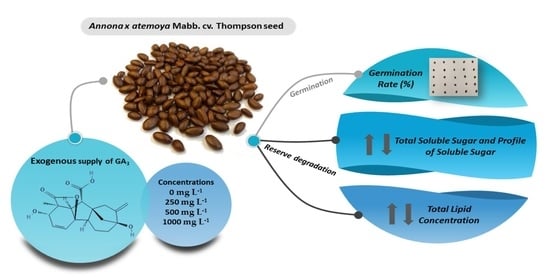
Impact of GA3 on Sugar and Lipid Degradation during Annona x atemoya Mabb. Seed Germination
Abstract
1. Introduction
2. Materials and Methods
2.1. Germination Test
2.2. Reserve Degradation
2.3. Extraction and Quantification of Total Lipids
2.4. Extraction and Quantification of Total Soluble Sugars and Sugar Profile
2.5. Statistical Analysis
3. Results
3.1. Germinability
3.2. Lipids
3.3. Sugars
3.4. Sugar Profile
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chatrou, L.W.; Erkens, R.H.J.; Richardson, J.E.; Saunders, R.M.K.; Fay, M.F. The Natural History of Annonaceae. Bot. J. Linn. Soc. 2012, 169, 1–4. [Google Scholar] [CrossRef]
- Mendes-Silva, I.; Lopes, J.C.; Silva, L.V.; Oliveira, M.L.B. Annona in Flora e Funga Do Brasil. Available online: https://floradobrasil.jbrj.gov.br/FB110235 (accessed on 3 March 2023).
- de Lemos, E.E.P. A Produção de Anonáceas No Brasil. Rev. Bras. de Frutic. 2014, 36, 77–85. [Google Scholar] [CrossRef]
- José, A.R.S.; Pires, M.D.M.; De Freitas, A.L.G.E.; Ribeiro, D.P.; Perez, L.A.A. Atualidades e Perspectivas Das Anonáceas No Mundo. Rev. Bras. de Frutic. 2014, 36, 86–93. [Google Scholar] [CrossRef]
- Li, H.; Li, W.; Zhang, T.; Zhong, J.; Liu, J.; Yuan, C.; Liu, K. Comparative Transcriptomic Analysis of Split and Non-Split Atemoya (Annona cherimola Mill. × Annona squamosa L.) Fruit to Identify Potential Genes Involved in the Fruit Splitting Process. Sci. Hortic. 2019, 248, 216–224. [Google Scholar] [CrossRef]
- Al-Ghazzawi, A.M. Anti-Cancer Activity of New Benzyl Isoquinoline Alkaloid from Saudi Plant Annona squamosa. BMC Chem. 2019, 13, 13. [Google Scholar] [CrossRef]
- Anaya-Esparza, L.M.; García-Magaña, M.D.L.; Domínguez-Ávila, J.A.; Yahia, E.M.; Salazar-López, N.J.; González-Aguilar, G.A.; Montalvo-González, E. Annonas: Underutilized Species as a Potential Source of Bioactive Compounds. Food Res. Int. 2020, 138, 109775. [Google Scholar] [CrossRef]
- Leite, D.O.D.; Nonato, C.D.F.A.; Camilo, C.J.; De Carvalho, N.K.G.; Da Nobrega, M.G.L.A.; Pereira, R.C.; Da Costa, J.G.M. Annona Genus: Traditional Uses, Phytochemistry and Biological Activities. Curr. Pharm. Des. 2020, 26, 4056–4091. [Google Scholar] [CrossRef]
- De-la-Cruz-Chacón, I.; Riley-Saldaña, C.A.; Arrollo-Gómez, S.; Sancristóbal-Domínguez, T.J.; Castro-Moreno, M.; González-Esquinca, A.R. Spatio-Temporal Variation of Alkaloids in Annona purpurea and the Associated Influence on Their Antifungal Activity. Chem. Biodivers. 2019, 16, e1800284. [Google Scholar] [CrossRef]
