Review of the Current Research Progress of Seed Germination Inhibitors
Abstract
1. Introduction
2. Definition of Germination Inhibitors
3. Classification of Germination Inhibitors
3.1. Classification by Function
3.2. Classification by Chemical Structure
3.2.1. Phenols
3.2.2. Alkaloids
3.2.3. Essential Oils
3.2.4. Other Types
4. The Core Problem of Germination Inhibitors Research
4.1. Determination of Dormant Seed Germination Inhibitors
4.2. Concentration of Germination Inhibitors
4.3. Examination of Germination Inhibitors
5. Research Prospects of Germination Inhibitors
5.1. Determine the Relationship between Germination Inhibitors and Inhibition of Germination
5.2. Tests and Examination of Germination Inhibitors
5.3. Study on the Mechanism of Action of Germination Inhibitors
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- El-Maarouf-Bouteau, H.; Sajjad, Y.; Bazin, J.; Langlade, N.; Cristescu, S.M.; Balzergue, S.; Baudouin, E.; Bailly, C. Reactive oxygen species, abscisic acid and ethylene interact to regulate sunflower seed germination. Plant Cell Environ. 2015, 38, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Bewley, J.D.; Black, M.; Bewley, J.D.; Black, M. Seeds: Germination, structure, and composition. Seeds Physiol. Dev. Germination 1994, 1–33. [Google Scholar] [CrossRef]
- Bewley, J.D.; Black, M. Physiology and Biochemistry of Seeds in Relation to Germination: 1 Development, Germination, and Growth; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Jacobsen, J.; Beach, L. Control of transcription of α-amylase and rRNA genes in barley aleurone protoplasts by gibberellin and abscisic acid. Nature 1985, 316, 275–277. [Google Scholar] [CrossRef]
- Jones, R.L.; Jacobsen, J.V. Regulation of synthesis and transport of secreted proteins in cereal aleurone. Int. Rev. Cytol. 1991, 126, 49–88. [Google Scholar]
- Weyers, J.D.; Paterson, N.W. Plant hormones and the control of physiological processes. New Phytol. 2001, 152, 375–407. [Google Scholar] [CrossRef]
- Bewley, J.D. Seed germination and dormancy. Plant Cell 1997, 9, 1055. [Google Scholar] [CrossRef]
- Shu, K.; Liu, X.-D.; Xie, Q.; He, Z.-H. Two faces of one seed: Hormonal regulation of dormancy and germination. Mol. Plant 2016, 9, 34–45. [Google Scholar] [CrossRef]
- Wareing, P. Endogenous inhibitors in seed germination and dormancy. In Differenzierung und Entwicklung/Differentiation and Development; Springer: Berlin/Heidelberg, Germany, 1965; pp. 2556–2571. [Google Scholar]
- Baskin, J.M.; Baskin, C.C. Some considerations for adoption of Nikolaeva’s formula system into seed dormancy classification. Seed Sci. Res. 2008, 18, 131–137. [Google Scholar] [CrossRef]
- Willis, C.G.; Baskin, C.C.; Baskin, J.M.; Auld, J.R.; Venable, D.L.; Cavender-Bares, J.; Donohue, K.; Rubio de Casas, R.; NESCent Germination Working Group. The evolution of seed dormancy: Environmental cues, evolutionary hubs, and diversification of the seed plants. New Phytol. 2014, 203, 300–309. [Google Scholar] [CrossRef]
- Penfield, S. Seed dormancy and germination. Curr. Biol. 2017, 27, R874–R878. [Google Scholar] [CrossRef]
- Evenari, M. Germination inhibitors. Bot. Rev. 1949, 15, 153–194. [Google Scholar] [CrossRef]
- Guo, H.; Liu, Y.; Wang, H.; Li, S. Study on the dormancy characteristics of Chinese pistache (Pistacia chinensis Bunge) seeds. Forests 2022, 13, 1521. [Google Scholar] [CrossRef]
