Effects of Pre-Treatments and Conservation Conditions on Seed Viability and Germination of Two Varieties of an Endangered Species Anacyclus pyrethrum (L.) Link (Asteraceae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Germination Study
2.2.1. Seed Cleaning
2.2.2. Determination of Fresh Weight (FW), Dry Weight (DW) and Moisture Content of Seeds
2.2.3. Seed Germination Assay
2.2.4. Calculation of Germination Parameters
- Germination kinetics
- Germination %(TG) (% germination) [78]
- Germination capacity (GPC) [79]
- Germination speed [78]
- Mean Daily Germination (MDG) [85]
- Germination index (GI) [17]
- Germination reduction percentage (RG%) [17]
2.2.5. Viability Assay for Preserved Seeds
3. Results
3.1. Seed Water Content
3.2. Germination Type
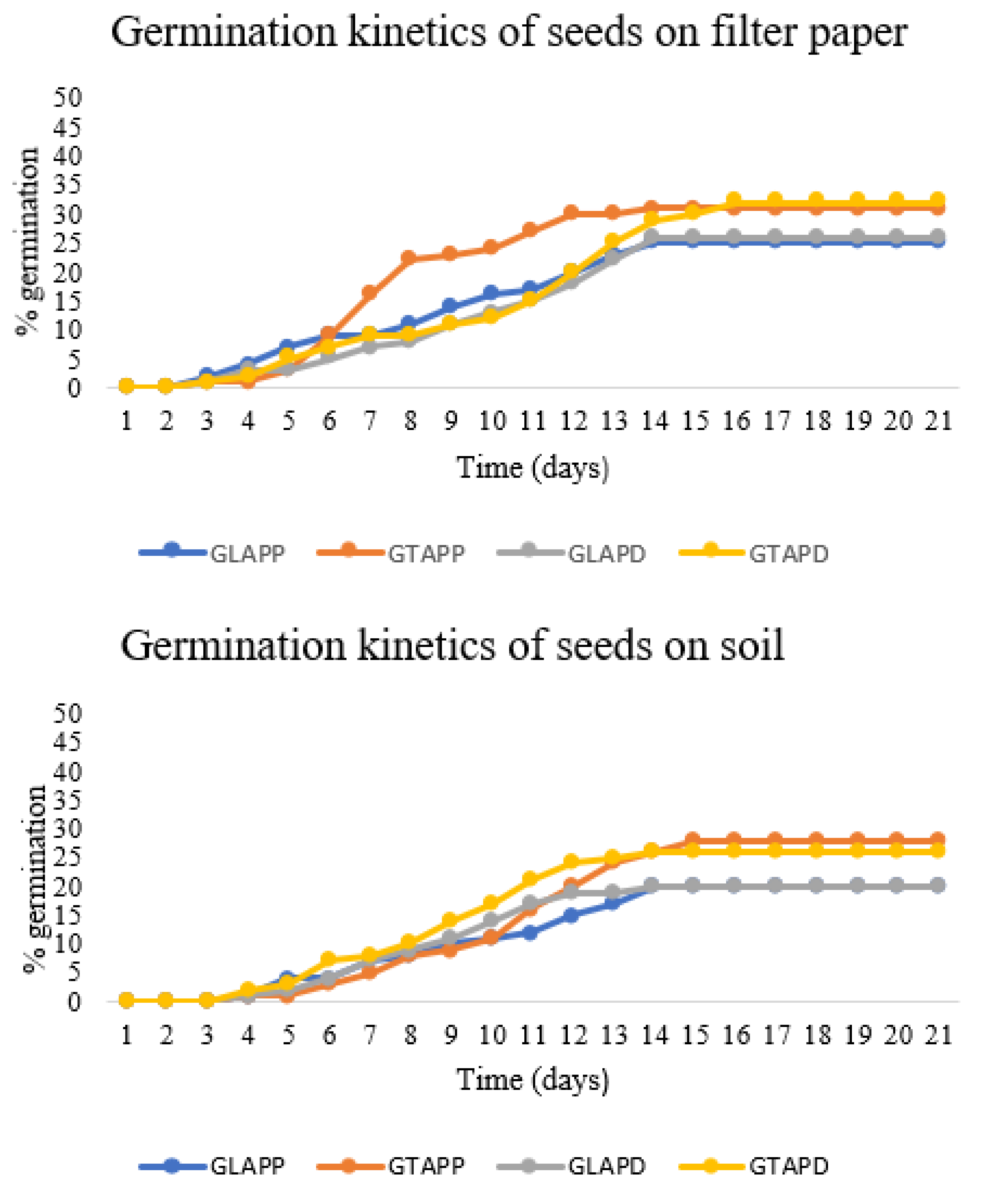
3.3. Germination Assays
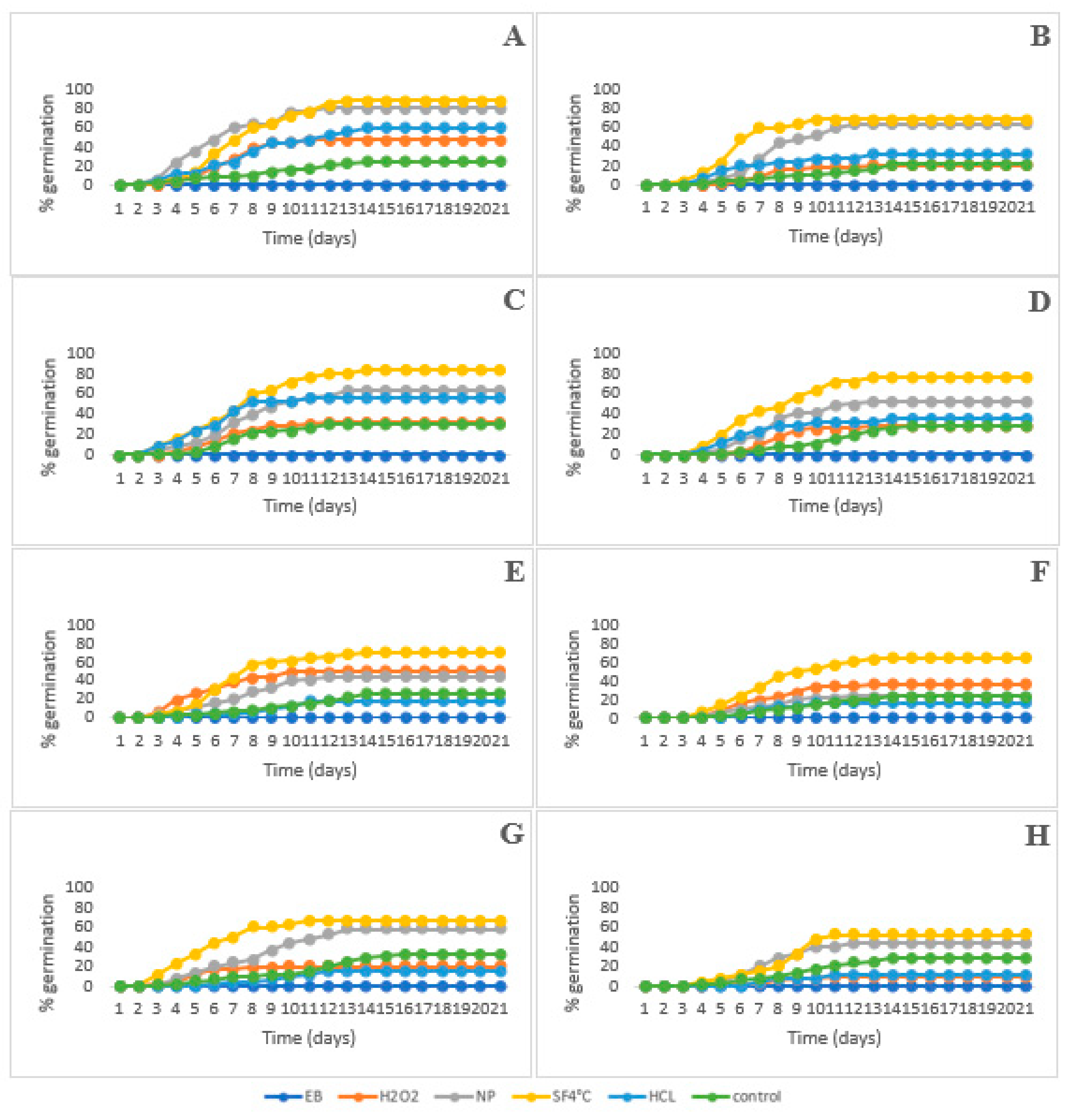
3.4. Influences of Pre-Treatment on Seed Germination Quality
3.5. Seed Viability Assay
Viability Assay for Preserved Seeds
3.6. Influences of Storage Conditions on Seed Germination Qualities
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Weitbrecht, K.; Müller, K.; Leubner-Metzger, G. First off the mark: Early seed germination. J. Exp. Bot. 2011, 62, 3289–3309. [Google Scholar] [CrossRef] [PubMed]
- El-Keblawy, A. Light and Temperature Requirements during Germination of Potential Perennial Grasses for Rehabilitation of Degraded Sandy Arabian Deserts. Land Degrad. Dev. 2017, 28, 1687–1695. [Google Scholar] [CrossRef]