- da Silva, E.A.A.; de Melo, D.L.B.; Davide, A.C.; de Bode, N.; Abreu, G.B.; Faria, J.M.R.; Hilhorst, H.W.M. Germination Ecophysiology of Annona crassiflora Seeds. Ann. Bot. 2007, 99, 823–830. [Google Scholar] [CrossRef]
- Dalanhol, S.J.; Mombach, T.C.; Toderke, M.L.; Nogueira, A.V.; Bortolini, M.F. DORMÊNCIA EM SEMENTES DE Annona cacans Warm. (ANNONACEAE) Dormancy in Seeds of Annona cacans Warm. (Annonaceae). Rev. Acad. Ciênc. Anim. 2013, 11, 183. [Google Scholar] [CrossRef]
- Ferreira, G.; De-La-Cruz-Chacón, I.; González-Esquinca, A.R. Superação Da Dormência de Sementes de Annona macroprophyllata e Annona purpurea Com o Uso de Reguladores Vegetais. Rev. Bras. de Frutic. 2016, 38, e-234. [Google Scholar] [CrossRef]
- Ferreira, G.; De-La-cruz-chacón, I.; González-Esquinca, A.R. Changes in Hormonal Balance as Key to Reserve Degradation after Dormancy Overcoming in Annona macroprophyllata and Annona purpurea Seeds. Rev. Bras. De Frutic. 2020, 42, 1–11. [Google Scholar] [CrossRef]
- Gómez-Cadenas, A.; Zentella, R.; Walker-Simmons, M.K.; Ho, T.-H.D. Gibberellin/Abscisic Acid Antagonism in Barley Aleurone Cells: Site of Action of the Protein Kinase PKABA1 in Relation to Gibberellin Signaling Molecules. Plant Cell 2001, 13, 667–679. [Google Scholar] [CrossRef]
- Small, C.C.; Degenhardt, D. Plant Growth Regulators for Enhancing Revegetation Success in Reclamation: A Review. Ecol. Eng. 2018, 118, 43–51. [Google Scholar] [CrossRef]
- Fleet, C.M.; Sun, T. A DELLAcate Balance: The Role of Gibberellin in Plant Morphogenesis. Curr. Opin. Plant Biol. 2005, 8, 77–85. [Google Scholar] [CrossRef]
- Rademacher, W. Plant Growth Regulators: Backgrounds and Uses in Plant Production. J. Plant Growth Regul. 2015, 34, 845–872. [Google Scholar] [CrossRef]
- Quettier, A.L.; Eastmond, P.J. Storage Oil Hydrolysis during Early Seedling Growth. Plant Physiol. Biochem. 2009, 47, 485–490. [Google Scholar] [CrossRef]
- Sun, J.; Jia, H.; Wang, P.; Zhou, T.; Wu, Y.; Liu, Z. Exogenous Gibberellin Weakens Lipid Breakdown by Increasing Soluble Sugars Levels in Early Germination of Zanthoxylum Seeds. Plant Sci. 2019, 280, 155–163. [Google Scholar] [CrossRef]
- Martínez, M.F.E.; Miranda, L.D.; Magnitskiy, S. Germinación de Semillas de Anón (Annona squamosa L.) Afectada Por La Aplicación de Giberelinas. Agron. Colomb. 2016, 34, 17–24. [Google Scholar] [CrossRef]
