Soilless Cultivation of Portulaca oleracea Using Medicinal and Aromatic Plant Residues for Partial Peat Replacement
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growing Media Preparation
2.2. Growing Media Characteristics
2.3. Plant Growth, Physiology, and Mineral Analysis
2.4. Total Phenolic Compounds, Total Flavonoids, and Antioxidant Activity
2.5. Lipid Peroxidation, Hydrogen Peroxide Content, and Enzyme Antioxidant Activity
2.6. Statistical Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Santana-Méridas, O.; González-Coloma, A.; Sánchez-Vioque, R. Agricultural residues as a source of bioactive natural products. Phytochem. Rev. 2012, 11, 447–466. [Google Scholar] [CrossRef]
- Saha, A.; Basak, B.B. Scope of value addition and utilization of residual biomass from medicinal and aromatic plants. Ind. Crops Prod. 2020, 145, 111979. [Google Scholar] [CrossRef]
- Olofsson, J.; Börjesson, P. Residual biomass as resource—Life-cycle environmental impact of wastes in circular resource systems. J. Clean. Prod. 2018, 196, 997–1006. [Google Scholar] [CrossRef]
- Ferhat, M.A.; Meklati, B.Y.; Chemat, F. Comparison of different isolation methods of essential oil from Citrus fruits: Cold pressing, hydrodistillation and microwave ‘dry’ distillation Mohamed. Flavour Fragr. J. 2008, 22, 494–504. [Google Scholar] [CrossRef]
- Saha, A.; Tripathy, V.; Basak, B.B.; Kumar, J. Entrapment of distilled palmarosa (Cymbopogon martinii) wastes in alginate beads for adsorptive removal of methylene blue from aqueous solution. Environ. Prog. Sustain. Energy 2018, 37, 1942–1953. [Google Scholar] [CrossRef]
- Sayed-Ahmad, B.; Talou, T.; Saad, Z.; Hijazi, A.; Merah, O. The Apiaceae: Ethnomedicinal family as source for industrial uses. Ind. Crops Prod. 2017, 109, 661–671. [Google Scholar] [CrossRef]
- Skendi, A.; Irakli, M.; Chatzopoulou, P.; Bouloumpasi, E.; Biliaderis, C.G. Phenolic extracts from solid wastes of the aromatic plant essential oil industry: Potential uses in food applications. Food Chem. Adv. 2022, 1, 100065. [Google Scholar] [CrossRef]
- Zhou, Y.; Manu, M.K.; Li, D.; Johnravindar, D.; Selvam, A.; Varjani, S.; Wong, J. Effect of Chinese medicinal herbal residues compost on tomato and Chinese cabbage plants: Assessment on phytopathogenic effect and nutrients uptake. Environ. Res. 2023, 216, 114747. [Google Scholar] [CrossRef] [PubMed]
- Gruda, N.S. Increasing sustainability of growing media constituents and stand-alone substrates in soilless culture systems. Agronomy 2019, 9, 298. [Google Scholar] [CrossRef]
- Ma, J.; Chen, Y.; Wang, K.; Huang, Y.; Wang, H. Re-utilization of Chinese medicinal herbal residues improved soil fertility and maintained maize yield under chemical fertilizer reduction. Chemosphere 2021, 283, 131262. [Google Scholar] [CrossRef] [PubMed]
- Ratiarisoa, R.V.; Magniont, C.; Ginestet, S.; Oms, C.; Escadeillas, G. Assessment of distilled lavender stalks as bioaggregate for building materials: Hygrothermal properties, mechanical performance and chemical interactions with mineral pozzolanic binder. Constr. Build. Mater. 2016, 124, 801–815. [Google Scholar] [CrossRef]
