Biocomposite Coatings Delay Senescence in Stored Diospyros kaki Fruits by Regulating Antioxidant Defence Mechanism and Delaying Cell Wall Degradation
Abstract
1. Introduction
2. Materials and Methods
2.1. Fruit Material
2.2. Edible Coatings Prpeparation
2.3. Application of Edible Coatings and Storage
2.4. Physiological Weight Loss (PWL)
2.5. Decay Incidence
2.6. Respiration Rate and Ethylene Production
2.7. Fruit Peel Colour
2.8. Total Carotenoid Contents
2.9. Electrolyte Leakage
2.10. Malondialdehyde (MDA) Content
2.11. Hydrogen Peroxide (H2O2) Content
2.12. Superoxide Anion Content
2.13. Enzymatic Antioxidants
2.14. Non-Enzymatic Antioxidants
2.14.1. Estimation of Antioxidant Activity
2.14.2. Estimation of Total Phenolic Content (TPC) and Total Flavonoid Content (TFC)
2.14.3. Soluble Tannin
2.15. Cell Wall Components
2.16. Fruit Firmness and Activity of Cell Wall Degrading Enzymes
2.17. Biochemical Analysis
2.17.1. Total Soluble Solids (TSS)
2.17.2. Titratable Acidity (TA)
2.17.3. Ascorbic Acid
2.18. Sensory Evaluation
2.19. Statistical Analysis
3. Results and Discussion
3.1. Physiological Weight Loss (PWL)
3.2. Decay Incidence
3.3. Respiration Rate and Ethylene Production
3.4. Fruit Peel Colour (Hue Angle and Chroma)
3.5. Total Carotenoid Contents
3.6. Electrolyte Leakage
3.7. Malondialdehyde (MDA) Content
3.8. Hydrogen Peroxide (H2O2) Content
3.9. Superoxide Anion Content
3.10. Antioxidant Enzyme Activities
3.11. Non-Enzymatic Antioxidants
3.11.1. Antioxidant Activity
3.11.2. Total Phenolic Content (TPC) and Total Flavonoid Content (TFC)
3.11.3. Soluble Tannins
3.12. Cell Wall Components
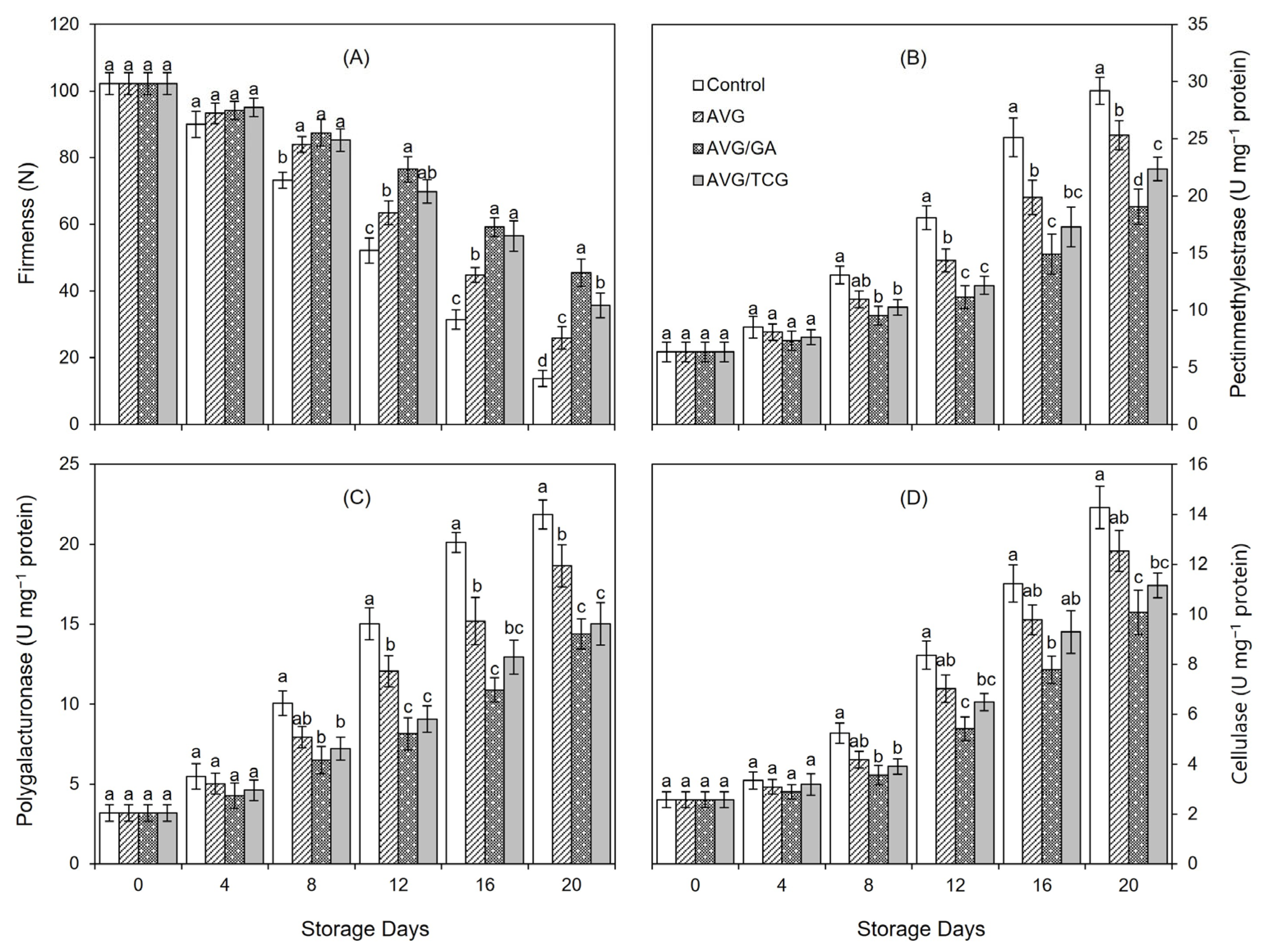
3.13. Fruit Firmness and Activity of Cell Wall Degrading Enzymes
3.14. Biochemical Analysis
3.14.1. Total Soluble Solids (TSS)
3.14.2. Titratable Acidity (TA)
3.14.3. Ascorbic Acid
3.15. Sensory Evaluation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maan, A.A.; Reiad Ahmed, Z.F.; Iqbal Khan, M.K.; Riaz, A.; Nazir, A. Aloe Vera Gel, an Excellent Base Material for Edible Films and Coatings. Trends Food Sci. Technol. 2021, 116, 329–341. [Google Scholar] [CrossRef]
- Sarker, A.; Grift, T.E. Bioactive Properties and Potential Applications of Aloe Vera Gel Edible Coating on Fresh and Minimally Processed Fruits and Vegetables: A Review. J. Food Meas. Charact. 2021, 15, 2119–2134. [Google Scholar] [CrossRef]