- Palepad, K.; Deshmukh Krishi Vidyapeeth, P.; Bharad, I.S.; Bansode, I.G.; Deshmukh Krishi, P.; Bharad, S.; Bansode, G. Effect of Seed Treatments on Germination, Seedling Vigour and Growth Rate of Custard Apple (Annona squamosa). J. Pharmacogn. Phytochem. 2017, 6, 20–23. [Google Scholar]
- De Carvalho, D.U.; Da Cruz, M.A.; Osipi, E.A.F.; Cossa, C.A.; Colombo, R.C.; Sorace, M.A.F. Plant growth regulators on atemoya seeds germination. Nucleus 2018, 15, 457–462. [Google Scholar] [CrossRef]
- Kim, D.H. Practical Methods for Rapid Seed Germination from Seed Coat-Imposed Dormancy of Prunus yedoensis. Sci. Hortic. 2019, 243, 451–456. [Google Scholar] [CrossRef]
- Ferreira, G.; Esquinca, A.R.G.; De-La-Cruz-Chacón, I. Water Uptake by Annona diversifolia Saff. and A. purpurea Moc. & Sessé Ex Dunal Seeds (Annonaceae). Rev. Bras. De Frutic. 2014, 36, 288–295. [Google Scholar] [CrossRef]
- Ferreira, G.; Guimarães, V.F.; De Pinho, S.Z.; De Oliveira, M.C.; Richart, A.; Braga, J.F.; Dias, G.B. Curva de Absorção de Água Em Sementes de Atemóia (Annona cherimola Mill. x Annona squamosa L.) Cv. Gefner. Rev. Bras. De Frutic. 2006, 28, 121–124. [Google Scholar] [CrossRef]
- Yan, D.; Duermeyer, L.; Leoveanu, C.; Nambara, E. The Functions of the Endosperm During Seed Germination. Plant Cell Physiol. 2014, 55, 1521–1533. [Google Scholar] [CrossRef]
- Nonogaki, H.; Bassel, G.W.; Bewley, J.D. Germination-Still a Mystery. Plant Sci. 2010, 179, 574–581. [Google Scholar] [CrossRef]
- Li, Z.; Gao, Y.; Zhang, Y.; Lin, C.; Gong, D.; Guan, Y.; Hu, J. Reactive Oxygen Species and Gibberellin Acid Mutual Induction to Regulate Tobacco Seed Germination. Front Plant Sci. 2018, 9, 1279. [Google Scholar] [CrossRef]
- Gimenez, J.I.; Ferreira, G.; Corsato, J.M. Soluble Sugars and Germination of Annona emarginata (Schltdl.) H. Rainer Seeds Submitted to Immersion in GA3 up to Different Water Contents. Rev. Bras. De Frutic. 2014, 36, 281–287. [Google Scholar] [CrossRef]
- Corsato, J.M.; Ferreira, G.; Barbedo, C.J. Desiccation Tolerance in Seeds of Annona emarginata (Schldtl.) H. Rainer and Action of Plant Growth Regulators on Germination. Braz. J. Plant Physiol. 2012, 24, 253–260. [Google Scholar] [CrossRef]
- De Oliveira, M.C.; Ferreira, G.; Guimarães, V.F.; Dias, G.B. Germinação de Sementes de Atemoia (Annona cherimola Mill. x A. squamosa L.) Cv “Gefner” Submetidas a Tratamentos Com Ácido Giberélico (GA3) e Ethephon. Rev. Bras. De Frutic. 2010, 32, 544–554. [Google Scholar] [CrossRef]
- Gomes, R.L.; Passos, J.R.D.S.; Gimenez, J.I.; Sousa, M.C.; De-Pieri-Oliveira, M.D.F.; Mimi, C.O.; Ferreira, G. Optimum Sample Size in the Germination of Atemoya Seeds (Annona × atemoya Mabb.). J. Agric. Sci. 2019, 11, 239. [Google Scholar] [CrossRef]