- Singh, P.; Hundal, J.S.; Patra, A.K.; Wadhwa, M.; Sharma, A. Sustainable utilization of Aloe vera waste in the diet of lactating cows for improvement of milk production performance and reduction of carbon footprint. J. Clean. Prod. 2021, 288, 125118. [Google Scholar] [CrossRef]
- Xiao, D.; Shao, H.; Huo, Y.; Agung Nugroho, W.; Ifeoluwa Ogunniran, B.; Fan, W.; Huo, M. Reclamation of ginseng residues using two-stage fermentation and evaluation of their beneficial effects as dietary feed supplements for piglets. Waste Manag. 2022, 154, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Chrysargyris, A.; Antoniou, O.; Xylia, P.; Petropoulos, S.; Tzortzakis, N. The use of spent coffee grounds in growing media for the production of Brassica seedlings in nurseries. Environ. Sci. Pollut. Res. 2021, 28, 24279–24290. [Google Scholar] [CrossRef] [PubMed]
- Chrysargyris, A.; Antoniou, O.; Athinodorou, F.; Vassiliou, R.; Papadaki, A.; Tzortzakis, N. Deployment of olive-stone waste as a substitute growing medium component for Brassica seedling production in nurseries. Environ. Sci. Pollut. Res. 2019, 26, 35461–35472. [Google Scholar] [CrossRef] [PubMed]
- Patsalou, M.; Chrysargyris, A.; Tzortzakis, N.; Koutinas, M. A biorefinery for conversion of citrus peel waste into essential oils, pectin, fertilizer and succinic acid via different fermentation strategies. Waste Manag. 2020, 113, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Bolechowski, A.; Moral, R.; Bustamante, M.A.; Bartual, J.; Paredes, C.; Pérez-Murcia, M.D.; Carbonell-Barrachina, A.A. Winery-distillery composts as partial substitutes of traditional growing media: Effect on the volatile composition of thyme essential oils. Sci. Hortic. 2015, 193, 69–76. [Google Scholar] [CrossRef]
- Lu, Q.; Li, C. Comprehensive utilization of Chinese medicine residues for industry and environment protection: Turning waste into treasure. J. Clean. Prod. 2021, 279, 123856. [Google Scholar] [CrossRef]
- Ilangovan, M.; Guna, V.; Hu, C.; Nagananda, G.S.; Reddy, N. Curcuma longa L. plant residue as a source for natural cellulose fibers with antimicrobial activity. Ind. Crops Prod. 2018, 112, 556–560. [Google Scholar] [CrossRef]
- Bistgani, Z.E.; Hashemi, M.; DaCosta, M.; Craker, L.; Maggi, F.; Morshedloo, M.R. Effect of salinity stress on the physiological characteristics, phenolic compounds and antioxidant activity of Thymus vulgaris L. and Thymus daenensis Celak. Ind. Crops Prod. 2019, 135, 311–320. [Google Scholar] [CrossRef]
- Bustamante, M.A.; Paredes, C.; Moral, R.; Agulló, E.; Pérez-Murcia, M.D.; Abad, M. Composts from distillery wastes as peat substitutes for transplant production. Resour. Conserv. Recycl. 2008, 52, 792–799. [Google Scholar] [CrossRef]
- Giménez, A.; Fernández, J.A.; Pascual, J.A.; Ros, M.; Saez-Tovar, J.; Martinez-Sabater, E.; Gruda, N.S.; Egea-Gilabert, C. Promising composts as growing media for the production of baby leaf lettuce in a floating system. Agronomy 2020, 10, 1540. [Google Scholar] [CrossRef]
