Abstract
Soilless crop production is spread worldwide. It is a cultivating technique that enhances yield quality and quantity, thus contributing to both food safety and food security. However, in closed-loop soilless crops, the risk of spreading soil-borne pathogens through the recycled nutrient solution makes the establishment of a disinfection strategy necessary. In the current study, sodium hypochlorite was applied to the recycled nutrient solution as a chemical disinfectant to assess its impact on plant growth, leaf gas exchange, fruit yield, tissue mineral composition, and possible accumulation of chlorate and perchlorate residues in tomato fruits. The application of 2.5, 5, and 7.5 mg L−1 of chlorine three times at fortnightly intervals during the cropping period had no impact on plant growth or gas exchange parameters. Furthermore, the application of 2.5 mg L−1 of chlorine led to a significant increase in the total production of marketable fruits (total fruit weight per plant). No consistent differences in nutrient concentrations were recorded between the treatments. Moreover, neither chlorate nor perchlorate residues were detected in tomato fruits, even though chlorate residues were present in the nutrient solution. Therefore, the obtained tomatoes were safe for consumption. Further research is needed to test the application of chlorine in combination with crop inoculation with pathogens to test the efficiency of chlorine as a disinfectant in soilless nutrient solutions.
1. Introduction
Independence from the soil as a means of rooting allows the optimization of both physical and chemical characteristics in the root environment, as well as a more effective control of the phytopathogenic microorganisms [1]. These characteristics result in higher crop yields with usually lower production costs, combined with reduced pesticide use and high product quality [2]. Given these shortcomings of soil-based production systems, crop production worldwide has shifted to soilless culture in greenhouses, and one of the main reasons for this development is the more efficient control of soil-borne pathogens [3]. Nevertheless, soilless cultivation provides a free start from pathogens but cannot exclude the incidence of a pathogen infection during the cropping period. Especially in closed soilless systems, there are reports on the spread of phytopathogens through the recycled nutrient solution (NS), leading to complete crop failure [4,5]. In closed-loop soilless cultivation systems, the spread of plant diseases associated with the recycling of the NS has been suggested to be among the most important problems for growers [6]. Hence, disinfection of the NS by applying chlorine is a common method of NS disinfection in closed-loop soilless crops. However, in the Mediterranean greenhouses, periodic application of chlorine as a NS disinfectant is a common practice also in open soilless crops, which constitute the standard type of soilless systems in the region. Nevertheless, the safety of chlorine application is still an open question, as there are limited data about the possible accumulation of chlorate/perchlorate residues in harvested vegetables originating from soilless cultivations treated with chlorine [7].
Soilless culture is a cultivation technique that can increase the quantity and quality of production while reducing water use [8], thus contributing to the requirements for food security and sustainability [9]. Moreover, many governmental and non-governmental organizations highlight its benefits for food security [10,11,12,13]. In fact, the imperative need to meet the increasing nutritional needs of the growing population has upgraded the importance of soilless cultivation as an efficient crop production system [14].
In addition, soilless cultivation is a controlled environment system of agricultural production that could enhance food safety in a crop, thanks to the lower need for application of agrochemicals against soil-borne plant diseases [1] and the much lower risk of contamination with heavy metals originating from polluted soils [13,15]. Nevertheless, it has been pointed out in the literature that there are safety risks also in soilless grown vegetables [16,17,18], although there are approaches towards resolving such issues [19]. Nonetheless, although soilless culture provides a free start from soil-borne pathogens [8], various soil pathogens have been detected in soilless crops, e.g., fungi, bacteria, viruses, and nematodes [4]. Hence, the need to install disinfection systems in soilless cultivation systems is imperative. The chemical methods of NS disinfection in a soilless culture include the use of chlorine (Cl), chlorine dioxide, bromine, ozone, hydrogen peroxide, etc. [4]. Regarding chlorine, it can be applied in liquid, solid, or gaseous form [20]. The most common method, especially in the Mediterranean basin [21], is the application of Cl in its liquid form as sodium hypochlorite, namely bleach [4,7]. In general, the recommended Cl concentration after the addition of sodium hypochlorite is between 2.5 and 5 mg L−1 for the treatment of various fungi and oomycetes, such as species of the genus Pythium sp. [22,23], Phytophthora sp. [24], Fusarium oxysporum f. sp. dianthii [25], bacteria such as Agrobacterium tumefaciens [26], CLSV (cucumber leaf spot virus) [27] and root-knot nematodes (Meloidogyne javanica) [28], so that no phytotoxicity is expected. Nevertheless, further research is necessary about the effect of bleach on cultivated plants when used as an NS disinfectant, as there are concerns for sodium (Na+) and Cl− accumulation [21] as well as the presence of residues and byproducts toxic to humans in the edible products [4].
Regarding disinfection with sodium hypochlorite, it has been reported that it is likely to lead to the formation of derivatives such as chloramines, organic halogens, and trihalomethanes (chloroform, dichlorobromomethane, bichromobromomethane, and bromoform) [4]. In addition, Bull et al. [29] highlighted that the use of the hypochlorite anion (ClO−) in the disinfection of NS may be associated with the production of chlorates that can cause acute toxicity in humans [29]. Nevertheless, this risk is associated with the dosage and the frequency of application, as well as the plant part consumed as an edible product.
Exposure of humans to chlorates (ClO3−) and perchlorates (ClO4−) is expected from residues occurring as by-products of the use of chlorinated disinfectants in food processing and water treatment [30]. From monitoring data collected between 2014 and 2018 [31], residues at quantitative levels were found in various commodities, leading to the establishment of maximum residue levels (MRLs) for chlorates, which were currently set to 0.05–0.7 mg kg−1 depending on the commodity [32]. These MRLs are tentative, and the discussion at EU level is still ongoing [33,34,35]. For tomatoes, the current MRL is set at 0.1 mg kg−1. In a previous investigation [7], the use of potassium hypochlorite (KClO) for disinfection of the NS supplied to a soilless tomato cultivation led to the accumulation of chlorate residues in fruits.
Globally, the tomato is one of the most important vegetables [36]. Additionally, it serves as an important model-organism in plant research [37]. In 2019, tomato was cultivated on 6,117,242.00 hectares with a production of 243,635,433.00 tons worldwide, and the countries with the highest production were China, India, and Turkey [38]. Within this context, the scope of the current work was to investigate the effect of sodium hypochlorite application in the NS supplied to an open soilless cultivation system of tomato on plant growth and total yield and detect possible safety risks for the consumer. Therefore, in addition to the agronomic parameters, residue analysis of chlorates and perchlorates in tomato fruits was performed.
2. Results and Discussion
2.1. Chloride Concentrations in the Drainage Solution
As shown in Table 1, the chloride concentration in the drainage solution was significantly higher in the treatment group of 7.5 mg L−1 compared to the control group and the 2.5 mg L−1 treatment group at 30 days after the first application (DAFA). This is due to the addition of sodium hypochlorite, which was not accompanied by a commensurate increase in its uptake by the plants after its application. This hypothesis is supported by measurements of the chloride concentration in leaf and root samples as well as fruit samples (results in Section 2.3), which were similar before and after sodium hypochlorite application to the NS.