- Egydio, A.P.M.; Santos, D.Y.A.C. dos Underutilized Annona Species from the Brazilian Cerrado and Amazon Rainforest: A Study on Fatty Acids Profile and Yield of Seed Oils. Econ. Bot. 2011, 65, 329–333. [Google Scholar] [CrossRef]
- Luzia, D.M.M.; Jorge, N. Bioactive Substance Contents and Antioxidant Capacity of the Lipid Fraction of Annona crassiflora Mart. Seeds. Ind. Crops Prod. 2013, 42, 231–235. [Google Scholar] [CrossRef]
- Arruda, H.S.; Pastore, G.M. Araticum (Annona crassiflora Mart.) as a Source of Nutrients and Bioactive Compounds for Food and Non-Food Purposes: A Comprehensive Review. Food Res. Int. 2019, 123, 450–480. [Google Scholar] [CrossRef]
- Stenzel, N.M.C.; Murata, I.M.; Neves, C.S.V.J. Superação Da Dormência Em Sementes de Atemóia e Fruta-Do-Conde. Rev. Bras. De Frutic. 2003, 25, 305–308. [Google Scholar] [CrossRef]
- Gimenez, J.I.; Ferreira, G.; Cavariani, C. Tetrazolium Test for Assessment of Seed Viability of Atemoya (Annona cherimola Mill. × A. squamosa L.). J. Seed Sci. 2014, 36, 357–361. [Google Scholar] [CrossRef]
- Brasil, Ministério da Agricultura, Pecuária e Abastecimento. Regras Para Análise de Sementes, 1st ed.; Mapa/ACS, Ed.; Ministério da Agricultura, Pecuária e Abastecimento, Secretaria de Defesa Agropecuária: Brasília, Brazil, 2009; ISBN 978-85-99851-70-8.
- Hadas, A. Water Uptake and Germination of Leguminous Seeds Under Changing External Water Potential in Osmotic Solutions. Source J. Exp. Bot. 1976, 27, 480–489. [Google Scholar] [CrossRef]
- Maguire, J.D. Speed of Germination—Aid In Selection And Evaluation for Seedling Emergence And Vigor. Crop Sci. 1962, 2, 176–177. [Google Scholar] [CrossRef]
- Edmond, J.B.; Drapala, W.J. The Effects of Temperature, Sand and Soil, and Acetone on Germination of Okra Seeds. Proc. Am. Soc. Hortic. Sci. Alex. 1958, 71, 428–434. [Google Scholar]
- Ranal, M.A.; de Santana, D.G. How and Why to Measure the Germination Process? Rev. Bras. Bot. 2006, 29, 1–11. [Google Scholar] [CrossRef]
- Manirakiza, P.; Covaci, A.; Schepens, P. Comparative Study on Total Lipid Determination Using Soxhlet, Roese-Gottlieb, Bligh & Dyer, and Modified Bligh & Dyer Extraction Methods. J. Food Compos. Anal. 2001, 14, 93–100. [Google Scholar] [CrossRef]
- Ambalkar, V.U.; Sapkal, V.S.; Talib, M.; Khandelwal, S.A. Soxhlet Extraction of Neem Seed (Azadirachta indica A. Juss) Using Hexane as Solvent. Int. J. Chem. Anal. Sci. 2011, 2, 2. [Google Scholar]