- Ceglie, F.G.; Bustamante, M.A.; Ben Amara, M.; Tittarelli, F. The challenge of peat substitution in organic seedling production: Optimization of growing media formulation through mixture design and response surface analysis. PLoS ONE 2015, 10, e0128600. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Antoniou, O.; Tzionis, A.; Prasad, M.; Tzortzakis, N. Alternative soilless media using olive-mill and paper waste for growing ornamental plants. Environ. Sci. Pollut. Res. 2018, 25, 35915–35927. [Google Scholar] [CrossRef]
- Petropoulos, S.; Fernandes, Â.; Stojković, D.; Pereira, C.; Taofiq, O.; Di Gioia, F.; Tzortzakis, N.; Soković, M.; Barros, L.; Ferreira, I.C.F.R. Cotton and cardoon byproducts as potential growing media components for Cichorium spinosum L. commercial cultivation. J. Clean. Prod. 2019, 240, 118254. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Stamatakis, A.; Moustakas, K.; Prasad, M.; Tzortzakis, N. Evaluation of Municipal Solid Waste Compost and/or Fertigation as Peat Substituent for Pepper Seedlings Production. Waste Biomass Valorization 2018, 9, 2285–2294. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Tzortzakis, N. Municipal solid waste compost as a peat substitute for vegetable seedling production. A case study on cucumber and endive seedlings. In Municipal Solid Waste: Management Strategies, Challenges and Future Directions; Tzortzakis, Ν., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2017; pp. 367–386. [Google Scholar]
- Mill, L.A.; Najar, B.; Demasi, S.; Caser, M.; Gaino, W.; Cioni, P.L.; Pistelli, L.; Scariot, V. Cultivation substrate composition influences morphology, volatilome and essential oil of Lavandula angustifolia Mill. Agronomy 2019, 9, 411. [Google Scholar]
- Fan, R.; Luo, J.; Yan, S.; Zhou, Y.; Zhang, Z. Effects of Biochar and Super Absorbent Polymer on Substrate Properties and Water Spinach Growth. Pedosphere 2015, 25, 737–748. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Saridakis, C.; Tzortzakis, N. Use of Municipal Solid Waste Compost as Growing Medium Component for Melon Seedlings Production. J. Plant Biol. Soil Health 2013, 2, 1–5. [Google Scholar]
- Chrysargyris, A.; Prasad, M.; Kavanagh, A.; Tzortzakis, N. Biochar type, ratio, and nutrient levels in growing media affects seedling production and plant performance. Agronomy 2020, 10, 1421. [Google Scholar] [CrossRef]
- Tubeileh, A.M.; Souikane, R.T. Effect of olive vegetation water and compost extracts on seed germination of four weed species. Curr. Plant Biol. 2020, 22, 100150. [Google Scholar] [CrossRef]
- Kelepesi, S.; Tzortzakis, N.G. Olive mill wastesA growing medium component for seedling and crop production of lettuce and chicory. Int. J. Veg. Sci. 2009, 15, 325–339. [Google Scholar] [CrossRef]
- Kuczmarski, D. Amending the cost of media. Am. Nurs. Manag. 1994, 179, 47–52. [Google Scholar]
- Tsakaldimi, M. Kenaf (Hibiscus cannabinus L.) core and rice hulls as components of container media for growing Pinus halepensis M. seedlings. Bioresour. Technol. 2006, 97, 1631–1639. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Hajisolomou, E.; Xylia, P.; Tzortzakis, N. Olive-mill and grape-mill waste as a substitute growing media component for unexploded vegetables production. Sustain. Chem. Pharm. 2023, 31, 100940. [Google Scholar] [CrossRef]