Table 1.
Chloride concentration in the drainage solution samples collected at 30, 37, and 48 DAFA of sodium hypochlorite in a soilless cultivation of tomato. Sodium hypochlorite was applied at concentrations of 2.5, 5.0, and 7.5 mg L−1 of chlorine, while in the control treatment no sodium hypochlorite was added.
Although chlorination is a popular method of disinfecting NS in soilless cultures, it is not considered to be used to its full potential, mainly due to technical issues related to the monitoring of free available chlorine [39]. Most phytopathogens are controlled by chlorine concentrations of 1–3 mg L−1, while higher initial concentrations (5–10 mg L−1) are required as chlorine reacts with various other substances in NS [20,39]. Other important factors affecting the success of chlorination are pH, temperature, and organic matter content in the NS, as well as pathogen type and pathogen load [4].
As shown in Table 1, the highest chloride concentration in the drainage (9.05 mg L−1) was detected in the treatment with a 7.5 mg L−1 chlorine application one day after the third application of sodium hypochlorite (30 DAFA). However, the difference was significant only in comparison with the control treatment and the treatment with the addition of sodium hypochlorite at 2.5 mg L−1 of chlorine.
2.2. Growth of Plant and Gas Exchange
The disinfection of the NS by applying sodium hypochlorite did not significantly affect the growth of the plants, as indicated by the absence of any statistical differences in the leaf biomass and total leaf area (Table 2). Furthermore, no phytotoxicity symptoms were observed in any of the treatments.

Table 2.
Estimation of fresh weight (fw), dry weight (dw), dry matter content (DMC, %), specific leaf area (SLA) and leaf area (cm2) in the collected leaf samples.
Generally, many studies have reported on the effect of chlorine on various plant species by applying different forms of chlorine (gas, chlorine dioxide, etc.) through different disinfection protocols [6,25,26,40,41,42,43,44]. Therefore, a direct comparison of their results is not possible.
In general, chlorine at concentrations higher than 5 mg L−1 can cause phytotoxicity in many plants, while some plants are sensitive even at much lower concentrations (0.05 mg L−1) [43]. In this study, disinfection with sodium hypochlorite was selected because it is economically affordable for producers, effective against important plant pathogens, and very common in agricultural practice. Unlike other chemical methods of disinfection, sodium hypochlorite does not degrade quickly, thereby resulting in a longer disinfection capacity. The results of the present study concerning the disinfection effect on tomato growth coincide with the 5 mg L−1 rule [43]. Nevertheless, chlorine application following the current disinfection protocol with sodium hypochlorite did not affect the growth of tomato plants cv. ‘ELPIDA’ even at 7.5 mg L−1 chlorine.
Gas exchange was not affected by the application of chlorine up to a concentration of 7.5 mg L−1, as indicated by the absence of any significant differences in the net photosynthetic rates, transpiration rate, stomatal conductance, intercellular CO2, and water use efficiency (Table 3). Nevertheless, there are examples in which chlorine, applied in gaseous form, affected the photosynthesis of Pinus plants by reducing their photosynthetic capacity [40]. Chlorine, as an anion, can be beneficial to plants by substituting for nitrates in vacuoles and positively impacting photosynthesis [44]. Therefore, some researchers used chlorine to replace part of the nitrates in the NS in tomato [45] and tobacco [46] and found that the stomatal conductivity was affected. However, the concentrations of chlorine tested in this experiment were lower than those in the above-mentioned studies and did not result in any significant differences.

Table 3.
Net photosynthetic rate, stomatal conductance, intercellular CO2 concentration, transpiration rate and water use efficiency in leaves (3rd or 4th fully developed leaf from the top) of tomato plants at 0, 31, and 43 DAFA of sodium hypochlorite at three different concentrations of chlorine in the supplied nutrient solution.
2.3. Fruit Yield and Fruit Mineral Composition
Analysis of variance showed that the disinfection of NS using sodium hypochlorite at a concentration of 2.5 mg L−1 of chlorine significantly boosted the total production of marketable fruits when considering total fruit weight per plant (Figure 1A). Nonetheless, it should be noted that the total production for the control treatment was relatively low due to a lower average weight per fruit than the typical weight for this variety. This is possible due to the small scale of this experiment compared to a commercial production.
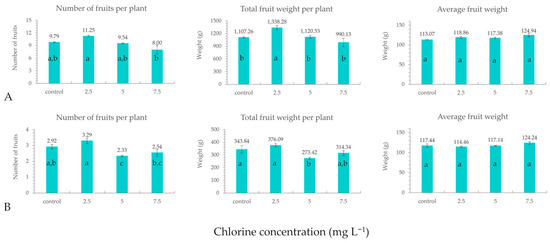
Figure 1.
(A) Total and (B) Extra class production of tomato fruit after disinfection of the nutrient solution using sodium hypochlorite in an open soilless system applying chlorine concentrations of 2.5, 5 and 7.5 mg L−1. Values (means of four replications, bars = SE), followed by different letter in each column indicate significant differences according to Duncan’s multiple range test (p < 0.05).
To the best of our knowledge, this is the first study in the relevant scientific literature indicating an enhancement of tomato yield after the addition of chlorine in the NS. Τhe increased yield in the treatment of 2.5 mg L−1 of chlorine was not accompanied by statistical differences between the treatments regarding gas exchange. Hence, it cannot be ascribed to an enhancement of anabolic functions. However, considering that the dose of chlorine that led to this yield increase was low, it could be attributed to hormetic effects [47,48]. An alternative explanation is that the presence of chlorine in the NS protected plants from low-impact infections of the root system, which did not cause symptoms detectable by visual observation, thus improving their performance compared to plants not treated with chlorine.
The mineral analysis of tomato fruits revealed a significantly higher Mg concentration when chlorine was applied at a concentration of 2.5 mg L−1, which correlates well with the higher yield in this treatment (Table 4). The chloride content in the fruit was increased by chlorine application only in the second harvest (43 DAFA) and only in the 7.5 mg L−1 treatment, and the difference was significant compared not only with the control but also with the other two chlorine treatments. In general, the concentration of nutrients in the fruits ranged between 2.4–4.1 mg g−1 for phosphorus, 41.5–98.0 mg g−1 for potassium, 2.5–3.5 mg g−1 for chlorine, 0.16–0.21 mg g−1 for calcium, and 1.5–1.8 mg g−1 for magnesium.

Table 4.
Mineral nutrient analysis in tomato fruit (mg g−1 dw) harvested 31 and 43 DAFA of sodium hypochlorite in an open soilless system at chlorine concentrations of 2.5, 5.0 and 7.5 mg L−1.