- Manzoor, S.; Hameed, A.; Khan, M.A.; Gul, B. Seed germination ecology of a medicinal halophyte Zygophyllum propinquum: Responses to abiotic factors. Flora 2017, 233, 163–170. [Google Scholar] [CrossRef]
- Hadi, S.M.S.; Ahmed, M.Z.; Hameed, A.; Khan, M.A.; Gul, B. Seed germination and seedling growth responses of toothbrush tree (Salvadora persica Linn.) to different interacting abiotic stresses. Flora 2018, 243, 45–52. [Google Scholar] [CrossRef]
- Elnaggar, A.; El-Keblawy, A.; Mosa, K.A.; Soliman, S. Drought tolerance during germination depends on light and temperature of incubation in Salsola imbricata, a desert shrub of Arabian deserts. Flora 2018, 249, 156–163. [Google Scholar] [CrossRef]
- Kaufmann, M.R.; Ross, K.J. Water potential, temperature, and kinetin effects on seed germination in soil and solute systems. Amer. J. Bot. 1970, 57, 413–419. Available online: https://scholar.google.com/scholar_lookup?title=Water+potential%2C+temperature%2C+and+kinetin+effects+on+seed+germination+in+soil+and+solute+systems&author=Kaufmann%2C+M.R.&publication_year=1970 (accessed on 6 February 2023). [CrossRef]
- Finch-Savage, W.E.; Leubner-Metzger, G. Seed dormancy and the control of germination. New Phytol. 2006, 171, 501–523. [Google Scholar] [CrossRef] [PubMed]
- Maiti, R.; Rodríguez, H.G.; Ivanova, N.S. (Eds.) Seed characteristics, seed dormancy, germination and plant propagation. In Autoecology and Ecophysiology of Woody Shrubs and Trees; John Wiley & Sons, Ltd: Chichester, UK, 2016; pp. 270–277. Available online: https://onlinelibrary.wiley.com/doi/10.1002/9781119104452.ch23 (accessed on 6 February 2023).
