Abstract
An adequate and balanced diet is fundamental in preserving the health of astronauts from several space-induced diseases. Therefore, the integration of a diet with fresh food, rich in bioactive compounds such as microgreens produced directly onboard, may be useful in space for human nutrition. However, ionizing radiation (IR) in space represents a significant hindrance for organisms, with potential critical outcomes on plant morpho-anatomical, eco-physiological, and biochemical aspects, depending on the plant and IR features (e.g., species, developmental stage, IR dose, and type). In this study, we analyzed the effect of different doses of X-rays (0-control, 0.3, 1, 10, 20, and 30 Gy) on the morpho-anatomical and nutritional traits of microgreens of Brassica rapa L., irradiated at the stage of germinated seeds. After the irradiation, microgreens were cultivated in controlled conditions. At harvest, the morpho-biometric traits were analyzed, along with the leaf functional anatomical traits and the phytochemical content of the aboveground biomass. The results showed that X-ray exposure does not induce detrimental effects on growth, while it stimulates the production of antioxidants, improving plant defense and nutritional value. The overall results support the idea of using this species in space as a supplemental functional food.
1. Introduction
There is an unbreakable link between human nutrition and health: a varied and balanced diet, combined with a healthy lifestyle, are effective tools for the prevention, management, and treatment of a wide range of pathological diseases, particularly for individuals in specific physiological and psychological conditions due to the exposure to stressful factors. Astronauts belong to this category, as they work in isolation and confinement conditions in space, and are constantly exposed to extreme environmental factors such as ionizing radiation (IR) and altered gravity [1,2,3].
The integration of a diet with appetible fresh-food from plant origin, being rich in bioactive compounds (e.g., antioxidants, vitamins, minerals, fibers), may be an effective countermeasure toward the occurrence of space-induced diseases in astronauts, which also induce a positive effect on their psychology [4,5,6,7,8,9].
Given the higher content of phytonutrients in microgreens compared to adult plants, they have become extremely popular in the last decades, being proposed as a supplement functional food for Earth and for space cultivation [10,11,12]. Microgreens, also called “vegetable confetti”, are young and tender greens harvested after 1–3 weeks from germination, developed from seeds of several crop species, with bright colors, delicate textures, and peculiar flavors. The use of microgreens in space could help enhance appetite and preserve the health and homeostasis of astronauts, who are used to relying on pre-packaged food as the one and only source of nourishment. Microgreens have a short, easy to manage production cycle, and need very low input resources for cultivation. Moreover, they are characterized by a high harvest index [10,13,14], making them suitable for direct cultivation onboard during space flights.
Currently, several families are candidates to be exploited in microgreen production. Among these, many species of the Brassicaceae family present high amounts of micronutrients such as polyphenols, ascorbic acid, carotenoids, tocopherols [15,16,17,18], selenium, sulfur, calcium, and glycosylates [19,20,21], which are very effective in neutralizing radiation-induced oxidative damage and the risk of the occurrence of several types of cancer [7,20,22,23,24]. These secondary metabolites are tightly linked to plant protection and defense pathways, and may be influenced by environmental conditions and agricultural practices [25,26,27].
Nevertheless, space farming must face and overcome several hurdles related to the same environmental constraints that affect the health of astronauts [28,29,30]. Among the space factors, ionizing radiation is considered to be the main constraint for exploratory class manned missions and long-term permanence on Moon and Mars. Indeed, in space, the galactic cosmic rays and solar particle events are responsible for harmful outcomes in both mammals and plants through direct and indirect mechanisms involving DNA and other chemical compounds as the main targets.
Ionizing radiation, interacting with living matter, induces radiolysis processes and the over-production of reactive oxygen species (ROS), thus resulting in oxidative stress to nucleic acids, lipids, and proteins, which are the main targets of ROS [31,32,33,34,35].
In the last decades, many studies have explored the effects of ionizing radiation on the morpho-anatomical and physiological traits of crops with different main purposes [34,36,37,38]: (i) explore the radio-tolerance of different species; (ii) understand if low levels of ionizing radiation could strengthen plant phytochemical and growth responses; (iii) simulate the effect of a space environment on plants.
Notwithstanding the fact that the impact of IR on plant fitness is not completely unraveled, it has emerged that in the plant response to IR, the radiation features (e.g., dose, exposure time, and type of IR delivered) may act as discriminators along with other traits strictly related to the plant (e.g., species, cultivar as well as phenological stage and type of tissue exposed) [39,40,41,42,43].
The exposure doses measured on the ISS, Moon, and Mars have been estimated to be about 0.5, 1.37, 0.64 mSv/day, respectively, which are about hundreds of times that of an the Earth dose. In the experiments of radiobiology performed on Earth, considering the reduced possibility to recreate all the radiation spectrum and their dose intensity, particle accelerators are used. In these facilities, dose rates of many orders of magnitude higher than those in space are normally used to reduce the duration of the trials and to estimate the possible effects that would occur with lower doses over a longer period of time [44].
In most experiments, the dry seeds were the irradiation target, enhancing the hypothesis of the major radio-resistance of plants, especially in this phenological stage, due to the structural and metabolic traits of the dry seed. However, testing the responses to radiation impact on actively growing tissues, as in the case of meristems during the seed germination, may represent a more useful way to clarify the mechanism behind the radiation-induced alterations in plants.
The breaking of the seed’s dormancy period due to imbibition, and subsequent hydration of the integuments, leads to a series of processes such as the activation of metabolic activity, the mobilization of the seed reserve, and the activation of the meristem cell proliferative pathways. At this stage, the young plant is very vulnerable to abiotic stress like IR, potentially compromising the maintenance of genome integrity, essential for meristem function, and consequently for seedling growth and establishment [45,46,47]. Thus, overcoming this stage may represent a bottleneck in the cultivation of adult plants in bioregenerative life support systems (BLSSs) to successfully support the human inhabitation of Moon and Mars [28,48].
To the best of our knowledge, very few studies have explored the effect of ionizing radiation on imbibed or germinated seeds of different species, but this effect has never been tested on microgreens.
For this purpose, we irradiated germinated seeds of Brassica rapa L. subsp. sylvestris var. Esculenta microgreens with X-rays at the doses of 0.3, 1, 10, 20, and 30 Gy (i.e., up to tens of times higher than the absorbed dose estimated for long-duration missions as those on Moon and Mars) plus a non-irradiated control (0), in order to evaluate the dose–response curves of plants as well as the possible occurrence of positive or negative effects. X-rays were chosen since they are considered as the reference radiation before exploring other radiation types and because they share a similarity in effectiveness with space-low-energy protons [49]. At the end of the cultivation cycle, morpho-biometric, anatomical, and biochemical analyses were conducted to evaluate the effect of X-rays on the growth and productivity performance of the microgreens to evaluate their possible use in the space environment as a food supplement.
