Abstract
Fertilization programs in lilium are suggested to start after shoot emergence or when the flower buds become visible because the nutrients stored in the bulb are adequate to meet plant demands at the transplant time. Defining plant nutrient uptake is essential to determine the periods of high demand and the amounts at which they should be provided. The objective of this study was to model the nutrients accumulated in Oriental lilium to provide insight into the design of environmentally sound fertilization programs. The most demanded macronutrient was K (1272.8 mg/plant), followed by N (719.1 mg/plant) and Ca (119.7 mg/plant), while Zn (140.7 mg/plant) and Fe (137.7 mg/plant) were the most demanded micronutrients. At the end of the season, most of the Fe (78.0%), P (55.0%) and N (54.3%) originated from the bulb, whereas most of the Ca (86.5%), Mn (84.8%) and Mg (62.9%) were uptaken by roots. During the first 15 days after transplant, 35.1% of the N in the shoot was absorbed from the substrate, as well as 91.0% Mg, 68.6% S, 49.6% K and 13.0% P, suggesting that fertilization for lilium should start at the transplant time. The results suggest that Ca, Fe, Zn and Cu were remobilized from the bulb.
1. Introduction
The main challenge facing agriculture in the near future focuses on the production of enough food, fiber and fuel for a growing population, balanced against acceptable environmental costs [1]. The production of food, fibers and industrial crops is currently dependent on the provision of mineral and/or organic fertilizers to support plant growth; however, society is becoming more concerned about the environmental fate of fertilizers and pesticides used during crop production [2].
One of the most important aspects of environmental sustainability is the efficient use of nutrients by crops while preventing losses from the soil and damage to the environment [3]. Excessive and deficient nitrogen (N) fertilization can cause growth impairment; for example, deficient N fertilization decreases the production and quality of cotton, whereas excess N prolongs the vegetative period, causing maturity delays, a decreased sugar content and increased pests attacks or diseases [4]. Phosphorus (P) and N runoff caused by excess fertilization has been reported to cause cyanobacteria to bloom in lakes, resulting in undrinkable water and negatively affecting municipal and industrial use [5,6].
Higher nutrient use efficiency needs to be addressed through the development of the best management practices that can increase productivity and profitability by optimizing the amounts of fertilizers and by matching the timing of nutrient applications to plant demand [3]. The use of the 4R fertilization technology is considered an improved management technique that may increase nutrient use efficiency [7], following the principles of the right time, type, amount and placement of fertilizers [2]. Therefore, it is essential to determine the rates of nutrient uptake during different periods of the crop production cycle [8].
Nutrient accumulation patterns follow dry matter accumulation according to the growth stages of crops [9]. According to Arunachalam and Chavan [10], the fertilizer requirements are calculated through models based on the total nutrient requirements at harvest; however, we feel that it should be calculated according to the nutrient demands at each phenological phase in order to synchronize the crop nutrient demand with the fertilizer rate and application method.
Nutrient accumulation through the growing season has been determined mainly for horticultural crops, including tomato [11], cabbage [12], onion [10] and potato [13], and for grain or cereal crops such as soybean [14,15,16], corn [17], dry bean [9] and sesame [18]. However, little information for nutrient accumulation in ornamental species has been published, although there are some studies that focus on poinsettia [19], chrysanthemum [20] and lisianthus [21].
Lilium is one of the most important cut flowers in the worldwide ornamental market [22]. It is a geophyte that develops an underground bulb with no external covering and is formed by scales, modified leaves and a basal plate [23,24]. This non-tunicate bulb is a storage organ that accumulates starch, polysaccharides, proteins, amino acids, phospholipids [25], hormones and mineral nutrients that can be used to sustain plant growth during the following season once cold requirements for vernalization have been met. Due to the mineral nutrient content in the bulb, it is suggested that fertilization programs for lilium should start after shoot emergence or when the flower bud becomes visible [26]. Fertigation with solutions containing 200 mg L−1 of N using a two-to-one ratio fertilizer of calcium nitrate and potassium nitrate is recommended [24]. However, no fertilization programs suggested for cut lilium flower production are based on the actual extraction of mineral nutrients from the plant.
The objective of the present study was to determine the amount of macro- and micronutrients accumulated in plants of Oriental lilium and to model the extraction of such nutrients in order to identify the critical phases at which they are most demanded, thus providing insight into the design of environmentally sound fertilization programs.
2. Materials and Methods
2.1. Study Site and Plant Material
The experiment was carried out from May to August 2020 in a tunnel-type greenhouse at Universidad Autónoma Agraria Antonio Narro in northeast México (lat. 25°21′24″ N, long. 101°02′05″ W, 1765 m above sea level). The average maximum/minimum temperature was 28/10 °C, and the maximum and minimum relative humidity for the experiment duration averaged 75% and 45%, respectively. The average photosynthetically active radiation measured at solar noon was 302 μmol m−2 s−1.
2.2. Cultural Conditions
Bulbs of Oriental lilium (Lilium orientalis ‘Sorbone’) which were 20–22 cm in circumference were planted in a mixture of sphagnum peat (PREMIER, Premier Tech, Toronto, ON, Canada) and horticultural-grade perlite (HORTIPERL, Termolita, Monterrey, Mexico) using rigid plastic containers (30 cm width × 48 cm length × 27 cm depth (38.8 L)). Mixture pH and electrical conductivity (EC) were adjusted to 5.5 and 0.35 dS m−1, respectively. A total of 10 bulbs per container were placed over a 7.5 cm substrate layer and then covered with an additional 15 cm layer on top of them. The plant density was maintained at 69 bulbs per m2.
The plants were irrigated with a complete nutrient solution (10 meq L−1 NO3−, 1 meq L−1 H2PO4−, 6 meq L−1 SO4−2, 7 meq L−1 K+, 7 meq L−1 Ca+2, 3 meq L−1 Mg+2), including micronutrients (EDTA chelates of iron, zinc, manganese, and copper at 5, 0.5, 0.01, and 0.02 ppm, respectively). The nutrient solution was prepared with potable water but considering its chemical properties for the supply of nutrients. The pH of the nutrient solution was adjusted to 5.3, and the EC was maintained at 2.5 dS m−1. The nutrient solution was applied through an automated drip irrigation system dispensing 4 L h−1, with four drip stakes installed per container.
2.3. Sampling and Processing of Samples
During the study period, four complete plants were sampled every 15 days starting from the transplanting day (day zero) and then at 15, 30, 45, 60, 75, 90 and 105 days after the transplant (DAT). Plants located in the intermediate zone of each container were sampled, thereby maintaining consistent competition. Sampled plants were then washed to remove the substrate from the root zone, separated by plant parts and placed in an oven, where they were dried at 75 °C for 72 h (the bulbs were kept from 120 to 168 h).
The dried material was ground to pass a 40-mesh sieve (Mini Willey Mill, Thomas Scientific, Swedesboro, NJ, USA), and 0.25 g of the bulb, roots, stem, leaves and flowers ground tissues from the four sampled plants at each sampling date were digested separately in a 5 mL mixture of H2SO4 and 1 mL H2O2 (and in a mixture of HNO3 and HClO4 for S analysis). The digests were made up to 25 mL and filtered prior to the determination of P, potassium (K), calcium (Ca), magnesium (Mg), sulfur (S), iron (Fe), zinc (Zn), copper (Cu) and manganese (Mn) with an Inductively Coupled Plasma Emission Spectrometer (Agilent 725-ES ICP-OES, Mulgrave, Victoria, Australia) [27]. The nitrogen concentration was determined with the semi-micro Kjeldalh procedure [28].
2.4. Modeling of Nutrient Accumulation
The results obtained from the nutrient analysis were transformed from concentration units to nutrient content per plant part and per total plant content. The data were then modeled with Sigma Plot 12.5 using the stepwise procedure, selecting those models that better fit the average of the four plant samples.
3. Results and Discussion
3.1. Nitrogen
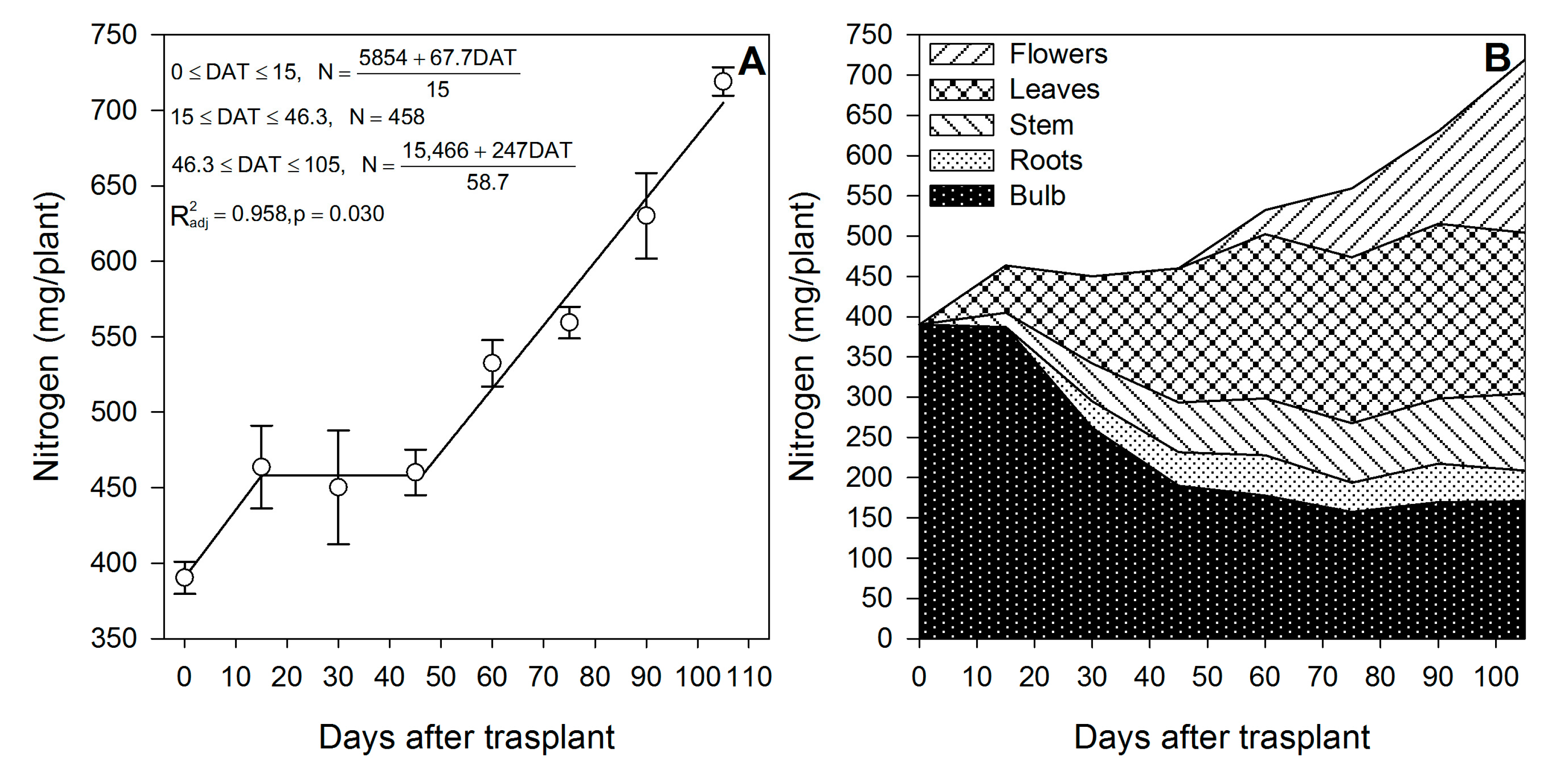
The models indicate that there are two phases in which N is absorbed by lilium (Figure 1A); the first phase is from 0 to 15 DAT, when bulbs sprouted and developed the first leaves and roots, while the second phase is estimated from ~46 DAT up to the end of the growing season, when plants continued developing leaves and flowers (Figure 1A). During the first 15 DAT, N was rapidly absorbed at a rate of 4.51 mg/plant/day; however, as observed in Figure 1B, there was not a significant change in the N content in the bulb, suggesting adequate N uptake from the substrate. This rapid but short N accumulation phase was followed by a phase in which N was not absorbed, from 15 to ~46 DAT (Figure 1A), indicating that the N for the formation of new leaves, stems and roots was remobilized from the bulb, as suggested by the sharp depletion of 51% of the N stored in the bulb during this time period (Figure 1B).