- Garcia, I.S.; Souza, A.; Barbedo, C.J.; Dietrich, S.M.C.; Figueiredo-Ribeiro, R.C.L. Changes in Soluble Carbohydrates during Storage of Caesalpinia echinata LAM. (Brazilwood) Seeds, an Endangered Leguminous Tree from the Brazilian Atlantic Forest. Braz. J. Biol. 2006, 66, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Suda, C.N.K.; Giorgini, J.F. Seed Reserve Composition and Mobilization during Germination and Initial Seedling Development of Euphorbia heterophylla. Braz. J. Plant Physiol. 2000, 12, 226–245. [Google Scholar] [CrossRef]
- Corte, V.B.; Borges, E.E.D.L.E.; Pontes, C.A.; Leite, I.T.D.A.; Ventrella, M.C.; Mathias, A.D.A. Mobilização de Reservas Durante a Germinação Das Sementes e Crescimento Das Plântulas de Caesalpinia peltophoroides Benth. (Leguminosae-Caesalpinoideae). Rev. Árvore 2006, 30, 941–949. [Google Scholar] [CrossRef]
- Corte, V.B.; Borges, E.E.D.L.; Ventrella, M.C.; Leite, I.T.D.A.; Braga, A.J.T. Histochemical Aspects of Reserves Mobilization of Caesalpinia peltophoroides (Leguminosae) Seeds during Germination and Seedlings Early Growth. Rev. Árvore 2008, 32, 641–650. [Google Scholar] [CrossRef]
- Caramori, S.S.; Lima, C.S.; Fernandes, K.F. Biochemical Characterization of Selected Plant Species from Brazilian Savannas. Braz. Arch. Biol. Technol. 2004, 47, 253–259. [Google Scholar] [CrossRef]
- Ribeiro, J.C.L.; Bruginski, E.; Zuccolotto, T.; Santos, A.D.D.C.; Bomfim, L.M.; Rocha, S.L.A.; Barison, A.; Sassaki, G.; Cavalcanti, S.C.D.H.; Costa, E.V.; et al. Chemical Composition, Larvicidal and Cytotoxic Activity of Annona salzmannii (Annonaceae) Seed Oil. Braz. J. Pharm. Sci. 2021, 57, e18479. [Google Scholar] [CrossRef]
- Borek, S.; Ratajczak, W.; Ratajczak, L. Ultrastructural and Enzymatic Research on the Role of Sucrose in Mobilization of Storage Lipids in Germinating Yellow lupine Seeds. Plant Sci. 2006, 170, 441–452. [Google Scholar] [CrossRef]
- Graham, I.A. Seed Storage Oil Mobilization. Annu. Rev. Plant Biol. 2008, 59, 115–142. [Google Scholar] [CrossRef]
- Theodoulou, F.L.; Eastmond, P.J. Seed Storage Oil Catabolism: A Story of Give and Take. Curr. Opin. Plant Biol. 2012, 15, 322–328. [Google Scholar] [CrossRef]
- Sheen, J.; Zhou, L.; Jang, J.-C. Sugars as Signaling Molecules. Curr. Opin. Plant Biol. 1999, 2, 410–418. [Google Scholar] [CrossRef]