- Chapman, A.G. Scarification of Black Locust Seed to Increase and Hasten Germination. J. For. 1936, 34, 66–74. [Google Scholar]
- Debroux, L.; Mbolo, M.; Delvingt, W.; Amougou, A. Régénération du Moabi et du Mukulungu au Cameroun. Prospectives pour l’aménagement. Bois Forets Trop. 1998, 255, 5–17. [Google Scholar]
- Toumi, M.; Barris, S.; Seghiri, M.; Cheriguene, H.; Aid, F. Effet de plusieurs méthodes de scarification et du stress osmotique sur la germination des graines de Robinia pseudoacacia L. Comptes Rendus Biol. 2017, 340, 264–270. [Google Scholar] [CrossRef]
- Dimri, K.; Sharma, N. Seed germination of Anacyclus pyrethrumd.c. in experimental field. Sci. Temper 2019, X, 45–50. [Google Scholar]
- Sheldon, J.C. The behaviour of seeds in soil: III. The influence of seed morphology and the behaviour of seedlings on the establishment of plants from surface-lying seeds. J. Ecol. 1974, 62, 47–66. [Google Scholar] [CrossRef]
- Torices, R.; Agudo, A.; Álvarez, I. Not only size matters: Achene morphology affects time of seedling emergence in three heterocarpic species of Anacyclus (Anthemideae, Asteraceae). An. Jardín Botánico Madr. 2013, 70, 48–55. [Google Scholar] [CrossRef]
- Bello, M.A.; Álvarez, I.; Torices, R.; Aguilar, J.F. Floral development and evolution of capitulum structure in Anacyclus (Anthemideae, Asteraceae). Ann. Bot. 2013, 112, 1597–1612. [Google Scholar] [CrossRef] [PubMed]
- Afonso, A.; Castro, S.; Loureiro, J.; Mota, L.; de Oliveira, J.C.; Torices, R. The effects of achene type and germination time on plant performance in the heterocarpic Anacyclus clavatus (Asteraceae). Am. J. Bot. 2014, 101, 892–898. [Google Scholar] [CrossRef] [PubMed]
- Mazliak, P. Physiologie Végétale. Croissance et Développement, nouvelle ed.; Hermann: Paris, France, 1998; Volume Tome 2, p. 468. [Google Scholar]
- Donohue, K.; De Casas, R.R.; Burghardt, L.; Kovach, K.; Willis, C.G. Germination, Postgermination Adaptation, and Species Ecological Ranges. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 293–319. [Google Scholar] [CrossRef]
- Mohammed, D.; Mohamed, T. Effect of Abiotic factors on seed germination of Anacyclus pyrethrum (L.) link, and modeling of habitat suitability in Saida (Algeria). Indian J. Ecol. 2019, 46, 777–782. [Google Scholar]
- Buza, L.; Young, A.; Thrall, P. Genetic erosion, inbreeding and reduced fitness in fragmented populations of the endangered tetraploid pea Swainsona recta. Biol. Conserv. 2000, 93, 177–186. [Google Scholar] [CrossRef]
- Young, A.; Boyle, T.; Brown, T. The population genetic consequences of habitat fragmentation for plants. Trends Ecol. Evol. 1996, 11, 413–418. [Google Scholar] [CrossRef]
- Menges, E.S. Seed Germination Percentage Increases with Population Size in a Fragmented Prairie Species. Conserv. Biol. 1991, 5, 158–164. [Google Scholar] [CrossRef]
- Morgan, J.W. Effects of Population Size on Seed Production and Germinability in an Endangered, Fragmented Grassland Plant. Conserv. Biol. 1999, 13, 266–273. [Google Scholar] [CrossRef]
- Costin, B.J.; Morgan, J.W.; Young, A.G. Reproductive success does not decline in fragmented populations of Leucochrysum albicans subsp. albicans var. tricolor (Asteraceae). Biol. Conserv. 2001, 98, 273–284. [Google Scholar] [CrossRef]
- Jawhari, F.Z.; Imtara, H.; El Moussaoui, A.; Khalis, H.; Es-Safi, I.; Al Kamaly, O.; Saleh, A.; Parvez, M.K.; Guemmouh, R.; Bari, A. Reproductive Biology of the Two Varieties of Anacyclus pyrethrum L.—Anacyclus pyrethrum var. pyrethrum (L.) Link and Anacyclus pyrethrum var. depressus (Ball.) Maire—An Endemic Endangered Species. Plants 2022, 11, 2299. [Google Scholar] [CrossRef] [PubMed]
- Sujith, K.; Darwin, C.R.; Suba, V. Antioxidant activity of ethanolic root extract of Anacyclus pyrethrum. Int. Res. J. Pharm. 2011, 5, 27. [Google Scholar]
- Jawhari, F.Z.; Moussaoui, A.E.; Bourhia, M.; Imtara, H.; Saghrouchni, H.; Ammor, K.; Ouassou, H.; Elamine, Y.; Ullah, R.; Ezzeldin, E.; et al. Anacyclus pyrethrum var. pyrethrum (L.) and Anacyclus pyrethrum var. depressus (Ball) Maire: Correlation between Total Phenolic and Flavonoid Contents with Antioxidant and Antimicrobial Activities of Chemically Characterized Extracts. Plants 2021, 10, 149. [Google Scholar]
- Pahuja, M.; Mehla, J.; Reeta, K.H.; Tripathi, M.; Gupta, Y.K. Effect of Anacyclus pyrethrum on Pentylenetetrazole-Induced Kindling, Spatial Memory, Oxidative Stress and Rho-Kinase II Expression in Mice. Neurochem. Res. 2013, 38, 547–556. [Google Scholar] [CrossRef]
- Benali, O.; Selles, C.; Salghi, R. Inhibition of acid corrosion of mild steel by Anacyclus pyrethrum L. extracts. Res. Chem. Intermed. 2014, 40, 259–268. [Google Scholar] [CrossRef]
- Selles, C.; Benali, O.; Tabti, B.; Larabi, L.; Harek, Y. Green corrosion inhibitor: Inhibitive action of aqueous extract of Anacyclus pyrethrum L. for the corrosion of mild steel in 0.5 M H2SO4. J. Mater. Environ. Sci. 2012, 3, 206–219. [Google Scholar]
- Manouze, H.; Bouchatta, O.; Gadhi, A.C.; Bennis, M.; Sokar, Z.; Ba-M’Hamed, S. Anti-inflammatory, Antinociceptive, and Antioxidant Activities of Methanol and Aqueous Extracts of Anacyclus pyrethrum Roots. Front. Pharmacol. 2017, 8, 598. [Google Scholar] [CrossRef] [PubMed]
- Rimbau, V.; Cerdan, C.; Vila, R.; Iglesias, J. Antiinflammatory activity of some extracts from plants used in the traditional medicine of North-African countries (II). Phytother. Res. 1999, 5, 128–132. [Google Scholar] [CrossRef]
- Jawhari, F.Z.; El Moussaoui, A.; Bourhia, M.; Imtara, H.; Mechchate, H.; Es-Safi, I.; Ullah, R.; Ezzeldin, E.; Mostafa, G.A.; Grafov, A.; et al. Anacyclus pyrethrum (L): Chemical Composition, Analgesic, Anti-Inflammatory, and Wound Healing Properties. Molecules 2020, 25, 5469. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.K.; Patel, R.V.; Venkatakrishna-Bhatt, H.; Gopalakrishna, G.; Devasankariah, G. A clinical appraisal of Anacyclus pyrethrum root extract in dental patients. Phytotherapy Res. 1992, 6, 158–159. [Google Scholar] [CrossRef]
- Sijelmassi, A. Les Plantes Médicinales du Maroc. 1993. Available online: https://sites.google.com/site/tiomenmafe/les-plantes-medicinales-du-maroc-badu (accessed on 23 August 2019).
- Van Hecken, L.; Practoner, G. Literature Review on Anacyclus pyrethrum and Profile of Company Jura in Germany who Supplies the Pyrethrum Root Powder Belgium. 2004, p. 28. Available online: https://docplayer.net/amp/47803539-Literature-revieuw-on-anacyclus-pyrethrum-and-profile-of-company-jura-in-germany-who-supplies-the-pyrethrum-root-powder.html (accessed on 8 July 2021).
