechet, K.; Frighetto, R.T.S.; Anjos, V.D.D.A.; Benato, E.A.; Halfeld-Vieira, B.D.A. Physical Postharvest Treatments in the Control of Stem-End Rot of Mango. J. Phytopathol. 2018, 166, 581–589. [Google Scholar] [CrossRef]
- Sripong, K.; Jitareerat, P.; Uthairatanakij, A. UV Irradiation Induces Resistance against Fruit Rot Disease and Improves the Quality of Harvested Mangosteen. Postharvest Biol. Technol. 2019, 149, 187–194. [Google Scholar] [CrossRef]
- Mpai, S.; Sivakumar, D. Stimulation of Light-Emitting Diode Treatment on Defence System and Changes in Mesocarp Metabolites of Avocados Cultivars (Hass and Fuerte) during Simulated Market Shelf Conditions. Agronomy 2020, 10, 1654. [Google Scholar] [CrossRef]
- Ge, Y.; Chen, Y.; Li, C.; Wei, M.; Li, X.; Tang, Q.; Duan, B. Effect of Trisodium Phosphate Treatment on Black Spot of Apple Fruit and the Roles of Anti-Oxidative Enzymes. Physiol. Mol. Plant Pathol. 2019, 106, 226–231. [Google Scholar] [CrossRef]
- Obianom, C.; Romanazzi, G.; Sivakumar, D. Effects of Chitosan Treatment on Avocado Postharvest Diseases and Expression of Phenylalanine Ammonia-Lyase, Chitinase and Lipoxygenase Genes. Postharvest Biol. Technol. 2019, 147, 214–221. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, J.; Lin, H.; Lin, M.; Lin, Y.; Wang, H.; Hung, Y.C. Salicylic Acid Treatment Suppresses Phomopsis Longanae Chi-Induced Disease Development of Postharvest Longan Fruit by Modulating Membrane Lipid Metabolism. Postharvest Biol. Technol. 2020, 164, 1–9. [Google Scholar] [CrossRef]
- Quaglia, M.; Baglivo, F.; Moretti, C. Postharvest β-Aminobutyric-Acid–Primed Resistance Is Not Effective in the Control of Penicillium Expansum Link. on ‘Golden Delicious’ Apple Fruit. Crop. Prot. 2017, 102, 43–48. [Google Scholar] [CrossRef]
- Jing, J.; Zhang, H.; Xue, Y.; Zeng, K. Effects of INA on postharvest blue and green molds and anthracnose decay in citrus fruit. J. Integr Agr. 2019, 19, 1396–1406. [Google Scholar] [CrossRef]
- Pan, L.; Chen, X.; Xu, W.; Fan, S.; Wan, T.; Zhang, J.; Cai, Y. Methyl Jasmonate Induces Postharvest Disease Resistance to Decay Caused by Alternaria Alternata in Sweet Cherry Fruit. Sci. Hortic. 2021, 292, 1–9. [Google Scholar] [CrossRef]
- Jin, L.; Cai, Y.; Sun, C.; Huang, Y.; Yu, T. Exogenous L-Glutamate Treatment Could Induce Resistance against Penicillium Expansum in Pear Fruit by Activating Defense-Related Proteins and Amino Acids Metabolism. Postharvest Biol. Technol. 2019, 150, 148–157. [Google Scholar] [CrossRef]
- Wang, F.; Deng, J.; Jiao, J.; Lu, Y.; Yang, L.; Shi, Z. The Combined Effects of Carboxymethyl Chitosan and Cryptococcus Laurentii Treatment on Postharvest Blue Mold Caused by Penicillium Italicum in Grapefruit Fruit. Sci. Hortic. 2019, 253, 35–41. [Google Scholar] [CrossRef]
- Shi, Z.; Wang, F.; Lu, Y.; Deng, J. Combination of Chitosan and Salicylic Acid to Control Postharvest Green Mold Caused by Penicillium Digitatum in Grapefruit Fruit. Sci. Hortic. 2018, 233, 54–60. [Google Scholar] [CrossRef]
- Vera-Guzmán, A.M.; Aispuro-Hernández, E.; Vargas-Arispuro, I.; Islas-Osuna, M.A.; Martínez-Téllez, M.Á. Expression of Antioxidant-Related Genes in Flavedo of Cold-Stored Grapefruit (Citrus Paradisi Macfad Cv. Rio Red) Treated with Pectic Oligosaccharides. Sci. Hortic. 2019, 243, 274–280. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuéllar-Torres, E.A.; Aguilera-Aguirre, S.; Hernández-Oñate, M.Á.; López-García, U.M.; Vega-Arreguín, J.; Montalvo-González, E.; Ortiz-Basurto, R.I.; Chacón-López, A. Molecular Aspects Revealed by Omics Technologies Related to the Defense System Activation in Fruits in Response to Elicitors: A Review. Horticulturae 2023, 9, 558. https://doi.org/10.3390/horticulturae9050558
Cuéllar-Torres EA, Aguilera-Aguirre S, Hernández-Oñate MÁ, López-García UM, Vega-Arreguín J, Montalvo-González E, Ortiz-Basurto RI, Chacón-López A. Molecular Aspects Revealed by Omics Technologies Related to the Defense System Activation in Fruits in Response to Elicitors: A Review. Horticulturae. 2023; 9(5):558. https://doi.org/10.3390/horticulturae9050558
Chicago/Turabian StyleCuéllar-Torres, Esther Angélica, Selene Aguilera-Aguirre, Miguel Ángel Hernández-Oñate, Ulises Miguel López-García, Julio Vega-Arreguín, Efigenia Montalvo-González, Rosa Isela Ortiz-Basurto, and Alejandra Chacón-López. 2023. "Molecular Aspects Revealed by Omics Technologies Related to the Defense System Activation in Fruits in Response to Elicitors: A Review" Horticulturae 9, no. 5: 558. https://doi.org/10.3390/horticulturae9050558
APA StyleCuéllar-Torres, E. A., Aguilera-Aguirre, S., Hernández-Oñate, M. Á., López-García, U. M., Vega-Arreguín, J., Montalvo-González, E., Ortiz-Basurto, R. I., & Chacón-López, A. (2023). Molecular Aspects Revealed by Omics Technologies Related to the Defense System Activation in Fruits in Response to Elicitors: A Review. Horticulturae, 9(5), 558. https://doi.org/10.3390/horticulturae9050558