- Aragão, V.P.M.; Navarro, B.V.; Passamani, L.Z.; Macedo, A.F.; Floh, E.I.S.; Silveira, V.; Santa-Catarina, C. Free Amino Acids, Polyamines, Soluble Sugars and Proteins during Seed Germination and Early Seedling Growth of Cedrela fissilis Vellozo (Meliaceae), an Endangered Hardwood Species from the Atlantic Forest in Brazil. Exp. Plant Physiol. 2015, 27, 157–169. [Google Scholar] [CrossRef]
- Sánchez-Linares, L.; Gavilanes-Ruíz, M.; Díaz-Pontones, D.; Guzmán-Chávez, F.; Calzada-Alejo, V.; Zurita-Villegas, V.; Luna-Loaiza, V.; Moreno-Sánchez, R.; Bernal-Lugo, I.; Sánchez-Nieto, S. Early Carbon Mobilization and Radicle Protrusion in Maize Germination. J. Exp. Bot. 2012, 63, 4513–4526. [Google Scholar] [CrossRef]
- Ataíde, G.M.; Borges, E.E.L.; Picoli, E.A.T.; Leite Filho, A.T.; Flores, A.V. Alterações Nas Reservas de Sementes de Melanoxylon brauna Schott. (Fabaceae Caesalpinoideae) Durante a Germinação Em Diferentes Temperaturas. Rev. Bras. Ciênc. Agrár. 2017, 12, 372–379. [Google Scholar] [CrossRef]

| GA3 [mg L–1] | G (%) | MGT (Days) | GSI | U |
|---|---|---|---|---|
| [0] | 51.00 ± 6.83 b | 8.39 ± 0.92 a | 1.74 ± 0.30 b | 0.14 ± 0.01 a |
| [250] | 59.00 ± 8.87 ab | 9.55 ± 0.50 a | 1.75 ± 0.38 b | 0.13 ± 0.02 a |
| [500] | 75.75 ± 5.91 a | 8.57 ± 0.83 a | 2.75 ± 0.45 a | 0.15 ± 0.01 a |
| [1000] | 71.00 ± 12.38 a | 9.06 ± 0.63 a | 2.15 ± 0.40 ab | 0.13 ± 0.02 a |
| p | 0.007 ** | 0.169 n.s | 0.010 ** | 0.275 n.s. |
| F | 6.48 | 1.99 | 6.03 | 1.46 |
| C.V. (%) | 13.79 | 8.30 | 18.38 | 13.94 |
| GA3 [mg L−1] | T1—Dry Seeds | T2—1 Day | T3—5 Days | T4—10 Days | T5—15 Days |
|---|---|---|---|---|---|
| [0] | 274.68 ± 7.17 a A | 210.65 ± 16.14 a BC | 211.03 ± 5.45 a BC | 187.73 ± 18.72 a C | 219.25 ± 6.08 a B |
| [250] | 274.68 ± 7.17 a A | 192.00 ± 15.58 a BC | 209.25 ± 13.48 ab B | 167.03 ± 13.31 ab C | 218.13 ± 25.06 a B |
| [500] | 274.68 ± 7.17 a A | 216.95 ± 7.21 a B | 221.73 ± 10.44 a B | 186.23 ± 3.56 a C | 179.53 ± 15.51 b C |
| [1000] | 274.68 ± 7.17 a A | 204.23 ± 25.08 a B | 185.10 ± 14.41 b BC | 160.70 ±11.34 b C | 170.13 ± 12.22 b C |
| GA3 p: 0.0001 **; F: 8.236 | Days p < 0.001 **; F: 109.095 | GA3 × Days p: 0.0003 **; F: 3.729 | |||
| GA3 [mg L−1] | T1—Dry Seeds | T2—1 Day | T3—5 Days | T4—10 Days | T5—15 Days |
|---|---|---|---|---|---|
| [0] | 19.90 ± 0.82 a A | 13.90 ± 1.41 b B | 9.52± 0.13 a C | 12.07 ± 1.42 bc BC | 11.07 ± 2.23 c BC |
| [250] | 19.90 ± 0.82 a A | 17.50 ± 0.53 a B | 8.88± 1.18 a C | 11.09 ± 1.20 c BC | 14.02 ± 1.94 b C |
| [500] | 19.90 ± 0.82 a A | 16.41± 1.43 ab B | 9.30± 1.94 a D | 14.81 ± 2.22 b BC | 13.11 ± 2.90 bc C |
| [1000] | 19.90 ± 0.82 a A | 16.33± 1.75 ab B | 9.22± 0.59 a C | 18.12 ± 2.47 a AB | 17.20 ± 1.62 a AB |