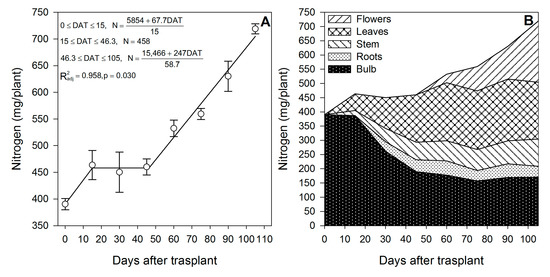
Figure 1.
Nitrogen accumulation by lilium: (A) Segmented analysis of nitrogen accumulation over the growing season; (B) Nitrogen distribution in the lilium plant parts. Day 0 = transplant day, days 0 to 15 = sprouting and initial vegetative growth, days 15 to 60 = rapid vegetative growth plus flower buds formation, days 60 to 105 = blooming. DAT = days after transplant.
After this period of initial N demand, there was a 30-day period in which there was no N accumulation (Figure 1A); however, from approximately day 46 up to the end of the growing season, there was an N accumulation rate similar to that of the first 15 DAT (4.21 mg/plant/day) (Figure 1A). This N was mobilized to the leaves and the flower buds once the plants started blooming at day 60 (Figure 1B). The increase in the N accumulation rate during the flower development may be associated with the synthesis of N-containing compounds reported in the petals of other lilium species such as L. candida [29,30]; in fact, the composition of volatile compounds produced by lilium flowers is reported to be affected by an adequate provision of N, as feeding lilium with 12 mM of N increased the proportion of 1,8 cineole, β ocimene and terpineol, while it decreased that of linalool, 2-methoxy p-cresol, 2-4 dimethylben zaldehyde and nonanal [31]. The N for flower development was provided by the uptake from the growing medium, as there seems to be limited N remobilization from the bulb. We suggest that this N probably also came from the bulb, but it was rapidly absorbed from the substrate, restoring the amount of N accumulated in this plant part (Figure 1B).
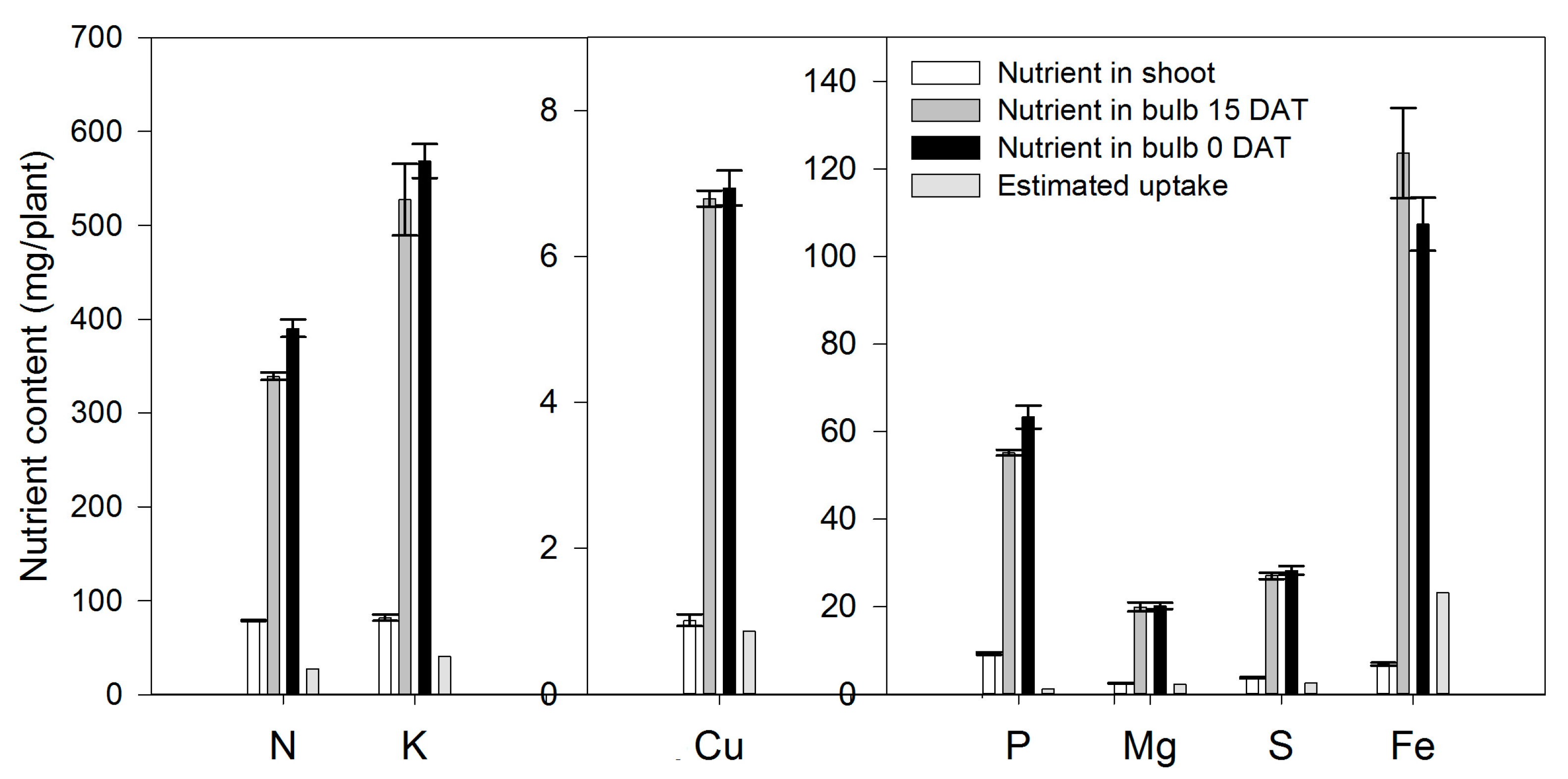
Lilium bulbs are considered a storage organ [32] in which nutrients, water, hormones and carbohydrates may be accumulated. According to our results, lilium plants rely on the bulb as a source of the N. At the end of the growing season, 54.3% of the total N measured in the whole plant was already present in the bulb (Table 1). In contrast to the general statement of no fertilization needed by lilium during the initial 21 days [33], our results indicate that, during the first 15 DAT, the aerial parts of the plant contained 78.84 mg N (Figure 2). However, only 64.9% originated from the bulb; therefore 35.1% of the N demanded for the shoot growth during sprouting had to be absorbed by the roots. These results suggest that fertigation with N should be started early in the season. Flowering was a developmental phase in which 78.8% of the total N was accumulated in lilium plants during this phase.

Table 1.
Total macronutrients accumulated by lilium plants (mean ± standard error) at the end of the growing season and the amount provided by the bulb or absorbed from the growing medium.
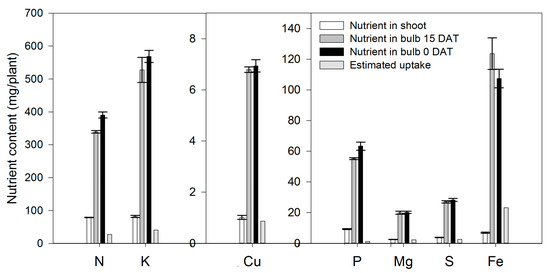
Figure 2.
Total nutrient content in the shoot (leaves + stem) and in the bulb of lilium plants at 15 days after transplant (15 DAT) and at transplant time (0 DAT). Estimated uptake was calculated by the difference between the nutrient content in the shoot and the change in nutrient content in the bulb from 0 to 15 DAT. Bars represent the standard error of the mean.
3.2. Phosphorus
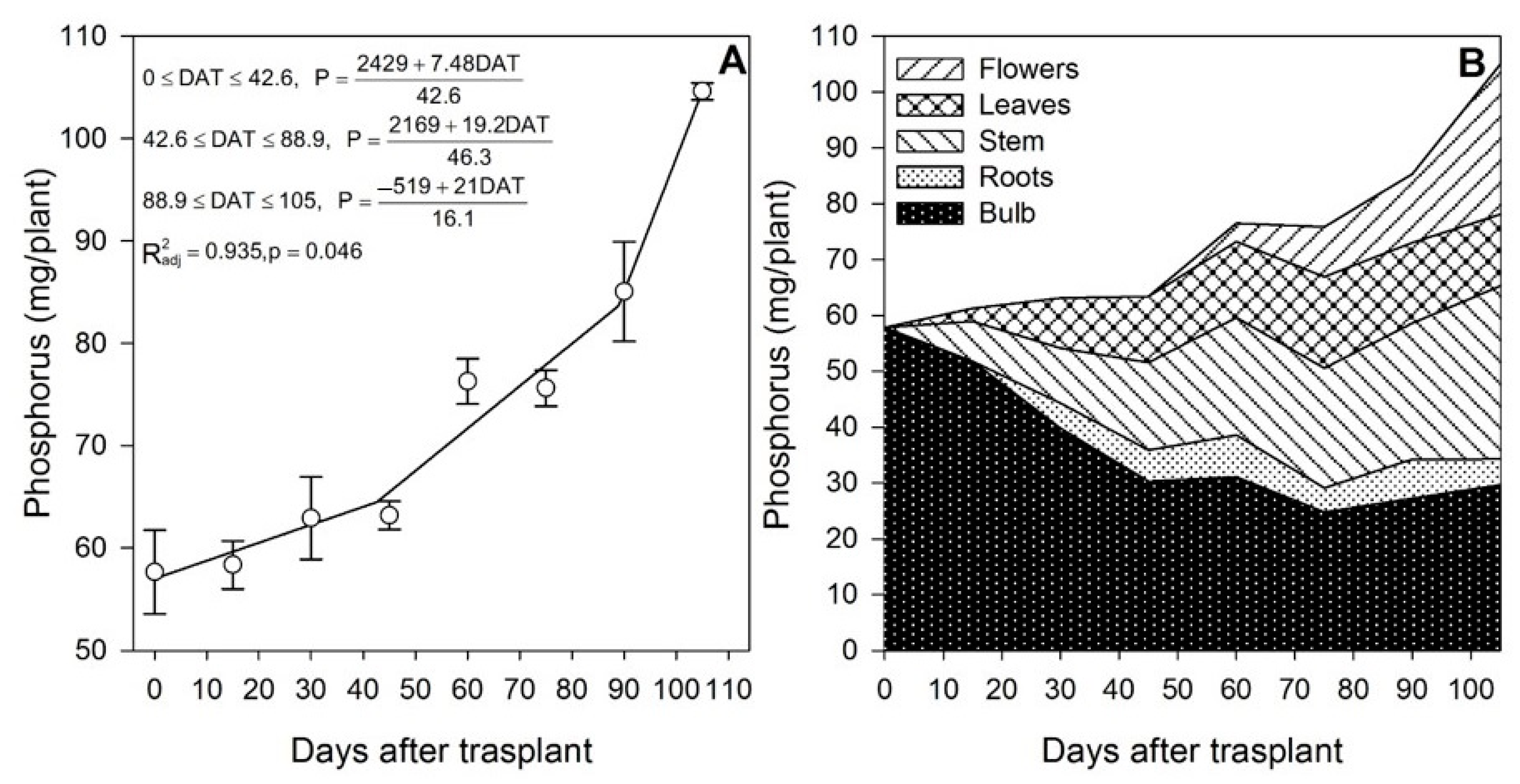
Lilium responds significantly to soil P, as when it was increased from 8.41 to 13.63 ppm, the plants increased the shoot length, the number of leaves and the bulblet size [34]. The results obtained in the present study show that P was accumulated at increasing rates throughout the growing season (Figure 3A). The uptake rate was 0.18 mg/plant/day during the initial vegetative development phase (0 to ~43 DAT), 0.41 mg/plant/day during the rapid vegetative development and the start of flower buds formation phase (~43 to ~90 DAT) and 1.30 mg/plant/day at the end of the flowering phase (~90 to 105 DAT). The higher uptake rate of P as the plants approached flower formation and development is in agreement with statements by Malhotra et al. [35], indicating that P stimulates flower and seed formation and that it is essential in all developmental stages.