- Oppenheimer, H. The absence of germination in the containers of the mother plant. Imperial Akad Wiss 1922, 131, 279–312. [Google Scholar]
- Bingöl, Ö.; Battal, A.; Aslan, A.; Emre, E. Investigation of the allelopathic effects of lyophilized ethanol extract of Xanthoparmelia somloensis (Gyelnik) Hale lichen on tomato plant. Anatol. J. Bot. 2022, 6, 39–43. [Google Scholar]
- Light, M.E.; Burger, B.V.; Staerk, D.; Kohout, L.; Van Staden, J. Butenolides from plant-derived smoke: Natural plant-growth regulators with antagonistic actions on seed germination. J. Nat. Prod. 2010, 73, 267–269. [Google Scholar] [CrossRef]
- Papenfus, H.B.; Kulkarni, M.G.; Pošta, M.; Finnie, J.F.; Van Staden, J. Smoke-isolated trimethylbutenolide inhibits seed germination of different weed species by reducing amylase activity. Weed Sci. 2015, 63, 312–320. [Google Scholar] [CrossRef]
- Surmont, R.; Verniest, G.; De Kimpe, N. Short synthesis of the seed germination inhibitor 3, 4, 5-trimethyl-2(5H)-furanone. J. Org. Chem. 2010, 75, 5750–5753. [Google Scholar] [CrossRef]
- Kockemann, A. About an anti-germination substance in fleshy fruits. Ber. Deut. Bot. Ges. 1934, 52, 523–526. [Google Scholar]
- Gao, Y.; Zhu, M.; Wang, H.; Li, S. Dynamic Changes to Endogenous Germination Inhibitors in Cercis chinensis Seeds during Dormancy Release. HortScience 2021, 56, 557–562. [Google Scholar] [CrossRef]
- Ke, B.Y.; Zhou, C.Y.; Li, J.Y.; Pan, J.; Zhou, P. Study on Methods of Seed Dormancy and Dormancy-release for Rare Species Acrocarpus fraxinifolius. Seed 2020, 39, 139–143. [Google Scholar]
- Song, J.; Zhang, H.; Zhang, Y.; Yue, H. Effect of Stratification after lmmersion of Gibberellin on Seed Germination of Plagiorhegma dubia Maxim. J. Northeast For. Univ. 2021, 12, 34–39. [Google Scholar]
- Chen, B.-X.; Peng, Y.-X.; Gao, J.-D.; Zhang, Q.; Liu, Q.-J.; Fu, H.; Liu, J. Coumarin-induced delay of rice seed germination is mediated by suppression of abscisic acid catabolism and reactive oxygen species production. Front. Plant Sci. 2019, 828. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.-X.; Peng, Y.-X.; Yang, X.-Q.; Liu, J. Delayed germination of Brassica parachinensis seeds by coumarin involves decreased GA4 production and a consequent reduction of ROS accumulation. Seed Sci. Res. 2021, 31, 224–235. [Google Scholar] [CrossRef]
- Oracz, K.; Bailly, C.; Gniazdowska, A.; Côme, D.; Corbineau, F.; Bogatek, R. Induction of oxidative stress by sunflower phytotoxins in germinating mustard seeds. J. Chem. Ecol. 2007, 33, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Cuypers, A.; Keunen, E.; Bohler, S.; Jozefczak, M.; Opdenakker, K.; Gielen, H.; Vercampt, H.; Bielen, A.; Schellingen, K.; Vangronsveld, J. Cadmium and copper stress induce a cellular oxidative challenge leading to damage versus signalling. In Metal Toxicity in Plants: Perception, Signaling and Remediation; Springer: Berlin/Heidelberg, Germany, 2011; pp. 65–90. [Google Scholar]
- Huybrechts, M.; Cuypers, A.; Deckers, J.; Iven, V.; Vandionant, S.; Jozefczak, M.; Hendrix, S. Cadmium and plant development: An agony from seed to seed. Int. J. Mol. Sci. 2019, 20, 3971. [Google Scholar] [CrossRef]
- Lawrence, J.G.; Colwell, A.; Sexton, O.J. The ecological impact of allelopathy in Ailanthus altissima (Simaroubaceae). Am. J. Bot. 1991, 78, 948–958. [Google Scholar] [CrossRef]