- Abbas Zaidi, S.M.; Pathan, S.A.; Singh, S.; Jamil, S.; Ahmad, F.J.; Khar, R.K. Anticonvulsant, Anxiolytic and Neurotoxicity Profile of Aqarqarha (Anacyclus pyrethrum) DC (Compositae) Root Ethanolic Extract. Pharmacol. Pharm. 2013, 4, 535–541. [Google Scholar] [CrossRef]
- Boonen, J.; Sharma, V.; Dixit, V.; Burvenich, C.; De Spiegeleer, B. LC-MS N-alkylamide Profiling of an Ethanolic Anacyclus pyrethrum Root Extract. Planta Med. 2012, 78, 1787–1795. [Google Scholar] [CrossRef] [PubMed]
- Boonen, J.; Sharma, V.; Dixit, V.; De Spiegeleer, B. New N-alkylamides from Anacyclus pyrethrum. Planta Med. 2011, 77, PG94. [Google Scholar] [CrossRef]
- Shahraki, S.; Rad, J.S.; Rostami, F.M.; Shahraki, M.R.; Arab, M.R. Effects of Aqueous Root Extracts of Anacyclus pyrethrum on Gonadotropins and Testosterone Serum in Adult Male Rats. Am. J. Phytomed. Clin. Ther. 2014, 6, 767–772. [Google Scholar]
- Sharma, V.; Thakur, M.; Chauhan, N.S.; Dixit, V.K. Evaluation of the Anabolic, Aphrodisiac and Reproductive Activity of Anacyclus pyrethrum DC in Male Rats. Sci. Pharm. 2009, 77, 97–110. [Google Scholar] [CrossRef]
- Bendjeddou, D.; Lalaoui, K.; Satta, D. Immunostimulating activity of the hot water-soluble polysaccharide extracts of Anacyclus pyrethrum, Alpinia galanga and Citrullus colocynthis. J. Ethnopharmacol. 2003, 88, 155–160. [Google Scholar] [CrossRef]
- Sharma, V.; Thakur, M.; Chauhan, N.S.; Dixit, V.K. Immunomodulatory activity of petroleum ether extract of Anacyclus pyrethrum. Pharm. Biol. 2010, 48, 1247–1254. [Google Scholar] [CrossRef]
- Annalakshmi, R.; Uma, R. A treasure of medicinal herb—Anacyclus pyrethrum: A review. Indian J. Drugs Dis. 2012, 3, 9. [Google Scholar]
- Gautam, O.P.; Verma, S.; Jain, S.K. Anticonvulsant and myorelaxation activity of Anacyclus pyrethrum DC. (Akarkara) root extract. Pharmacologyonline 2011, 5, 121–125. [Google Scholar]
- Amine, D.; Mohamed, B.; Jamal, I.; Laila, N. Antibacterial Activity of Aqueous Extracts of Anacyclus pyrethrum (L) Link and Corrigiola Telephiifolia Pourr. From the Middle Atlas Region-Morocco. Eur. Sci. J. ESJ 2017, 13, 116. [Google Scholar] [CrossRef][Green Version]
- Jalayer Naderi, N.; Niakan, M.; Khodadadi, E.; Mohamadi-Motlagh, M. The antibacterial activity of methanolic Anacyclus pyrethrum and Pistacia lentiscus L. extract on Escherichia coli. Iran. J. Microbiol. 2016, 6, 372. [Google Scholar]
- Jalayer Naderi, N.; Niakan, M.; Khodadadi, E. Determination of Antibacterial Activity of Anacyclus pyrethrum Extract against Some of the Oral Bacteria: An In Vitro Study. J. Dent. Shiraz. Univ. Med. Scien. 2012, 13, 5. [Google Scholar]
- Selles, C.; Dib, M.E.A.; Djabou, N.; Beddou, F.; Muselli, A.; Tabti, B.; Costa, J.; Hammouti, B. Antimicrobial activity and evolution of the composition of essential oil from Algerian Anacyclus pyrethrum L. through the vegetative cycle. Nat. Prod. Res. 2013, 27, 2231–2234. [Google Scholar] [CrossRef]
- Selles, C. Valorisation D’une Plante Médicinale à Activité Antidiabétique de la Région de Tlemcen: Anacyclus pyrethrum L. Application de L’extrait Aqueux à L’inhibition de Corrosion D’un Acier Doux Dans H2SO4 0.5M. Ph.D. Thesis, Universite Abou Bekr Belkaid, Chetouane, Algeria, 30 June 2012. [Google Scholar]
- Hamimed, S. Caractérisation Chimique des Principes à Effet Antidermatophyte des Racines d’Anacyclus pyrethrum L. Master’s Thesis, Universite Mentouri Constantine, Constantine, Algeria, 24 June 2009. [Google Scholar]
- Doudach, L.; Meddah, B.; Alnamer, R.; Chibani, F.; Cherrah, Y. In vitro antibacterial activity of the methanolic and aqueous extracts of Anacyclus pyrethrum used in moroccan traditional medicine. Int. J. Pharm. Pharm. Sci. 2012, 4, 4. [Google Scholar]
- Elazzouzi, H.; Soro, A.; Elhilali, F.; Bentayeb, A.; Belghiti, M.A.E. Phytochemical study of Anacyclus pyrethrum (L.) of Middle Atlas (Morocco), and in vitro study of antibacterial activity of pyrethrum. Adv. Nat. Appl. Sci. 2014, 10, 131–141. [Google Scholar]
- Kushwaha, M.; Jatav, V.S.; Pandey, S. Plant Anacyclus pyrethrum—A Review. Res. J. Pharmacogn. Phytochem. 2012, 4, 164–170. [Google Scholar]
- Muralikrishnan, K.; Asokan, S.; Priya, P.G.; Ahmed, K.S.Z.; Ayyappadasan, G. Comparative evaluation of the local anesthetic activity of root extract of Anacyclus pyrethrum and its interaction at the site of injection in guinea pigs. Anesth. Essays Res. 2017, 11, 444–448. [Google Scholar] [CrossRef]
- Manouze, H.; Bouchatta, O.; Bennis, M.; Sokar, Z.; Ba-M’Hamed, S. Anticonvulsive and neuroprotective effects of aqueous and methanolic extracts of Anacyclus pyrethrum root in kainic acid-induced-status epilepticus in mice. Epilepsy Res. 2019, 158, 106225. [Google Scholar] [CrossRef]