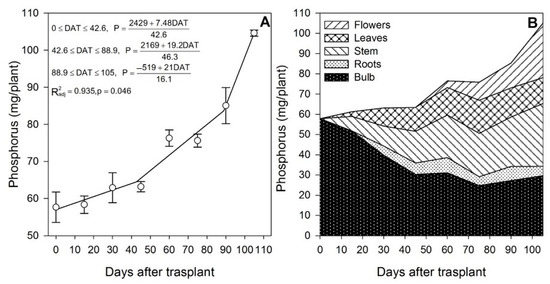
Figure 3.
Phosphorus accumulation by lilium: (A) Segmented analysis of phosphorus accumulation over the growing season; (B) Phosphorus distribution in the lilium plant parts. Day 0 = transplant day, days 0 to 15 = sprouting and initial vegetative growth, days 15 to 60 = rapid vegetative growth plus flower buds formation, days 60 to 105 = blooming. DAT = days after transplant.
Phosphorus stored in the bulb decreased sharply since the transplant date (Figure 3B), suggesting that P accumulated by the plant throughout the season came from bulb remobilization and P root acquisition. In fact, during the first 15 DAT, the aerial parts of the plant contained 9.31 mg P; 87.0% of this P was supplied by the bulb and 13.0% had to be absorbed by the roots (Figure 2).
During the last 30 days of the growing season, it appears that either no more P was remobilized from the bulb or the bulb P that was remobilized was rapidly restored by the uptake of new P from the substrate. Other plant parts did not seem to provide significant remobilization of P (Figure 3B). As for N, at the end of the growing season, slightly higher than half of the total P measured in the plant originated from the bulb (Table 1), and flowering was a developmental phase during which 89.6% of the total P was accumulated by lilium plants.
3.3. Potassium
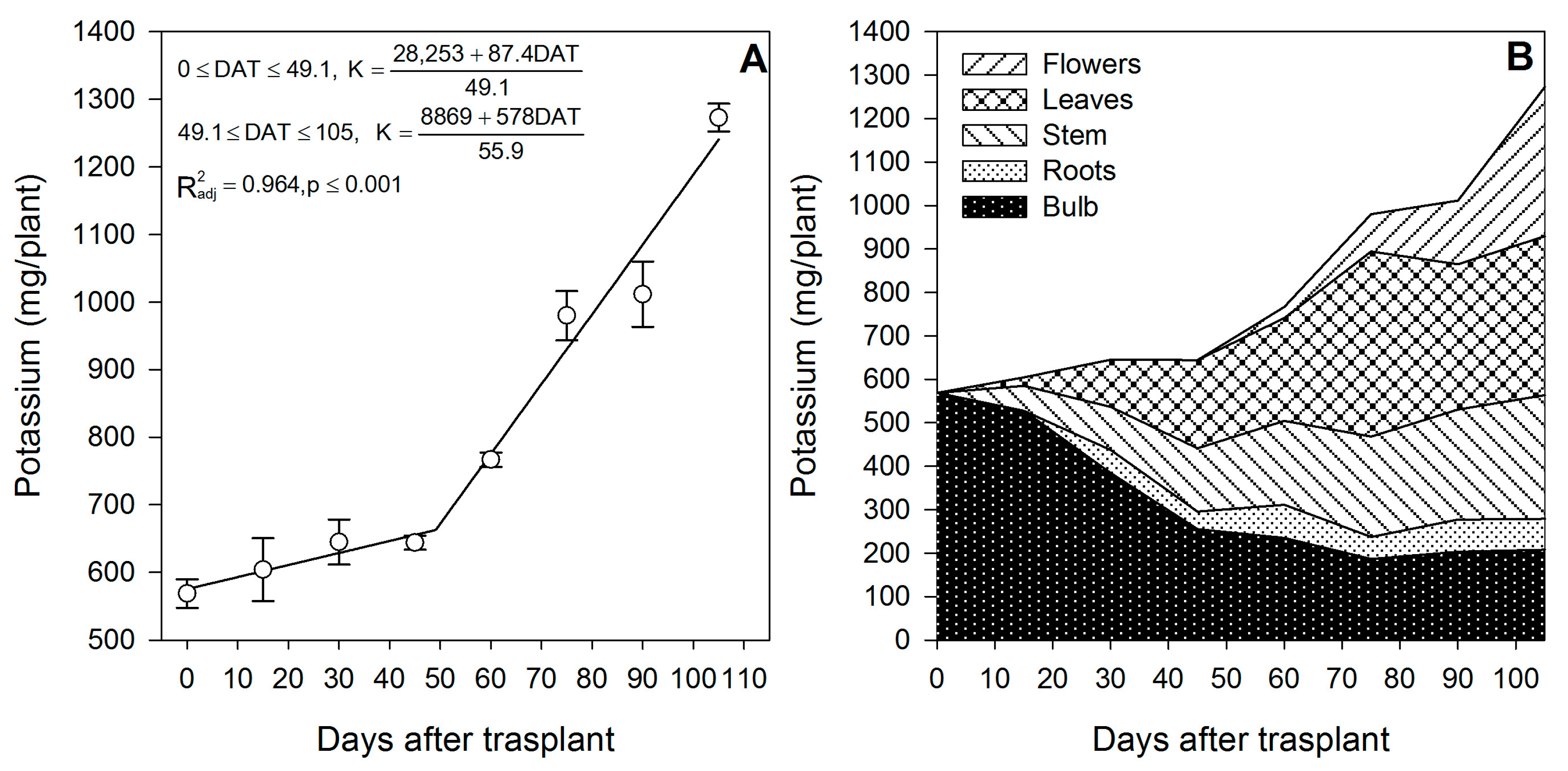
Potassium was reported to affect the shoot mass, stem length, flower diameter, water status and photosynthesis rate in lilium [36]. Potassium at 5.6 mmol·L−1 is considered the optimum rate during cultivation in soilless media [36]. As reported for gladiolus [37], K was the nutrient most demanded by lilium (Table 1); however, there is a contrasting accumulation pattern between both species. In lilium, the models indicate that K is demanded at two different rates (Figure 4A). In that, during the sprouting up to the initial leaf formation (from 0 to ~49 DAT), the accumulation rate was 1.78 mg/plant/day. However, after ~49 DAT up to study termination, K was markedly demanded for the development of leaves and flowers, as the accumulation rate was increased by 5.8 times (10.33 mg/plant/day) and remained so until flowering was completed (Figure 3A). In contrast, for gladiolus, K was accumulated at three different rates [37].
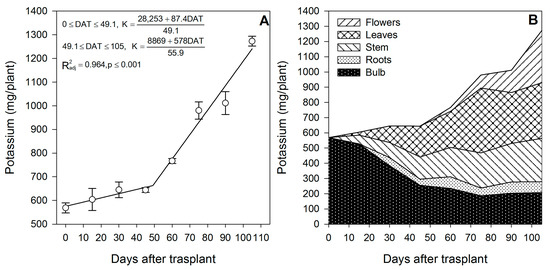
Figure 4.
Potassium accumulation in lilium: (A) Segmented analysis of potassium accumulation over the growing season; (B) Potassium distribution in the lilium plant parts. Day 0 = transplant day, days 0 to 15 = sprouting and initial vegetative growth, days 15 to 60 = rapid vegetative growth plus flower buds formation, days 60 to 105 = blooming. DAT = days after transplant.
The increase in the uptake rate after ~49 DAT was associated with the initiation phase of flowering and a marked increase in the growth of leaves, suggesting that K was used for flower and leaf expansion through the control of water relations [38].
During the first 15 DAT, the aerial parts of the plant contained 82.0 mg/plant K, 51.4% of which originated from bulb remobilization and 49.6% was absorbed by the roots (Figure 2). As for other nutrients, these results suggest that fertigation with K should be started early in the season. This initial high K accumulation may be associated with the synthesis and accumulation of polysaccharides in the bulbs, as reported by Sha et al. [39] in L. davidii. At the end of the season, 44.3% of the total K measured in the plant came from the bulb, while the remaining 55.4% was absorbed from the growing medium (Table 1), and flowering was the developmental phase with the highest accumulation, as 89.3% of the total K was absorbed in this phase.
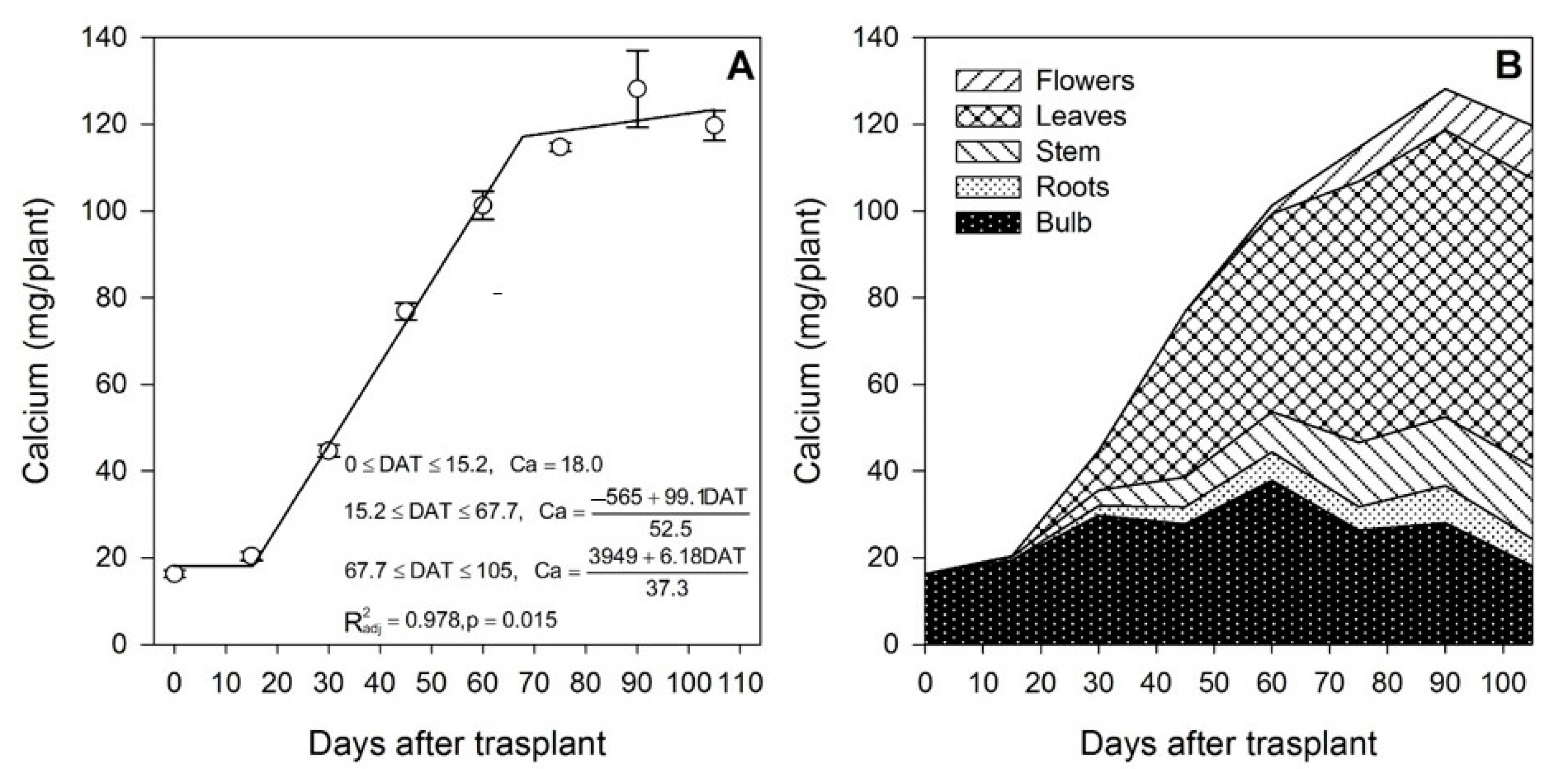
3.4. Calcium
Calcium affects the plant quality in lilium, as leaf necrosis and other related disorders have been ascribed to deficiencies of this nutrient [40]. Salazar-Orozco et al. [31] reported that, in lilium under soilless cultivation, a nutrient solution containing 2 to 4 mmol L−1 of Ca resulted in increased stem length, flower diameter and leaf area. However, Whipker et al. [33] reported that the leaf Ca concentration decreased after transplanting from week 8 to week 12 in L. longiflorum.