- Tebenkova, D.N.; Lukina, N.V.; Vorobyev, R.A.; Orlova, M.A.; Gagarin, Y.N. Germination and biometric parameters of seedlings grown on solid pulp and paper waste medium. Contemp. Probl. Ecol. 2015, 8, 892–900. [Google Scholar] [CrossRef]
- Chugh, V.; Mishra, V.; Vishal Chugh, C.; Dwivedi, S.; Sharma, K. Purslane (Portulaca oleracea L.): An underutilized wonder plant with potential pharmacological value. Pharma Innov. J. 2019, 8, 236–246. [Google Scholar]
- Kumar, A.; Sreedharan, S.; Kashyap, A.K.; Singh, P.; Ramchiary, N. A review on bioactive phytochemicals and ethnopharmacological potential of purslane (Portulaca oleracea L.). Heliyon 2022, 8, e08669. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Fernandes, Â.; Calhelha, R.C.; Rouphael, Y.; Petrović, J.; Soković, M.; Ferreira, I.C.F.R.; Barros, L. Antimicrobial properties, cytotoxic effects, and fatty acids composition of vegetable oils from purslane, linseed, luffa, and pumpkin seeds. Appl. Sci. 2021, 11, 5738. [Google Scholar] [CrossRef]
- Carrascosa, A.; Pascual, J.A.; Ros, M.; Petropoulos, S.A.; Alguacil, M. Agronomical Practices and Management for Commercial Cultivation of Portulaca oleracea as a Crop: A Review. Plants 2023, 12, 1246. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Karkanis, A.; Martins, N.; Ferreira, I.C.F.R. Edible halophytes of the Mediterranean basin: Potential candidates for novel food products. Trends Food Sci. Technol. 2018, 74, 69–84. [Google Scholar] [CrossRef]
- Thalassinos, G.; Petropoulos, S.A.; Antoniadis, V. The Response of Purslane (Portulaca oleracea) to Soil-Added Pb: Is It Suitable as a Potential Phytoremediation Species? Toxics 2023, 11, 153. [Google Scholar] [CrossRef]
- Eliasson, J. Sand/Media Specifications; Washington State Department of Health: Tumwater, WA, USA, 2002. [Google Scholar]
- European Standard EN 13041; Soil Improvers and Growing Media—Determination of Physical Properties—Dry Bulk Density, Air Volume, Water Volume, Shrinkage Value and Total Pore Space. European Committee for Standardization: Brussels, Belgium, 1999.
- Chrysargyris, A.; Nikolaidou, E.; Stamatakis, A.; Tzortzakis, N. Vegetative, physiological, nutritional and antioxidant behavior of spearmint (Mentha spicata L.) in response to different nitrogen supply in hydroponics. J. Appl. Res. Med. Aromat. Plants 2017, 6, 52–61. [Google Scholar] [CrossRef]
- Tzortzakis, N.; Pitsikoulaki, G.; Stamatakis, A.; Chrysargyris, A. Ammonium to Total Nitrogen Ratio Interactive Effects with Salinity Application on Solanum lycopersicum Growth, Physiology, and Fruit Storage in a Closed Hydroponic System. Agronomy 2022, 12, 386. [Google Scholar] [CrossRef]
- Meyers, K.J.; Watkins, C.B.; Pritts, M.P.; Liu, R.H. Antioxidant and antiproliferative activities of strawberries. J. Agric. Food Chem. 2003, 51, 6887–6892. [Google Scholar] [CrossRef]
- Loreto, F.; Velikova, V. Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol. 2001, 127, 1781–1787. [Google Scholar] [CrossRef] [PubMed]
- De Azevedo Neto, A.D.; Prisco, J.T.; Enéas-Filho, J.; De Abreu, C.E.B.; Gomes-Filho, E. Effect of salt stress on antioxidative enzymes and lipid peroxidation in leaves and roots of salt-tolerant and salt-sensitive maize genotypes. Environ. Exp. Bot. 2006, 56, 87–94. [Google Scholar] [CrossRef]