- Bauer, J.T.; Shannon, S.M.; Stoops, R.E.; Reynolds, H.L. Context dependency of the allelopathic effects of Lonicera maackii on seed germination. Plant Ecol. 2012, 213, 1907–1916. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, B.; Zhang, S.; Tang, J.; Tu, C.; Hu, S.; Yong, J.W.H.; Chen, X. Enhanced allelopathy and competitive ability of invasive plant Solidago canadensis in its introduced range. J. Plant Ecol. 2012, 6, 253–263. [Google Scholar] [CrossRef]
- Butcko, V.M.; Jensen, R.J. Evidence of tissue-specific allelopathic activity in Euthamia graminifolia and Solidago canadensis (Asteraceae). Am. Midl. Nat. 2002, 148, 253–262. [Google Scholar] [CrossRef]
- Zwanenburg, B.; Blanco-Ania, D. Strigolactones: New plant hormones in the spotlight. J. Exp. Bot. 2018, 69, 2205–2218. [Google Scholar] [CrossRef]
- Dorning, M.; Cipollini, D. Leaf and root extracts of the invasive shrub, Lonicera maackii, inhibit seed germination of three herbs with no autotoxic effects. Plant Ecol. 2006, 184, 287–296. [Google Scholar] [CrossRef]
- Koocheki, A.; Lalegani, B.; Hosseini, S. Ecological consequences of allelopathy. In Allelopathy: Current Trends and Future Applications; Springer: Berlin/Heidelberg, Germany, 2013; pp. 23–38. [Google Scholar]
- McEwan, R.W.; Arthur-Paratley, L.G.; Rieske, L.K.; Arthur, M.A. A multi-assay comparison of seed germination inhibition by Lonicera maackii and co-occurring native shrubs. Flora-Morphol. Distrib. Funct. Ecol. Plants 2010, 205, 475–483. [Google Scholar] [CrossRef]
- Williams, R.D.; Hoagland, R.E. The effects of naturally occurring phenolic compounds on seed germination. Weed Sci. 1982, 30, 206–212. [Google Scholar] [CrossRef]
- Miller, H.G.; Ikawa, M.; Peirce, L.C. Caffeic acid identified as an inhibitory compound in asparagus root filtrate. HortScience 1991, 26, 1525–1527. [Google Scholar] [CrossRef]
- Li, X.; Gruber, M.Y.; Hegedus, D.D.; Lydiate, D.J.; Gao, M.-J. Effects of a coumarin derivative, 4-methylumbelliferone, on seed germination and seedling establishment in Arabidopsis. J. Chem. Ecol. 2011, 37, 880–890. [Google Scholar] [CrossRef]
- Muzaffar, S.; Ali, B.; Wani, N.A. Effect of catechol, gallic acid and pyrogallic acid on the germination, seedling growth and the level of endogenous phenolics in cucumber (Cucumis sativus L.). Inter J. Life Sci. Biotechnol. Pharma. Res. 2012, 1, 50–55. [Google Scholar]
- Reigosa, M.; Souto, X.; Gonz´lez, L. Effect of phenolic compounds on the germination of six weeds species. Plant Growth Regul. 1999, 28, 83–88. [Google Scholar] [CrossRef]
- Shirley, B.W. Flavonoids in seeds and grains: Physiological function, agronomic importance and the genetics of biosynthesis. Seed Sci. Res. 1998, 8, 415–422. [Google Scholar] [CrossRef]
- Jia, L.; Wu, Q.; Ye, N.; Liu, R.; Shi, L.; Xu, W.; Zhi, H.; Rahman, A.R.B.; Xia, Y.; Zhang, J. Proanthocyanidins inhibit seed germination by maintaining a high level of abscisic acid in Arabidopsis thaliana F. J. Integr. Plant Biol. 2012, 54, 663–673. [Google Scholar] [CrossRef]
- Samanta, A.; Das, G.; Das, S.K. Roles of flavonoids in plants. Carbon 2011, 100, 12–35. [Google Scholar]
- Chotsaeng, N.; Laosinwattana, C.; Charoenying, P. Inhibitory Effects of a Variety of Aldehydes on Amaranthus tricolor L. and Echinochloa crus-galli (L.) Beauv. Molecules 2018, 23, 471. [Google Scholar] [CrossRef] [PubMed]
- Bradow, J.M.; Connick, W.J. Volatile seed germination inhibitors from plant residues. J. Chem. Ecol. 1990, 16, 645–666. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, S.F.; Boydston, R.A. Volatile allelochemicals released by crucifer green manures. J. Chem. Ecol. 1997, 23, 2107–2116. [Google Scholar] [CrossRef]