Calcium acquisition from the growing medium started after 15 DAT (Figure 5A); however, the bulb was a sink organ for Ca during the first 60 DAT, as it was accumulated in this plant part (Figure 5B). When flower initiation started after 60 DAT, some Ca from the bulb was mobilized to other plant parts, mainly to the leaves, as suggested by the marked decrease in the Ca content. Similar trends were reported by Chang and Miller [40], as the bulbs of lilium Star Gazer accumulated Ca during the first 40 DAT, probably through the bulb scales. Calcium then continued to decline from 40 to 70 DAT. In contrast to the results observed in the present study, gladiolus exhibited minimal modifications in the Ca content in the corm throughout the growing season, although similar to our results, at the end of the study, it was accumulated mainly in the leaves [37].
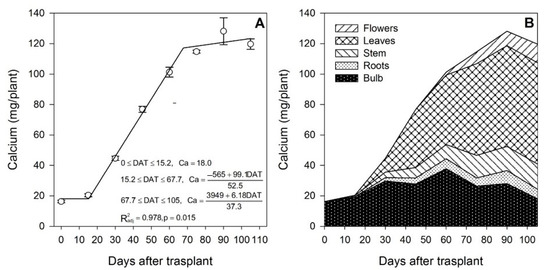
Figure 5.
Calcium accumulation in lilium: (A) Segmented analysis of calcium accumulation over the growing season; (B) Calcium distribution in the lilium plant parts. Day 0 = transplant day, days 0 to 15 = sprouting and initial vegetative growth, days 15 to 60 = rapid vegetative growth plus flower buds formation, days 60 to 105 = blooming. DAT = days after transplant.
Calcium is considered a nutrient of very low mobility in the phloem; however, Chang and Miller [40] stated that the mobilization of Ca between organs may not apply to storage organs such as bulbs. In the present study, it was observed that Ca mobilization, uptake and distribution among plant organs had a different pattern compared to that of N, P and K. In this case, Ca was not supplied primarily by the bulb, as just 13.5% of the total Ca accumulated in the plant at the end of the growing season originated from it, and the remaining 86.5% was taken up from the growing medium. Compared to other nutrients, the Ca content in the bulbs was the lowest (Figure 5B). This trait has been described in other below-ground storage organs such as potatoes by Subramanian et al. [41], and it has been associated with the low transpiration rate of this plant part and the low mobility of Ca from the shoot.
The uptake of Ca from the growing medium occurred from day 15 to ~68 DAT at a rate of 1.89 mg/plant/day, but after approximately day 68, the rate decreased to 0.16 mg/plant/day. In contrast to N, P and K, flowering was not the developmental phase in which most of the nutrient was accumulated, as only 41.4% of the total Ca was absorbed in this phase.
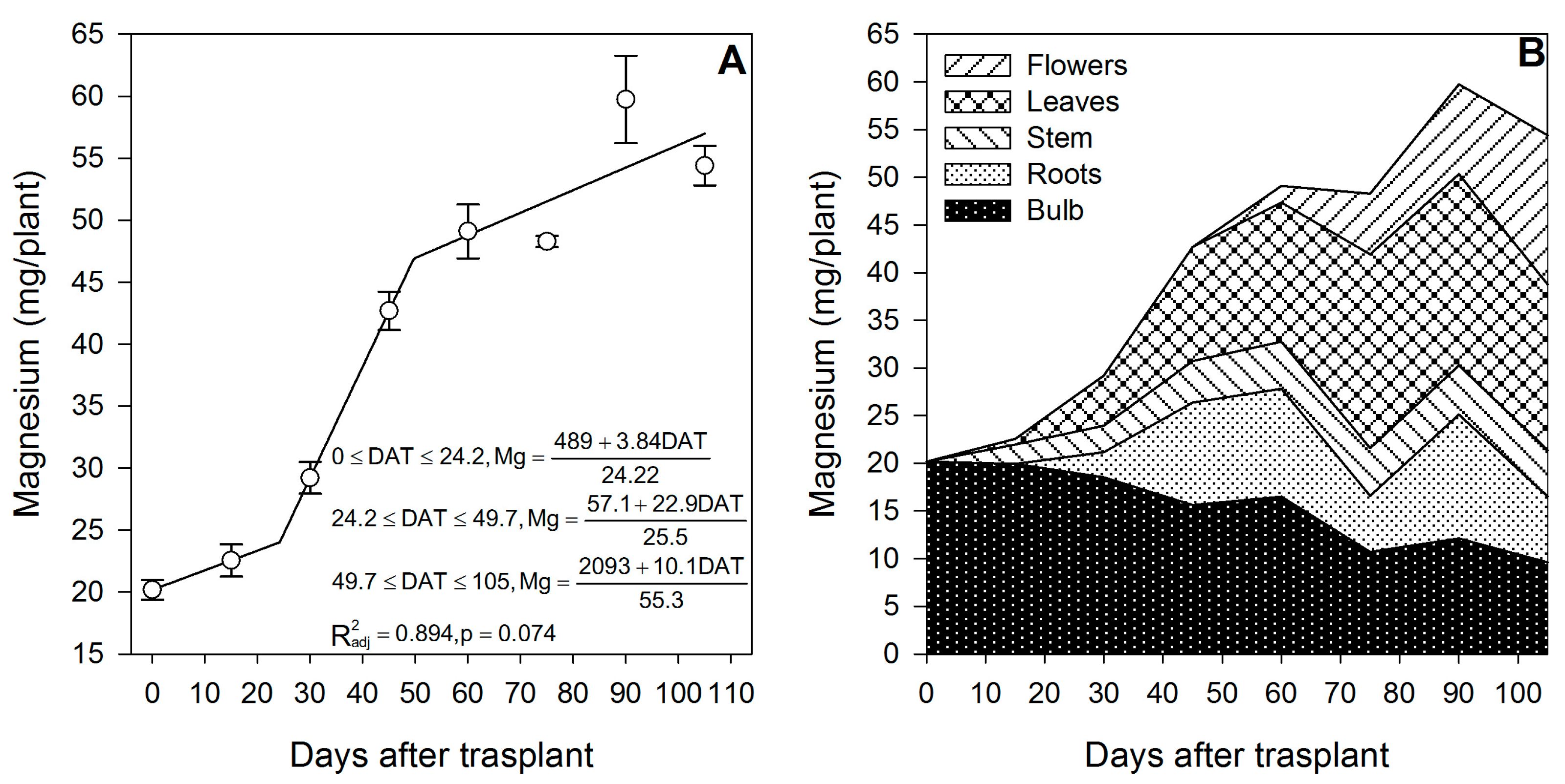
3.5. Magnesium
Compared to other nutrients, Mg exhibited a very slow initial accumulation rate (0.16 mg/plant/day) from 0 to ~24 DAT, that is, during the slow vegetative development phase (Figure 6A); however, after day ~24, that is, during the rapid vegetative growth phase, the accumulation rate increased by 5.6× (0.90 mg/plant/day) (Figure 6A). After day ~57, when flowers were developing, the uptake decreased to a rate comparable to that of the slow vegetative development phase (0.18 mg/plant/day).
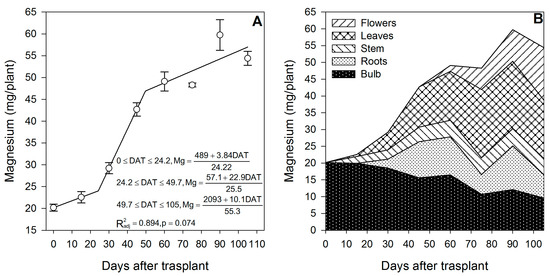
Figure 6.
Magnesium accumulation in lilium: (A) Segmented analysis of magnesium accumulation over the growing season; (B) Magnesium distribution in the lilium plant parts. Day 0 = transplant day, days 0 to 15 = sprouting and initial vegetative growth, days 15 to 60 = rapid vegetative growth plus flower buds formation, days 60 to 105 = blooming. DAT = days after transplant.
As for Ca, Mg was not supplied primarily by the bulb, as only 37.1% of the total Mg in the plant at the end of the growing season proceeded from this plant part, while 62.9% of the Mg had to be supplied through the uptake of external Mg (Figure 6B). At 15 DAT, the Mg content in the developing shoot was 2.54 mg/plant, 9.01% of which originated from bulb remobilization, so 91.0% was absorbed by the roots (Figure 2). As for other nutrients, this suggests that fertigation with Mg should be started early in the season.
Comparable to Ca, at the end of the season, most of the Mg was absorbed from the growing medium. Even though it is considered as a readily mobile nutrient in the phloem [41], only 37.1% of the total Mg accumulated came from the bulb; the remaining 62.9% was absorbed by the roots (Table 1). Likewise, the corm of gladiolus has been reported to provide very little amounts of Mg to the plants, even if the corms are of a large size [37]. Similar to Ca, flowering was not the developmental phase in which most of the nutrient was accumulated, as only 45.2% of the total Mg was absorbed during this phase.