Chlorine is usually reported for its phytotoxic action when absorbed by the roots at excessively high rates in the form of chloride ions [49]. However, chlorine is an essential trace element in chloroplasts, where it participates in the oxygen evolution process in photosystem II [50] and possibly controls the function of some enzymes involved in cell division [51]. Chlorine is absorbed by plants as the chloride ion at concentrations ranging from low [0.1–0.2 mg g−1 [44]] to high [2–20 mg g−1 [51]]. At higher concentrations than those required for its essential functions, chloride may have beneficial functions such as osmoregulation [52,53]. However, high concentrations of chloride in the plant tissues are considered to limit the growth of plants [54,55]. The reasons behind this phenomenon are not clear [52] and probably differ in the various susceptible plants such as Lotus corniculatus [56], citrus [57] and grapevine [54], which show phytotoxicity at concentrations of 4–7 mg g−1 dw [58]. Tomatoes have a high tolerance to chloride concentration compared to other vegetables grown in greenhouses [45]. More specifically, in a closed soilless system, when part of the nitrogen was replaced by chloride, no effect on total yield was reported [45]. Nonetheless, reduction of physiological abnormalities in tomato fruits (white and green spots, blossom end rot) has been indicated when chloride levels are high (8–10 mM) [59,60]. Finally, it should be noted that high chloride concentrations under stress (NaCl) could increase soluble solids and dry matter content and also affect fruit firmness [61].
2.4. Chlorates and Perchlorates in the Nutrient Solution
2.4.1. Stability of Chlorate and Perchlorate Residues in the Nutrient Solution
Perchlorate residues were not detected in any of the fruit samples. Regarding chlorate, which was formed at the beginning (0 day), residues were found at 0.45 and 1.45 mg L−1 in the solutions with 2.5 and 5% ammonia, respectively. On the first day, a recovery of 62% compared to day 0 was observed in the 5% ammonia solution, but in the next sample points, recoveries were all above 70%, indicating that no degradation above 30% or formation of perchlorate was observed during the 28-day period of the study (Table 5).

Table 5.
Summary results of the 28-day storage stability study of chlorate residues in the nutrient solutions with 2.5 and 5% ammonia concentration. The nutrient solution was composed to support the fruiting of an open soilless system cultivation of tomato.
2.4.2. Detection of Chlorate Residues in the Nutrient Solution
Chlorate residues were detected in the NS of all treatments (Figure 2 and Figure S1), whilst no perchlorate residues were found in any treatment. Furthermore, the concentration of chlorate residues was increasing in the different treatments following the increased sodium hypochlorite addition in the NS. More specifically, the highest concentration of chlorates determined was 0.325 mg L−1 in the treatment of 7.5 mg L−1 of chlorine.
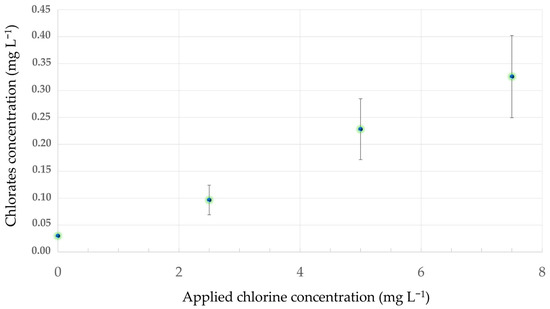
Figure 2.
Chlorate residues detected in the nutrient solution of an open soilless cultivation of tomato after application of chlorine at concentrations of 2.5, 5 and 7.5 mg L−1. The bars represent standard deviations.
Industry quality controls and official food safety controls have detected chlorate residues in various vegetables. Relatively high concentrations have been found in tomato (0.2 mg kg−1) and carrot (0.3 mg kg−1) samples, which exceeded the MRLs set by Reg. 2020/749 (0.1 mg kg−1 and 0.15 mg kg−1, respectively) [30]. Nonetheless, it has been suggested that these high levels derive from post-harvest handling where bleach is used as a disinfectant [63,64,65].
2.5. Chlorates and Perchlorates in Tomato Fruit
After the fortification and analysis of QC samples in the LC/MS/MS, recovery values and relative standard deviations for each substance and charge level were calculated. The accuracy of the method was expressed as a percent recovery. Chlorate recovery values ranged from 111 to 119%, while for perchlorates the range was from 116 to 119%. Repeatability was expressed as % RSD, which ranged from 7–19% for chlorates and from 2–7% for perchlorates. The results are considered acceptable and prove that the method was reliable.
Chlorate and perchlorate residues were not detected in tomato fruit samples of the extra class grade (Figure S2). These results indicate that the fruits were safe for consumption. Chlorates are generally an important inorganic derivative of chlorine used for disinfection, and the key question is if they can be absorbed and accumulated in edible parts during plant production [66]. Here, in all analyzed fruit samples, the residues were lower than the limit of quantification (LOQ = 0.01 mg kg−1). In contrast, Dannehl et al. (2016) found that chlorate residues were detected after disinfection of the NS with KClO in a closed soilless tomato growing system [7]. Furthermore, chlorate residues have been recorded in “baby” lettuce and spinach samples when the crops were irrigated with chlorinated water [67,68]. However, chlorates and perchlorates do not coexist [7]. Finally, Lonigro et al. (2017) suggested that organochlorine compounds in soil, roots, and leaves are linked to the chlorine concentration in the irrigation water or nutrient solution [42].
Disinfection of vegetables using chlorine has many applications, e.g., production, harvest, and post-harvest handling of fresh fruits and vegetables for many decades [69,70,71]. In the past, high concentrations of chlorine were applied since there was no awareness about possible toxic residues in the final products [72,73]. Nevertheless, it is now clear that chlorine can react with organic matter, leading to derivatives such as chloroform (CHCl3) or trihalomethanes that are carcinogenic at high doses [74]. Therefore, low doses of chlorine, when using bleach, are recommended for disinfection of NSs in soilless crops to produce safe products free from chlorine byproducts, in addition to avoiding possible symptoms of phytotoxicity [66].
3. Conclusions
Disinfection of the nutrient solution in open soilless cropping systems by applying chlorine up to a dose of 7.5 mg L−1 three times at fortnightly intervals did not lead to phytotoxicity in tomato. However, the application of chlorine at a concentration of 2.5 mg L−1 increased the total fruit production, due mainly to an increase in the number of fruits per plant. Additionally, no residues of chlorates or perchlorates were detected in tomato fruits. Nonetheless, further research is necessary to address the gap in the literature regarding the disinfection methods applied and their efficiency in controlling economically important pathogens.
4. Materials and Methods
4.1. Experimental Design, Biological Material and Cropping Conditions
4.1.1. Greenhouse Cultivation
An experiment with tomato (Solanum lycopersicum cv. “ELPIDA”) cultivated in bags containing perlite was carried out in a glasshouse at the Agricultural University of Athens in Greece (37°59′10″ N, 23°42′29″ E, altitude 24 m) from May to July 2019.