- Bialy, Z.; Oleszek, W.; Lewis, J.; Fenwick, G. Allelopathic potential of glucosinolates (mustard oil glycosides) and their degradation products against wheat. Plant Soil 1990, 129, 277–281. [Google Scholar] [CrossRef]
- Azirak, S.; Karaman, S. Allelopathic effect of some essential oils and components on germination of weed species. Acta Agric. Scand. Sect. B-Soil Plant Sci. 2008, 58, 88–92. [Google Scholar] [CrossRef]
- Wink, M. Inhibition of seed germination by quinolizidine alkaloids: Aspects of allelopathy in Lupinus albus L. Planta 1983, 158, 365–368. [Google Scholar] [CrossRef]
- Chiji, H.; Giga, T.; Izawa, M.; Kiriyama, S. Two phenolic amides in the seed balls of sugar beet (Beta vulgaris L. var saccharifera Alefeld). Agric. Biol. Chem. 1984, 48, 1653–1654. [Google Scholar] [CrossRef]
- Laura, A.; Moreno-Escamilla, J.O.; Rodrigo-García, J.; Alvarez-Parrilla, E. Phenolic compounds. In Postharvest Physiology and Biochemistry of Fruits and Vegetables; Elsevier: Amsterdam, The Netherlands, 2019; pp. 253–271. [Google Scholar]
- Noperi-Mosqueda, L.C.; López-Moreno, F.J.; Navarro-León, E.; Sánchez, E.; Blasco, B.; Moreno, D.A.; Soriano, T.; Ruiz, J.M. Effects of asparagus decline on nutrients and phenolic compounds, spear quality, and allelopathy. Sci. Hortic. 2020, 261, 109029. [Google Scholar] [CrossRef]
- Li, H.-H.; Inoue, M.; Nishimura, H.; Mizutani, J.; Tsuzuki, E. Interactions of trans-cinnamic acid, its related phenolic allelochemicals, and abscisic acid in seedling growth and seed germination of lettuce. J. Chem. Ecol. 1993, 19, 1775–1787. [Google Scholar] [CrossRef]
- Cutillo, F.; D’Abrosca, B.; DellaGreca, M.; Fiorentino, A.; Zarrelli, A. Lignans and neolignans from Brassica fruticulosa: Effects on seed germination and plant growth. J. Agric. Food Chem. 2003, 51, 6165–6172. [Google Scholar] [CrossRef]
- Ng, P.; Ferrarese, M.; Huber, D.; Ravagnani, A.; Ferrarese-Filho, O. Canola (Brassica napus L.) seed germination influenced by cinnamic and benzoic acids and derivatives: Effects on peroxidase. Seed Sci. Technol. 2003, 31, 39–46. [Google Scholar] [CrossRef]
- Radwan, A.M.; Alghamdi, H.A.; Kenawy, S.K. Effect of Calotropis procera L. plant extract on seeds germination and the growth of microorganisms. Ann. Agric. Sci. 2019, 64, 183–187. [Google Scholar] [CrossRef]
- Einhellig, F.A.; Rasmussen, J.A. Synergistic inhibitory effects of vanillic and p-hydroxybenzoic acids on radish and grain sorghum. J. Chem. Ecol. 1978, 4, 425–436. [Google Scholar] [CrossRef]
- Demos, E.; Woolwine, M.; Wilson, R.; McMillan, C. The effects of ten phenolic compounds on hypocotyl growth and mitochondrial metabolism of mung bean. Am. J. Bot. 1975, 62, 97–102. [Google Scholar] [CrossRef]
- Levin, D.A.; York, B.M., Jr. The toxicity of plant alkaloids: An ecogeographic perspective. Biochem. Syst. Ecol. 1978, 6, 61–76. [Google Scholar] [CrossRef]
- Levitt, J.; Lovett, J. Activity of allelochemicals of Datura stramonium L.(thorn-apple) in contrasting soil types. Plant Soil 1984, 79, 181–189. [Google Scholar] [CrossRef]
- Levitt, J.; Lovett, J. Alkaloids, antagonisms and allelopathy. Biol. Agric. Hortic. 1985, 2, 289–301. [Google Scholar] [CrossRef]
- Aerts, R.J.; Snoeijer, W.; van der Meijden, E.; Verpoorte, R. Allelopathic inhibition of seed germination by Cinchona alkaloids? Phytochemistry 1991, 30, 2947–2951. [Google Scholar] [CrossRef]