2. Materials and Methods
2.1. Experimental Design
Seeds of Brassica rapa L. subsp. sylvestris var. Esculenta were purchased from a local provider (Bioseme s.c.a.r.l., Piombino, Italy) and before the irradiations, preliminary germination tests were carried out to evaluate the rate of the germination and growth of the radicles in order to establish the most suitable target length of seedlings for irradiation. The tests were conducted in Petri dishes by placing the seeds on three layers of filter paper imbibed with distilled water and incubating them in a growth chamber at 24 °C in the dark. Eventually, a radicle length of 0.3 cm, reached after 30 h from the start of germination, was chosen as the target length for the irradiation test, and the seed incubation time and environmental parameters were modulated to have seedlings of the target length at the time of irradiation.
2.2. Irradiation Procedure and Microgreens Cultivation
The irradiation took place at the National Cancer Institute IRCCS Fondazione G. Pascale in Naples (Italy), using a linear accelerator Linac Synergy (Elekta) and carried out at the pre-established doses of 0.3, 1, 10, 20, and 30 Gy X-rays. The instrument delivered 6 MV photon beams with a dose rate of 200 MU/min, with the 3D-CRT (three-dimensional conformal radiotherapy technique), which provided the exposure of the sample to two opposing fields of size 20 × 20 cm2 at the isocenter. For each dose, two thousand germinated seeds, with a 0.3 cm radicle, were placed between two Perspex blocks of 2.5 cm and 5 cm, leaned on filter paper soaked in distilled water.
After the irradiation, germinated seeds were immediately transferred to the laboratory, divided into 5 replicates per dose (400 seeds used per replicates), sowed in pots (12 × 9 × 4 cm) filled with standard gardening soil, and placed in a growth chamber under controlled conditions of temperature (24 ± 2 °C), relative humidity (RH 70 ± 10%), and light (white LED light of 200 ± 20 μmol photons m−2 s−1 photosynthetic photon flux density, PPFD; 12 h photoperiod).
During the transfer to and from the irradiation center, the germinated seeds were kept under controlled temperature conditions, together with the not-irradiated control. At 14 days from seeding, microgreens were harvested and divided into groups to perform morpho-biometric, anatomical, and biochemical analyses.
2.3. Morpho-Biometric Analyses and Sampling
At harvest, the survival percentage of microgreens was evaluated for each dose and control as the percentage of seedlings that grow up to the “stage” of microgreens with two expanded leaves after 14 days from sowing [50,51,52].
On 10 microgreens per replicate, the total length was measured, and at the same time, pictures of the 10 microgreens were taken in order to calculate their leaf area by using ImageJ software (U.S. National Institutes of Health, Bethesda, MS, USA). Moreover, on the same microgreens used to calculate the length and leaf area, the leaf fresh and dry weight were evaluated using a fine scale. The dry weight was obtained after drying the samples in an oven at 60 °C until they reached a constant weight.
Microgreens were then sampled for microscopy and biochemical analyses. Then, the fresh and dry biomass of the whole canopy was calculated as the amount of biomass produced per a growing area of 0.018 m2 and reported as Kg m−2 for the fresh biomass and g m−2 for the dry biomass.
2.4. Anatomical Analyses
Microgreens collected for microscopy analyses were fixed in the F.A.A. chemical fixative (5 mL of 40% formaldehyde, 5 mL of glacial acetic acid, 90 mL of 50% ethanol) to prevent decomposition phenomena and the loss of chemical-physical properties. Subsequently, the leaf samples (n = 3) were dissected in order to obtain sub-samples of leaf lamina of about 5 × 6 mm, which were then subjected to dehydration, infiltration, and inclusion in an acrylic resin (JB4®, Polysciences, Eppelheim, Germany). These sub-samples were sectioned with a rotary microtome, obtaining cross sections, 5 μm thick, which were stained with Toluidine Blue at 0.5% in distilled water (Feder and O’Brien, 1968). Then, the sections were mounted with mineral oil and observed under a transmitted light microscope (BX 51, Olympus, Hamburg, Germany). Digital images were acquired with a camera (Olympus EP50) and the software CellSens 2.3 (Olympus, Tokyo, Japan) was used to analyze and quantify some of the functional anatomical parameters. Specifically, in each section, the total thicknesses of the leaf lamina (TLLT), the upper epidermis thickness (UET), lower epidermis thickness (LET), the palisade parenchyma thickness (PPT), and the spongy parenchyma thickness (SPT) were measured at three points of the section. Furthermore, the adaxial and abaxial stomatal density (SDad; SDab) per linear transect unit as well as the percentage of intercellular spaces in the spongy parenchyma (SPIS) were also measured in three regions of the section.
2.5. Antioxidant, Pigment and Protein Determination
These analyses were performed on 8 microgreens per treatment. The antioxidant capacity was determined using the Ferric Reducing Antioxidant Power (FRAP) assay according to the protocol of George et al. [53], modified by Costanzo et al. [54], which was performed on microgreens previously powdered in liquid nitrogen. The samples were treated with a 60:40 methanol/water solution in a centrifuge at 14,000 rpm for 15 min at 4 °C. The FRAP reagents (300 mM acetate buffer pH 3.6, 1:16.6 v/v; 10 mM tripyridyl triazine, TPTZ, in 40 mM HCl, 1:11.6 v/v; 12 mM FeCl3, 1:11.6 v/v) were combined with the extracts and incubated for 1 h in the dark. Then, the absorbance was read at 593 nm by a spectrophotometer (UV-VIS Cary 100; Agilent Technologies, Palo Alto, CA, USA). The antioxidant capacity was calculated using a Trolox standard curve and expressed as μmol Trolox equivalents g−1 FW (μmol Trolox eq g−1 FW).
The total content of chlorophylls (a + b) and carotenoids (x + c) was assessed according to Lichtenthaler [55]; leaf disks of 0.283 cm2 area were homogenized in ice-cold 100% acetone using a mortar and pestle. The extracts were centrifuged at 5000 rpm for 5 min in a Labofuge GL (Heraeus Sepatech, Hanau, Germany). Afterward, the sample absorbance was determined using a UV-VIS Cary 100 spectrophotometer from Agilent Technologies at wavelengths of 470, 645, and 662 nm.
The amount of total polyphenols was determined as reported in Vitale et al. [56]. Fresh samples were powdered in liquid nitrogen, treated with 80% methanol at 4 °C and centrifuged at 11,000 rpm for 5 min. The soluble fraction was mixed with 10% Folin–Ciocalteu solution in a ratio of 1:1 v/v for three minutes and then treated with 700 mM Na2CO3 solution in a ratio of 1:5, v/v. Samples were incubated for 2 h in the darkness. The absorbance was measured at 765 nm with a spectrophotometer (UV-VIS Cary 100; Agilent Technologies) and the content of total polyphenol was expressed as mg of gallic acid equivalents g−1 FW (mg GAE g−1 FW) using a gallic acid standard curve.
The total protein content was determined on five fresh leaf samples ground in liquid nitrogen according to Wang et al. [57]. The protein amount was quantified by the Bradford colorimetric assay [58], measuring the sample absorbance at 595 nm by a spectrophotometer (UV-VIS Cary 100, Agilent Technologies, Palo Alto, CA, USA). The protein concentration was expressed as μg BSA equivalents g−1 FW using BSA (bovine serum albumin) to build a standard curve.