The soilless cultivation system comprised 10 channels, eight of which were used to apply the experimental treatments (2 channels per treatment), while the other two were used as border lines (Figure 3). A completely randomized design was followed. Six bags of perlite (33 L, Perlite Hellas, Athens, Greece) were placed in each channel, and two tomato plants were planted in each bag. Prior to transplanting, the perlite bags were irrigated with NS up to saturation, and subsequently their bottoms were slit to allow for free drainage of the NS from the root zone. The composition of the NS was computed using the algorithm developed by Savvas and Adamidis (1999) [75] after setting as target nutrient values those suggested by Savvas et al. (2013) [76] for open soilless cultivations of tomato. The pH of the supplied NS was adjusted to 5.6 using nitric acid. The nutrient solution compositions applied at different cropping stages are shown in Table S1. One day after wetting the substrate (10 May 2019), the tomato seedlings, which were at the 8-leaf stage, were transplanted. The channels had a slope of 1–2% to facilitate drainage, and every two days the values of pH and electrical conductivity (EC) were recorded. The NS collected at the lower end of each channel was discharged. To aid fruit setting, a bumblebee hive (Bombus terrestris) (Bio Insecta, Thessaloniki, Greece) was placed inside the experimental greenhouse compartment, and the usual pruning [2,77] and plant protection practices were followed [78,79]. More specifically regarding plant protection, integrated pest management (IPM) principles were applied. Protective nets were put on the greenhouse openings as well as adhesive traps to prevent infestation by insects. In addition, seven days after transplanting the plants, the beneficial insect Macrolophus pygmaeus was released as a plant protection measure against Tuta absoluta.

Figure 3.
Experimental pipeline for the study of the impact of sodium hypochlorite applied as a nutrient solution disinfectant on growth, nutritional status, yield, and consumer safety of tomato fruit produced in a soilless cultivation.
Corrective recipes were also used during the cropping period whenever they were necessary. The irrigation frequency was automatically adjusted using a heliometer to measure the solar radiation intensity and an irrigation controller, aiming to achieve a drainage percentage of about 30%.
4.1.2. Disinfection Methodology
Sodium hypochlorite was applied through the last irrigation cycle of the day, while no irrigation was applied during the night. During the cropping period, sodium hypochlorite was applied to the tomato crop three times at fortnightly intervals. Common bleach containing 4.5% w/v sodium hypochlorite (Ostria Ultra Power, Lamia, Greece) was used. More specifically, the amounts of bleach needed to apply three different chlorine concentrations, namely 2.5, 5, and 7.5 mg L−1, were calculated and added to the NS supplied. These chlorine concentrations were selected to range around the average concentration of chlorine that is not supposed to negatively affect the plants according to the EPA (5 mg L−1) [43]. The final concentration of chloride was not tested in the NS after the application of sodium hypochlorite, but it was measured at the drainage the following day (Table 1). Furthermore, the pH was tested on site in the NS after the addition of sodium hypochlorite and in the drainage solutions. The first application took place on 11 June 2019, i.e., about one month after the beginning of fruit set (16 May 2019). All applications of sodium hypochlorite are shown in detail in Table S2.
4.1.3. Sample Collection
In order to estimate the impact of the treatments on plant growth, nutrient concentrations and the possible formation of chlorates and/or perchlorates in plant tissues, including leaves, fruits, and roots, were sampled. Leaf samples (3rd and 4th fully developed leaves from the top) were collected 0, 31, and 43 days after the first application of sodium hypochlorite (DAFA—days after first application). Fruit samples were collected at DAFA 31 and 43. The time points of sampling were chosen in an effort to discover the effect of sodium hypochlorite on tomato plants after the third chlorine application (31 DAFA) and almost ten days later, namely slightly before the end of the culture. At the end of the cultivation (48 DAFA), the whole root system was also selected, separated from perlite grains, and used as root samples. A total of two plant tissue samples were collected from two different plants in each channel, thereby obtaining four replicate samples per treatment. Prior to the nutrient analysis, the fresh weight of each sample was recorded, and then all samples were placed in an oven at 65°C for drying until their weight was stabilized. Finally, the dry matter content (DMC) and specific leaf area (SLA, on fresh leaves) were calculated [80].
In addition, samples of NS were collected from the drainage solution before the first harvest (30 DAFA), 7 days later (37 DAFA), and at the end of the cultivation (48 DAFA) to determine the level of chloride and nutrients. Two drainage solution samples were collected from each channel, thereby obtaining four replicates per treatment.
4.2. Gas Exchange Measurements
The treatment impact on net photosynthetic rate, stomatal conductance, intercellular CO2 concentration, and transpiration rate were determined using a Li-6400 instrument (Li-Cor, Inc., Lincoln, NE, USA) [81]. The measurements were conducted in leaves of the same physiological stage (the most recent fully expanded leaf) at the same time of the day (between 09:00 am and 12:00 pm) prior to their sampling, as described in Section 4.1.3. All measurements were conducted under natural light conditions and ambient CO2 atmospheric concentration on sunny days to exclude light intensity effects. Furthermore, water use efficiency was estimated as previously described [82].
4.3. Plant Mineral Status
Initially, the dried plant samples were ground using a ball mill and sieved (40-mesh) until they obtained the texture of powder. The procedure was followed by dry burning at 500 °C for 8 h and the extraction of minerals using 10 mL of 1 M HCl to determine tissue P, K, Ca, and Mg concentrations. The extracts were filtered and stored at 4 °C until further processing. Phosphorus was estimated photometrically (Anthos Zenyth 200, Biochrom, Holliston, MA, USA) by applying the ammonium phosphomolybdate method [83]. Potassium was estimated through flame photometry using a Sherwood Model 410 (Sherwood Scientific Ltd., Cambridge, UK). Ca and Mg were measured by employing atom absorption spectrophotometry using a Shimadzu AA-7000 instrument (Shimadzu Europa GmbH, Duisburg, Germany). Chloride was measured using a chloridometer (Thermo Scientific Orion Star A214, Thermo Fisher Scientific, Waltham, MA, USA) in aqueous extracts of the powdered leaf samples after filtering.
4.4. Estimation of Total Fruit Production
Fruit was harvested twice, on 12 July 2019 and 24 July 2019, i.e., 2 and 14 days after the last application of sodium hypochlorite, respectively. At each harvest date, all ripe fruits were collected and graded into extra class, class I, class II, and non-marketable produce in accordance with the EU Regulation (543/2011) [84]. The fruit yield was estimated by recording the total number of fruits per plant (for both time points together and for each tomato class separately) and the total fruit weight per plant, while calculating the average fruit weight.
4.5. Residue Determinations
In order to investigate the possible contamination of harvested tomato fruits with chlorates (ClO3−) and perchlorates (ClO4−) due to disinfection of the NS with sodium hypochlorite, residue analysis was performed as described below:
4.5.1. Chemicals and Reagents
Chlorate, perchlorate, and internal standards (IS) (perchlorate 18O4 and chlorate 18O3) stock solutions at 80 mg L−1 in MeOH were obtained from the EU-Reference Laboratory for pesticides requiring single residue methods. Methanol (HPLC gradient grade) and water (LC-MS grade) were bought from Fischer chemicals. Acetic acid (99% for analysis) and formic acid (99%, for analysis) were bought from Carlo-Erba reagents.