- Rinaldi, S.; De Lucia, B.; Salvati, L.; Rea, E. Understanding complexity in the response of ornamental rosemary to different substrates: A multivariate analysis. Sci. Hortic. 2014, 176, 218–224. [Google Scholar] [CrossRef]
- Chand, S.; Anwar, M.; Patra, D.D.; Khanuja, S.P.S. Effect of Mint Distillation Waste on Soil Microbial Biomass in a Mint-Mustard Cropping Sequence. Commun. Soil Sci. Plant Anal. 2004, 35, 243–254. [Google Scholar] [CrossRef]
- Abad, M.; Noguera, P.; Burés, S. National inventory of organic wastes for use as growing media for ornamental potted plant production: Case study in Spain. Bioresour. Technol. 2001, 77, 197–200. [Google Scholar] [CrossRef]
- Lasaridi, K.; Protopapa, I.; Kotsou, M.; Pilidis, G.; Manios, T.; Kyriacou, A. Quality assessment of composts in the Greek market: The need for standards and quality assurance. J. Environ. Manag. 2006, 80, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Selvam, A.; Wong, J.W.C. Effect of Chinese medicinal herbal residues on microbial community succession and anti-pathogenic properties during co-composting with food waste. Bioresour. Technol. 2016, 217, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhan, S.; Yu, H.; Xue, X.; Hong, N. The effects of temperature and catalysts on the pyrolysis of industrial wastes (herb residue). Bioresour. Technol. 2010, 101, 3236–3241. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, H.M.; Romero, A.M.; Pereira, H.; Borges, P.; Cabral, F.; Vasconcelos, E. Evaluation of a compost obtained from forestry wastes and solid phase of pig slurry as a substrate for seedlings production. Bioresour. Technol. 2007, 98, 3294–3297. [Google Scholar] [CrossRef] [PubMed]
- Patra, D.D.; Anwar, M.; Chand, S. Integrated nutrient management and waste recycling for restoring soil fertility and productivity in Japanese mint and mustard sequence in Uttar Pradesh, India. Agric. Ecosyst. Environ. 2000, 80, 267–275. [Google Scholar] [CrossRef]
- Tüzel, Y.; Ekinci, K.; Öztekin, G.B.; Erdal, I.; Varol, N.; Merken, Ö. Utilization of olive oil processing waste composts in organic tomato seedling production. Agronomy 2020, 10, 797. [Google Scholar] [CrossRef]
- Papafotiou, M.; Kargas, G.; Lytra, I. Olive-mill waste compost as a growth medium component for foliage potted plants. HortScience 2005, 40, 1746–1750. [Google Scholar] [CrossRef]
- Ouzounidou, G.; Asfi, M.; Sotirakis, N.; Papadopoulou, P.; Gaitis, F. Olive mill wastewater triggered changes in physiology and nutritional quality of tomato (Lycopersicon esculentum Mill.) depending on growth substrate. J. Hazard. Mater. 2008, 158, 523–530. [Google Scholar] [CrossRef]
- Kiarostami, K.; Mohseni, R.; Saboora, A. Biochemical changes of Rosmarinus officinalis under salt stress. J. Stress Physiol. Biochem. 2010, 6, 114–122. [Google Scholar]
- Carmona, E.; Moreno, M.T.; Avilés, M.; Ordovas, J. Composting of wine industry wastes and their use as a substrate for growing soilless ornamental plants. Span. J. Agric. Res. 2012, 10, 482. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Maggini, R.; Incrocci, L.; Pardossi, A.; Tzortzakis, N. Copper tolerance and accumulation on Pelargonium graveolens l’hér. Grown in hydroponic culture. Plants 2021, 10, 1663. [Google Scholar] [CrossRef] [PubMed]