| GA3 p < 0.001 **; F: 11.544 | Days p < 0.001 **; F: 96.985 | GA3 × Days p < 0.001 **; F: 4.769 | |||
| Arabinose | |||||
|---|---|---|---|---|---|
| GA3 [mg L−1] | T1—Dry Seeds | T2—1 Day | T3—5 Days | T4—10 Days | T5—15 Days |
| [0] | 2.67 ± 0.76 a B | 1.33 ± 1.12 a BC | 0.76 ± 0.25 a C | 1.66 ± 0.89 c BC | 7.77 ± 1.49 a A |
| [250] | 2.67 ± 0.76 a C | 1.63 ± 0.88 a C | 2.26 ± 0.28 a C | 7.06 ± 1.11 a A | 4.70 ± 1.03 b B |
| [500] | 2.67 ± 0.76 a BC | 1.36 ± 0.67 a C | 0.98 ± 0.44 a C | 3.55 ± 0.30 b B | 8.09 ± 1.30 a A |
| [1000] | 2.67 ± 0.76 a AB | 1.17 ± 0.67 a B | 2.26 ± 0.40 a AB | 3.36 ± 1.44 bc A | 3.86 ± 1.56 b A |
| GA3 p: 0.077 **; F: 4.349 | Days p < 0.001 **; F: 81.009 | GA3 × Days p < 0.001 **; F: 13.547 | |||
| Fructose | |||||
| GA3 [mg L−1] | T1—Dry Seeds | T2—1 day | T3—5 days | T4—10 days | T5—15 days |
| [0] | 1.43 ± 0.12 a A | 0.08 ± 0.01 a D | 0.22 ± 0.08 b CD | 0.47 ± 0.03 a B | 0.37 ± 0.09 a BC |
| [250] | 1.43 ± 0.12 a A | 0.09 ± 0.05 a C | 0.31 ± 0.07 b B | 0.29 ± 0.06 b B | 0.36 ± 0.06 a B |
| [500] | 1.43 ± 0.12 a A | 0.08 ± 0.03 a C | 0.34 ± 0.08 b B | 0.26 ± 0.05 b B | 0.26 ± 0.09 a B |
| [1000] | 1.43 ± 0.12 a A | 0.08 ± 0.03 a D | 0.54 ± 0.11 a B | 0.28 ± 0.08 b B | 0.38 ± 0.02 a B |
| GA3 p < 0.001 **; F: 12.273 | Days p < 0.001 **; F: 1310.822 | GA3 × Days p < 0.001 **; F: 10.442 | |||
| Glucose | |||||
| GA3 [mg L−1] | T1—Dry Seeds | T2—1 day | T3—5 days | T4—10 days | T5—15 days |
| [0] | 2.97 ± 1.14 a B | 2.25 ± 0.62 b B | 4.07 ± 0.42 a B | 6.74 ± 1.69 b A | 6.29 ± 0.96 a A |
| [250] | 2.97 ± 1.14 a B | 2.54 ± 0.89 b B | 3.34 ± 0.67 a B | 6.72 ± 1.48 b A | 7.23 ± 1.82 a A |
| [500] | 2.97 ± 1.14 a B | 4.38 ± 1.24 ab AB | 4.24 ± 0.53 a AB | 5.94 ± 2.21 b A | 6.13 ± 0.71 a A |
| [1000] | 2.97 ± 1.14 a C | 5.49 ± 1.00 b BC | 5.12 ± 1.42 a BC | 12.73 ± 3.30 a A | 6.49 ± 1.42 a B |
| GA3 p < 0.001 **; F: 10.972 | Days p < 0.001 **; F: 42.219 | GA3 × Days p < 0.001 **; F: 5.648 | |||
| Mannose | |||||
| GA3 [mg L−1] | T1—Dry Seeds | T2—1 day | T3—5 days | T4—10 days | T5—15 days |
| [0] | 6.90 ± 2.07 a B | 11.72 ± 0.52 a B | 12.45 ± 0.86 ab B | 30.57 ± 1.30 b A | 27.51 ± 2.08 a A |
| [250] | 6.90 ± 2.07 a B | 9.44 ± 3.30 a B | 11.89 ± 2.11 b B | 29.28 ± 3.32 b A | 32.98 ± 1.75 a A |
| [500] | 6.90 ± 2.07 a C | 12.84 ± 1.08 a BC | 16.18 ± 1.82 ab B | 24.56 ± 9.04 b A | 30. 99 ± 4.17 a A |
| [1000] | 6.90 ± 2.07 a D | 13.38 ± 1.36 a CD | 18.67 ± 2.82 a C | 38.01 ± 6.47 a A | 29.77 ± 4.38 a B |