4.5.2. Investigation of the Stability of Chlorates and Perchlorates in the NS Samples
During the final application of sodium hypochlorite in the different treatments, two 0.5 L samples of NS were collected from the drippers, and their chlorate and perchlorate concentrations were determined.
The nutrient solution, which had previously been analyzed to confirm that no detectable residues of chlorates and perchlorates were present, served as a control to investigate the stability of chlorate and perchlorate residues in NS under freezing conditions. Two solutions with 2.5 and 5% ammonia concentrations were prepared and stored at 4 °C. During this time, successive sampling of the fortified NS was performed at days 0, 1, 2, 4, 6, 8, 11, 21, 22, and 28 and was followed by injection into the LC/MS/MS chromatographic system.
4.5.3. Extraction Procedure of Tomato Fruit Samples
A laboratory sample of 2 kg of the extra class tomato fruits was collected and homogenized 8 h after harvest, after removal of the calyx. The homogenized samples were stored at −20 °C until further processing.
Residues analysis was performed following the in-house laboratory method M17, which is based on the QuPPe protocol [85]. A brief description of the procedures is described below:
An aliquot of 10 ± 1 g of homogenized sample was weighted, and 10 mL of the extraction solvent methanol (acidified 1% HCOOH) was added. The mixture was allowed to soak for 30 min before the addition of the extraction solvent. The mixture was shaken by hand for 1 min and centrifuged at 4000 rpm (1792× g) for 5 min. An aliquot of the extract was transferred into a screw cup-top storage vial. Before injecting it into the chromatographic system, the final solution was filtered through a 0.45 μm disposable cellulose syringe filter.
4.5.4. Chromatographic Analysis of Fruit Samples—Instrumentation
For the chromatographic analysis of the samples, a Varian liquid chromatography LC-MS/MS system consisting of two Varian Prostar 210 pumps and a Prostar 420 autosampler using a 100 mL syringe was used, combined with a triple quadrupole mass spectrometer (Varian model 1200 L) and equipped with an electrospray ionization (ESI) interface, operating in the negative mode. Separation was performed on a Hypercarb 2.1 × 100 mm 5 μm at a flow rate of 0.4 mL min−1. The column was at room temperature. Eluent A consisted of an aqueous solution of 1% CH3COOH, while eluent B was MeOH with 1% CH3COOH. The LC gradient started at 100% A and was linearly decreased to 70% B, over 10 min. Finally, the gradient was instantly switched to 100% A and equilibrated for 5 min before the next injection took place. The injection volume was 10 mL.
The following instrumental settings were used: the source temperature was set at 50 °C and the drying gas temperature at 300 °C. Drying gas and nebulizing gas were nitrogen generated from a high purity generator at 18 psi and synthetic air (purity > = 99.99%) at 40 psi, respectively. For the operation in MS/MS mode, Argon 99.999% was used as a collision gas with a pressure of 1.8 mTorr. The scanning of the transitions was conducted with a dwell time of 50 ms per transition. The number of data points across the peaks was at least ten. Capillary voltage (CV) and collision energy (CE) varied depending on the precursor ion and product ion and are presented in Table S3.
4.5.5. Method Performance
The analytical method applied to determine chlorates and perchlorates in tomato fruit samples of extra class has been proposed by the EURLs (European Reference Laboratories for Single Residue Methods), hence method validation data are available. In order to ensure the performance of the present analysis and verify the obtained results, a brief validation of the method was performed. Considering that an internal standard was used to quantify the results, calibration standards were applied to the solvent. Specifically, quality control samples containing 0.01 mg kg−1 (in 2 replicates, n = 3), 0.1 mg kg−1 (in 2 replicates, n = 3), and 1 mg kg−1 (in 2 replicates, n = 2) in chlorates—perchlorates were prepared by fortifying the corresponding concentration in control tomato samples to determine the accuracy of the method. The samples used as controls had previously been analyzed, and it was confirmed that they had no residues from or interferences with the substances under investigation. Accuracy was checked by assessing the recovery for the selected concentrations, and repeatability was verified by measuring the relative standard deviation (RSD).
4.6. Statistical Analysis
The experiment was set up as a completely randomized design examining one factor (sodium hypochlorite concentration) with four levels and four replicates per treatment. The statistical analysis was performed using the Rstudio program (Version: 1.3.1093, Boston, MA, USA) and the Agricolae package [86]. First, box plots were made to ensure the lack of outliers in the various parameters studied for each treatment separately. The normality of the data was checked in terms of skewness and kurtosis [87], and Levene’s test was employed to test the equality of the variances. When an ANOVA analysis rendered a significant difference at the significance level of 95%, post-hoc comparisons using the Duncan’s Multiple Range Test with p < 0.05 were performed [88].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae9030352/s1, Figure S1: Chlorate residue analysis in a nutrient solution sample (red) of an open soilless system cultivation of tomato and in HPLC water (green). The nutrient solution sample corresponds to the 7.5 mg L−1 of chlorine treatment after the application of sodium hypochlorite in which chlorates residues were detected; Figure S2: LC-MS/MS scan data of chlorate (red) and perchlorate (green) residue analysis in tomato fruit samples collected from the 5 mg L−1 chlorine treatment; Table S1: Recipes of nutrient solutions used in tomato cultivated in an open soilless system; Table S2: Details about the three disinfection applications using sodium hypochlorite in the nutrient solution supplied to a tomato cultivated in an open soilless system; Table S3: Precursor ion, product ion, Capillary Voltage (CV) and Collision Energy (CE) for the chlorates and perchlorates analytes examined with LC/MS/MS for the determination of their residues in tomato fruits and nutrient solution samples of an open soilless system cultivation.
Author Contributions
Conceptualization: D.S. and E.B.; funding acquisition: D.S.; investigation: M.L., E.B. and I.K.; methodology: M.L., E.B., I.K., C.A. and D.S.; project administration: D.S.; Supervision: D.S. and K.A.A.; writing—original draft; M.L. and E.B.; writing—review and editing: M.L., E.B., I.K., C.A., K.A.A. and D.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Hellenic Foundation for Research and Innovation (H.F.R.I.) under the “First Call for H.F.R.I. Research Projects to support Faculty members and Researchers and the procurement of high-cost research equipment grant” (Project Number: HFRI-FM17-3196—NUTRISENSE).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to thank the students Maria Theodorakopoulou, Nikos Andreou, and Athanasia Papakosta for their significant contribution in the field work and analysis of the nutrients.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jan, S.; Rashid, Z.; Ahngar, T.A.; Iqbal, S.; Naikoo, M.A.; Majeed, S.; Bhat, T.A.; Gull, R.; Nazir, I. Hydroponics–A Review. Int. J. Curr. Microbiol. App. Sci. 2020, 9, 1779–1787. [Google Scholar] [CrossRef]
- Gruda, N.; Savvas, D.; Colla, G.; Rouphael, Y. Impacts of genetic material and current technologies on product quality of selected greenhouse vegetables–A review. Eur. J. Hortic. Sci. 2018, 83, 319–328. [Google Scholar] [CrossRef]
- Critten, D.L.; Bailey, B.J. A review of greenhouse engineering developments during the 1990s. Agric. For. Meteorol. 2002, 112, 1–22. [Google Scholar] [CrossRef]
- Stewart-Wade, S.M. Plant pathogens in recycled irrigation water in commercial plant nurseries and greenhouses: Their detection and management. Irrig. Sci. 2011, 29, 267–297. [Google Scholar] [CrossRef]
- Martínez, F.; Castillo, S.; Carmona, E.; Avilés, M. Dissemination of Phytophthora cactorum, cause of crown rot in strawberry, in open and closed soilless growing systems and the potential for control using slow sand filtration. Sci. Hortic. 2010, 125, 756–760. [Google Scholar] [CrossRef]
- Zheng, Y.; Cayanan, D.F.; Dixon, M. Control of pathogens in irrigation water using chlorine without injury to plants. Comb. Proc. Int. Plant Propag. Soc. 2008, 58, 248–258. Available online: http://admin.ipps.org/uploads/58_051.pdf (accessed on 20 December 2022).