- Khaliq, G.; Ramzan, M.; Baloch, A.H. Effect of Aloe Vera Gel Coating Enriched with Fagonia Indica Plant Extract on Physicochemical and Antioxidant Activity of Sapodilla Fruit during Postharvest Storage. Food Chem. 2019, 286, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Mendy, T.K.; Misran, A.; Mahmud, T.M.M.; Ismail, S.I. Application of Aloe Vera Coating Delays Ripening and Extend the Shelf Life of Papaya Fruit. Sci. Hortic. 2019, 246, 769–776. [Google Scholar] [CrossRef]
- Hassanpour, H. Effect of Aloe Vera Gel Coating on Antioxidant Capacity, Antioxidant Enzyme Activities and Decay in Raspberry Fruit. LWT—Food Sci. Technol. 2015, 60, 495–501. [Google Scholar] [CrossRef]
- Rehman, M.A.; Asi, M.R.; Hameed, A.; Bourquin, L.D. Effect of Postharvest Application of Aloe Vera Gel on Shelf Life, Activities of Anti-Oxidative Enzymes, and Quality of “gola” Guava Fruit. Foods 2020, 9, 1361. [Google Scholar] [CrossRef]
- Saleem, M.S.; Ejaz, S.; Anjum, M.A.; Ali, S.; Hussain, S.; Nawaz, A.; Naz, S.; Maqbool, M.; Abbas, A.M. Aloe Vera Gel Coating Delays Softening and Maintains Quality of Stored Persimmon (Diospyros Kaki Thunb.) Fruits. J. Food Sci. Technol. 2022, 59, 3296–3306. [Google Scholar] [CrossRef]
- Saha, A.; Tyagi, S.; Gupta, R.K.; Tyagi, Y.K. Natural Gums of Plant Origin as Edible Coatings for Food Industry Applications. Crit. Rev. Biotechnol. 2017, 37, 959–973. [Google Scholar] [CrossRef]
- Zare, E.N.; Makvandi, P.; Tay, F.R. Recent Progress in the Industrial and Biomedical Applications of Tragacanth Gum: A Review. Carbohydr. Polym. 2019, 212, 450–467. [Google Scholar] [CrossRef]
- Saleem, M.S.; Ejaz, S.; Anjum, M.A.; Nawaz, A.; Naz, S.; Hussain, S.; Ali, S.; Canan, İ. Postharvest Application of Gum Arabic Edible Coating Delays Ripening and Maintains Quality of Persimmon Fruits during Storage. J. Food Process. Preserv. 2020, 44, 14583. [Google Scholar] [CrossRef]
- Ali, S.; Akbar Anjum, M.; Nawaz, A.; Naz, S.; Ejaz, S.; Shahzad Saleem, M.; Tul-Ain Haider, S.; Ul Hasan, M. Effect of Gum Arabic Coating on Antioxidative Enzyme Activities and Quality of Apricot (Prunus armeniaca L.) Fruit during Ambient Storage. J. Food Biochem. 2021, 45, 13656. [Google Scholar] [CrossRef]
- Nasiri, M.; Barzegar, M.; Sahari, M.A.; Niakousari, M. Tragacanth Gum Containing Zataria Multiflora Boiss. Essential Oil as a Natural Preservative for Storage of Button Mushrooms (Agaricus bisporus). Food Hydrocoll. 2017, 72, 202–209. [Google Scholar] [CrossRef]
- Ali, S.; Anjum, M.A.; Nawaz, A.; Naz, S.; Ejaz, S.; Sardar, H.; Saddiq, B. Tragacanth Gum Coating Modulates Oxidative Stress and Maintains Quality of Harvested Apricot Fruits. Int. J. Biol. Macromol. 2020, 163, 2439–2447. [Google Scholar] [CrossRef]