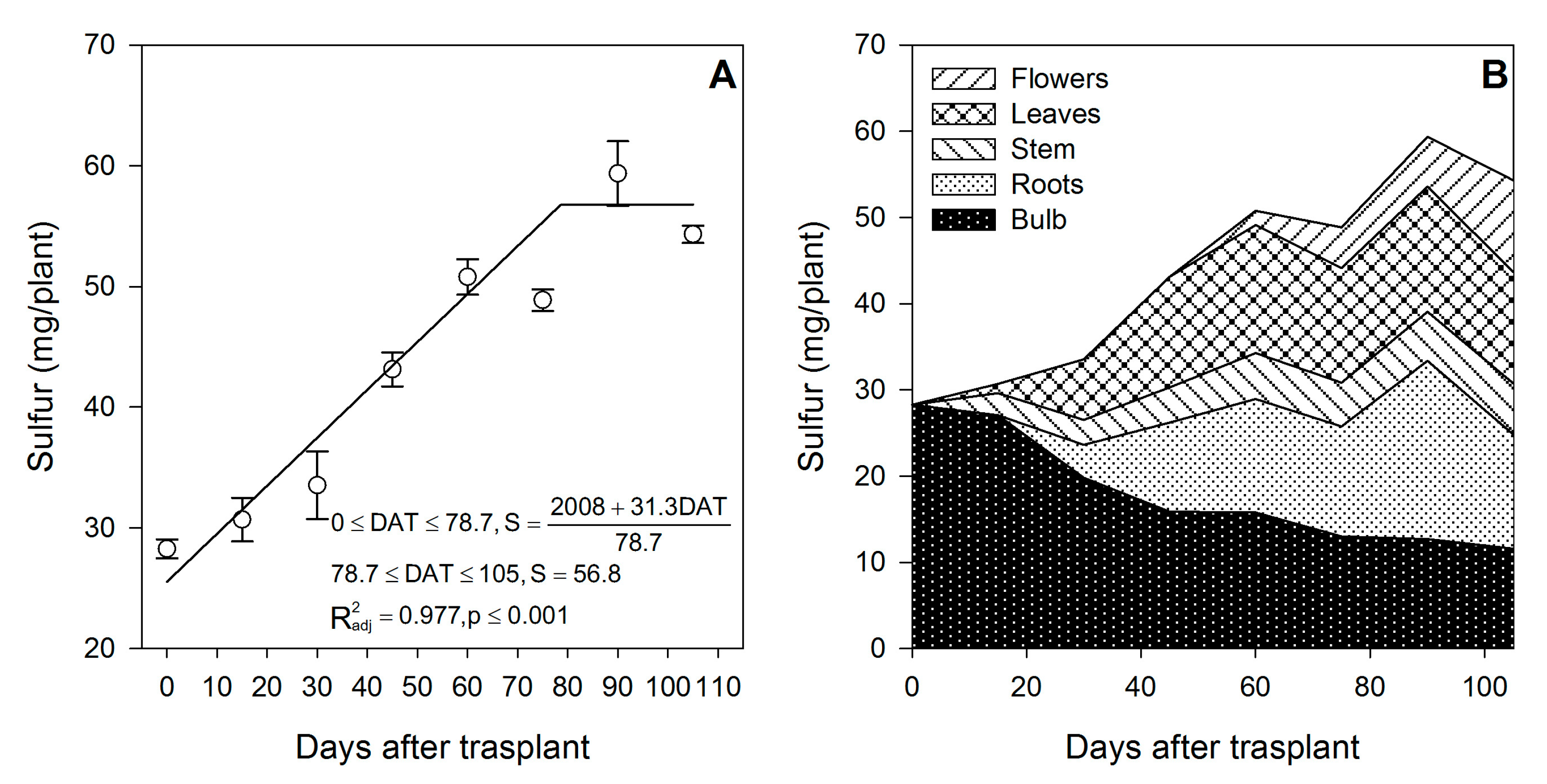
3.6. Sulfur
Sulfur was accumulated from the transplant day until ~79 DAT at a rate of 0.40 mg/plant/day (Figure 7A), while during the final flower development phase, there was no more S accumulation. Sulfur was provided at similar proportions by the external supply (51.6%) or by the bulb (48.4%) (Table 1). Similar to N, P and K, flowering was the developmental phase in which S was most accumulated on lilium plants, as 69.7% of the total S was absorbed in this time period (Figure 7B). Sulfur was demanded since the transplant day, as during the first 15 DAT, 31.4% of the total S accumulated in the shoot proceeded from the bulb, while the remaining 68.6% had to be uptaken from the growing medium (Figure 2).
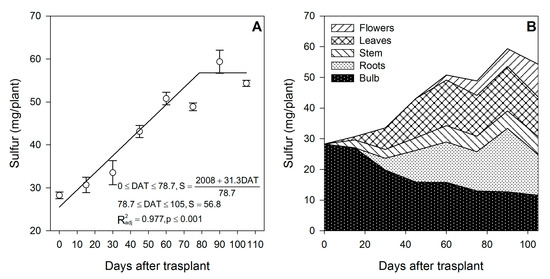
Figure 7.
Sulfur accumulation in lilium: (A) Segmented analysis of sulfur accumulation over the growing season; (B) Sulfur distribution in the lilium plant parts. Day 0 = transplant day, days 0 to 15 = sprouting and initial vegetative growth, days 15 to 60 = rapid vegetative growth plus flower buds formation, days 60 to 105 = blooming. DAT = days after transplant.
3.7. Micronutrients
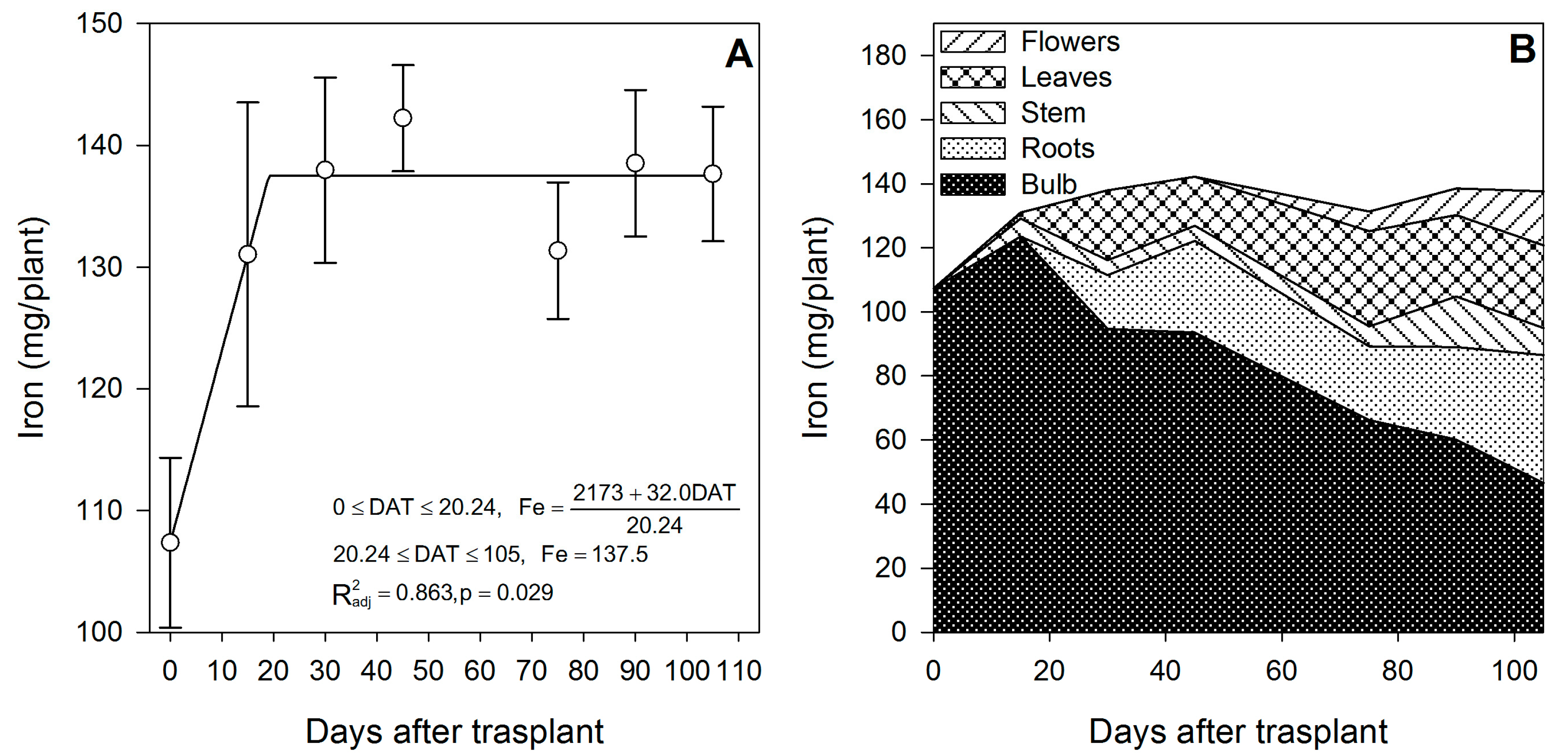
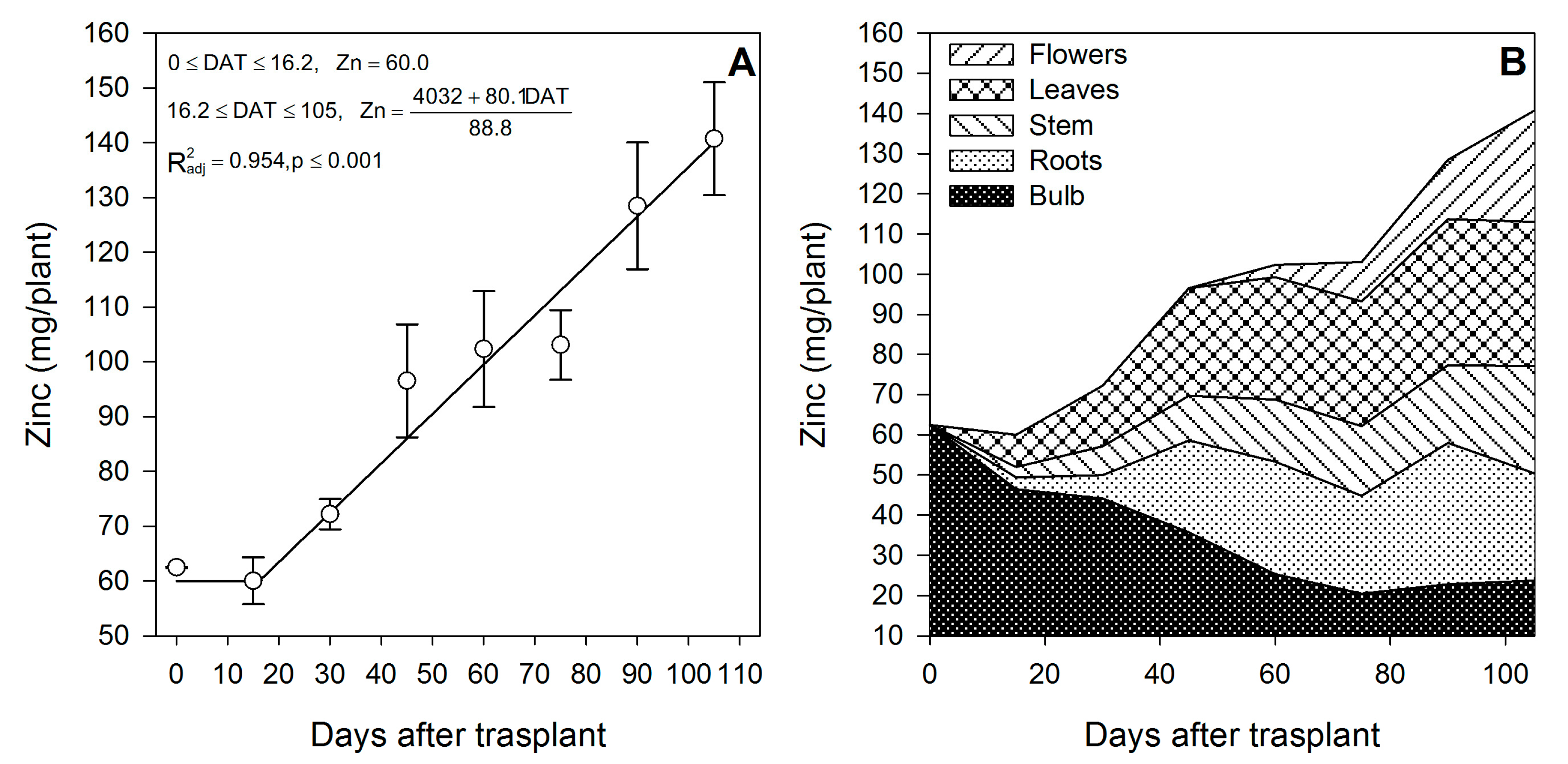
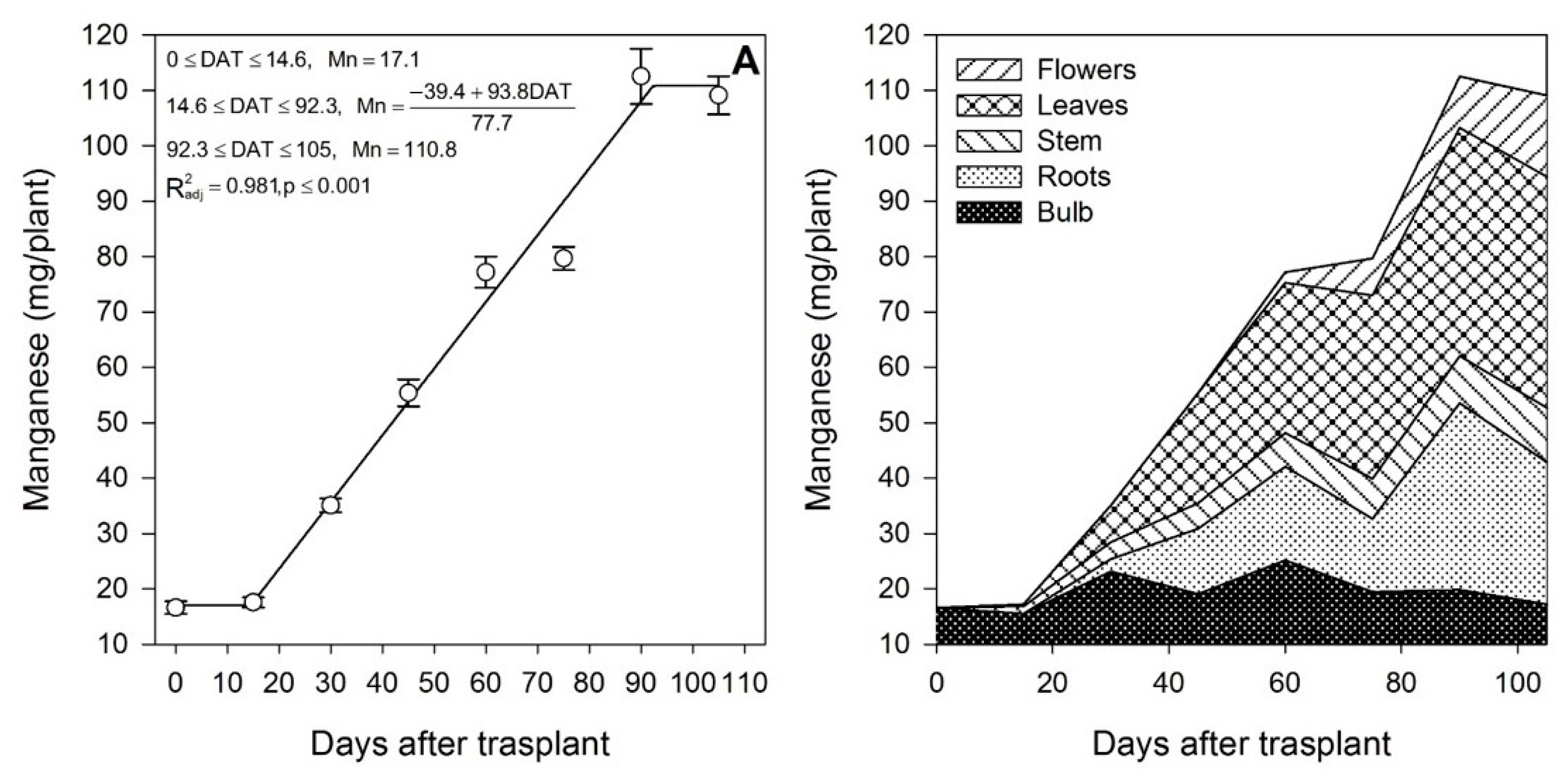
The most demanded micronutrients by lilium were Zn, Fe and Mn, followed by Cu (Table 2). Our results differ from those reported by Sharma et al. [42] for potato, as the accumulation pattern was in the order: Fe > Mn > Zn > Cu. Iron (Figure 8A), Zn (Figure 9A) and Mn (Figure 10A) exhibited only one phase in which there was nutrient accumulation.

Table 2.
Total micronutrients accumulated by lilium plants (mean ± standard error) at the end of the growing season and the amount provided by the bulb or absorbed from the growing medium.
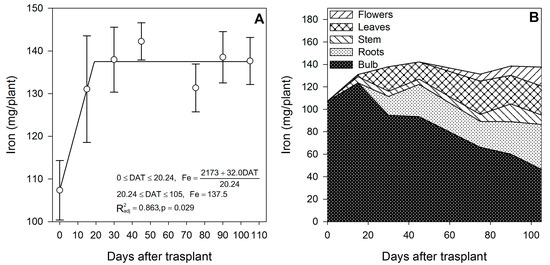
Figure 8.
Iron accumulation in lilium: (A) Segmented analysis of iron accumulation over the growing season; (B) Iron distribution in the lilium plant parts. Day 0 = transplant day, days 0 to 15 = sprouting and initial vegetative growth, days 15 to 60 = rapid vegetative growth plus flower buds formation, days 60 to 105 = blooming. DAT = days after transplant.
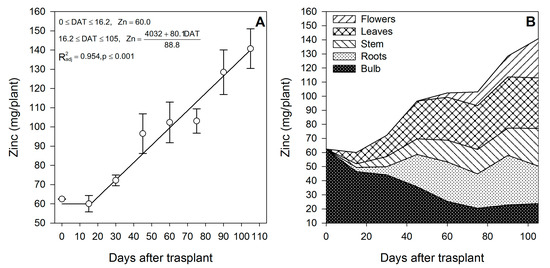
Figure 9.
Zinc accumulation in lilium: (A) Segmented analysis of phosphorus accumulation over the growing season; (B) Phosphorus distribution in the lilium plant parts. Day 0 = transplant day, days 0 to 15 = sprouting and initial vegetative growth, days 15 to 60 = rapid vegetative growth plus flower buds formation, days 60 to 105 = blooming. DAT = days after transplant.
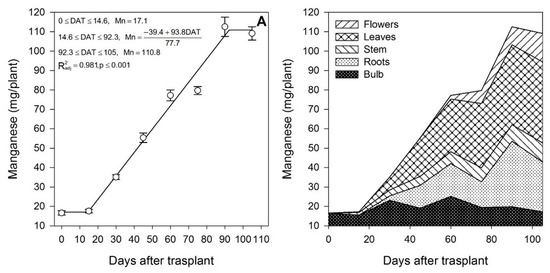
Figure 10.