- Dannehl, D.; Schuch, I.; Gao, Y.; Cordiner, S.; Schmidt, U. Effects of hypochlorite as a disinfectant for hydroponic systems on accumulations of chlorate and phytochemical compounds in tomatoes. Eur. Food Res. Technol. 2016, 242, 345–353. [Google Scholar] [CrossRef]
- Savvas, D.; Gruda, N. Application of soilless culture technologies in the modern greenhouse industry—A review. Eur. J. Hortic. Sci. 2018, 83, 280–293. [Google Scholar] [CrossRef]
- FAO; IFAD; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World 2022. Repurposing Food and Agricultural Policies to Make Healthy Diets More Affordable; FAO: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- MIT. Mission 2014: Feeding the World. Available online: http://12.000.scripts.mit.edu/mission2014/solutions/hydroponics (accessed on 21 November 2022).
- Bank, W. The Road to a Greener Future. Available online: https://blogs.worldbank.org/climatechange/road-greener-future (accessed on 21 November 2022).
- World Food Programme. How2grow. Available online: https://innovation.wfp.org/project/h2grow-hydroponics (accessed on 21 November 2022).
- Giro, A.; Ciappellano, S.; Ferrante, A. Vegetable production using a simplified hydroponics system inside city of Dead (Cairo). In Advances in Horticultural Science; Mancuso, S., Ed.; Department of Agri-Food Production and Environmental Sciences, University of Florence: Florence, Italy, 2016; pp. 23–29. [Google Scholar]
- Mordor Intelligence. Hydroponic Market–Growth, Trends, COVID-19 Impact and Forecasts (2022–2027). Available online: https://www.mordorintelligence.com/industry-reports/hydroponics-market (accessed on 30 October 2022).
- Eden Green Technology. How Hydroponics & Vertical Farming Can Improve Food Safety and Protect Our Food Supply. Available online: https://www.edengreen.com/blog-collection/how-hydroponics-and-vertical-farming-can-improve-food-safety (accessed on 30 October 2022).
- Riggio, G.M.; Wang, Q.; Kniel, K.E.; Gibson, K.E. Microgreens—A review of food safety considerations along the farm to fork continuum. Int. J. Food Microbiol. 2019, 290, 76–85. [Google Scholar] [CrossRef]
- Sawyer, T. Food safety and E. coli in aquaponic and hydroponic systems. Horticulturae 2021, 7, 36. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Deering, A.J.; Kim, H.-J. The occurrence of shiga toxin-producing E. coli in aquaponic and hydroponic systems. Horticulturae 2020, 6, 1. [Google Scholar] [CrossRef]
- Ilic, S.; LeJeune, J.; Lewis Ivey, M.L.; Miller, S. Delphi expert elicitation to prioritize food safety management practices in greenhouse production of tomatoes in the United States. Food Control 2017, 78, 108–115. [Google Scholar] [CrossRef]
- Clark, G.A.; Smajstrla, A.G. Treating irrigation systems with chlorine. Foliage Dig. 1992, 15, 3–5. [Google Scholar]
- Postma, J.; Van Os, E.; Bonants, P.J.M. 10-Pathogen detection and management strategies in soilless plant growing systems. In Soilless Culture; Raviv, M., Lieth, J.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 425–457. [Google Scholar] [CrossRef]
- Cayanan, D.F.; Zheng, Y.; Zhang, P.; Graham, T.; Dixon, M.; Chong, C.; Llewellyn, J. Sensitivity of five container-grown nursery species to chlorine in overhead irrigation water. HortScience 2008, 43, 1882–1887. [Google Scholar] [CrossRef]
- Cayanan, D.F.; Dixon, M.; Zheng, Y.; Llewellyn, J. Response of container-grown nursery plants to chlorine used to disin-fest irrigation water. HortScience 2009, 44, 164–167. [Google Scholar] [CrossRef]
- Hong, C.X.; Richardson, P.A.; Kong, P.; Bush, E.A. Efficacy of chlorine on multiple species of Phytophthora in recycled nursery irrigation water. Plant Dis. 2003, 87, 1183–1189. [Google Scholar] [CrossRef]
- Price TV, F.P. Behaviour of fungicides in recirculating nutrient film hydroponic systems. In Proceedings of the 6th in-Ternational Congress on Soilless Culture, Lunteren, The Netherlands, 28 April–5 May 1984; pp. 511–522. [Google Scholar]
- Poncet, C.; Offroy, M.; Bonnet, G.; Brun, R. Disinfection of recycling water in rose cultures. Acta Hortic. 2001, 547, 121–127. [Google Scholar] [CrossRef]
- Rosner, A.; Lachman, O.; Pearlsman, M.; Feigelson, L.; Maslenin, L.; Antignus, Y. Characterisation of cucumber leaf spot virus isolated from recycled irrigation water of soil-less cucumber cultures. Ann. Appl. Biol. 2006, 149, 313–316. [Google Scholar] [CrossRef]
- Stanton, J.M.; O’Donnell, W.E. Hatching, motility, and infectivity of root-knot nematode (Meloidogyne javanica) following exposure to sodium hypochlorite. Aust. J. Exp. Agric. 1994, 34, 105–108. [Google Scholar] [CrossRef]
- Bull, R.J.; Gerba, C.; Trussell, R.R. Evaluation of the health risks associated with disinfection. Crit. Rev. Environ. Control 1990, 20, 77–113. [Google Scholar] [CrossRef]
- Kaufmann-Horlacher, I. Chlorat-Rüchstände in Pflanzlichen Lebensmitteln-Ein Update. CVUA, Stuttgart. 2014. Available online: https://www.ua-bw.de/pub/beitrag.asp?subid=1&Thema_ID=5&ID=2008 (accessed on 1 December 2022).
- European Commission. Chlorate. Available online: https://ec.europa.eu/food/plants/pesticides/maximum-residue-levels/chlorate_en (accessed on 30 October 2022).