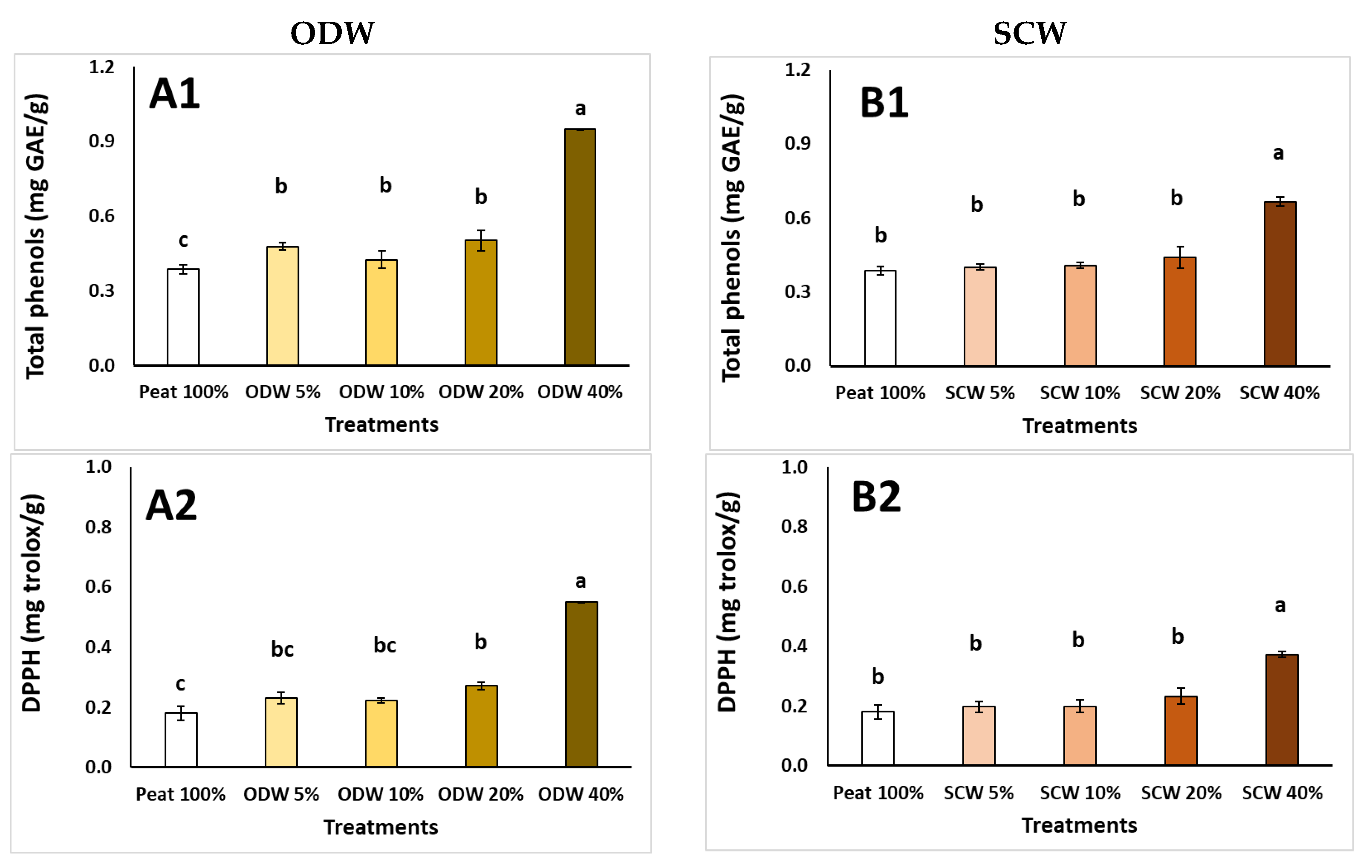
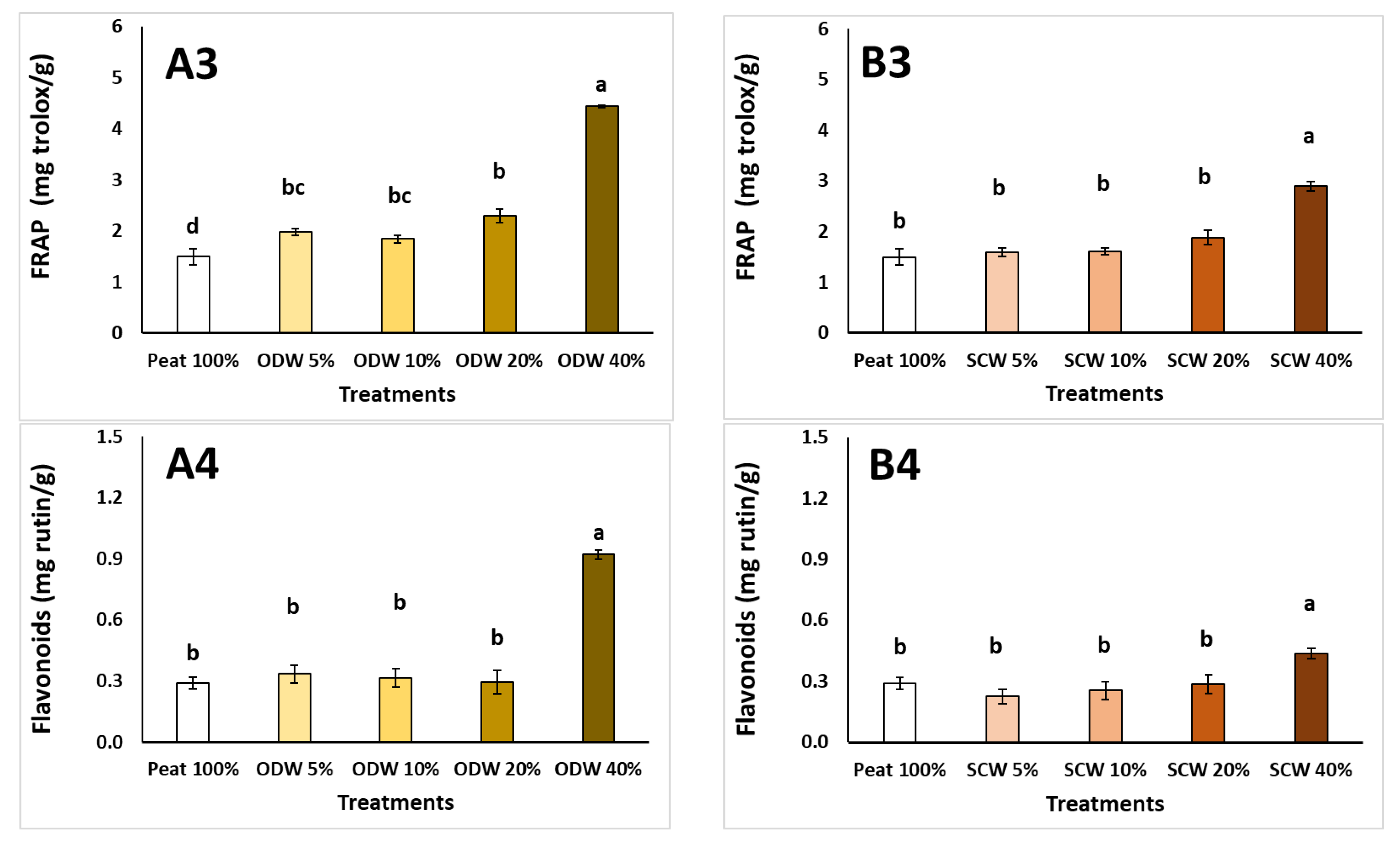
| Peat 100% | ODW 5% | ODW 10% | ODW 20% | ODW 40% | ODW 100% | |
|---|---|---|---|---|---|---|
| pH | 6.32 ± 0.17 b | 6.34 ± 0.05 b | 6.31 ± 0.09 b | 6.62 ± 0.01 b | 7.51 ± 0.12 a | 5.96 ± 0.07 c |
| EC (mS/cm) | 0.84 ± 0.04 c | 1.13 ± 0.07 b,c | 0.89 ± 0.00 b,c | 1.12 ± 0.04 b | 1.70 ± 0.18 a | 1.92 ± 0.01 a |
| Organic matter (%) | 72.38 ± 1.28 c,d | 73.03 ± 0.36 c | 73.30 ± 0.73 c | 70.16 ± 0.38 d | 76.91 ± 1.08 b | 92.79 ± 0.25 a |
| Organic C (%) | 41.98 ± 0.74 c,d | 42.36 ± 0.21 c | 42.51 ± 0.42 c | 40.69 ± 0.22 d | 44.61 ± 0.63 b | 53.82 ± 0.14 a |
| C/N ratio | 50.36 ± 2.12 a | 42.91 ± 0.99 b | 40.92 ± 3.35 b | 26.22 ± 0.67 c | 28.33 ± 0.79 c | 51.21 ± 0.23 a |
| N (g/kg) | 8.35 ± 0.19 c | 9.88 ± 0.20 b | 10.51 ± 0.76 b | 15.53 ± 0.33 a | 15.77 ± 0.59 a | 10.51 ± 0.05 b |
| K (g/kg) | 2.03 ± 0.05 d | 3.86 ± 0.31 c | 3.96 ± 0.41 c | 4.69 ± 0.13 c | 7.35 ± 0.31 b | 13.46 ± 0.11 a |
| P (g/kg) | 1.12 ± 0.04 c | 1.61 ± 0.29 b,c | 1.72 ± 0.09 b | 1.91 ± 0.18 b | 2.62 ± 0.18 a | 2.83 ± 0.02 a |
| Ca (g/kg) | 15.01 ± 0.56 b | 21.52 ± 2.43 a | 17.62 ± 0.98 b | 20.41 ± 0.21 a | 20.51 ± 0.49 a | 7.66 ± 0.27 c |
| Mg (g/kg) | 0.79 ± 0.04 e | 1.51 ± 0.21 d | 1.50 ± 0.09 d | 2.22 ± 0.02 c | 3.29 ± 0.06 a | 2.67 ± 0.10 b |
| Na (g/kg) | 0.97 ± 0.03 c | 1.12 ± 0.12 b | 1.12 ± 0.08 a,b | 1.24 ± 0.05 a,b | 1.31 ± 0.03 a | 1.22 ± 0.08 a,b |
| Total porosity % | 84.97 ± 0.76 a | 72.68 ± 3.31 b | 77.19 ± 4.48 a,b | 53.13 ± 1.37 c | 48.60 ± 3.67 c | 69.87 ± 3.89 b |
| Air-filled porosity (% v/v) | 18.43 ± 1.00 a | 10.47 ± 2.39 b | 9.14 ± 2.26 b | 7.90 ± 0.50 b | 5.52 ± 0.99 b,c | 1.57 ± 0.71 c |
| Bulk density (g/cm3) | 0.15 ± 0.00 c | 0.16 ± 0.00 b,c | 0.17 ± 0.00 b | 0.17 ± 0.00 b | 0.18 ± 0.00 b | 0.29 ± 0.01 a |
| Available water holding capacity (% v/v) | 66.54 ± 1.75 a | 62.21 ± 1.28 a | 68.04 ± 2.57 a | 45.41 ± 1.84 b | 43.07 ± 3.00 b | 68.30 ± 3.17 a |
| Peat 100% | SCW 5% | SCW 10% | SCW 20% | SCW 40% | SCW 100% | |
|---|---|---|---|---|---|---|
| pH | 6.32 ± 1.79 d | 6.27 ± 1.12 d | 6.53 ± 0.00 c,d | 6.91 ± 0.04 b | 7.54 ± 0.01 a | 6.75 ± 0.08 b,c |
| EC (mS/cm) | 0.84 ± 0.04 b | 1.26 ± 0.07 a | 1.24 ± 0.11 a | 1.10 ± 0.06 a | 1.18 ± 0.03 a | 1.19 ± 0.14 a |
| Organic matter (%) | 72.38 ± 1.28 b | 77.64 ± 2.26 b | 75.39 ± 2.64 b | 75.23 ± 1.45 b | 76.11 ± 1.22 b | 92.79 ± 0.25 a |
| Organic C (%) | 41.98 ± 0.74 b | 45.03 ± 1.31 b | 43.72 ± 1.53 b | 43.63 ± 0.84 b | 44.14 ± 0.71 b | 53.82 ± 0.14 a |
| C/N ratio | 50.36 ± 2.12 a | 50.89 ± 1.44 a | 38.38 ± 1.21 b,c | 36.33 ± 1.54 c | 30.04 ± 0.88 d | 42.57 ± 1.19 b |
| N (g/kg) | 8.35 ± 0.19 c | 8.87 ± 0.45 c | 11.40 ± 0.46 b | 12.04 ± 0.44 b | 14.73 ± 0.65 a | 12.66 ± 0.37 b |
| K (g/kg) | 2.03 ± 0.05 f | 3.11 ± 0.25 e | 4.20 ± 0.32 d | 6.31 ± 0.25 c | 8.75 ± 0.25 b | 14.06 ± 0.35 a |
| P (g/kg) | 1.12 ± 0.04 c | 1.39 ± 0.05 bc | 1.70 ± 0.06 b | 1.75 ± 0.07 b | 2.51 ± 0.28 a | 1.65 ± 0.10 b |