Manganese accumulation in lilium: (A) Segmented analysis of manganese accumulation over the growing season; (B) Manganese distribution in the lilium plant parts. Day 0 = transplant day, days 0 to 15 = sprouting and initial vegetative growth, days 15 to 60 = rapid vegetative growth plus flower buds formation, days 60 to 105 = blooming. DAT = days after transplant.
Iron was accumulated mainly during the initial vegetative growth phase from day 0 to day ~20, and the uptake rate was 1.58 mg/plant/day (Figure 8A). Iron accumulated after the transplant was stored mainly in the bulb and could have entered through direct uptake by the epidermis of the outer scales. Comparable tendencies were observed in potato, as the content of Fe and other micronutrients was higher in the peel than in the flesh of the tubers [42].
After day ~20, there was no further Fe accumulation, so the supply of this element to the new developing organs appears to rely on the remobilization from the bulb, as suggested by the pronounced decrease in the Fe content in the bulb (−62.3%) from 15 DAT up to the study termination (Figure 8B). This observation is contrary to the well-documented reports that Fe, as well as Zn and Cu, have low-to-intermediate mobility in the phloem. The limited mobility of Fe, Zn and Cu has been ascribed to their incorporation into cell structures and organic compounds of a high molecular weight [43]. We suggest that the mobility of Fe observed in the current study may be because it is accumulated into the vacuoles of the outer scales, and it was not incorporated into cell structures or an organic compound of a large molecular weight; thus, the low mobility may not apply to storage organs such as bulbs, as suggested by Chang and Miller [40] for Ca. White [43] suggested that the remobilization of micronutrients may be surprisingly high during the reproductive stages.
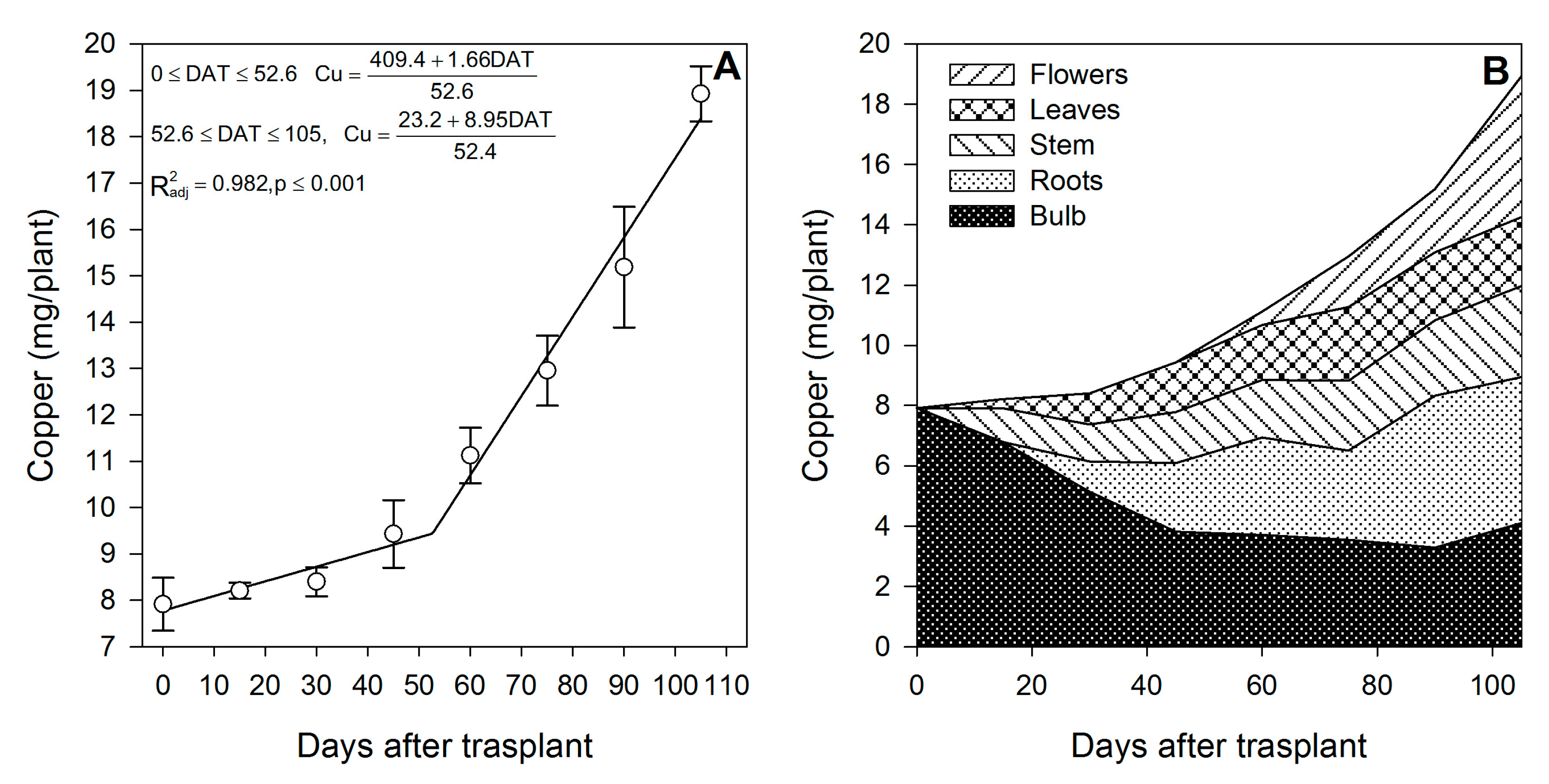
Zinc (Figure 9A) and Mn (Figure 10A) were mainly accumulated during the rapid vegetative growth and flowering phases at a rate of 0.90 and 1.21 mg/plant/day, respectively, after approximately days 16 and 15, respectively. Copper (Figure 11A) exhibited an accumulation pattern very similar to that of K (Figure 4A), as there was a slow remobilization or uptake rate during the early vegetative growth (0.03 mg/plant/day), although it was markedly increased after approximately day 53 during the rapid vegetative growth and flowering phases (0.17 mg/plant/day). Similar to Fe (Figure 8B) and Zn (Figure 9B), Cu also exhibited a sharp remobilization from the bulb, as its total content decreased by 83.3% and 56.2%, respectively, when compared to the original content in the bulb at the transplant (Figure 11B).