- Regulation 2020/749. Commission Regulation (EU) 2020/749 of 4 June 2020 Amending Annex III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as Regards Maximum Residue Levels for Chlorate in or on Certain Products. Available online: http://data.europa.eu/eli/reg/2020/749/oj (accessed on 30 October 2022).
- BfR Recommendations for the Health Assessment of Chlorate Residues in Food, BfR Opinion No. 028/2014; The German Federal Institute for Risk Assessment: Berlin, Germany, 2014.
- EFSA. Trilateral Meeting on Perchlorate Risk Assessment; Report of the Meeting on 12 February 2014; European Food Safety Authority: Parma, Italy, 2014. [Google Scholar]
- World Health Organization. Joint FAO/WHO Expert Committee on Food Additives. Meeting (67th: 2006: Geneva, Switzerland) & International Programme on Chemical Safety. Safety Evaluation of Certain Food Additives and Contaminants/Prepared by the Sixty-Eighth Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JEFCA). 2008. Available online: https://apps.who.int/iris/handle/10665/43823 (accessed on 30 October 2022).
- Rodríguez-Ortega, W.M.; Martínez, V.; Nieves, M.; Simón, I.; Lidón, V.; Fernandez-Zapata, J.C.; Martinez-Nicolas, J.J.; Cámara-Zapata, J.M.; García-Sánchez, F. Agricultural and physiological responses of tomato plants grown in different soilless culture systems with saline water under greenhouse conditions. Sci. Rep. 2019, 9, 6733. [Google Scholar] [CrossRef]
- Razifard, H.; Ramos, A.; Della Valle, A.L.; Bodary, C.; Goetz, E.; Manser, E.J.; Li, X.; Zhang, L.; Visa, S.; Tieman, D.; et al. Genomic evidence for complex domestication history of the cultivated tomato in latin america. Mol. Biol. Evol. 2020, 37, 1118–1132. [Google Scholar] [CrossRef]
- FAOSTAT. Crops and Livestock Products. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 30 October 2022).
- De Hayr, R.; Bodman, K.; Forsberg, L. Bromine and chlorine disinfestation of nursery water supplies. Comb. Proc. Int. Plant Propag. Soc. 1994, 44, 60–66. [Google Scholar]
- Schreuder, M.D.J.; Brewer, C.A. Persistent effects of short-term, high exposure to chlorine gas on physiology and growth of Pinus ponderosa and Pseudotsuga menziesii. Ann. Bot. 2001, 88, 197–206. [Google Scholar] [CrossRef]
- Ehret, D.; Alsanius, B.; Wohanka, W.; Menzies, J.; Utkhede, R. Disinfestation of recirculating nutrient solutions in greenhouse horticulture. Agron. EDP Sci. 2001, 21, 323–339. [Google Scholar] [CrossRef]
- Lonigro, A.; Montemurro, N.; Laera, G. Effects of residual disinfectant on soil and lettuce crop irrigated with chlorinated water. Sci. Total Environ. 2017, 584–585, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Environmental Protection Agency (USA). Guidelines for Water Reuse. 2012. Available online: https://www.epa.gov/sites/default/files/2019-08/documents/2012-guidelines-water-reuse.pdf (accessed on 17 December 2022).
- Wege, S.; Gilliham, M.; Henderson, S.W. Chloride: Not simply a ‘cheap osmoticum’, but a beneficial plant macronutrient. J. Exp. Bot. 2017, 68, 3057–3069. [Google Scholar] [CrossRef]
- Neocleous, D.; Nikolaou, G.; Ntatsi, G.; Savvas, D. Nitrate supply limitations in tomato crops grown in a chloride-amended recirculating nutrient solution. Agric. Water Manag. 2021, 258, 107163. [Google Scholar] [CrossRef]
- Franco-Navarro, J.D.; Brumós, J.; Rosales, M.A.; Cubero-Font, P.; Talón, M.; Colmenero-Flores, J.M. Chloride regulates leaf cell size and water relations in tobacco plants. J. Exp. Bot. 2015, 67, 873–891. [Google Scholar] [CrossRef] [PubMed]
- Agathokleous, E.; Kitao, M.; Calabrese, E.J. Hormesis: A compelling platform for sophisticated plant science. Trends Plant Sci. 2019, 24, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Erofeeva, E.A. Hormesis in plants: Its common occurrence across stresses. Curr. Opin. Toxicol. 2022, 30, 100333. [Google Scholar] [CrossRef]
- Geilfus, C.-M. Chloride: From Nutrient to Toxicant. Plant Cell Physiol. 2018, 59, 877–886. [Google Scholar] [CrossRef]
- Bové, J.M.; Bové, C.; Whatley, F.R.; Arnon, D.I. Chloride requirement for oxygen evolution in photosynthesis. Z. Für Nat.-B 1963, 18, 683–688. [Google Scholar] [CrossRef]
- Colmenero-Flores, J.M.; Franco-Navarro, J.D.; Cubero-Font, P.; Peinado-Torrubia, P.; Rosales, M.A. Chloride as a bene-ficial macronutrient in higher plants: New roles and regulation. Int. J. Mol. 2019, 20, 4686. [Google Scholar] [CrossRef]
- Geilfus, C.-M. Review on the significance of chlorine for crop yield and quality. Plant Sci. 2018, 270, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Raven, J.A. Chloride: Essential micronutrient and multifunctional beneficial ion. J. Exp. Bot. 2017, 68, 359–367. [Google Scholar] [CrossRef]
- Henderson, S.W.; Baumann, U.; Blackmore, D.H.; Walker, A.R.; Walker, R.R.; Gilliham, M. Shoot chloride exclusion and salt tolerance in grapevine is associated with differential ion transporter expression in roots. BMC Plant Biol. 2014, 14, 273. [Google Scholar] [CrossRef] [PubMed]
- Teakle, N.L.; Tyerman, S.D. Mechanisms of Cl- transport contributing to salt tolerance. Plant Cell Environ. 2010, 33, 566–589. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, D.H.; Pieckenstain, F.L.; Escaray, F.; Erban, A.; Kraemer, U.T.E.; Udvardi, M.K.; Kopka, J. Comparative ionomics and metabolomics in extremophile and glycophytic Lotus species under salt stress challenge the metabolic pre-adaptation hypothesis. Plant Cell Environ. 2011, 34, 605–617. [Google Scholar] [CrossRef]
- Brumos, J.; Talon, M.; Bouhlai, R.; Colmenero-Flores, J.M. Cl- homeostasis in includer and excluder citrus rootstocks: Transport mechanisms and identification of candidate genes. Plant Cell Environ. 2010, 33, 2012–2027. [Google Scholar] [CrossRef]
- Xu, G.; Magen, H.; Tarchitzky, J.; Kafkafi, U. Advances in chloride nutrition of plants. In Advances in Agronomy, Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 1999; Volume 68, pp. 97–150. [Google Scholar] [CrossRef]
- Gruda, N. Do Soilless Culture Systems Have an Influence on Product Quality of Vegetables? Available online: https://edoc.hu-berlin.de/handle/18452/10085 (accessed on 30 October 2022).