| Ca (g/kg) | 15.01 ± 0.56 b | 16.37 ± 1.19 b | 20.02 ± 0.33 a | 22.57 ± 1.07 a | 21.27 ± 0.73 a | 11.58 ± 0.50 c |
| Mg (g/kg) | 0.79 ± 0.04 d | 1.02 ± 0.08 d | 1.30 ± 0.03 c | 1.82 ± 0.08 b | 2.25 ± 0.06 a | 1.70 ± 0.11 b |
| Na (g/kg) | 0.97 ± 0.03 e | 1.03 ± 0.07 e | 1.20 ± 0.05 d | 1.65 ± 0.03 c | 2.03 ± 0.01 b | 5.79 ± 0.04 a |
| Total porosity % | 84.97 ± 0.76 a,b | 91.82 ± 3.87 a | 77.63 ± 3.41 b,c | 69.01 ± 3.70 c,d | 62.88 ± 5.28 d | 98.19 ± 1.62 a |
| Air-filled porosity (% v/v) | 18.43 ± 1.00 a | 15.52 ± 2.21 a,b | 14.28 ± 1.46 a,b | 13.42 ± 2.65 a,b | 9.62 ± 2.91 b | 17.14 ± 1.43 a,b |
| Bulk density (g/cm3) | 0.15 ± 0.00 b | 0.16 ± 0.00 a | 0.17 ± 0.00 a | 0.17 ± 0.00 a | 0.15 ± 0.00 b | 0.12 ± 0.00 c |
| Available water holding capacity (% v/v) | 66.54 ± 1.75 b | 76.30 ± 1.68 a | 63.35 ± 2.56 b | 55.57 ± 1.44 c | 53.25 ± 2.37 c | 81.05 ± 0.19 a |
| Height | Leaf No. | Fresh Weight | Dry Weight | |
|---|---|---|---|---|
| Peat 100% | 22.74 ± 0.79 a Y | 41.00 ± 3.11 a | 12.52 ± 0.60 a | 0.56 ± 0.01 a |
| ODW 5% | 18.12 ± 3.31 a,b | 27.60 ±5.80 a,b | 6.43 ± 2.30 b | 0.54 ± 0.20 a |
| ODW 10% | 15.48 ± 3.03 b | 21.60 ±8.50 b | 6.28 ± 2.76 b | 0.39 ± 0.17 a,b |
| ODW 20% | 9.34 ± 0.66 c | 7.60 ± 0.74 c | 0.98 ± 0.12 c | 0.07 ± 0.01 b |
| ODW 40% | 7.30 ± 0.99 c | 4.00 ± 0.71 c | 0.34 ± 0.09 c | 0.03 ± 0.00 b |
| Peat 100% | 22.74 ± 0.79 a | 41.00 ± 3.11 a | 12.52 ± 0.60 a | 0.56 ± 0.01 a |
| SCW 5% | 22.48 ± 1.74 a | 39.40 ± 3.04 a | 10.36 ± 1.64 a,b | 0.46 ± 0.11 a |
| SCW 10% | 17.04 ± 2.77 b | 27.60 ± 8.80 a,b | 5.55 ± 2.27 bc | 0.49 ± 0.16 a |
| SCW 20% | 15.90 ± 1.79 b,c | 21.00 ± 3.17 b,c | 3.93 ± 0.67 c | 0.30 ± 0.11 a,b |
| SCW 40% | 11.24 ± 1.56 c | 10.80 ± 0.96 c | 1.51 ± 0.32 c | 0.11 ± 0.01 b |
| Stomatal Conductance | Chlorophyll Fluorescence | Chl a | Chl b | Total Chls | Carotenoids | |
|---|---|---|---|---|---|---|
| Peat 100% | 110.16 ± 8.67 a Y | 0.80 ± 0.00 a | 0.39 ± 0.02 a | 0.10 ± 0.00 a | 0.49 ± 0.03 a | 0.07 ± 0.00 a |
| ODW 5% | 82.00 ± 5.50 a,b | 0.77 ± 0.00 b | 0.36 ± 0.06 a | 0.08 ± 0.01 a | 0.45 ± 0.07 a | 0.08 ± 0.01 a |
| ODW 10% | 65.00 ± 5.85 b | 0.75 ± 0.00 b,c | 0.20 ±0.03 b | 0.04 ± 0.01 b | 0.24 ± 0.04 b | 0.04 ± 0.00 b |
| ODW 20% | 62.5 ± 0.50 b | 0.74 ± 0.00 c | 0.18 ±0.04 b | 0.03 ± 0.01 b,c | 0.21 ± 0.05 b | 0.04 ± 0.01 b |
| ODW 40% | n.m. | n.m. | 0.09 ±0.00 b | 0.01 ± 0.00 c | 0.09 ± 0.00 b | 0.03 ± 0.00 b |
| Peat 100% | 110.16 ± 8.67 a | 0.80 ± 0.00 a | 0.39 ± 0.02 a | 0.10 ± 0.00 a | 0.49 ± 0.03 a | 0.07 ± 0.00 a |
| SCW 5% | 69.00 ± 6.80 b | 0.72 ± 0.03 b | 0.28 ± 0.03 b | 0.06 ± 0.01 b | 0.35 ± 0.04 b | 0.05 ± 0.01 b |
| SCW 10% | 59.50 ± 3.50 b,c | 0.75 ± 0.01 a,b | 0.18 ± 0.00 c,d | 0.04 ± 0.00 c,d | 0.22 ± 0.00 c,d | 0.04 ± 0.00 b,c |
| SCW 20% | 55.00 ± 5.85 b,c | 0.74 ± 0.01 a,b | 0.24 ± 0.01 b,c | 0.05 ± 0.00 b,c | 0.29 ± 0.01 b,c | 0.05 ± 0.00 b,c |
| SCW 40% | 33.50 ± 0.50 c | 0.65 ± 0.01 c | 0.14 ± 0.00 d | 0.03 ± 0.00 d | 0.17 ± 0.00 d | 0.03 ± 0.00 c |
| N | K | P | Na | Ca | Mg | |
|---|---|---|---|---|---|---|
| Peat 100% | 35.22 ± 0.27 a Y | 86.96 ± 1.10 b | 11.40 ± 0.31 a,b | 12.12 ± 0.09 c | 6.26 ± 0.16 a | 5.20 ± 0.18 a |