- Arnon, H.; Granit, R.; Porat, R.; Poverenov, E. Development of Polysaccharides-Based Edible Coatings for Citrus Fruits: A Layer-by-Layer Approach. Food Chem. 2015, 166, 465–472. [Google Scholar] [CrossRef]
- Souza, M.P.; Vaz, A.F.M.; Cerqueira, M.A.; Texeira, J.A.; Vicente, A.A.; Carneiro-da-Cunha, M.G. Effect of an Edible Nanomultilayer Coating by Electrostatic Self-Assembly on the Shelf Life of Fresh-Cut Mangoes. Food Bioprocess Technol. 2015, 8, 647–654. [Google Scholar] [CrossRef]
- Anjum, M.A.; Akram, H.; Zaidi, M.; Ali, S. Effect of Gum Arabic and Aloe Vera Gel Based Edible Coatings in Combination with Plant Extracts on Postharvest Quality and Storability of ‘Gola’ Guava Fruits. Sci. Hortic. 2020, 271, 109506. [Google Scholar] [CrossRef]
- Ebrahimi, F.; Rastegar, S. Preservation of Mango Fruit with Guar-Based Edible Coatings Enriched with Spirulina Platensis and Aloe Vera Extract during Storage at Ambient Temperature. Sci. Hortic. 2020, 265, 109258. [Google Scholar] [CrossRef]
- Shakir, M.S.; Ejaz, S.; Hussain, S.; Ali, S.; Sardar, H.; Azam, M.; Ullah, S.; Khaliq, G.; Saleem, M.S.; Nawaz, A.; et al. Synergistic Effect of Gum Arabic and Carboxymethyl Cellulose as Biocomposite Coating Delays Senescence in Stored Tomatoes by Regulating Antioxidants and Cell Wall Degradation. Int. J. Biol. Macromol. 2022, 201, 641–652. [Google Scholar] [CrossRef]
- Alvarado-González, J.S.; Chanona-Pérez, J.J.; Welti-Chanes, J.S.; Calderón-Domínguez, G.; Arzate-Vázquez, I.; Pacheco-Alcalá, S.U.; Garibay-Febles, V.; Gutiérrez-López, G.F. Optical, Microstructural, Functional and Nanomechanical Properties of Aloe Vera Gel/Gellan Gum Edible Films. Rev. Mex. Ing. Quim. 2012, 11, 193–202. [Google Scholar]
- Ortega-Toro, R.; Collazo-Bigliardi, S.; Roselló, J.; Santamarina, P.; Chiralt, A. Antifungal Starch-Based Edible Films Containing Aloe Vera. Food Hydrocoll. 2017, 72, 1–10. [Google Scholar] [CrossRef]
- Nourozi, F.; Sayyari, M. Enrichment of Aloe Vera Gel with Basil Seed Mucilage Preserve Bioactive Compounds and Postharvest Quality of Apricot Fruits. Sci. Hortic. 2020, 262, 109041. [Google Scholar] [CrossRef]
- Mohebbi, M.; Ansarifar, E.; Hasanpour, N.; Amiryousefi, M.R. Suitability of Aloe Vera and Gum Tragacanth as Edible Coatings for Extending the Shelf Life of Button Mushroom. Food Bioprocess Technol. 2012, 5, 3193–3202. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, X.; Luo, Z.; Sun, J.; Li, L.; Yin, X.; Li, J.; Xu, Y. Effects of Inside-out Heat-Shock via Microwave on the Fruit Softening and Quality of Persimmon during Postharvest Storage. Food Chem. 2021, 349, 129161. [Google Scholar] [CrossRef] [PubMed]