To evaluate the ascorbic acid (AsA) content, we used the Ascorbic Acid Assay Kit (MAK074, Sigma-Aldrich, St. Louis, MO, USA), following the procedure reported by Costanzo et al. [54]. Briefly, 10 mg of the sample was homogenized in 4 volumes of cold AsA buffer before being centrifuged at 12,850× g for 10 min at 4 °C. To a final volume of 120 L, the supernatant was mixed with AsA assay buffer. In this assay, the AsA concentration was determined using a coupled enzyme reaction that produced a colorimetric (570 nm) product equal to the amount of ascorbic acid in the sample. The concentration of ascorbic acid was expressed in ng mL−1 and calculated in relation to a standard curve.
2.6. Statistical Analyses
Data were analyzed through one-way analysis of variance (ANOVA) using the SPSS statistical package (SPSS Inc., Chicago, IL, USA) and the SNK (Student–Newman–Keuls) multiple comparison tests (p ≤ 0.05). To check for normality, the Kolmogorov–Smirnov test was performed; the arcsine transformation function was applied to percent data before statistical analysis.
3. Results
3.1. Morpho-Biometric Traits
The different doses of X-rays did not induce statistically significant changes in terms of the survival percentage of microgreens. Control and microgreens from seeds irradiated at doses from 0.3 up to 20 Gy showed a survival percentage ranging between 62% and 65%, whilst the 30 Gy microgreens showed a tendency to increase the survival, reaching 71%, which was still not significantly different from the other doses. No morphological aberrations were detected in the microgreens from irradiated seedlings compared to the controls.
All doses induced a significant increase in fresh biomass (Figure 1a) compared to the control. The highest value was observed at the dose of 30 Gy, whose microgreens were significantly heavier than those at 0.3 and 20 Gy, which in turn showed significantly higher values that at 1 and 10 Gy. In contrast to the dry biomass (Figure 1b), only the dose of 20 Gy induced a significant increase in weight compared to all the other doses and to the control.
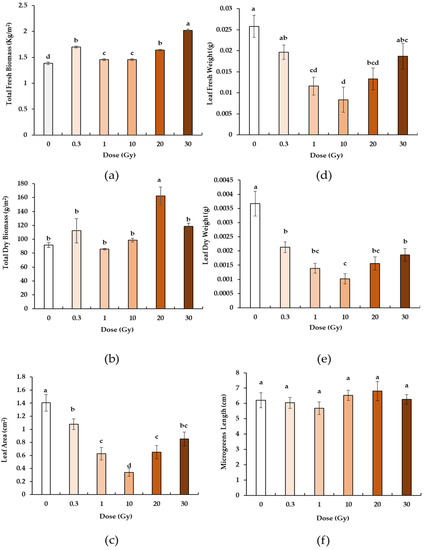
Figure 1.
Fresh (a) and dry biomass (b), leaf area (c), fresh (d) and dry leaf weight (e), and total length of the microgreens (f) of B. rapa microgreens from the control (0) and irradiated seedlings with increasing doses (0.3, 1, 10, 20, 30 Gy) of X-rays. Mean values and standard errors are shown (n = 5 for biomass; n = 10 for leaf weight, area, and microgreens length). Different letters correspond to significant differences among irradiation doses according to the Student–Newman–Keuls multiple comparison tests (p ≤ 0.05).
Concerning the microgreen leaves, the leaf area (Figure 1c), the leaf fresh weight (Figure 1d) and dry weight (Figure 1e) showed the same trend of variation. Compared to the control, all microgreens from the irradiated seeds showed significantly lower values. More specifically, the irradiation induced a significant decrease in all parameters up to 10 Gy, followed by an increase again up to values at 30 Gy comparable to the control (for leaf fresh weight) and to 0.3 Gy (for leaf dry weight and leaf area). No radiation-induced variations in terms of the microgreen length (Figure 1f) were observed.
3.2. Anatomical Traits
The exposure to increasing doses of X-rays did not cause evident aberrations in the organization of tissues of the typical leaf lamina with dorsiventral anatomy (Figure 2). From a quantitative viewpoint, irradiation induced statistically significant changes in all of the analyzed anatomical parameters (Figure 2 and Table 1), except for the upper epidermis thickness (UET), lower epidermis thickness (LET), and abaxial stomatal density (SDab). No unique trend of variation was found in the different analyzed parameters after irradiation. More specifically, the total thicknesses of the leaf lamina (TLLT) and the spongy parenchyma thickness (SPT) were significantly higher at the doses of 1 and 30 Gy compared to all of the others, with 10 Gy showing the lowest values. The palisade parenchyma thickness (PPT) was significantly higher at 1 Gy than the control and 30 Gy microgreens, which in turn showed significantly higher values than all the others.
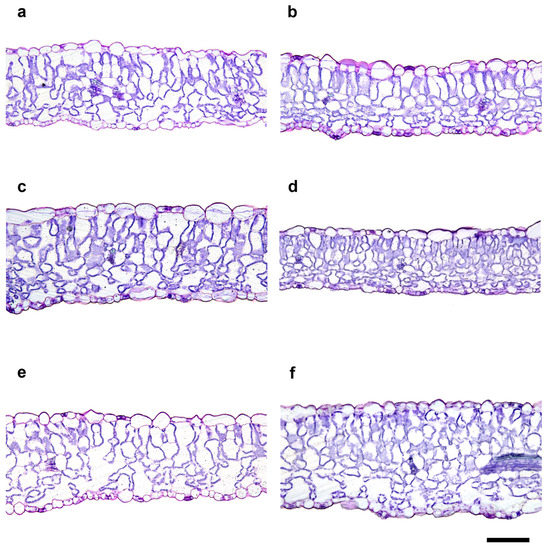
Figure 2.
Light microscopy views of cross sections of B. rapa leaf lamina from the control (0 Gy, (a)) and irradiated seedlings with increasing doses (0.3 Gy, (b); 1 Gy, (c); 10 Gy, (d); 20 Gy, (e); 30 Gy, (f)) of X-rays. Images are all at the same magnification. Bars = 100 μm.

Table 1.
Effect of irradiation on the leaf anatomical traits: upper epidermis thickness (UET), palisade parenchyma thickness (PPT), spongy parenchyma thickness (SPT), lower epidermis thickness (LET), total leaf lamina thickness (TLLT), percentage of spongy parenchyma intercellular spaces (SPIS), and stomatal density of the adaxial and the abaxial surface (SDad; SDab) in the leaves of B. rapa microgreens from the control (0) and seedlings irradiated with increasing doses (0.3, 1, 10, 20, 30 Gy) of X-rays. The mean values and standard errors are shown (n = 27 for tissue thicknesses and SPIS%; n = 9 for SD).
Concerning the percentage of intercellular spaces in the spongy parenchyma (SPIS), all doses except for 0.3 Gy induced a significant increase compared to the control. In contrast, for the stomatal density on the adaxial lamina (SDad) at 20 Gy, the values were significantly higher than at 1 and 30 Gy.
3.3. Antioxidants, Photosynthetic Pigment, and Proteins
The X-rays elicited a significant effect on all of the analyzed biochemical parameters, with differences depending on the dose. Compared to the control, irradiation induced a significant increase in antioxidant capacity (Figure 3a), with no significant differences among doses. The total polyphenol content (Figure 3b) significantly increased in the irradiated samples starting from 1 Gy compared to the control and 0.3 Gy plants, which exhibited comparable values. The highest polyphenol concentration was found in the 20 Gy plants, while intermediate values were detected at 1, 10, and 30 Gy.