- Weston, L.A.; Duke, S.O. Weed and crop allelopathy. Crit. Rev. Plant Sci. 2003, 22, 367–389. [Google Scholar] [CrossRef]
- Singh, H.; Batish, D.; Setia, N.; Kohli, R. Herbicidal activity of volatile oils from Eucalyptus citriodora against Parthenium hysterophorus. Ann. Appl. Biol. 2005, 146, 89–94. [Google Scholar] [CrossRef]
- Ramezani, S.; Saharkhiz, M.J.; Ramezani, F.; Fotokian, M.H. Use of essential oils as bioherbicides. J. Essent. Oil Bear. Plants 2008, 11, 319–327. [Google Scholar] [CrossRef]
- Benvenuti, S.; Cioni, P.L.; Flamini, G.; Pardossi, A. Weeds for weed control: Asteraceae essential oils as natural herbicides. Weed Res. 2017, 57, 342–353. [Google Scholar] [CrossRef]
- Bell, E.A. ’Uncommon’amino acids in plants. Febs Lett. 1976, 64, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, G.A.; Hughes, C.G.; Janzen, D.H. L-Canavanine, a dietary nitrogen source for the seed predator Caryedes brasiliensis (Bruchidae). Science 1982, 217, 353–355. [Google Scholar] [CrossRef]
- Bell, E.; Janzen, D.H. Medical and ecological considerations of L-dopa and 5-HTP in seeds. Nature 1971, 229, 136–137. [Google Scholar] [CrossRef]
- Elmore, C. Inhibition of turnip (Brassica rapa) seed germination by velvetleaf (Abutilon theophrasti) seed. Weed Sci. 1980, 28, 658–660. [Google Scholar] [CrossRef]
- Wilson, M.F.; Bell, E.A. The determination of the changes in the free amino acid content of the eluate from germinating seeds of Glycine wightii (L.) and its effect on the growth of lettuce fruits. J. Exp. Bot. 1978, 29, 1243–1247. [Google Scholar] [CrossRef]
- Vurro, M.; Boari, A.; Pilgeram, A.L.; Sands, D.C. Exogenous amino acids inhibit seed germination and tubercle formation by Orobanche ramosa (broomrape): Potential application for management of parasitic weeds. Biol. Control 2006, 36, 258–265. [Google Scholar] [CrossRef]
- Amor, Y.; Babiychuk, E.; Inzé, D.; Levine, A. The involvement of poly (ADP-ribose) polymerase in the oxidative stress responses in plants. Febs Lett. 1998, 440, 1–7. [Google Scholar] [CrossRef]
- Doucet-Chabeaud, G.; Godon, C.; Brutesco, C.; De Murcia, G.; Kazmaier, M. Ionising radiation induces the expression of PARP-1 and PARP-2 genes in Arabidopsis. Mol. Genet. Genom. 2001, 265, 954–963. [Google Scholar] [CrossRef]
- Zheng, X.-Q.; Hayashibe, E.; Ashihara, H. Changes in trigonelline (N-methylnicotinic acid) content and nicotinic acid metabolism during germination of mungbean (Phaseolus aureus) seeds. J. Exp. Bot. 2005, 56, 1615–1623. [Google Scholar] [CrossRef]
- Hunt, L.; Holdsworth, M.J.; Gray, J.E. Nicotinamidase activity is important for germination. Plant J. 2007, 51, 341–351. [Google Scholar] [CrossRef]
- Oster, U.; Bios, I.; Rüdiger, W. Natural Inhibitors of germination and growth IV compounds from fruit and seeds of mountain ash (Sorbus aucuparia). Z. Nat. C 1987, 42, 1179–1184. [Google Scholar] [CrossRef]
- Levi-Minzi, R.; Saviozzi, A.; Riffaldi, R. Organic acids as seed germination inhibitors. J. Environ. Sci. Health Part A 1994, 29, 2203–2217. [Google Scholar] [CrossRef]
- Uygur, S. Effects of mustard oil on germination and growth of yellow starthistle (Centaurea solstitialis L.). Allelopath. J. 2011, 27, 23–32. [Google Scholar]
- Bian, F.; Su, J.; Liu, W.; Li, S. Dormancy release and germination of Taxus yunnanensis seeds during wet sand storage. Sci. Rep. 2018, 8, 3205. [Google Scholar] [CrossRef]