- Sujith, K.; Suba, V.; Darwin, C.R. Neuropharmacological profile of ethanolic extract of Anacyclus pyrethrum in albino Wistar rats. Int. J. Pharm. Sci. Res. 2011, 2, 6. [Google Scholar]
- Azzi, R.; Djaziri, R.; Lahfa, F.; Sekkal, F.Z.; Benmehdi, H.; Belkacem, N. Ethnopharmacological survey of medicinal plants used in the traditional treatment of diabetes mellitus in the North Western and South Western Algeria. J. Med. Plants Res. 2012, 10, 2041–2050. [Google Scholar]
- Tyagi, S.; Mansoori, M.H.; Singh, N.K.; Shivhare, M.K.; Bhardwaj, P.; Singh, R.K. Antidiabetic Effect of Anacyclus pyrethrum DC in Alloxan Induced Diabetic Rats. Eur. J. Biol. Sci. 2011, 4, 117–120. [Google Scholar]
- Usmani, A.; Mujahid, M.D.; Khushtar, M.; Siddiqui, H.H.; Rahman, A. Hepatoprotective effect of Anacyclus pyrethrum Linn. against antitubercular drug-induced hepatotoxicity in SD rats. J. Complement. Integr. Med. 2016, 13, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Boonen, J.; Spiegeleer, B.D.; Dixit, V.K. Androgenic and Spermatogenic Activity of Alkylamide-Rich Ethanol Solution Extract of Anacyclus pyrethrum DC: Androgenic and spermatogenic activity of Anacyclus pyrethrum. Phytother. Res. 2013, 27, 99–106. [Google Scholar] [CrossRef]
- Mohammadi, A.; Mansoori, B.; Baradaran, P.C.; Baradaran, S.C.; Baradaran, B. Anacyclus pyrethrum Extract Exerts Anticancer Activities on the Human Colorectal Cancer Cell Line (HCT) by Targeting Apoptosis, Metastasis and Cell Cycle Arrest. J. Gastrointest. Cancer 2017, 48, 333–340. [Google Scholar] [CrossRef]
- Sujith, K.; Darwin, C.R.; Suba, V. Memory-enhancing activity of Anacyclus pyrethrum in albino Wistar rats. Asian Pac. J. Trop. Dis. 2012, 2, 307–311. [Google Scholar] [CrossRef]
- Humphries, C.J. A revision of the genus Anacyclus L. (Compositae: Anthemidaea). Bull. Br. Mus. Nat. Hist. Bot. 1979, 7, 83–142. [Google Scholar]
- Ouarghidi, A.; Powell, B.; Martin, G.J.; Abbad, A. Traditional sustainable harvesting knowledge and distribution of a vulnerable wild medicinal root (Anacyclus pyrethrum var. pyrethrum) in Ait M’hamed valley, Morocco. Econ. Bot. 2017, 71, 83–95. [Google Scholar]
- Ouarghidi, A.; Abbad, A. Étude Ethnobotanique, Ethno-Taxonomique et Ethnoécologique de Anacyclus pyrethrum var. pyrethrum (L.) Link. (Asteraceae) Dans la Vallée d’Ait Mhamed (Région d’Azilal, Maroc). Rev D’ethnoécologie. 22 November 2019. Available online: http://journals.openedition.org/ethnoecologie/5546. (accessed on 8 July 2021).
- Sanogo, R.; Maiga, A.; Diallo, D. Activites analgesique et anti-iniflammatoire des extraits de Maytenus senegalensis, Stereospermum kuntrianum et Tricrilia emetica utilisees dans le traitement traditionnel des dysmenorrhees au mali. Pharm. Med. Trad. Afr. 2006, 14, 123–136. [Google Scholar]
- Willan, R.L. Guide de Manipulation des Semences Forestieres dans le cas Particulier des Regions Tropicales; Food & Agriculture Organization: Rome, Italy, 1992; p. 516. [Google Scholar]
- Hoareau, D. Ecologie de la Germination des Espèces Indigènes de La Réunion. Ph.D. Thesis, Université de la Réunion, Saint Denis, France, 2012. Available online: https://agritrop.cirad.fr/567793/1/document_567793.pdf (accessed on 8 July 2021).
- Nivot, N. Essais de Germination et de Bouturage de Six Espèces Indigènes Sciaphytes du Canada. Master’s Thesis, Université Laval, Québec, QC, Canada, April 2005. [Google Scholar]
- Rao, N.K.; Hanson, J.; Dulloo, M.E.; Ghosh, K.; Nowell, D.; Larinde, M. Manuel de Manipulation des Semences dans les Banques de Gènes; Bioversity International: Rome, Italy, 2006; p. 181. [Google Scholar]
- Foley, M.E. Seed dormancy: An update on terminology, physiological genetics, and quantitative trait loci regulating germinability. Weed Sci. 2001, 49, 305–317. [Google Scholar] [CrossRef]
- Geneve, R.L. Impact of Temperature on Seed Dormancy. HortScience 2003, 38, 336–341. [Google Scholar] [CrossRef]
- Niang-Diop, F.; Sambou, B.; Lykke, A. Contraintes de régénération naturelle de Prosopis africana: Facteurs affectant la germination des graines. Int. J. Biol. Chem. Sci. 2011, 4, 1693–1705. Available online: http://www.ajol.info/index.php/ijbcs/article/view/65578 (accessed on 8 July 2021). [CrossRef]
- Benamar, S. Contribution à la Réhabilitation au Maroc de L’espèce Forestière Alnus Glutinosa par son Étude Éco Physiologique et la Caractérisation Moléculaire de Son Microsymbiote Diazotophe Frankia [Internet] [Physiologie végétale]. [Meknès]: Université Moulay Ismail, Faculté des Sciences. 2005. Available online: http://hdl.handle.net/123456789/7510. (accessed on 8 July 2021).