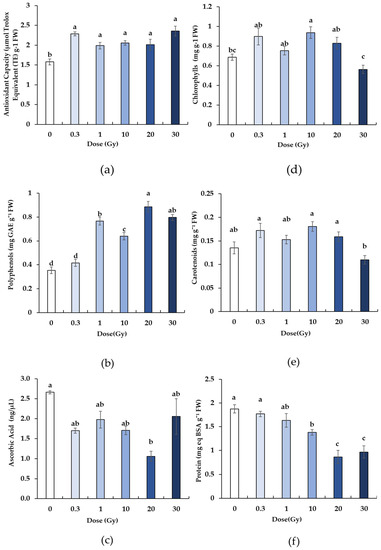
Figure 3.
Total antioxidant capacity (a) and the content of polyphenols (b), ascorbic acid (c) chlorophylls (d), carotenoids (e), and protein (f) in B. rapa microgreens from the control (0) and irradiated seedlings with increasing doses (0.3, 1, 10, 20, 30 Gy) of X-rays. The mean values and standard errors are shown (n = 8). Different letters correspond to significant differences among irradiation doses according to the Student–Newman–Keuls multiple comparison tests (p ≤ 0.05).
Concerning the production of ascorbic acid, the irradiation at 20 Gy induced a significant reduction compared to the control, while all of the other treatments showed intermediate values (Figure 3c).
The total content of chlorophylls and carotenoids was also modulated by the radiation treatments, but this modulation was not dependent on the dose (Figure 3d,e). For the chlorophylls, 10 Gy seedlings showed values significantly higher than 0 and 30 Gy, while 0.3, 1, and 20 seedlings exhibited intermediate values (Figure 3c). For the carotenoids, an apparent increase was confirmed at 0.3 and 10 Gy, but it was not significant compared to the control and other doses, except for the dose of 30 Gy, at which the plants exhibited a significantly lower amount of carotenoids compared to other treatments (Figure 3e).
The protein content decreased with increasing doses. More specifically, it was similar in the control, 0.3, and 1 Gy, while it was statistically lower at 20 and 30 Gy (Figure 3f).
4. Discussion
This study indicated that B. rapa has valuable radio-resistance, since all X-ray doses tested, from 0.3 up to 30 Gy, did not prevent the seedling survival and growth when the target of irradiation was the germinated seed. This stage represents a very delicate phase in the plant life cycle because after the break of dormancy, the plant is characterized by active meristems and morphogenesis, particularly vulnerable to abiotic stress, which may weaken the seedling establishment [59]. In space, the presence of ionizing radiation [29,30,44] may influence early morphogenesis in plants [45,46,47]. Previous research has demonstrated that low doses of X-rays may generally induce positive effect on the viability, germination, and growth of the seeds, while high doses may reduce or hamper these delicate phases [35,60,61].
Despite the few published works focusing on the effects of IR on plants at the stage of germinated seeds, our results are consistent with previous findings on Vigna radiata L. sprouts, in which X-rays did not cause growth aberrations as well as premature senescence or death in irradiated seedlings [62].
In our study, the morpho-anatomical and biochemical responses observed in B. rapa microgreens irradiated at the stage of germinated seed were dependent on the dose, but no univocal tendency was found for all of the measured parameters. Specifically, the relationships appeared to be nonlinear, confirming what was found in previous studies [62,63].
In terms of the morphological parameters, despite the length of the microgreens not changing within the different doses, it is noteworthy that the variations observed in the fresh and dry biomass (leaves and hypocotyls) of the microgreens were not in agreement to those found in the leaves alone, indicating that the effect of radiation on biomass accumulation and photosynthate allocation may already be organ-specific in the first stages of development. This result suggests a greater allocation of resources toward the hypocotyls, resulting in a probable greater diameter and robustness. Concerning the whole canopy biomass, here, all doses showed enhanced fresh biomass compared to the control, while the dry biomass high values were observed only at the dose of 20 Gy. In a previous study on tomato cultivar ‘microtom’ irradiated with X-rays [64], 20 Gy was found to be a threshold dose for the occurrence of positive outcomes on plant growth, which became null or negative at higher doses.
Regarding the leaf parameters, increasing X-ray doses induced a decrease in the fresh and dry weight and in the leaf area up to 10 Gy, which would appear as a new threshold dose for these parameters in brassica. A similar reduction in the same parameters was observed by [35] on juvenile leaves of Phaseolus vulgaris L. exposed to increasing doses of X-rays, probably highlighting a greater radiosensitivity of the leaves compared to the whole canopy in different species.
The anatomical analysis underlined that exposure to X-rays, particularly at 1 and 30 Gy, induced the formation of a thicker leaf lamina, with leaves at 1 Gy also having a thicker palisade parenchyma tissue. It can be speculated that if such a trend is maintained in the further emerging leaves, adult plants may have a greater photosynthetic gain. With respect to the percentage of intercellular spaces in the spongy parenchyma (SPIS), with the exception of the 0.3 and 10 Gy dose, all of the other doses showed comparable or even significantly higher values compared to the control. The increase in SPIS may respond to the need by irradiated seedlings to better control transpiration, since a higher presence of intercellular spaces indicates reduced cell connection, and consequently slowed flow [65,66].
The effect induced by increasing doses of X-ray on stomatal density was different between the two epidermal layers: for the upper epidermis, it resulted in an increase or the maintenance of stomatal density compared to the control, consistent with the behavior of the intercellular spaces. Conversely, the upper epidermis was not affected by the irradiation, suggesting that stomatal density is a stable trait, in agreement with other recent studies [41,67].
At the biochemical level, X-rays induced positive responses in microgreens, significantly increasing the antioxidant capacity and total polyphenol content compared to the control plants. This may suggest a strategy employed by plants to increase the synthesis of antioxidants to protect plant cells from radiation-induced oxidative stress [36,37], which also had the useful consequence of increasing the nutritional profile of plants. It has been demonstrated that the increase in phenolic compounds, beyond exerting a detoxifying action against free radicals, also acts as a natural screen against radiation including IR, preferentially distributing along photosynthetic membranes [63,64].
Our findings are partially in agreement with previous works [39,62,67,68,69,70] in which the irradiation of various plants species with different types and doses of IR determined the maintenance, reduction, or increase in polyphenols compared to the control plants, remarking on the difficulty in highlighting an unequivocal behavior in response to IR. Conversely, the ascorbic acid (AsA) concentration showed a tendency to decrease, even if its reduction was not statistically significant compared to the control, except for the dose of 20 Gy. These data suggest that AsA does not contribute to the rise in total antioxidant capacity observed in response to the increasing X-ray doses. Ascorbic acid is recognized as one of the most powerful “oxidant scavengers “and is quickly used by ascorbate peroxidase (APX) as a co-factor for the detoxification of ROS [71]. The significant decrease in its concentration at 20 Gy could depend on the strong request by the detoxification system at this specific dose.