- Denoya, G.I.; Pataro, G.; Ferrari, G. Effects of Postharvest Pulsed Light Treatments on the Quality and Antioxidant Properties of Persimmons during Storage. Postharvest Biol. Technol. 2020, 160, 111055. [Google Scholar] [CrossRef]
- Naser, F.; Rabiei, V.; Razavi, F.; Khademi, O. Effect of Calcium Lactate in Combination with Hot Water Treatment on the Nutritional Quality of Persimmon Fruit during Cold Storage. Sci. Hortic. 2018, 233, 114–123. [Google Scholar] [CrossRef]
- Sanchís, E.; González, S.; Ghidelli, C.; Sheth, C.C.; Mateos, M.; Palou, L.; Pérez-Gago, M.B. Browning Inhibition and Microbial Control in Fresh-Cut Persimmon (Diospyros Kaki Thunb. Cv. Rojo Brillante) by Apple Pectin-Based Edible Coatings. Postharvest Biol. Technol. 2016, 112, 186–193. [Google Scholar] [CrossRef]
- Xue, J.; Huang, L.; Zhang, S.; Sun, H.; Gao, T. Study on the Evaluation of Carboxymethyl-Chitosan Concentration and Temperature Treatment on the Quality of “Niuxin” Persimmon during Cold Storage. J. Food Process. Preserv. 2020, 44, e14560. [Google Scholar] [CrossRef]
- Saleem, M.; Ejaz, S.; Anjum, M.; Hussain, S.; Ercisli, S.; Ilhan, G.; Marc, R.; Skrovankova, S.; Mlcek, J. Improvement of Postharvest Quality and Bioactive Compounds Content of Persimmon Fruits after Hydrocolloid-Based Edible Coating Application. Horticulturae 2022, 8, 1045. [Google Scholar] [CrossRef]
- Ejaz, S.; Jezik, K.M.; Stumpf, W.; Gosch, C.; Halbwirth, H.; Stich, K. Amelioration of an Open Soilless Cultivation System for Microgardening Spinach (Spinacia Oleracea L.). Zemdirb.-Agric. 2015, 102, 201–208. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Yang, H.; Wu, F.; Cheng, J. Reduced Chilling Injury in Cucumber by Nitric Oxide and the Antioxidant Response. Food Chem. 2011, 127, 1237–1242. [Google Scholar] [CrossRef]
- Sun, J.; You, X.; Li, L.; Peng, H.; Su, W.; Li, C.; He, Q.; Liao, F. Effects of a Phospholipase D Inhibitor on Postharvest Enzymatic Browning and Oxidative Stress of Litchi Fruit. Postharvest Biol. Technol. 2011, 62, 288–294. [Google Scholar] [CrossRef]
- Velikova, V.; Loreto, F. On the Relationship between Isoprene Emission and Thermotolerance in Phragmites Australis Leaves Exposed to High Temperatures and during the Recovery from a Heat Stress. Plant Cell Environ. 2005, 28, 318–327. [Google Scholar] [CrossRef]