- Li, X.; Baskin, J.M.; Baskin, C.C. Anatomy of two mechanisms of breaking physical dormancy by experimental treatments in seeds of two North American Rhus species (Anacardiaceae). Am. J. Bot. 1999, 86, 1505–1511. [Google Scholar] [CrossRef] [PubMed]
- Hajlaoui, H.; Denden, M.; Bouslama, M. Etude de la variabilité intraspécifique de tolérance au stress salin du pois chiche (Cicer arietinum L.) au stade germination. Tropicultura 2007, 25, 168–173. [Google Scholar]
- Côme, D. Les Obstacles à la Germination. Publ. Société. Linn. Lyon 1971, 40, 108. [Google Scholar]
- Labouriau, L.G. A Germinacao das Sementes; Secretaria-Geral da Organização dos Estados Americanos, Programa Regional de Desenvolvimento Científico e Tecnológico: Washington, DC, USA, 1983; p. 188. [Google Scholar]
- Salehzade, H.; Izadkhah Shishvan, M.; Ghiyasi, M.; Forouzin, F.; Abbasi Siyahjani, A. Effect of Seed Priming on Germination and Seedling Growth of Wheat (Triticum aestivum L.). Res. J. Biol. Sci. 2009, 4, 629–631. [Google Scholar]
- Czabator, F.J. Germination Value: An Index Combining Speed and Completeness of Pine Seed Germination. Forest Sci. 1962, 4, 386–396. [Google Scholar]
- Gaudreault, M. Amorçage et Séparation des Graines D’épinette Noire {Picea Mariana [mill.] B.s.p.): Amélioration de la Germination des Lots de Semences Forestières. Bachelor’s Thesis, Université du Québec à Chicoutimi, Chicoutimi, QC, Canada, May 2005. [Google Scholar]
- Ranal, M.A.; de Santana, D.G. How and why to measure the germination process? Rev. Bras. Botânica 2006, 29, 1–11. [Google Scholar] [CrossRef]
- Carvalho, M.P.; Santana, D.G.; Ranal, M.A. Emergência de plântulas de Anacardium humile A. St.-Hil. (Anacardiaceae) avaliada por meio de amostras pequenas. Braz. J. Bot. 2005, 28, 627–633. [Google Scholar] [CrossRef]
- Osborne, J.M.; Fox, J.E.D.; Mercer, S. Germination response under elevated salinities of six semi-arid bluebush species (Western Australia). In Towards the Rational Use of High Salinity Tolerant Plants: Deliberations about High Salinity Tolerant Plants and Ecosystems; Lieth, H., Al Masoom, A.A., Eds.; Springer: Dordrecht, The Netherlands, 1993; Volume 1, pp. 323–338. [Google Scholar] [CrossRef]
- Ferradous, A.; Lamhamedi, M.S.; Ouhammou, A.; Alifriqui, M. Mise en application opérationnelle du test de viabilité au tétrazolium chez les semences d’arganier (Argania spinosa L. Skeels) stockées pendant plusieurs années. Can. J. For. Res. 2017, 29, 1286–1292. [Google Scholar] [CrossRef]
- Dedi, J.; Allou, K. Etude du pouvoir germinatif de quatre variétés de riz que sont giza 178, wab 56–50, lohinini, danane et identification des champignons présents sur les grains en germination. Afr. Sci. 2015, 11, 161–171. [Google Scholar]
- N’Dri, A.A.; Vroh-Bi, I.; Kouamé, P.L.; Bi, I.Z. Bases génétiques et biochimiques de la capacité germinative des graines: Implications pour les systèmes semenciers et la production alimentaire. Sci. Nat. 2011, 8, 119–137. [Google Scholar]
- Imbert, E.; Escarré, J.; Lepart, J. Achene Dimorphism and Among-Population Variation in Crepis sancta (Asteraceae). Int. J. Plant Sci. 1996, 157, 309–315. [Google Scholar] [CrossRef]
- Imbert, E.; Escarré, J.; Lepart, J. Seed Heteromorphism in Crepis sancta (Asteraceae): Performance of Two Morphs in Different Environments. Oikos 1997, 79, 325. [Google Scholar] [CrossRef]
- van Mölken, T.; Jorritsma-Wienk, L.D.; van Hoek, P.H.; de Kroon, H. Only seed size matters for germination in different populations of the dimorphic Tragopogon pratensis subsp. pratensis (Asteraceae). Am. J. Bot. 2005, 92, 432–437. [Google Scholar] [CrossRef]
- Ming, L.C.; Dias, M.C.; Ventrella, M.C. Effect of five substrates and three seed types on Calendula officinalis (Asteraceae) germination and seedling development. In II WOCMAP Congress Medicinal and Aromatic Plants, Part 3: Agricultural Production, Post Harvest Techniques, Biotechnology; International Society for Horticultural Science: Mendoza, Argentina, 1997; Volume 502, pp. 99–104. [Google Scholar]
- Valencia-Díaz, S.; Montaña, C. Effects of seed age, germination substrate, gibberelic acid, light, and temperature on seed germination in Flourensia cernua (Asteraceae), a Chihuahuan desert shrub. Southwest Nat. 2003, 48, 1–13. [Google Scholar] [CrossRef]
- Nelson, S. Noni seed handling and seedling production. Fruit Nut Beverage Crops 2005, 10, 4. [Google Scholar]