It is interesting to note that the total chlorophyll and carotenoid content was not negatively affected by radiation. Specifically, the significant increase in the values observed in both parameters at doses of 0.3, 10, and 20 Gy could be the result of a sort of “surviving strategy” by plants to enhance photosynthesis in response to the decrease in the expansion and thickness of the leaf lamina, especially of the palisade parenchyma observed at the same doses. This evidence, also found in other works [41,67], can be considered as a positive characteristic, given the pivotal role of these pigments in the photosynthetic process. Furthermore, being that chlorophyll and carotenoids are also employed in antioxidant activity, their importance extends to the nutritional sphere in protecting the human body from oxidative stress [72,73,74]
Medium and high IR doses have been reported to also induce an accumulation of soluble proteins in the tissues of plants grown from irradiated seeds [75,76,77,78,79]. On the other hand, nonlinear effects, both positive and negative, were observed at low IR doses [75,80,81,82]. In our experiment, the soluble protein content decreased as the irradiation dose increased, suggesting a stressful condition for microgreens. Ionizing radiation may cause protein oxidation, shortening their functional life and altering the processes of protein synthesis, degradation as well as protein profile [32]. As mentioned before, to avoid oxidative stress, plants increase or decrease the content of various secondary metabolites with antioxidant activity such as phenols, terpenoids, and proteins [83].
In conclusion, the results presented in this paper indicated that the responses of B. rapa microgreens to X-rays were dose- and parameter-dependent. Irradiation did not induce detrimental effects at the morphological, anatomical, or biochemical level, allowing for the normal growth and establishment of seedlings. However, some variations in functional anatomical traits, if maintained in adult leaves, could lead to variations in the functional efficiency of the photosynthetic process. Nevertheless, at this stage of development, which is primarily aimed at consumption for diet integration, the exposure to X-rays has induced stimulatory outcomes like an overproduction of antioxidants, probably due to the implementation of plant defense. Indeed, the production of these secondary metabolites improved the nutritional profile of microgreens as well as the ability of plant cells to counteract oxidative stress, supporting the hypothesis of using this species for cultivation in space as a supplemental functional food. Further studies are still needed to test the radioresistance of this species as well as to other types of ionizing radiation, especially high-LET radiation and chronic radiation doses as well as further testing their palatability and safety attributes for future crews to confirm the suitability of Brassica microgreens for cultivation in space.
Author Contributions
Conceptualization, C.A. (Carmen Arena) and V.D.M.; Methodology, M.P., C.A. (Cecilia Arrichiello), G.A., P.M., C.A. (Carmen Arena) and V.D.M.; Formal analysis, S.D.F., C.A. (Chiara Amitrano), E.V. and G.C.; Investigation, S.D.F., C.A. (Chiara Amitrano), E.V., G.C., C.A. (Carmen Arena) and V.D.M.; Resources, C.A. (Carmen Arena) and V.D.M.; Data curation, S.D.F., C.A. (Chiara Amitrano), E.V., G.C., C.A. (Carmen Arena) and V.D.M.; Writing—original draft preparation, S.D.F.; Writing—review and editing, S.D.F., C.A. (Chiara Amitrano), E.V., G.C., M.P., C.A. (Cecilia Arrichiello), G.A., P.M., C.A. (Carmen Arena) and V.D.M.; Supervision, C.A. (Carmen Arena) and V.D.M.; Project administration, V.D.M.; Funding acquisition, V.D.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work benefited from support from the project In situ REsource Bio-Utilization for life Support system (ReBUS), unique project code (CUP) F74I16000000005 financed by the Italian Space Agency.
Data Availability Statement
Data are available upon reasonable request to the corresponding author.
Acknowledgments
We would like to acknowledge Cristina Illiano for her technical assistance in the laboratory. The work by S.D.F. was supported by the European Space Agency and the Italian Space Agency within the MELiSSA (Micro-Ecological Life Support System Alternative) PhD POMP program with the project ‘Radiation effect on plants’ at the University of Naples PhD Sustainable Agricultural and Forestry Systems and Food Security (XXXVII Cycle). We are also grateful to Stefania De Pascale for coordinating the Italian Space Agency ReBUS project and for being responsible for the University of Naples PhD projects within the MELiSSA PhD POMP program.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cucinotta, F.A. Space Radiation Risks for Astronauts on Multiple International Space Station Missions. PLoS ONE 2014, 9, e96099. [Google Scholar] [CrossRef] [PubMed]
- Kononikhin, A.S.; Starodubtseva, N.L.; Pastushkova, L.K.; Kashirina, D.N.; Fedorchenko, K.Y.; Brhozovsky, A.G.; Popov, I.A.; Larina, I.M.; Nikolaev, E.N. Spaceflight Induced Changes in the Human Proteome. Expert Rev. Proteom. 2017, 14, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Hellweg, C.E.; Baumstark-Khan, C. Getting Ready for the Manned Mission to Mars: The Astronauts’ Risk from Space Radiation. Naturwissenschaften 2007, 94, 517–526. [Google Scholar] [CrossRef]
- Smith, T.A.; Kirkpatrick, D.R.; Smith, S.; Smith, T.K.; Pearson, T.; Kailasam, A.; Herrmann, K.Z.; Schubert, J.; Agrawal, D.K. Radioprotective Agents to Prevent Cellular Damage Due to Ionizing Radiation. J. Transl. Med. 2017, 15, 232. [Google Scholar] [CrossRef] [PubMed]
- Mukwevho, E.; Ferreira, Z.; Ayeleso, A. Potential Role of Sulfur-Containing Antioxidant Systems in Highly Oxidative Environments. Molecules 2014, 19, 19376–19389. [Google Scholar] [CrossRef]