- Ali, S.; Khan, A.S.; Malik, A.U.; Shahid, M. Effect of Controlled Atmosphere Storage on Pericarp Browning, Bioactive Compounds and Antioxidant Enzymes of Litchi Fruits. Food Chem. 2016, 206, 18–29. [Google Scholar] [CrossRef]
- Bradford, M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Krings, U.; Berger, R.G. Antioxidant Activity of Some Roasted Foods. Food Chem. 2001, 72, 223–229. [Google Scholar] [CrossRef]
- Taira, S. Astringency in Persimmon. In Modern Method of Plant Analysis, Fruit Analysis; Linskens, H.F., Jackson, J.F., Eds.; Springer: Berlin/Heidelberg, Germany, 1996; pp. 97–110. [Google Scholar]
- Wang, H.; Chen, Y.; Lin, H.; Lin, M.; Chen, Y.; Lin, Y. 1-Methylcyclopropene Containing-Papers Suppress the Disassembly of Cell Wall Polysaccharides in Anxi Persimmon Fruit during Storage. Int. J. Biol. Macromol. 2020, 151, 723–729. [Google Scholar] [CrossRef]
- Yoshida, O.; Nakagawa, H.; Ogura, N.; Sato, T. Effect of Heat Treatment on the Development of Polygalacturonase Activity in Tomato Fruit during Ripening. Plant Cell Physiol. 1984, 25, 505–509. [Google Scholar] [CrossRef]
- Deng, Y.; Wu, Y.; Li, Y. Changes in Firmness, Cell Wall Composition and Cell Wall Hydrolases of Grapes Stored in High Oxygen Atmospheres. Food Res. Int. 2005, 38, 769–776. [Google Scholar] [CrossRef]
- Nasiri, M.; Barzegar, M.; Sahari, M.A.; Niakousari, M. Application of Tragacanth Gum Impregnated with Satureja Khuzistanica Essential Oil as a Natural Coating for Enhancement of Postharvest Quality and Shelf Life of Button Mushroom (Agaricus Bisporus). Int. J. Biol. Macromol. 2018, 106, 218–226. [Google Scholar] [CrossRef]
- Dong, F.; Wang, X. Guar Gum and Ginseng Extract Coatings Maintain the Quality of Sweet Cherry. LWT—Food Sci. Technol. 2018, 89, 117–122. [Google Scholar] [CrossRef]
- Kou, J.; Wei, C.; Zhao, Z.; Guan, J.; Wang, W. Effects of Ethylene and 1-Methylcyclopropene Treatments on Physiological Changes and Ripening-Related Gene Expression of ‘Mopan’ Persimmon Fruit during Storage. Postharvest Biol. Technol. 2020, 166, 111185. [Google Scholar] [CrossRef]
- Kou, J.; Zhao, Z.; Wang, W.; Wei, C.; Guan, J.; Ference, C. Comparative Study of Ripening Related Gene Expression and Postharvest Physiological Changes between Astringent and Nonastringent Persimmon Cultivars. J. Am. Soc. Hortic. Sci. 2020, 145, 203–212. [Google Scholar] [CrossRef]
- Sousa, F.F.; Pinsetta Junior, J.S.; Oliveira, K.T.E.F.; Rodrigues, E.C.N.; Andrade, J.P.; Mattiuz, B.H. Conservation of ‘Palmer’ Mango with an Edible Coating of Hydroxypropyl Methylcellulose and Beeswax. Food Chem. 2021, 346, 128925. [Google Scholar] [CrossRef]