| GA3 p: 0.0149 *; F: 3.781 | Days p < 0.001 **; F: 181.337 | GA3 × Days p: 0.0001 **; F: 4.161 | |||
| Sucrose | |||||
| GA3 [mg L−1] | T1—Dry Seeds | T2—1 day | T3—5 days | T4—10 days | T5—15 days |
| [0] | 153.01 ± 17.42 a AB | 142.75 ± 16.37 c AB | 196.63 ± 56.74 a A | 133.89 ± 14.86 a B | 113.23 ± 13.79 a B |
| [250] | 153.01 ± 17.42 a B | 153.21 ± 26.32 bc B | 216.94 ± 34.25 a A | 115.48 ± 29.03 a B | 103.09 ± 11.99 a B |
| [500] | 153.01 ± 17.42 a ABC | 206.70 ± 62.69 ab A | 160.65 ± 46.19 ab AB | 103.54 ± 21.94 a BC | 97.57 ± 4.37 a C |
| [1000] | 153.01 ± 17.42 a B | 256.32 ± 52.21 a A | 102.95 ± 9.63 b B | 129.22 ± 46.17 a B | 138.42 ± 15.72 a B |
| GA3 p: 0.7590 n.s.; F: 0.392 | Days p < 0.001 **; F: 18.100 | GA3 × Days p < 0.001 **; F: 6.462 | |||
| Stachyose | |||||
| GA3 [mg L−1] | T1—Dry Seeds | T2—1 day | T3—5 days | T4—10 days | T5—15 days |
| [0] | 0.33 ± 0.04 a B | 0.33 ± 0.09 a B | 0.62 ± 0.14 a B | 2.38 ± 0.72 ab A | 2.76 ± 0.51 a A |
| [250] | 0.33 ± 0.04 a B | 0.21 ± 0.02 a B | 0.59 ± 0.01 a B | 1.81 ± 0.84 ab A | 2.73 ± 0.48 a A |
| [500] | 0.33 ± 0.04 a BC | 0.19 ± 0.05 a C | 0.16 ± 0.04 a C | 1.28 ± 0.43 b B | 2.64 ± 1.46 a A |
| [1000] | 0.33 ± 0.04 a B | 0.28 ± 0.03 a B | 1.07 ± 0.69 a AB | 2.00 ± 0.47 a A | 1.13 ± 0.41 b AB |
| GA3 p: 0.0698 n.s.; F: 2.478 | Days p < 0.001 **; F: 58.636 | GA3 × Days p: 0.0009 **; F: 3.368 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mimi, C.O.; Sousa, M.C.; Corrêa, P.L.C.; De-la-Cruz-Chacón, I.; Boaro, C.S.F.; Ferreira, G. Impact of GA3 on Sugar and Lipid Degradation during Annona x atemoya Mabb. Seed Germination. Horticulturae 2023, 9, 388. https://doi.org/10.3390/horticulturae9030388
Mimi CO, Sousa MC, Corrêa PLC, De-la-Cruz-Chacón I, Boaro CSF, Ferreira G. Impact of GA3 on Sugar and Lipid Degradation during Annona x atemoya Mabb. Seed Germination. Horticulturae. 2023; 9(3):388. https://doi.org/10.3390/horticulturae9030388
Chicago/Turabian StyleMimi, Carolina Ovile, Marília Caixeta Sousa, Patrícia Luciana Carriel Corrêa, Ivan De-la-Cruz-Chacón, Carmen Sílvia Fernandes Boaro, and Gisela Ferreira. 2023. "Impact of GA3 on Sugar and Lipid Degradation during Annona x atemoya Mabb. Seed Germination" Horticulturae 9, no. 3: 388. https://doi.org/10.3390/horticulturae9030388
APA StyleMimi, C. O., Sousa, M. C., Corrêa, P. L. C., De-la-Cruz-Chacón, I., Boaro, C. S. F., & Ferreira, G. (2023). Impact of GA3 on Sugar and Lipid Degradation during Annona x atemoya Mabb. Seed Germination. Horticulturae, 9(3), 388. https://doi.org/10.3390/horticulturae9030388