- Chaloulakou, S.; Poulia, K.A.; Karayiannis, D. Physiological Alterations in Relation to Space Flight: The Role of Nutrition. Nutrients 2022, 14, 4896. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Goyal, N.; Pramanik, J.; Prajapati, B.; Patel, J. Nutritional and Alternative Approaches to Treatment in Space. In Handbook of Space Pharmaceuticals; Springer International Publishing: Cham, Switzerland, 2022; pp. 935–953. [Google Scholar]
- Bychkov, A.; Reshetnikova, P.; Bychkova, E.; Podgorbunskikh, E.; Koptev, V. The Current State and Future Trends of Space Nutrition from a Perspective of Astronauts’ Physiology. Int. J. Gastron. Food Sci. 2021, 24, 100324. [Google Scholar] [CrossRef]
- Carter, K.; Campbell, J.; Shoemaker, P.; Dichiara, E.; Patel, N.; Caruso, J. Dietary Needs, Approaches and Recommendations to Meet the Demands of Future Manned Space Flights. Recent Prog. Nutr. 2021, 2, 1. [Google Scholar] [CrossRef]
- Mishra, G.P.; Priti; Dikshit, H.K.; Aski, M.; Sangwan, S.; Stobdan, T.; Singh, A.; Kumar, R.R.; Praveen, S. Microgreens: A Novel Food for Nutritional Security. In Conceptualizing Plant-Based Nutrition; Springer Nature: Singapore, 2022; pp. 123–156. [Google Scholar]
- Kyriacou, M.C.; de Pascale, S.; Kyratzis, A.; Rouphael, Y. Microgreens as a Component of Space Life Support Systems: A Cornucopia of Functional Food. Front. Plant Sci. 2017, 8, 1587. [Google Scholar] [CrossRef]
- Teng, Z.; Luo, Y.; Pearlstein, D.J.; Wheeler, R.M.; Johnson, C.M.; Wang, Q.; Fonseca, J. Microgreens for Home, Commercial, and Space Farming: A Comprehensive Update of the Most Recent Developments. Annu. Rev. Food Sci. Technol. 2023, 14, 539–562. [Google Scholar] [CrossRef]
- Teng, J.; Liao, P.; Wang, M. The Role of Emerging Micro-Scale Vegetables in Human Diet and Health Benefits—An Updated Review Based on Microgreens. Food Funct. 2021, 12, 1914–1932. [Google Scholar] [CrossRef] [PubMed]
- Kyriacou, M.C.; Rouphael, Y.; di Gioia, F.; Kyratzis, A.; Serio, F.; Renna, M.; de Pascale, S.; Santamaria, P. Micro-Scale Vegetable Production and the Rise of Microgreens. Trends Food Sci. Technol. 2016, 57, 103–115. [Google Scholar] [CrossRef]
- Jahangir, M.; Kim, H.K.; Choi, Y.H.; Verpoorte, R. Health-Affecting Compounds in Brassicaceae. Compr. Rev. Food Sci. Food Saf. 2009, 8, 31–43. [Google Scholar] [CrossRef]
- Ramirez, D.; Abellán-Victorio, A.; Beretta, V.; Camargo, A.; Moreno, D.A. Functional Ingredients From Brassicaceae Species: Overview and Perspectives. Int. J. Mol. Sci. 2020, 21, 1998. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Shi, J.; Wan, J.; Pham, Q.; Zhang, Z.; Sun, J.; Yu, L.; Luo, Y.; Wang, T.T.Y.; Chen, P. Profiling of Polyphenols and Glucosinolates in Kale and Broccoli Microgreens Grown under Chamber and Windowsill Conditions by Ultrahigh-Performance Liquid Chromatography High-Resolution Mass Spectrometry. ACS Food Sci. Technol. 2022, 2, 101–113. [Google Scholar] [CrossRef]
- Marchioni, I.; Martinelli, M.; Ascrizzi, R.; Gabbrielli, C.; Flamini, G.; Pistelli, L.; Pistelli, L. Small Functional Foods: Comparative Phytochemical and Nutritional Analyses of Five Microgreens of the Brassicaceae Family. Foods 2021, 10, 427. [Google Scholar] [CrossRef]
- Abellán, Á.; Domínguez-Perles, R.; García-Viguera, C.; Moreno, D.A. Evidence on the Bioaccessibility of Glucosinolates and Breakdown Products of Cruciferous Sprouts by Simulated In Vitro Gastrointestinal Digestion. Int. J. Mol. Sci. 2021, 22, 11046. [Google Scholar] [CrossRef] [PubMed]
- Miękus, N.; Marszałek, K.; Podlacha, M.; Iqbal, A.; Puchalski, C.; Świergiel, A.H. Health Benefits of Plant-Derived Sulfur Compounds, Glucosinolates, and Organosulfur Compounds. Molecules 2020, 25, 3804. [Google Scholar] [CrossRef]
- Elena Cartea, M.; Cámara-Martos, F.; Obregón, S.; Rubén Badenes-Pérez, F.; de Haro, A. Advances in Breeding in Vegetable Brassica rapa Crops. In Brassica Breeding and Biotechnology; IntechOpen: London, UK, 2021. [Google Scholar]
- Cartea, M.E.; Velasco, P. Glucosinolates in Brassica Foods: Bioavailability in Food and Significance for Human Health. Phytochem. Rev. 2008, 7, 213–229. [Google Scholar] [CrossRef]
- Traka, M.; Mithen, R. Glucosinolates, Isothiocyanates and Human Health. Phytochem. Rev. 2009, 8, 269–282. [Google Scholar] [CrossRef]
- Fimognari, C.; Turrini, E.; Ferruzzi, L.; Lenzi, M.; Hrelia, P. Natural Isothiocyanates: Genotoxic Potential versus Chemoprevention. Mutat. Res. Rev. Mutat. Res. 2012, 750, 107–131. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; El-Nakhel, C.; Pannico, A.; Graziani, G.; Soteriou, G.A.; Giordano, M.; Zarrelli, A.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Genotype-Specific Modulatory Effects of Select Spectral Bandwidths on the Nutritive and Phytochemical Composition of Microgreens. Front. Plant. Sci. 2019, 10, 1501. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; El-Nakhel, C.; Pannico, A.; Graziani, G.; Soteriou, G.A.; Giordano, M.; Palladino, M.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Phenolic Constitution, Phytochemical and Macronutrient Content in Three Species of Microgreens as Modulated by Natural Fiber and Synthetic Substrates. Antioxidants 2020, 9, 252. [Google Scholar] [CrossRef]
- di Stasio, E.; Rouphael, Y.; Colla, G.; Raimondi, G.; Giordano, M.; Pannico, A.; El-Nakhel, C.; De Pascale, S. The Influence of Ecklonia Maxima Seaweed Extract on Growth, Photosynthetic Activity and Mineral Composition of Brassica rapa L. Subsp. Sylvestris under Nutrient Stress Conditions. Eur. J. Hortic. Sci. 2018, 82, 286–293. [Google Scholar] [CrossRef]
- Cazalis, R. Plants under the Moonlight: The Biology and Installation of Industrial Plants for Lunar Settlements; Springer International Publishing: Cham, Switzerland, 2021; pp. 75–96. [Google Scholar]
- Prasad, B.; Richter, P.; Vadakedath, N.; Haag, F.W.M.; Strauch, S.M.; Mancinelli, R.; Schwarzwälder, A.; Etcheparre, E.; Gaume, N.; Lebert, M. How the Space Environment Influences Organisms: An Astrobiological Perspective and Review. Int. J. Astrobiol. 2021, 20, 159–177. [Google Scholar] [CrossRef]