- Etemadipoor, R.; Mirzaalian Dastjerdi, A.; Ramezanian, A.; Ehteshami, S. Ameliorative Effect of Gum Arabic, Oleic Acid and/or Cinnamon Essential Oil on Chilling Injury and Quality Loss of Guava Fruit. Sci. Hortic. 2020, 266, 109255. [Google Scholar] [CrossRef]
- Imahori, Y.; Bai, J.; Baldwin, E. Antioxidative Responses of Ripe Tomato Fruit to Postharvest Chilling and Heating Treatments. Sci. Hortic. 2016, 198, 398–406. [Google Scholar] [CrossRef]
- Khaliq, G.; Muda Mohamed, M.T.; Ghazali, H.M.; Ding, P.; Ali, A. Influence of Gum Arabic Coating Enriched with Calcium Chloride on Physiological, Biochemical and Quality Responses of Mango (Mangifera indica L.) Fruit Stored under Low Temperature Stress. Postharvest Biol. Technol. 2016, 111, 362–369. [Google Scholar] [CrossRef]
- Ali, S.; Khan, A.S.; Nawaz, A.; Anjum, M.A.; Naz, S.; Ejaz, S.; Hussain, S. Aloe Vera Gel Coating Delays Postharvest Browning and Maintains Quality of Harvested Litchi Fruit. Postharvest Biol. Technol. 2019, 157, 110960. [Google Scholar] [CrossRef]
- Saleem, M.S.; Anjum, M.A.; Naz, S.; Ali, S.; Hussain, S.; Azam, M.; Sardar, H.; Khaliq, G.; Canan, İ.; Ejaz, S. Incorporation of Ascorbic Acid in Chitosan-Based Edible Coating Improves Postharvest Quality and Storability of Strawberry Fruits. Int. J. Biol. Macromol. 2021, 189, 160–169. [Google Scholar] [CrossRef]
- Hassan, B.; Chatha, S.A.S.; Hussain, A.I.; Zia, K.M.; Akhtar, N. Recent Advances on Polysaccharides, Lipids and Protein Based Edible Films and Coatings: A Review. Int. J. Biol. Macromol. 2018, 109, 1095–1107. [Google Scholar] [CrossRef]
- Nasr, F.; Pateiro, M.; Rabiei, V.; Razavi, F.; Formaneck, S.; Gohari, G.; Lorenzo, J.M. Chitosan-Phenylalanine Nanoparticles (Cs-Phe nps) Extend the Postharvest Life of Persimmon (Diospyros kaki) Fruits under Chilling Stress. Coatings 2021, 11, 819. [Google Scholar] [CrossRef]






| Storage Days | Treatments | TSS (%) | TA (%) | Ascorbic Acid (mg 100 g−1 FW) | Taste (Score) | Aroma (Score) | Overall Acceptability (Score) |
|---|---|---|---|---|---|---|---|
| D0 | 12.95 | 0.553 | 56.62 | 5.17 | 5.42 | 5.17 | |
| D4 | Control | 14.40 a | 0.516 a | 51.20 a | 5.58 a | 5.67 a | 5.58 a |
| AVG | 14.14 a | 0.520 a | 52.12 a | 5.25 a | 5.50 a | 5.42 a | |
| AVG/GA | 14.05 a | 0.529 a | 52.64 a | 5.17 a | 5.50 a | 5.33 a | |
| AVG/TCG | 14.15 a | 0.526 a | 51.50 a | 5.25 a | 5.50 a | 5.25 a | |
| D8 | Control | 16.86 a | 0.409 b | 43.17 b | 6.75 a | 6.50 a | 6.58 a |
| AVG | 16.03 ab | 0.492 a | 46.18 ab | 5.83 b | 5.58 b | 6.08 ab | |