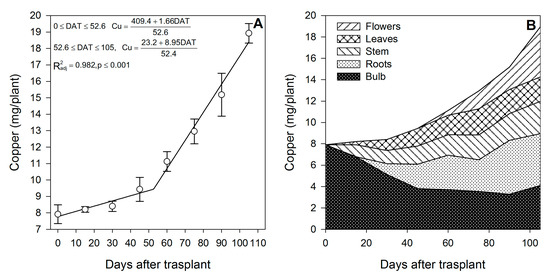
Figure 11.
Copper accumulation in lilium: (A) Segmented analysis of copper accumulation over the growing season; (B) Copper distribution in the lilium plant parts. Day 0 = transplant day, days 0 to 15 = sprouting and initial vegetative growth, days 15 to 60 = rapid vegetative growth plus flower buds formation, days 60 to 105 = blooming. DAT = days after transplant.
At the end of the growing season, most of the Fe in the plants proceeded from the bulb, so there was little Fe uptake from the growing medium (Table 2); in contrast, Mn was mainly obtained from the growing medium, as little Mn was provided by the bulb (Table 2). This was probably associated with its very low mobility [43]. Lilium seems to be a very low Cu accumulator (Table 2), and most of it was absorbed from the growing medium, as the bulb supplied only 41.9%. At the end of the growing season, most of the Fe (Figure 8B) and Cu (Figure 11B) were accumulated in the bulb and roots, whereas Zn (Figure 9B) and Mn (Figure 10B) were accumulated in the leaves.
During the first 15 DAT, the shoot Fe was 6.91 mg/plant, while the bulb also exhibited increased Fe accumulation (+16.24 mg/plant) (Figure 2); this implies that the Fe root uptake is very high and, therefore, this micronutrient has to be supplied early in the season to meet the demands of lilium plants. Copper in the shoot at 15 DAT was provided primarily by nutrient uptake from the bulb (85.5%) (Figure 2).
3.8. Nutrient Allocation
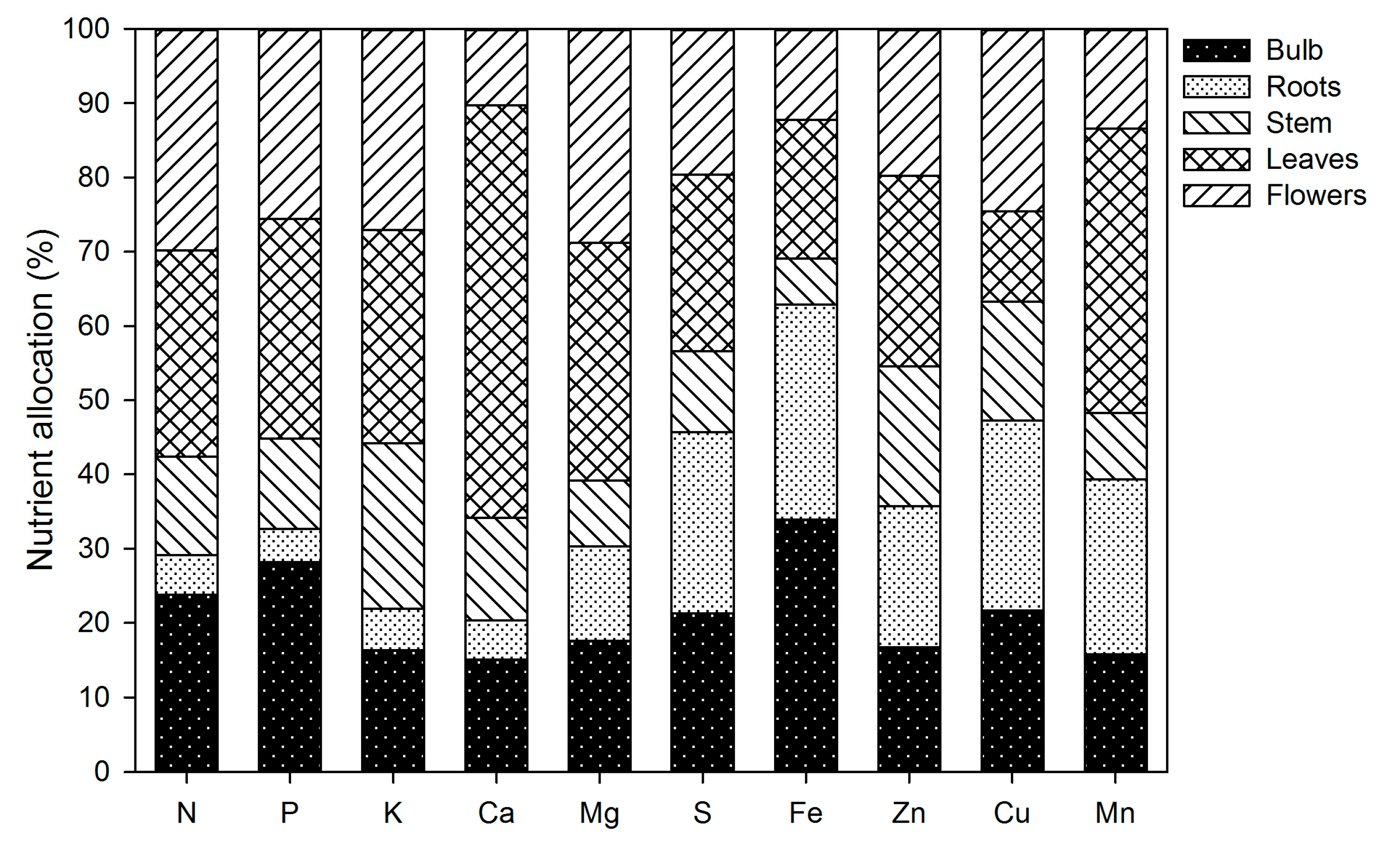
At the end of the growing season, N and P were accumulated primarily in the flowers, leaves and bulb, whereas the stem and the roots contained the lowest amounts (Figure 12). Potassium was present primarily in the aerial parts of the plants (Figure 12). More than half of the Ca accumulated by plants was in the leaves, whereas Mg was primarily present in the leaves and flowers (Figure 12). The accumulation of S was distributed at similar proportions in the flowers, leaves, roots and bulb, while the stem contained the lowest S (Figure 12). Iron and Cu were mainly accumulated in the below-ground plant parts, whereas Zn and Mn were mainly in the leaves (Figure 12).
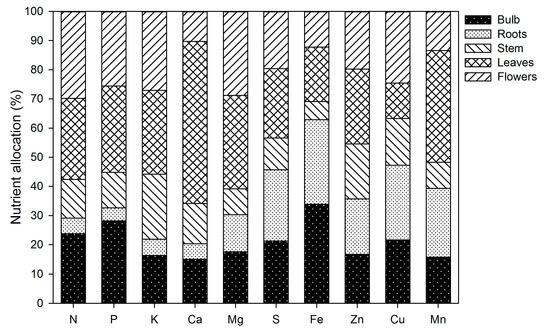
Figure 12.
Nutrient allocation to the different plant parts of lilium at the end of the growing season.
4. Conclusions
The accumulation of macro and micronutrients in lilium was properly modeled by segmented analysis. The models indicate the amounts of nutrients demanded by plants and when they are required. The macronutrient in the highest demand was K, followed by N and Ca, while Zn and Fe were the most demanded micronutrients. At the end of the season, most of the Fe, P and N required by the plants originated from the bulb, whereas most of the Ca, Mn and Mg were taken up from the growing medium. During the first 15 days after transplant, 35.1% of the N in the developing above-ground plant parts was absorbed from the growing medium, as well as 91.0% of Mg, 68.6% of S, 49.6% of K and 13.0% of P, suggesting that supplying nutrients through fertilization should start early at the transplant time. Our results suggest that there is a remobilization of Ca, Fe, Zn and Cu from the bulb.