- De Pascale, S.; Arena, C.; Aronne, G.; De Micco, V.; Pannico, A.; Paradiso, R.; Rouphael, Y. Biology and Crop Production in Space Environments: Challenges and Opportunities. Life Sci. Space Res. 2021, 29, 30–37. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Hossain, M.A.; da Silva, J.A.T.; Fujita, M. Plant Response and Tolerance to Abiotic Oxidative Stress: Antioxidant Defense Is a Key Factor. In Crop Stress and Its Management: Perspectives and Strategies; Springer: Dordrecht, The Netherlands, 2012; pp. 261–315. [Google Scholar]
- Gudkov, S.V.; Grinberg, M.A.; Sukhov, V.; Vodeneev, V. Effect of Ionizing Radiation on Physiological and Molecular Processes in Plants. J. Environ. Radioact. 2019, 202, 8–24. [Google Scholar] [CrossRef]
- Le Caër, S. Water Radiolysis: Influence of Oxide Surfaces on H2 Production under Ionizing Radiation. Water 2011, 3, 235–253. [Google Scholar] [CrossRef]
- Caplin, N.; Willey, N. Ionizing Radiation, Higher Plants, and Radioprotection: From Acute High Doses to Chronic Low Doses. Front. Plant. Sci. 2018, 9, 847. [Google Scholar] [CrossRef]
- Arena, C.; de Micco, V.; Aronne, G.; Pugliese, M.; Virzo De Santo, A.; de Maio, A. Response of Phaseolus vulgaris L. Plants to Low-Let Ionizing Radiation: Growth and Oxidative Stress. Acta Astronaut. 2013, 91, 107–114. [Google Scholar] [CrossRef]
- De Micco, V.; Arena, C.; Pignalosa, D.; Durante, M. Effects of Sparsely and Densely Ionizing Radiation on Plants. Radiat. Environ. Biophys. 2011, 50, 1–19. [Google Scholar] [CrossRef]
- Arena, C.; de Micco, V.; Macaeva, E.; Quintens, R. Space Radiation Effects on Plant and Mammalian Cells. Acta Astronaut. 2014, 104, 419–431. [Google Scholar] [CrossRef]
- Mousseau, T.A.; Møller, A.P. Plants in the Light of Ionizing Radiation: What Have We Learned From Chernobyl, Fukushima, and Other “Hot” Places? Front. Plant Sci. 2020, 11, 552. [Google Scholar] [CrossRef]
- Rezk, A.A.; Al-Khayri, J.M.; Al-Bahrany, A.M.; El-Beltagi, H.S.; Mohamed, H.I. X-ray Irradiation Changes Germination and Biochemical Analysis of Two Genotypes of Okra (Hibiscus esculentus L.). J. Radiat. Res. Appl. Sci. 2019, 12, 393–402. [Google Scholar] [CrossRef]
- Arena, C.; de Micco, V.; de Maio, A. Growth Alteration and Leaf Biochemical Responses in Phaseolus Vulgaris Exposed to Different Doses of Ionising Radiation. Plant Biol. 2014, 16, 194–202. [Google Scholar] [CrossRef]
- Albarzinji, I.M.; Anwar, A.M.; Karim, H.H.; Ahmed, M.O. Photosynthetic Pigments and Stomata Characteristics of Cowpea (Vigna Sinensis Savi) under the Effect of X-ray Radiation. UHD J. Sci. Technol. 2022, 6, 58–64. [Google Scholar] [CrossRef]
- De Micco, V.; De Francesco, S.; Amitrano, C.; Arena, C. Comparative Analysis of the Effect of Carbon- and Titanium-Ions Irradiation on Morpho-Anatomical and Biochemical Traits of Dolichos Melanophthalmus DC. Seedl. Aimed Space Explor. Plants 2021, 10, 2272. [Google Scholar] [CrossRef]
- Jan, S.; Parween, T.; Siddiqi, T.O. Mahmooduzzafar Effect of Gamma Radiation on Morphological, Biochemical, and Physiological Aspects of Plants and Plant Products. Environ. Rev. 2012, 20, 17–39. [Google Scholar] [CrossRef]
- De Micco, V.; Arena, C.; Di Fino, L.; Narici, L. Radiation Environment in Exploration-Class Space Missions and Plants’ Responses Relevant for Cultivation in Bioregenerative Life Support Systems. Front. Plant Sci. 2022, 13, 1001158. [Google Scholar] [CrossRef]
- Nisa, M.-U.; Huang, Y.; Benhamed, M.; Raynaud, C. The Plant DNA Damage Response: Signaling Pathways Leading to Growth Inhibition and Putative Role in Response to Stress Conditions. Front. Plant Sci. 2019, 10, 653. [Google Scholar] [CrossRef]
- Kathpalia, R.; Bhatla, S.C. Seed Dormancy and Germination. In Plant Physiology, Development and Metabolism; Springer: Singapore, 2018; pp. 885–906. [Google Scholar]
- Bewley, J.D.; Bradford, K.J.; Hilhorst, H.W.M.; Nonogaki, H. Seeds; Springer: New York, NY, USA, 2013; ISBN 978-1-4614-4692-7. [Google Scholar]
- Wamelink, G.W.W.; Frissel, J.Y.; Krijnen, W.H.J.; Verwoert, M.R. Crop Growth and Viability of Seeds on Mars and Moon Soil Simulants. In Terraforming Mars; Wiley: New York, NY, USA, 2021; pp. 313–329. [Google Scholar]
- Durante, M.; Cucinotta, F.A. Heavy Ion Carcinogenesis and Human Space Exploration. Nat. Rev. Cancer 2008, 8, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Hase, Y.; Shimono, K.; Inoue, M.; Tanaka, A.; Watanabe, H. Biological Effects of Ion Beams in Nicotiana tabacum L. Radiat. Environ. Biophys. 1999, 38, 111–115. [Google Scholar] [CrossRef]
- Kumagai, J.; Katoh, H.; Kumada, T.; Tanaka, A.; Tano, S.; Miyazaki, T. Strong Resistance of Arabidopsis Thaliana and Raphanus Sativus Seeds for Ionizing Radiation as Studied by ESR, ENDOR, ESE Spectroscopy and Germination Measurement: Effect of Long-Lived and Super-Long-Lived Radicals. Radiat. Phys. Chem. 2000, 57, 75–83. [Google Scholar] [CrossRef]
- Wu, L.; Yu, Z. Radiobiological Effects of a Low-Energy Ion Beam on Wheat. Radiat. Environ. Biophys. 2001, 40, 53–57. [Google Scholar] [CrossRef] [PubMed]
- George, B.; Kaur, C.; Khurdiya, D.S.; Kapoor, H.C. Antioxidants in Tomato (Lycopersium Esculentum) as a Function of Genotype. Food Chem. 2004, 84, 45–51. [Google Scholar] [CrossRef]
- Costanzo, G.; Iesce, M.R.; Naviglio, D.; Ciaravolo, M.; Vitale, E.; Arena, C. Comparative Studies on Different Citrus Cultivars: A Revaluation of Waste Mandarin Components. Antioxidants 2020, 9, 517. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. [34] Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. In Methods in Enzymology; Academic Press: Orlando, FL, USA, 1987; pp. 350–382. [Google Scholar]
- Vitale, E.; Velikova, V.; Tsonev, T.; Ferrandino, I.; Capriello, T.; Arena, C. The Interplay between Light Quality and Biostimulant Application Affects the Antioxidant Capacity and Photosynthetic Traits of Soybean (Glycine max L. Merrill). Plants 2021, 10, 861. [Google Scholar] [CrossRef]