- Le Grand, E. Etude Expérimentale des Propriétés Germinatives de Quelques Semences Sahéliennes; ORSTOM: Ouagadougou, Burkina Faso, 1979. [Google Scholar]
- Hilhorst, H.W.M.; Karssen, C.M. Seed dormancy and germination: The role of abscisic acid and gibberellins and the importance of hormone mutants. Plant Growth Regul. 1992, 11, 225–238. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M.; Baskin, C.C.; Baskin, J.M. Seeds. Ecology, Biogeography, and Evolution of Dormancy and Germination; Academic Press: San Diego, CA, USA, 1998; p. 666. [Google Scholar]
- Frasier, G.W. Characterization of Seed Germination and Seedling Survival during the Initial Wet-Dry Periods following Planting. J. Range Manag. 1989, 42, 299. Available online: https://pubag.nal.usda.gov/catalog/5975 (accessed on 8 July 2021). [CrossRef]

| Harvested Stations | Population No. | Latitude | Longitude | Altitude | Varieties |
|---|---|---|---|---|---|
| Timahdite (Tassemagt el maadane) | P3 P4 | 33.14311626° N | 5.15923206° W | 1948 m | A.P var. depressus A.P var. pyrethrum |
| Samples | PMF (g) | PMS(g) | TE (%) | |
|---|---|---|---|---|
| A.P var. depressus | GL | 0.056 | 0.047 | 16.07 |
| GT | 0.061 | 0.052 | 14.75 | |
| A.P var. pyrethrum | GL | 0.122 | 0.099 | 18.85 |
| GT | 0.147 | 0.124 | 15.64 | |
| Germination Characteristic | Control | EB | H2O2 | NP (KNO3) | SF 4 °C | H2SO4 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GT | GL | GT | GL | GT | GL | GT | GL | GT | GL | GT | GL | |
| TGF (%) | 31 ± 0.011 | 25 ± 0.020 | 0 | 0 | 48 ± 0.011 | 32 ± 0.02 | 80 ± 0.025 | 64 ± 0.005 | 88 ± 0.020 | 84 ± 0.005 | 60 ± 0.011 | 56 ± 0.015 |
| T50 (j) | 4.1 ± 0.26 | 6.83 ± 0.005 | 0 | 0 | 4.12 ± 0.003 | 4.87 ± 0.020 | 5 ± 0.1 | 5.11 ± 0.179 | 4.215 ± 0.003 | 3.83 ± 0.004 | 5.5 ± 0.011 | 3.6 ± 0.040 |
| No. of germination days total | 14 ± 0 | 14.66 ± 1.15 | 0 | 0 | 12 ± 1 | 12 ± 0 | 12 ± 1 | 13.33 ± 0.577 | 13 ± 1 | 14 ± 0 | 14 ± 1 | 11.33 ± 0.57 |
| MDG | 2.21 ± 0.002 | 1.78 ± 0.003 | 0 | 0 | 4 ± 0.066 | 2.66 ± 0.004 | 6.66 ± 0.006 | 4.92 ± 0.006 | 6.76 ± 0.002 | 6.04 ± 0.052 | 4.28 ± 0.002 | 5.09 ± 0.006 |
| CV | 7 ± 0.004 | 6.95 ± 0.003 | 0 | 0 | 7.16 ± 0.004 | 7.20 ± 0.001 | 7.42 ± 0.005 | 7.06 ± 0.003 | 7.05 ± 0.003 | 7.18 ± 0.008 | 7.04 ± 0.004 | 7.45 ± 0.005 |
| IG | 6.55 ± 0.007 | 6.36 ± 0.003 | 0 | 0 | 10.89 ± 0.020 | 7.63 ± 0.002 | 26.96 ± 0.007 | 15.89 ± 0.011 | 19.72 ± 0.003 | 24.48 ± 0.001 | 15.48 ± 0.003 | 20.14 ± 0.002 |
| Germination Characteristic | Control | EB | H2O2 | NP (KNO3) | SF 4 °C | H2SO4 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GT | GL | GT | GL | GT | GL | GT | GL | GT | GL | GT | GL | |
| TGF (%) | 28 ± 0.015 | 22 ± 0.005 | 0 | 0 | 29 ± 0.025 | 20 ± 0.01 | 64 ± 0.015 | 53 ± 0.005 | 68 ± 0.015 | 76 ± 0.02 | 32 ± 0.015 | 36 ± 0.005 |
| T50 (j) | 8.3 ± 0.006 | 3.66 ± 0.015 | 0 | 0 | 6.07 ± 0.059 | 6.37 ± 0.002 | 5.75 ± 0.050 | 6.25 ± 0.004 | 4.5 ± 0.052 | 4.28 ± 0.020 | 4.27 ± 0.015 | 4 ± 0.167 |
| No. of germination days total | 15.33 ± 0.577 | 14 ± 1 | 0 | 0 | 13 ± 1 | 13.33 ± 0.577 | 12.33 ± 0.577 | 13.33 ± 1.527 | 10.66 ± 0.577 | 13 ± 0 | 13.66 ± 1.15 | 14.66 ± 1.154 |
| MDG | 1.86 ± 0.009 | 1.57 ± 0.018 | 0 | 0 | 2.23 ± 0.004 | 1.53 ± 0.002 | 5.33 ± 0.029 | 4.07 ± 0.002 | 6.8 ± 0.022 | 5.84 ± 0.005 | 2.46 ± 0.009 | 2.57 ± 0.002 |
| CV | 6.46 ± 0.002 | 6.66 ± 0.002 | 0 | 0 | 6.86 ± 0.003 | 7.01 ± 0.003 | 6.95 ± 0.001 | 6.95 ± 0.001 | 7.45 ± 0.002 | 7.13 ± 0.014 | 7.30 ± 0.006 | 5.25 ± 0.001 |
| IG | 4.53 ± 0.002 | 7.05 ± 0.007 | 0 | 0 | 6.06 ± 0.003 | 6.75 ± 0.003 | 12.14 ± 0.004 | 13.44 ± 0.001 | 21.47 ± 0.010 | 26.41 ± 0.005 | 8.78 ± 0.006 | 5.67 ± 0.004 |
| Germination Characteristic | Control | EB | H2O2 | NP (KNO3) | SF 4 °C | H2SO4 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GT | GL | GT | GL | GT | GL | GT | GL | GT | GL | GT | GL | |
| TGF (%) | 32 ± 0.01 | 26 ± 0.02 | 0 | 0 | 51 ± 0.04 | 20 ± 0.006 | 58 ± 0.004 | 44 ± 0.015 | 70 ± 0.007 | 66 ± 0.012 | 18 ± 0.01 | 15 ± 0.008 |
| T50 (j) | 8.53 ± 0.05 | 4.51 ± 0.04 | 0 | 0 | 3.48 ± 0.07 | 3.66 ± 0.002 | 5.51 ± 0.16 | 5.04 ± 0.051 | 4.91 ± 0.001 | 3.56 ± 0.05 | 7.07 ± 0.064 | 7.17 ± 0.017 |
| No. of germination days total | 16.33 ± 0.57 | 14 ± 1 | 0 | 0 | 13.33 ± 1.52 | 10 ± 1 | 13.33 ± 0.577 | 12.33 ± 1.52 | 14 ± 1 | 11 ± 2 | 11.66 ± 1.15 | 12.33 ± 1.52 |
| MDG | 2.03 ± 0.05 | 1.85 ± 0.002 | 0 | 0 | 3.84 ± 0.002 | 2.06 ± 0.1 | 4.46 ± 0.001 | 3.66 ± 0.005 | 5.04 ± 0.052 | 6.06 ± 0.055 | 1.63 ± 0.002 | 1.25 ± 0.004 |