| ODW 5% | 27.87 ± 1.09 c | 84.13 ± 4.11 b | 9.27 ± 1.56 b,c | 11.78 ± 0.56 c | 5.56 ± 0.05 b,c | 3.14 ± 0.46 b |
| ODW 10% | 25.34 ± 0.48 d | 99.95 ± 4.05 a | 12.17 ± 0.59 a | 12.41 ± 0.22 c | 5.33 ± 0.30 c | 3.29 ± 0.40 b |
| ODW 20% | 32.74 ± 0.32 b | 86.31 ± 1.69 b | 10.92 ± 0.21 a,b | 14.61 ± 0.28 b | 6.10 ± 0.12 a,b | 3.61 ± 0.07 b |
| ODW 40% | 31.23 ± 0.24 b | 60.35 ± 1.18 c | 7.15 ± 0.14 c | 16.96 ± 0.33 a | 5.40 ± 1.10 c | 2.81 ± 0.05 b |
| Peat 100% | 35.22 ± 0.27 a | 86.96 ± 1.10 b,c | 11.40 ± 0.31 b | 12.12 ± 0.09 b | 6.26 ± 0.16 a | 5.20 ± 0.18 a |
| SCW 5% | 28.56 ± 0.15 b | 84.55 ± 0.54 b,c | 8.51 ± 0.64 c | 10.71 ± 0.05 c | 5.60 ± 0.04 b | 3.96 ± 0.06 b |
| SCW 10% | 25.95 ± 0.87 c | 89.59 ± 3.90 b | 11.51 ± 0.27 b | 11.42 ± 0.33 c | 6.15 ± 0.21 a | 3.92 ± 0.28 b |
| SCW 20% | 25.92 ± 0.12 c | 100.43 ± 1.96 a | 12.85 ± 0.25 a | 11.17 ± 0.22 c | 4.99 ± 0.09 c | 3.55 ± 0.07 b |
| SCW 40% | 25.89 ± 0.14 c | 81.78 ± 1.60 c | 5.49 ± 0.11 d | 13.69 ± 0.26 a | 4.86 ± 0.09 c | 4.05 ± 0.08 b |
| H2O2 | MDA | SOD | CAT | POD | |
|---|---|---|---|---|---|
| Peat 100% | 0.20 ± 0.01 b,c Y | 8.84 ± 0.74 b | 0.94 ± 0.12 b | 3.61 ± 0.71 b,c | 0.54 ± 0.05 c |
| ODW 5% | 0.23 ± 0.01 b,c | 8.68 ± 0.59 b,c | 1.15 ± 0.07 b | 11.65 ± 0.85 a | 0.81 ± 0.06 b |
| ODW 10% | 0.19 ± 0.00 c | 7.46 ± 0.86 c | 0.82 ± 0.11 b | 5.10 ± 0.60 b | 0.68 ± 0.03 b,c |
| ODW 20% | 0.24 ± 0.01 b | 10.93 ± 0.91 a,b | 1.08 ± 0.08 b | 1.68 ± 0.41 c | 0.86 ± 0.09 b |
| ODW 40% | 0.46 ± 0.03 a | 12.87 ± 0.71 a | 1.85 ± 0.04 a | 3.19 ± 0.35 b,c | 1.30 ± 0.16 a |
| Peat 100% | 0.15 ± 0.00 b,c | 7.85 ± 0.57 a | 0.94 ± 0.12 | 3.61 ± 0.71 b,c | 0.54 ± 0.05 c |
| SCW 5% | 0.17 ± 0.01 a,b | 5.25 ± 0.67 b | 1.13 ± 0.04 | 4.69 ± 0.22 b | 0.86 ± 0.03 a,b |
| SCW 10% | 0.12 ± 0.00 c | 5.11 ± 0.78 b,c | 1.01 ± 0.12 | 9.03 ± 0.91 a | 0.84 ± 0.07 a,b |
| SCW 20% | 0.15 ± 0.02 b,c | 3.73 ± 0.14 b,c | 1.14 ± 0.07 | 2.04 ± 0.58 c | 0.76 ± 0.02 b |
| SCW 40% | 0.21 ± 0.03 a | 3.27 ± 0.52 c | 1.15 ± 0.07 | 2.38 ± 0.85 b,c | 0.99 ± 0.08 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chrysargyris, A.; Louka, S.; Petropoulos, S.A.; Tzortzakis, N. Soilless Cultivation of Portulaca oleracea Using Medicinal and Aromatic Plant Residues for Partial Peat Replacement. Horticulturae 2023, 9, 474. https://doi.org/10.3390/horticulturae9040474
Chrysargyris A, Louka S, Petropoulos SA, Tzortzakis N. Soilless Cultivation of Portulaca oleracea Using Medicinal and Aromatic Plant Residues for Partial Peat Replacement. Horticulturae. 2023; 9(4):474. https://doi.org/10.3390/horticulturae9040474
Chicago/Turabian StyleChrysargyris, Antonios, Stavros Louka, Spyridon A. Petropoulos, and Nikolaos Tzortzakis. 2023. "Soilless Cultivation of Portulaca oleracea Using Medicinal and Aromatic Plant Residues for Partial Peat Replacement" Horticulturae 9, no. 4: 474. https://doi.org/10.3390/horticulturae9040474
APA StyleChrysargyris, A., Louka, S., Petropoulos, S. A., & Tzortzakis, N. (2023). Soilless Cultivation of Portulaca oleracea Using Medicinal and Aromatic Plant Residues for Partial Peat Replacement. Horticulturae, 9(4), 474. https://doi.org/10.3390/horticulturae9040474