- Prudente, D.d.O.; Paiva, R. Seed dormancy and germination: Physiological considerations. J. Cell Dev. Biol. 2018, 2, 2. [Google Scholar]
- Zehhar, N.; Ingouff, M.; Bouya, D.; Fer, A. Possible involvement of gibberellins and ethylene in Orobanche ramosa germination. Weed Res. 2002, 42, 464–469. [Google Scholar] [CrossRef]
- Footitt, S.; Douterelo-Soler, I.; Clay, H.; Finch-Savage, W.E. Dormancy cycling in Arabidopsis seeds is controlled by seasonally distinct hormone-signaling pathways. Proc. Natl. Acad. Sci. USA 2011, 108, 20236–20241. [Google Scholar] [CrossRef]
- Zhao, T.; Qian, C.; Gao, Y.; Chen, L.; Zhu, M.; Pan, Y.; Li, S. Germination inhibitors detected in Sapium sebiferum seeds. J. For. Res. 2019, 30, 2305–2312. [Google Scholar] [CrossRef]
- Ali, A.S.; Elozeiri, A.A. Metabolic processes during seed germination. Adv. Seed Biol. 2017, 2017, 141–166. [Google Scholar]
- Labbafi, M.; Mehrafarin, A.; Badi, H.; Ghorbani, M.; Tavakoli, M. Improve germination of caper (Capparis spinosa L.) seeds by different induction treatments of seed dormancy breaking. Trakia J. Sci. 2018, 16, 71–74. [Google Scholar] [CrossRef]
- Hendricks, S.; Taylorson, R. Promotion of seed germination by nitrates and cyanides. Nature 1972, 237, 169–170. [Google Scholar] [CrossRef]
- Bogatek, R.; Dziewanowska, K.; Lewak, S. Hydrogen cyanide and embryonal dormancy in apple seeds. Physiol. Plant. 1991, 83, 417–421. [Google Scholar] [CrossRef]
- Roberts, E. Seed Dormancy and Oxidation Processes. Symp. Soc. Exp. Biol. 1969, 23, 161–192. [Google Scholar]
- Oracz, K.; El-Maarouf-Bouteau, H.; Kranner, I.; Bogatek, R.; Corbineau, F.; Bailly, C. The mechanisms involved in seed dormancy alleviation by hydrogen cyanide unravel the role of reactive oxygen species as key factors of cellular signaling during germination. Plant Physiol. 2009, 150, 494–505. [Google Scholar] [CrossRef]
- Mullick, P.; Chatterji, U. Effect of sodium cyanide on germination of two leguminous seeds. Osterr. Bot. Z. 1967, 114, 88–91. [Google Scholar] [CrossRef]
- Taylorson, R.; Hendricks, S. Promotion of seed germination by cyanide. Plant Physiol. 1973, 52, 23–27. [Google Scholar] [CrossRef]
- Devlin, H.R.; Harris, I.J. Mechanism of the oxidation of aqueous phenol with dissolved oxygen. Ind. Eng. Chem. Fundam. 1984, 23, 387–392. [Google Scholar] [CrossRef]
- Yang, Z.H.; Chen, X.M.; Chen, C.H. Characteristics of Taxodinm distichum var imbricatum Seed Coat and Their Effects on Seed Dormancy. Sci. Silvae Sin. 2022, 222, 11–21. [Google Scholar]
- Li, W.; Tang, H.; He, L. Effects of Crude Extracts of Paeonia rockii Seeds on Germination and Seedling Growth of receptor Plant. J. Northeast For. Univ. 2020, 48, 8–12. [Google Scholar]
- Yan, F.; Zhang, E.; Wang, Q.; Mao, D. The Mechanisms of Seed Dormancy of Wild Daphne giraldii and Methods for Dormancy Breaking. Sci. Silvae Sin. 2016, 52, 30–37. [Google Scholar]
- Li, Q.; Liu, Y.; Liu, G.; Liu, Y.; Hou, L.; Hu, J. Study on germination inhibitors of seeds of 7 species of Quercus species. Acta Ecol. Sin. 2013, 7, 2104–2112. [Google Scholar]
- Thines, B.; Katsir, L.; Melotto, M.; Niu, Y.; Mandaokar, A.; Liu, G.; Nomura, K.; He, S.Y.; Howe, G.A.; Browse, J. JAZ repressor proteins are targets of the SCFCOI1 complex during jasmonate signalling. Nature 2007, 448, 661–665. [Google Scholar] [CrossRef]