Author Contributions
Conceptualization, G.C.-M., D.A.-C. and L.A.V.-A.; methodology, P.P.-R. and L.A.V.-A.; software, A.D.C.; validation, A.D.C.; formal analysis, G.C.-M. and L.A.V.-A.; investigation, G.C.-M., D.A.-C. and L.A.V.-A.; resources, P.P.-R. and A.D.C.; data curation, G.C.-M.; writing—original draft preparation, G.C.-M. and D.A.-C.; writing—review and editing, G.C.-M., D.A.-C., L.A.V.-A., P.P.-R. and A.D.C.; visualization, P.P.-R. and A.D.C.; supervision, D.A.-C. and L.A.V.-A.; project administration, L.A.V.-A.; funding acquisition, P.P.-R. and A.D.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Robertson, G.P.; Swinton, S.M. Reconciling agricultural productivity and environmental integrity: A grand challenge for agriculture. Front. Ecol. Environ. 2005, 3, 38–46. [Google Scholar] [CrossRef]
- Johnston, A.M.; Bruulsema, T.W. 4R nutrient stewardship for improved nutrient use efficiency. Procedia Eng. 2014, 83, 365–370. [Google Scholar] [CrossRef]
- Schut, A.G.; Giller, K.E. Soil-based, field-specific fertilizer recommendations are a pipe-dream. Geoderma 2020, 380, 114680. [Google Scholar] [CrossRef]
- Anas, M.; Liao, F.; Verma, K.K.; Sarwar, M.A.; Mahmood, A.; Chen, Z.L.; Qiang, L.; Zeng, X.P.; Liu, Y.; Li, Y.R. Fate of nitrogen in agriculture and environment: Agronomic, eco-physiological and molecular approaches to improve nitrogen use efficiency. Biol. Res. 2020, 53, 47. [Google Scholar] [CrossRef] [PubMed]
- Scavia, D.; Kalcic, M.; Muenich, R.L.; Read, J.; Aloysius, N.; Bertani, I.; Boles, C.; Confesor, R.; De Pinto, J.; Gildow, M.; et al. Multiple models guide strategies for agricultural nutrient reductions. Front. Ecol. Environ. 2017, 15, 126–132. [Google Scholar] [CrossRef]
- Bullerjahn, G.S.; McKay, R.M.; Davis, T.W.; Baker, D.B.; Boyer, G.L.; D’Anglada, L.V.; Doucette, G.J.; Ho, J.C.; Irwin, E.G.; Kling, C.L.; et al. Global solutions to regional problems: Collecting global expertise to address the problem of harmful cyanobacterial blooms, a Lake Erie case study. Harmful Algae 2016, 54, 223–238. [Google Scholar] [CrossRef]
- Ju, X.; Gu, B.; Wu, Y.; Galloway, J.N. Reducing China’s fertilizer use by increasing farm size. Glob. Environ. Chang. 2016, 41, 26–32. [Google Scholar] [CrossRef]
- Fernandes, A.M.; Soratto, R.P.; Silva, B.L. Nutrient extraction and exportation by potato cultivars: I-macronutrients. Rev. Bras. Cienc. Solo 2011, 35, 2039–2056. [Google Scholar] [CrossRef]
- Fageria, N.K.; Baligar, V.C. Enhancing nitrogen use efficiency in crop plants. Adv. Agron. 2005, 88, 97–185. [Google Scholar] [CrossRef]
- Arunachalam, T.; Chavan, K.M. Dry matter accumulation and nutrient uptake patterns of onion seed crop. J. Plant Nutr. 2018, 41, 1879–1889. [Google Scholar] [CrossRef]
- de Moraes, C.C.; Factor, T.L.; de Araújo, H.S.; Purquerio, L.F.V. Plant growth and nutrient accumulation in two tomato hybrids under tropical conditions. Aust. J. Crop Sci. 2018, 12, 1419–1425. [Google Scholar] [CrossRef]
- Duarte, L.O.; Clemente, J.; Caixeta, I.A.B.; Senoski, M.D.P.; Aquino, L.A.D. Dry matter and nutrient accumulation curve in cabbage crop. Rev. Caatinga 2019, 32, 679–689. [Google Scholar] [CrossRef]
- Gómez, M.I.; Magnitskiy, S.; Rodríguez, L.E. Nitrogen, phosphorus and potassium accumulation and partitioning by the potato group Andigenum in Colombia. Nutr. Cycl. Agroecosyst. 2019, 113, 349–363. [Google Scholar] [CrossRef]
- Araújo, W.A.D.; Santana, R.S.; Mauad, M.; da Silva, R.S. Dry matter accumulation and nutrient uptake in determinate and indeterminate soybeans. J. Plant Nutr. 2020, 44, 508–522. [Google Scholar] [CrossRef]
- Bender, R.R.; Haegele, J.W.; Below, F.E. Nutrient uptake, partitioning, and remobilization in modern soybean varieties. Agron. J. 2015, 107, 563–573. [Google Scholar] [CrossRef]
- Marques Pires, M.F.; de Souza, H.A.; Medeiros, J.C.; Rosa, J.D.; de Souza Martins, R.V.; Sales Sobral, A.H.; Carvalho, S.P.; de Sousa Vera, G.; de Melo, P.F.; Vieira, J.; et al. Nutrient uptake by soybean plants in succession of cover crops in northeast of Brazil. Commun. Soil Sci. Plant Anal. 2023, 54, 945–963. [Google Scholar] [CrossRef]
- Bender, R.R.; Haegele, J.W.; Ruffo, M.L.; Below, F.E. Nutrient uptake, partitioning, and remobilization in modern, transgenic insect-protected maize hybrids. Agron. J. 2013, 105, 161–170. [Google Scholar] [CrossRef]
- Couch, A.; Jani, A.; Mulvaney, M.; Hochmuth, G.; Bennett, J.; Gloaguen, R.; Langham, R.; Rowland, D. Nitrogen accumulation, partitioning, and remobilization by diverse sesame cultivars in the humid southeastern USA. Field Crops Res. 2017, 203, 55–64. [Google Scholar] [CrossRef]
- Galindo-Garcia, D.V.; Alia-Tejacal, I.; Valdez-Aguilar, L.A.; Colinas-Leon, M.T.; Villegas-Torres, O.G.; Lopez-Martinez, V.; Sainz-Aispuro, M.J.; Guillen-Sanchez, D. Macronutrient extraction and growth of mexican native sun poinsettia varieties. Rev. Fitotec. Mex. 2015, 38, 305–312. [Google Scholar]
- Valdez-Aguilar, L.A.; Hernández-Pérez, A.; Alvarado-Camarillo, D.; Cruz-Altunar, Á. Diseño de un programa de fertilización para crisantemo en base a extracción de macronutrimentos. Rev. Mex. Cienc. Agríc. 2015, 6, 2263–2276. [Google Scholar]
- Castillo-González, A.M.; Avitia-García, E.; Valdez-Aguilar, L.A.; Velázquez-Maldonado, J. Extracción nutrimental en lisianthus (Eustoma grandiflorum [Raf.] Shinn) cv. Mariachi Pink. Rev. Mex. Cienc. Agríc. 2017, 8, 345–354. [Google Scholar]
- van Tuyl, J.M.; Arens, P.; Ramanna, M.S.; Shahin, A.; Khan, N.; Xie, S.; Marasek-Ciolakowska, A.; Lim, K.B.; Barba-Gonzalez, R. Lilium. In Wild Crop Relatives: Genomic and Breeding Resources; Kole, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 161–183. [Google Scholar] [CrossRef]
- Suh, J.K.; Wu, X.W.; Lee, A.K.; Roh, M.S. Growth and flowering physiology, and developing new technologies to increase the flower numbers in the Genus Lilium. Hortic. Environ. Biotechnol. 2013, 54, 373–387. [Google Scholar] [CrossRef]
- Faust, J.E.; Dole, J.M. (Eds.) Major cut flowers. In Cut Flowers and Foliages; CAB International: Boston, MA, USA, 2021; pp. 48–149. [Google Scholar] [CrossRef]
- Zhao, K.; Xiao, Z.; Zeng, J.; Xie, H. Effects of different storage conditions on the browning degree, PPO activity, and content of chemical components in fresh lilium bulbs (Lilium brownii FE Brown var. viridulum Baker.). Agriculture 2020, 11, 184. [Google Scholar] [CrossRef]
- Dole, J.M.; Wilkins, H.F. Floriculture, Principles and Species, 2nd ed.; Prentice Hall: Englewood Cliffs, NJ, USA, 2005. [Google Scholar]
- Soltanpour, P.N.; Johnson, G.W.; Workman, S.M.; Jones, J.B.; Miller, R.O. Inductively coupled plasma emission spectrometry and inductively coupled plasma-mass spectrometry. In Methods of Soil Analysis Part 3 Chemical Methods; Sparks, D.L., Ed.; Soil Science Society of America: Madison, WI, USA, 1996; pp. 91–139. [Google Scholar] [CrossRef]
- Bremner, J.M. Nitrogen—Total. In Methods of Soil Analysis Part III Chemical Methods; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1996; pp. 1085–1086. [Google Scholar] [CrossRef]
- Eisenreichova, E.; Haladova, M.; Buckova, A.; Ubik, K.; Uhrin, D. Derivatives of pyrroline in Lilium candidum L. Chem. Pap.-Chem. Zvesti 1991, 45, 709–711. [Google Scholar]
- Haladová, M.; Eisenreichová, E.; Bučková, A.; Tomko, J.; Uhrín, D. New nitrogen-containing compounds in Lilium candidum L. Collect. Czechoslov. Chem. Commun. 1988, 53, 157–160. [Google Scholar] [CrossRef]
- Salazar-Orozco, G.; Ruíz-Sánchez, M.C.; Valdez-Aguilar, L.A.; Pistelli, L.; Ruíz-Olmos, C.; Grassotti, A. Influencia de la fertilización nitrogenada y potásica en la calidad aromática de flores de Lilium “Starfighter”. Inf. Téc. Econ. Agrar. 2013, 109, 3–12. [Google Scholar]
- Kamenetsky, R. Flower biology in Lilium: Achievements and research challenges. Acta Hortic. 2014, 1027, 65–74. [Google Scholar] [CrossRef]
- Whipker, B.E.; Barnes, J.; McCall, I.; Gibson, J.; Poole, H. Nitrogen concentration and form effects on leaf tissue concentrations and lower leaf expansion of Lilium longiflorum ‘Nellie White’. Acta Hortic. 2010, 900, 125–132. [Google Scholar] [CrossRef]
- Varshney, A.; Sharma, M.P.; Adholeya, A.; Dhawan, V.; Srivastava, P.S. Enhanced growth of micropropagated bulblets of Lilium sp. inoculated with arbuscular mycorrhizal fungi at different P fertility levels in an alfisol. J. Hortic. Sci. Biotechnol. 2002, 77, 258–263. [Google Scholar] [CrossRef]
- Malhotra, H.; Vandana, S.; Pandey, R. Phosphorus nutrition: Plant growth in response to deficiency and excess. In Plant Nutrients and Abiotic Stress Tolerance; Hasanuzzaman, M., Fujita, M., Oku, H., Nahar, K., Hawrylak-Nowak, B., Eds.; Springer: Singapore, 2018; pp. 269–295. [Google Scholar] [CrossRef]
- Barrera-Aguilar, E.; Valdez-Aguilar, L.A.; Castillo-González, A.M.; Cartmill, A.D.; Cartmill, D.L.; Avitia-García, E.; Ibarra-Jímenez, L. Potassium nutrition in lilium: Critical concentrations, photosynthesis, water potential, leaf anatomy, and nutrient status. HortScience 2013, 48, 1537–1542. [Google Scholar] [CrossRef]
- Gómez-Pérez, L.; Valdez-Aguilar, L.A.; Cadena-Zapata, M.; Cartmill, D.L.; Cartmill, A.D.; Benavides-Mendoza, A. Biomass and accumulation of potassium, calcium, and magnesium in gladiolus as affected by heat units and corm size. Commun. Soil Sci. Plant Anal. 2018, 49, 344–357. [Google Scholar] [CrossRef]
- Hawkesford, M.; Horst, W.; Kichey, T.; Lambers, H.; Schjoerring, J.; Møller, I.S.; White, P. Functions of macronutrients. In Marschner’s Mineral Nutrition of Higher Plants, 4th ed.; Rengel, Z., Cakmak, I., White, P.J., Eds.; Academic Press: San Diego, CA, USA, 2023; pp. 201–260. [Google Scholar]
- Sha, X.; Zhang, P.; Yang, Y.; Bu, H.; Ma, Y.; Jin, L. Effects of potassium application on Lilium davidii var. unicolor growth, polysaccharide accumulation, and metabolism. Horticulturae 2022, 8, 940. [Google Scholar] [CrossRef]
- Chang, Y.C.; Miller, W.B. Growth and calcium partitioning in lilium Star Gazer in relation to leaf calcium deficiency. J. Am. Soc. Hortic. Sci. 2003, 128, 788–796. [Google Scholar] [CrossRef]
- Subramanian, N.K.; White, P.J.; Broadley, M.R.; Ramsay, G. The three-dimensional distribution of minerals in potato tubers. Ann. Bot. 2011, 107, 681–691. [Google Scholar] [CrossRef]
- Sharma, J.; Dalamu; Sharma, V.; Dua, V.K.; Gupta, V.K.; Kumar, D. Variations in micronutrient content in tubers of Indian potato varieties. Potato J. 2017, 44, 101–109. [Google Scholar]
- White, P.J.; Ding, G. Long-distance transport in the xylem and phloem. In Marschner’s Mineral Nutrition of Higher Plants, 4th ed.; Rengel, Z., Cakmak, I., White, P.J., Eds.; Academic Press: San Diego, CA, USA, 2023; pp. 73–104. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).