| CV | 6.58 ± 0.004 | 6.66 ± 0.007 | 0 | 0 | 7.52 ± 0.004 | 7.59 ± 0.09 | 6.97 ± 0.004 | 7.14 ± 0.006 | 7.18 ± 0.001 | 7.55 ± 0.004 | 6.81 ± 0.003 | 6.66 ± 0.005 |
| IG | 5.65 ± 0,.005 | 4.96 ± 0.002 | 0 | 0 | 19.15 ± 0.001 | 7.45 ± 0.001 | 12.47 ± 0.003 | 12.28 ± 0.005 | 17.72 ± 0.002 | 27.07 ± 0.002 | 3.30 ± 0.005 | 2.24 ± 0.002 |
| Germination Characteristic | Control | EB | H2O2 | NP (KNO3) | SF 4 °C | H2SO4 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GT | GL | GT | GL | GT | GL | GT | GL | GT | GL | GT | GL | |
| TGF (%) | 28 ± 0.015 | 23 ± 0.01 | 0 | 0 | 36 ± 0.02 | 10 ± 0.015 | 43 ± 0.02 | 23 ± 0.005 | 64 ± 0.006 | 52 ± 0.015 | 16 ± 0.005 | 12 ± 0.006 |
| T50 (j) | 6.02 ± 0.06 | 5.82 ± 0.03 | 0 | 0 | 4.33 ± 0.011 | 6.75 ± 0.01 | 5.07 ± 0.064 | 3.50 ± 0.017 | 4.81 ± 0.004 | 6.84 ± 0.002 | 5.71 ± 0.010 | 6.5 ± 0.01 |
| No. of germination days total | 14.66 ± 1.15 | 14.33 ± 0.57 | 0 | 0 | 13.66 ± 0.15 | 11.33 ± 0.57 | 12.66 ± 1.15 | 12.33 ± 0.577 | 14.33 ± 0.577 | 11.33 ± 0.57 | 10.66 ± 0.57 | 11 ± 1 |
| MDG | 2.06 ± 0.11 | 1.78 ± 0.12 | 0 | 0 | 2.76 ± 0.005 | 0.91 ± 0.001 | 3.58 ± 0.002 | 1.91 ± 0.003 | 4.57 ± 0.01 | 4.72 ± 0.005 | 1.6 ± 0.004 | 1.09 ± 0.02 |
| CV | 6.74 ± 0.002 | 6.68 ± 0.02 | 0 | 0 | 7.06 ± 0.001 | 1.98 ± 0.005 | 6.98 ± 0.004 | 7.13 ± 0.002 | 7.07 ± 0.011 | 6.89 ± 0.06 | 7.01 ± 0.005 | 5.79 ± 0.012 |
| IG | 6.78 ± 0.003 | 5.05 ± 0.005 | 0 | 0 | 10.28 ± 0.002 | 2.38 ± 0.01 | 10.4 ± 0.007 | 12.14 ± 0.004 | 20.26 ± 0.004 | 13.91 ± 0.003 | 3.75 ± 0.001 | 2.31 ± 0.014 |
| The Period | A.P var. pyrethrum | A.P var. depressus | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GL | GT | GL | GT | |||||||||
| Ambient T° | 4 °C | −17 °C | Ambient T° | 4 °C | −17 °C | Ambient T° | 4 °C | −17 °C | Ambient T° | 4 °C | −17 °C | |
| At harvest | 96% | 95% | 95% | 97% | ||||||||
| 6 months | 96% | 94% | 96% | 95% | 92% | 95% | 95% | 93% | 95% | 97% | 95% | 97% |
| 1 Year | 90% | 59% | 93% | 91% | 63% | 94% | 93% | 61% | 95% | 95% | 67% | 96% |
| 2 Years | 88% | 19% | 92% | 90% | 21% | 94% | 91% | 20% | 93% | 92% | 23% | 93% |
| The Period | A.P var. pyrethrum | A.P var. depressus | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GL | GT | GL | GT | ||||||||||
| Ambient T° | 4 °C | −17 °C | Ambient T° | 4 °C | −17 °C | Ambient T° | 4 °C | −17 °C | Ambient T° | 4 °C | −17 °C | ||
| 6 Months | CG | 27% | 79% | 68% | 31% | 76% | 62% | 28% | 73% | 62% | 32% | 69% | 60% |
| PR | 73% | 21% | 33% | 70% | 24% | 38% | 72% | 27% | 39% | 69% | 31% | 40% | |
| 1 Year | CG | 26% | 22% | 19% | 31% | 19% | 17% | 26% | 20% | 16% | 31% | 17% | 15% |
| PR | 74% | 78% | 81% | 69% | 81% | 83% | 74% | 80% | 84% | 69% | 83% | 85% | |
| 2 Years | CG | 26% | 0% | 0% | 30% | 0% | 0% | 27% | 0% | 0% | 30% | 0% | 0% |
| PR | 74% | 100% | 100% | 70% | 100% | 100% | 73% | 100% | 100% | 70% | 100% | 100% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jawhari, F.Z.; Imtara, H.; El Moussaoui, A.; Khalis, H.; Es-safi, I.; Saleh, A.; Al kamaly, O.; Parvez, M.K.; Bari, A. Effects of Pre-Treatments and Conservation Conditions on Seed Viability and Germination of Two Varieties of an Endangered Species Anacyclus pyrethrum (L.) Link (Asteraceae). Horticulturae 2023, 9, 472. https://doi.org/10.3390/horticulturae9040472
Jawhari FZ, Imtara H, El Moussaoui A, Khalis H, Es-safi I, Saleh A, Al kamaly O, Parvez MK, Bari A. Effects of Pre-Treatments and Conservation Conditions on Seed Viability and Germination of Two Varieties of an Endangered Species Anacyclus pyrethrum (L.) Link (Asteraceae). Horticulturae. 2023; 9(4):472. https://doi.org/10.3390/horticulturae9040472
Chicago/Turabian StyleJawhari, Fatima Zahra, Hamada Imtara, Abdelfattah El Moussaoui, Hind Khalis, Imane Es-safi, Asmaa Saleh, Omkulthom Al kamaly, Mohammad Khalid Parvez, and Amina Bari. 2023. "Effects of Pre-Treatments and Conservation Conditions on Seed Viability and Germination of Two Varieties of an Endangered Species Anacyclus pyrethrum (L.) Link (Asteraceae)" Horticulturae 9, no. 4: 472. https://doi.org/10.3390/horticulturae9040472
APA StyleJawhari, F. Z., Imtara, H., El Moussaoui, A., Khalis, H., Es-safi, I., Saleh, A., Al kamaly, O., Parvez, M. K., & Bari, A. (2023). Effects of Pre-Treatments and Conservation Conditions on Seed Viability and Germination of Two Varieties of an Endangered Species Anacyclus pyrethrum (L.) Link (Asteraceae). Horticulturae, 9(4), 472. https://doi.org/10.3390/horticulturae9040472