- Miersch, O.; Neumerkel, J.; Dippe, M.; Stenzel, I.; Wasternack, C. Hydroxylated jasmonates are commonly occurring metabolites of jasmonic acid and contribute to a partial switch-off in jasmonate signaling. New Phytol. 2008, 177, 114–127. [Google Scholar] [CrossRef]
- Oh, E.; Kang, H.; Yamaguchi, S.; Park, J.; Lee, D.; Kamiya, Y.; Choi, G. Genome-wide analysis of genes targeted by PHYTOCHROME INTERACTING FACTOR 3-LIKE5 during seed germination in Arabidopsis. Plant Cell 2009, 21, 403–419. [Google Scholar] [CrossRef]
- Graeber, K.; Linkies, A.; Müller, K.; Wunchova, A.; Rott, A.; Leubner-Metzger, G. Cross-species approaches to seed dormancy and germination: Conservation and biodiversity of ABA-regulated mechanisms and the Brassicaceae DOG1 genes. Plant Mol. Biol. 2010, 73, 67–87. [Google Scholar] [CrossRef]
- Pal, D.; Mukherjee, S. Tamarind (Tamarindus indica) seeds in health and nutrition. In Nuts and Seeds in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2020; pp. 171–182. [Google Scholar]
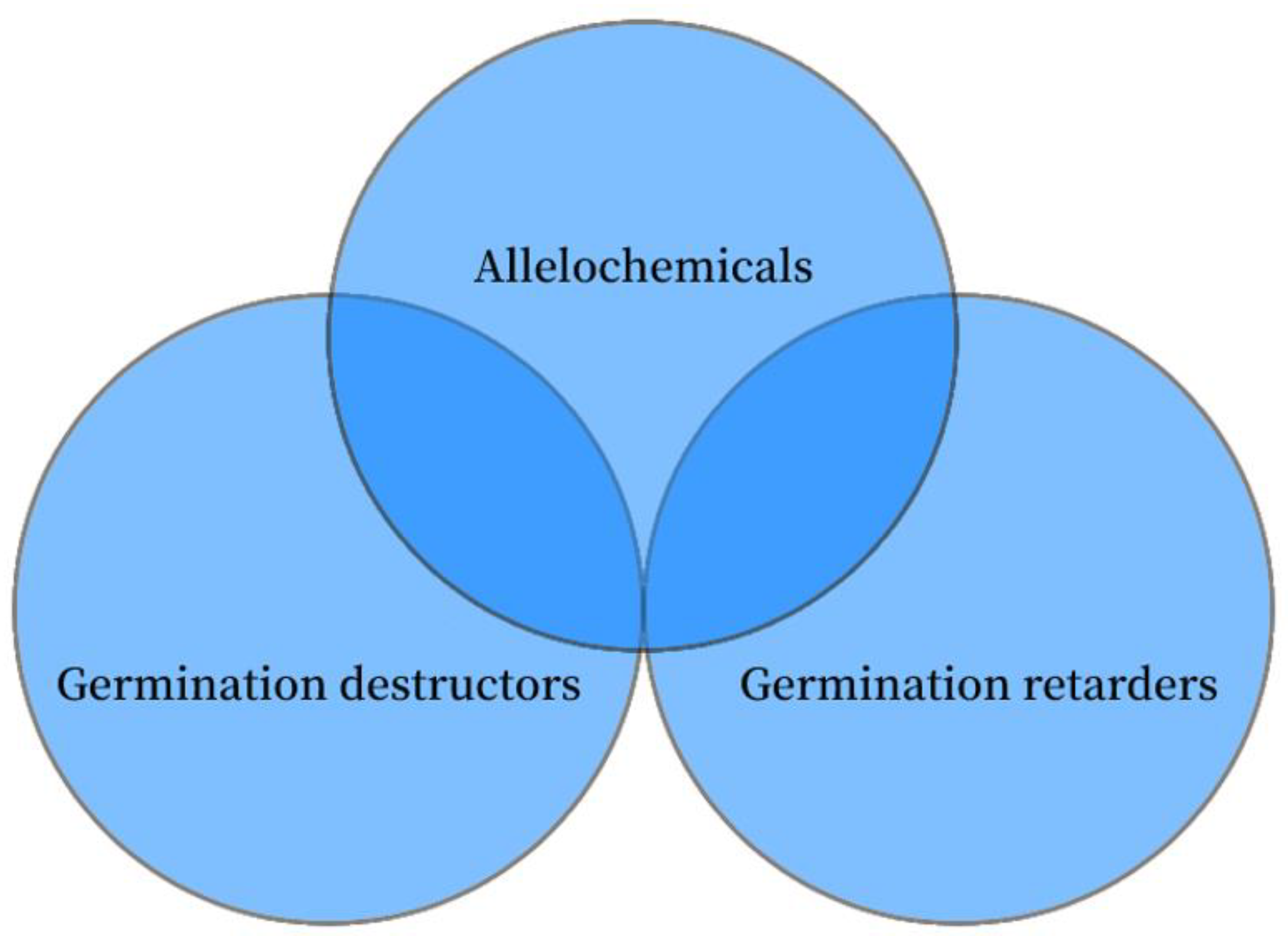

| Classification | Subdivision | Definition |
|---|---|---|
| Germination retarder | Endogenous germination retarder | Plant-produced substances that inhibit seed germination |
| Exogenous germination retarder | Non-plant substances that inhibit seed germination | |
| Germination destructor | Selective germination destructor | Substances that inflict physiological harm on the seeds of one or more plants |
| Unselective germination destructor | Substances that cause general seed damage |
| Name of Germination Inhibitors | Locations of Existence | Form of Existence | Particular Plants | Inhibitory Effects | ||
|---|---|---|---|---|---|---|
| Phenols | Caffeic acid | Seeds, fruits, and other plant tissues | Either in a free state or conjugated with sugars as glucosides and esters | Cucumis melo [37] | retarder | Caffeic acid delayed germination rather than destroying it. |
| Asparagus [38] | destructor | Caffeic Acid Identified as an inhibitory compound in Asparagus root filtrate. | ||||
| Chlorogenic acid | Sida spinosa, Sorghum bicolor [37] | destructor | It slightly reduced germination of Sida spinosa and Sorghum bicolor seeds. | |||
| Coumarin | Amaranthus retroflexus, Sida spinosa [37] | retarder | Germination was delayed rather than destructed by chlorogenic acid. | |||
| Arabidopsis thaliana [39] | destructor | It reduces germination of Arabidopsis thaliana seeds and led to reduced primary radicle elongation. | ||||
| Oryza sativa [24] | retarder | Coumarin induced delay of Oryza sativa seed germination is mediated by suppression of ABA catabolism and reduced production of ROS. | ||||
| Brassica parachinensis [25] | retarder | It delayed germination of Brassica parachinensis seeds by decreased GA4 production and a consequent reduction of ROS accumulation. | ||||
| p-Coumarie acid | Zea mays [37] | destructor | It significantly decreased the germination of Zea mays seeds. | |||
| Ferulic acid | Gossypiumhirsutum [37] | destructor | It significantly decreased the germination of Gossypium hirsutum seeds. | |||
| Fumaric acid | Sida spinosa [37] | destructor | It reduced the germination rate of Sida spinosa seedlings slightly. | |||
| Gallic acid | Cucumis sativus [40] | destructor | It greatly reduced germination rate, radicle and hypocotyl growth, and seedling fresh and dry weight. | |||
| Hydrocinnamic acid | Amaranthus retroflexus [37] | retarder | It delayed germination rather than destroying it. | |||