| AVG/GA | 15.48 b | 0.498 a | 51.15 a | 5.33 b | 5.42 b | 5.42 b | |
| AVG/TCG | 15.71 b | 0.490 a | 49.59 a | 5.75 b | 5.50 b | 5.58 b | |
| D12 | Control | 18.83 a | 0.323 b | 36.95 b | 8.17 a | 8.42 a | 8.00 a |
| AVG | 17.85 ab | 0.396 ab | 40.95 ab | 7.17 ab | 7.33 b | 7.33 ab | |
| AVG/GA | 16.39 c | 0.454 a | 44.87 a | 6.00 b | 6.17 c | 6.25 c | |
| AVG/TCG | 17.10 bc | 0.437 a | 41.49 ab | 6.75 b | 6.60 bc | 6.67 bc | |
| D16 | Control | 20.65 a | 0.232 c | 29.58 b | 8.42 a | 8.17 a | 7.83 a |
| AVG | 19.72 ab | 0.310 b | 34.64 ab | 7.92 a | 7.83 ab | 7.92 a | |
| AVG/GA | 18.61 b | 0.375 a | 39.77 a | 6.67 b | 6.83 c | 7.33 a | |
| AVG/TCG | 18.78 b | 0.355 ab | 36.06 a | 7.58 ab | 7.25 bc | 7.42 a | |
| D20 | Control | 21.35 a | 0.180 b | 24.01 c | 6.75 b | 6.83 b | 6.83 b |
| AVG | 20.53 ab | 0.225 b | 28.88 bc | 7.58 ab | 7.67ab | 7.58 ab | |
| AVG/GA | 19.65 b | 0.298 a | 34.89 a | 8.33 a | 8.33 a | 8.17 a | |
| AVG/TCG | 20.23 ab | 0.243 ab | 31.94 ab | 7.83 a | 8.25 a | 7.83 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saleem, M.S.; Ejaz, S.; Mosa, W.F.A.; Ali, S.; Sardar, H.; Ali, M.M.; Ullah, S.; Ali, H.M.; Lisek, A.; Anjum, M.A. Biocomposite Coatings Delay Senescence in Stored Diospyros kaki Fruits by Regulating Antioxidant Defence Mechanism and Delaying Cell Wall Degradation. Horticulturae 2023, 9, 351. https://doi.org/10.3390/horticulturae9030351
Saleem MS, Ejaz S, Mosa WFA, Ali S, Sardar H, Ali MM, Ullah S, Ali HM, Lisek A, Anjum MA. Biocomposite Coatings Delay Senescence in Stored Diospyros kaki Fruits by Regulating Antioxidant Defence Mechanism and Delaying Cell Wall Degradation. Horticulturae. 2023; 9(3):351. https://doi.org/10.3390/horticulturae9030351
Chicago/Turabian StyleSaleem, Muhammad Shahzad, Shaghef Ejaz, Walid F. A. Mosa, Sajid Ali, Hasan Sardar, Muhammad Moaaz Ali, Sami Ullah, Hayssam M. Ali, Anna Lisek, and Muhammad Akbar Anjum. 2023. "Biocomposite Coatings Delay Senescence in Stored Diospyros kaki Fruits by Regulating Antioxidant Defence Mechanism and Delaying Cell Wall Degradation" Horticulturae 9, no. 3: 351. https://doi.org/10.3390/horticulturae9030351
APA StyleSaleem, M. S., Ejaz, S., Mosa, W. F. A., Ali, S., Sardar, H., Ali, M. M., Ullah, S., Ali, H. M., Lisek, A., & Anjum, M. A. (2023). Biocomposite Coatings Delay Senescence in Stored Diospyros kaki Fruits by Regulating Antioxidant Defence Mechanism and Delaying Cell Wall Degradation. Horticulturae, 9(3), 351. https://doi.org/10.3390/horticulturae9030351