- Wang, W.; Vignani, R.; Scali, M.; Cresti, M. A Universal and Rapid Protocol for Protein Extraction from Recalcitrant Plant Tissues for Proteomic Analysis. Electrophoresis 2006, 27, 2782–2786. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Fenner, M.; Thompson, K. The Ecology of Seeds; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Sax, K. The Stimulation of Plant Growth by Ionizing Radiation. Radiat. Bot. 1963, 3, 179–186. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Baldwin, L.A. Radiation Hormesis: Its Historical Foundations as a Biological Hypothesis. Hum. Exp. Toxicol. 2000, 19, 41–75. [Google Scholar] [CrossRef] [PubMed]
- De Micco, V.; Amitrano, C.; Vitaglione, P.; Ferracane, R.; Pugliese, M.; Arena, C. Effect of Light Quality and Ionising Radiation on Morphological and Nutraceutical Traits of Sprouts for Astronauts’ Diet. Acta Astronaut. 2021, 185, 188–197. [Google Scholar] [CrossRef]
- de Micco, V.; Arena, C.; Aronne, G. Anatomical Alterations of P Haseolus vulgaris L. Matur. Leaves Irradiat. X-rays. Plant. Biol. 2014, 16, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Arena, C.; Turano, M.; Mele, B.; Cataletto, P.R.; Furia, M.; Pugliese, M.; Micco, V. Anatomy, Photochemical Activity, and DNA Polymorphism in Leaves of Dwarf Tomato Irradiated with X-rays. Biol. Plant. 2017, 61, 305–314. [Google Scholar] [CrossRef]
- Amitrano, C.; Arena, C.; Rouphael, Y.; De Pascale, S.; De Micco, V. Vapour Pressure Deficit: The Hidden Driver behind Plant Morphofunctional Traits in Controlled Environments. Ann. Appl. Biol. 2019, 175, 313–325. [Google Scholar] [CrossRef]
- Amitrano, C.; Junker, A.; D’Agostino, N.; de Pascale, S.; de Micco, V. Integration of High-Throughput Phenotyping with Anatomical Traits of Leaves to Help Understanding Lettuce Acclimation to a Changing Environment. Planta 2022, 256, 68. [Google Scholar] [CrossRef]
- Arena, C.; Vitale, E.; Hay Mele, B.; Cataletto, P.R.; Turano, M.; Simoniello, P.; De Micco, V. Suitability of Solanum lycopersicum L. ‘Microtom’ for Growth in Bioregenerative Life Support Systems: Exploring the Effect of High-LET Ionising Radiation on Photosynthesis, Leaf Structure and Fruit Traits. Plant Biol. 2019, 21, 615–626. [Google Scholar] [CrossRef]
- Vitale, E.; Vitale, L.; Costanzo, G.; Velikova, V.; Tsonev, T.; Simoniello, P.; De Micco, V.; Arena, C. Light Spectral Composition Influences Structural and Eco-Physiological Traits of Solanum lycopersicum L. Cv. ‘Microtom’ Response High-LET Ioniz. Radiation. Plants 2021, 10, 1752. [Google Scholar] [CrossRef]
- Aly, A.; Eliwa, N.; AbdEl-Megid, M. Stimulating Effect of Gamma Radiation on Some Active Compounds in Eggplant Fruits. Egypt. J. Radiat. Sci. Appl. 2019, 32, 61–73. [Google Scholar] [CrossRef]
- ben Salem, I.; Fekih, S.; Sghaier, H.; Bousselmi, M.; Saidi, M.; Landoulsi, A.; Fattouch, S. Effect of Ionising Radiation on Polyphenolic Content and Antioxidant Potential of Parathion-Treated Sage (Salvia Officinalis) Leaves. Food Chem. 2013, 141, 1398–1405. [Google Scholar] [CrossRef]
- Akram, N.A.; Shafiq, F.; Ashraf, M. Ascorbic Acid-A Potential Oxidant Scavenger and Its Role in Plant Development and Abiotic Stress Tolerance. Front. Plant. Sci. 2017, 8, 613–630. [Google Scholar] [CrossRef] [PubMed]
- Bendich, A.; Olson, J.A. Biological Actions of Carotenoids 1. FASEB J. 1989, 3, 1927–1932. [Google Scholar] [CrossRef] [PubMed]
- Britton, G. Structure and Properties of Carotenoids in Relation to Function. FASEB J. 1995, 9, 1551–1558. [Google Scholar] [CrossRef] [PubMed]
- Oshima, S.; Ojima, F.; Sakamoto, H.; Ishiguro, Y.; Terao, J. Supplementation with Carotenoids Inhibits Singlet Oxygen-Mediated Oxidation of Human Plasma Low-Density Lipoprotein. J. Agric. Food Chem. 1996, 44, 2306–2309. [Google Scholar] [CrossRef]
- Kiong, A.L.P.; Lai, A.G.; Hussein, S.; Harun, A.R. Physiological Responses of Orthosiphon Stamineus Plantles to Gamma Irradiation. Am. Eurasian J. Sustain. Agric. 2008, 2, 135–149. [Google Scholar]
- Kim, J.-H.; Lee, M.H.; Moon, Y.R.; Kim, J.-S.; Wi, S.G.; Kim, T.H.; Chung, B.Y. Characterization of Metabolic Disturbances Closely Linked to the Delayed Senescence of Arabidopsis Leaves after γ Irradiation. Environ. Exp. Bot. 2009, 67, 363–371. [Google Scholar] [CrossRef]
- Alikamanoglu, S.; Yaycili, O.; Sen, A. Effect of Gamma Radiation on Growth Factors, Biochemical Parameters, and Accumulation of Trace Elements in Soybean Plants (Glycine max L. Merrill). Biol. Trace Elem. Res. 2011, 141, 283–293. [Google Scholar] [CrossRef]
- Mohammed, A.H.; Mohamed, H.I.; Zaki, L.M.; Mogazy, A.M. Pre-Exposure to Gamma Rays Alleviates the Harmful Effect of Salinity on Cowpea Plants. J. Stress Physiol. Biochem. 2012, 8, 199–217. [Google Scholar]
- Desai Aruna, S. Rao Srinath Effect of Gamma Radiation on Germination and Physiological Aspects of Pigeon Pea (Cajanus cajan (L.) Millsp) Seedlings. IMPACT Int. J. Res. Appl. Nat. Social Sci. (IMPACT IJRANSS) 2014, 2, 47–52. [Google Scholar]
- Kim, J.-H.; Baek, M.-H.; Chung, B.Y.; Wi, S.G.; Kim, J.-S. Alterations in the Photosynthetic Pigments and Antioxidant Machineries of Red Pepper (Capsicum annuum L.) Seedlings from Gamma-Irradiated Seeds. J. Plant Biol. 2004, 47, 314–321. [Google Scholar] [CrossRef]
- Ling Anna Pick Kiong; Chia Jing Yi; Sobri Hussein; Abdul Rahim Harun Physiological Responses of Citrus Sinensis to Gamma Irradiation. World Appl. Sci. J. 2008, 5, 12–19.
- Kumar, P.; Sharma, V.; Atmaram, C.K.; Singh, B. Regulated Partitioning of Fixed Carbon (14C), Sodium (Na+), Potassium (K+) and Glycine Betaine Determined Salinity Stress Tolerance of Gamma Irradiated Pigeonpea [Cajanus cajan (L.) Millsp]. Environ. Sci. Pollut. Res. 2017, 24, 7285–7297. [Google Scholar] [CrossRef] [PubMed]
- Vardhan, P.V.; Shukla, L.I. Gamma Irradiation of Medicinally Important Plants and the Enhancement of Secondary Metabolite Production. Int. J. Radiat. Biol. 2017, 93, 967–979. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).