| Pyrocatechol | Amaranthus retroflexus [37] | retarder | It delayed germination rather than destroying it. | |||
| p-Hydroxybenzoic acid | Chenopodium album, Plantago lanceolata, Amaranthus retroflexus, Solanum nigrum, Cirsium, Rumex crispus [41] | retarder | A significant concentration of p-hydroxybenzoic acid inhibited the germination of all of these weeds. | |||
| Juglone | Zea mays [37] | retarder | It delayed germination rather than destroying it. | |||
| Abutilon theophrasti, Sida spinosa, Amaranthus retroflexus [37] | destructor | Seed growth is destroyed by juglone. | ||||
| Flavonoids | Flavonols | Seeds | Low molecular weight polyphenolic secondary metabolic compounds | Zea mays [42] | retarder | It takes part in seeds maturation and dormancy. |
| Proanthocyanidins | Arabidopsis thaliana [43] | retarder | It contributes to the maintenance of seed dormancy by promotion of ABA. | |||
| Dihydroflavonoids | Brassica campestris and Echinochloa crusgalli [44] | destructor | It inhibits embryo growth. | |||
| Aldehydes | Crotonaldehyde | Stem and root | Natural metabolic secretions of plants | Amaranthus tricolor [45] | destructor | The substance inhibits the growth of the germ and radicle |
| (E)-2-hexenal | Trifolium alexandrinum [46] | destructor | It inhibits germination and seedling development | |||
| 3-methylbutanal | Trifolium alexandrinum [46] | destructor | It inhibits germination and seedling development | |||
| Benzaldehyde | Brassicaceae [47] | destructor | It inhibits seed germination. | |||
| Mustard oil | Mustard oil glycosides | Seeds, and other plant tissues | Phytochemical of plants | Triticumturgidumvar. durum [48] | destructor | It completely inhibited Triticum turgidum var. durum seeds germination at 500 ppm. |
| Aromatic oils | Carum carvi | Most of plant tissues | Natural metabolic secretions of plants | Amaranthus retroflexus, Centaurea salsotitialis and Raphanus raphanistrum [49] | destructor | Even at low concentrations, thymol, carvacrol, and carvone showed significant inhibition of these seeds. |
| Mentha spicata | ||||||
| Origanum onites | ||||||
| Thymbra spicata | ||||||
| Alkaloid | Quinolizidine alkaloids | Seeds | Present as ester alkaloids | Lactuca sativa [50] | destructor | The alkaloid esters resulted in the strongest inhibition: 6 mM 13-tigloyloxylupanine inhibited germination by 100%. |
| Amide | Feruloylputrescine | Seeds and leaves | Natural secondary metabolites | Beta vulgaris [51] | retarder | It hinders germination of seeds. |
| Feruloylserotonin | ||||||
| N-trans-Feruloyltyramine | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chenyin, P.; Yu, W.; Fenghou, S.; Yongbao, S. Review of the Current Research Progress of Seed Germination Inhibitors. Horticulturae 2023, 9, 462. https://doi.org/10.3390/horticulturae9040462
Chenyin P, Yu W, Fenghou S, Yongbao S. Review of the Current Research Progress of Seed Germination Inhibitors. Horticulturae. 2023; 9(4):462. https://doi.org/10.3390/horticulturae9040462
Chicago/Turabian StyleChenyin, Peng, Wu Yu, Shi Fenghou, and Shen Yongbao. 2023. "Review of the Current Research Progress of Seed Germination Inhibitors" Horticulturae 9, no. 4: 462. https://doi.org/10.3390/horticulturae9040462
APA StyleChenyin, P., Yu, W., Fenghou, S., & Yongbao, S. (2023). Review of the Current Research Progress of Seed Germination Inhibitors. Horticulturae, 9(4), 462. https://doi.org/10.3390/horticulturae9040462