- Schnitzler, W.H.; Gruda, N. Hydroponics and product quality. In Hydroponic Production of Vegetables and Ornamentals; Savvas, D., Passam, H.C., Eds.; Embryo Publications: Athens, Greece, 2002; pp. 373–414. [Google Scholar]
- Leonardi, C.; Martorana, M.; Giuffrida, F.; Fogliano, V.; Pernice, R. Tomato fruit quality in relation to the content of sodium chloride in the nutrient solution. Acta Hortic. 2004, 659, 769–774. [Google Scholar] [CrossRef]
- European Commission. Guidance Document on Analytical Quality Control and Method Validation Procedures for Pesticide Residues and Analysis in Food and Feed; European Commission: Brussels, Belgium, 2019; SANTE/12682/2019. [Google Scholar]
- Goodburn, C.; Wallace, C.A. The microbiological efficacy of decontamination methodologies for fresh produce: A review. Food Control 2013, 32, 418–427. [Google Scholar] [CrossRef]
- Ramos, B.; Miller, F.A.; Brandão, T.R.S.; Teixeira, P.; Silva, C.L.M. Fresh fruits and vegetables—An overview on applied methodologies to improve its quality and safety. Innov. Food Sci. Emerg. 2013, 20, 1–15. [Google Scholar] [CrossRef]
- Joshi, K.; Mahendran, R.; Alagusundaram, K.; Norton, T.; Tiwari, B.K. Novel disinfectants for fresh produce. Trends Food Sci. Technol. 2013, 34, 54–61. [Google Scholar] [CrossRef]
- Leonel, L.P.; Tonetti, A.L. Wastewater reuse for crop irrigation: Crop yield, soil and human health implications based on giardiasis epidemiology. Sci. Total Environ. 2021, 775, 145833. [Google Scholar] [CrossRef]
- Garrido, Y.; Marín, A.; Tudela, J.A.; Truchado, P.; Allende, A.; Gil, M.I. Chlorate accumulation in commercial lettuce cultivated in open field and irrigated with reclaimed water. Food Control 2020, 114, 107283. [Google Scholar] [CrossRef]
- López-Gálvez, F.; Gil, M.I.; Meireles, A.; Truchado, P.; Allende, A. Demonstration tests of irrigation water disinfection with chlorine dioxide in open field cultivation of baby spinach. J. Sci. Food Agric. 2018, 98, 2973–2980. [Google Scholar] [CrossRef]
- Bartz, J.A. Potential for postharvest disease in tomato fruit infiltrated with chlorinated water and chlorination of asparagus hydrocooling water for the control of post-harvest decay organisms. Plant Dis. 1988, 72, 9–13. [Google Scholar] [CrossRef]
- Goodin, P.L. Chlorine for sick tomatoes (Erwinia carotovora). Agric. Res 1977, 26, 8–10. [Google Scholar]
- Winston, J.R.; Johnson, H.B.; Harvey, E.M. Using chemicals to stop spoilage. In Yearbook of Agriculture; USDA Printing Office: Washington, DC, USA, 1953; pp. 842–843. [Google Scholar]
- Combrink, J.C.; Visagie, T.R. Chlorination of dump tank water to reduce postharvest rot in apples. Deciduous Fruit Grow. 1982, 32, 61–63. [Google Scholar]
- Rabin, J. Pack tomatoes for higher profits-chlorinating packing shed wash water improves quality. Am. Veg. Grow. 1986, 34, 12. [Google Scholar]
- Suslow, T. Chlorination in the production and postharvest handling of fresh fruits and vegetables. In Use of Chlorine-Based Sanitizers and Disinfectants in the Food Manufacturing Industry; University of California-Davis: Davis, CA, USA, 2000; Available online: https://www.siphidaho.org/env/pdf/Chlorination_of_fruits_and_veggies.PDF (accessed on 20 December 2022).
- Savvas, D.; Adamidis, K. Automated management of nutrient solutions based on target electrical conductivity, pH, and nutrient concentration ratios. J. Plant Nut. 1999, 22, 1415–1432. [Google Scholar] [CrossRef]
- Savvas, D.; Gianquinto, G.P.; Tüzel, Y.; Gruda, N. Soilless Culture. In Good Agricultural Practices for Greenhouse Vegetable Crops. Principles for Mediterranean Climate Areas; Food and Agriculture Organization of the United Nations, Plant Production and Protection Paper 217: Rome, Italy, 2013; pp. 303–354. Available online: http://www.fao.org/3/a-i3284e.pdf (accessed on 20 December 2022).
- Navarrete, M.; Jeannequin, B. Effect of frequency of axillary bud pruning on vegetative growth and fruit yield in greenhouse tomato crops. Sci. Hortic. 2000, 86, 197–210. [Google Scholar] [CrossRef]
- Kalozoumis, P.; Vourdas, C.; Ntatsi, G.; Savvas, D. Can Biostimulants Increase Resilience of Hydroponically-Grown Tomato to Combined Water and Nutrient Stress? Horticulturae 2021, 7, 297. [Google Scholar] [CrossRef]
- Lykogianni, M.; Bempelou, E.; Ntatsi, G.; Karavidas, I.; Ropokis, A.; Aliferis, K.A.; Savvas, D. Spinosad residues in hydroponically grown tomato fruits. Acta Hortic. 2021, 1320, 197–204. [Google Scholar] [CrossRef]
- Garnier, E.; Shipley, B.; Roumet, C.; Laurent, G. A standardized protocol for the determination of specific leaf area and leaf dry matter content. Funct. Ecol. 2001, 15, 688–695. [Google Scholar] [CrossRef]
- He, Y.; Zhu, Z.; Yang, J.; Ni, X.; Zhu, B. Grafting increases the salt tolerance of tomato by improvement of photosynthesis and enhancement of antioxidant enzymes activity. Environ. Experim. Bot. 2009, 66, 270–278. [Google Scholar] [CrossRef]
- Medrano, H.; Tomás, M.; Martorell, S.; Flexas, J.; Hernández, E.; Rosselló, J.; Pou, A.; Escalona, J.M.; Bota, J. From leaf to whole-plant water use efficiency (WUE) in complex canopies: Limitations of leaf WUE as a selection target. Crop J. 2015, 3, 220–228. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Regulation 543/2011. EN 15.6.2011 Official Journal of the European Union L 157/109. Brussels: European Union. Available online: https://eur-lex.europa.eu/legal-content/GA/TXT/?uri=CELEX%3A32011R0543 (accessed on 30 October 2022).
- Anastassiades, M.; Kolberg, D.I.; Eichhorn, E.; Benkenstein, A.; Lukačević, S.; Mack, D.; Wildgrube, C.; Sigalov, I.; Dörk, D.; Barth, A. Quick Method for the Analysis of Numerous Highly Polar Pesticides in Foods of Plant Origin via LC-MS/MS Involving Simultaneous Extraction with Methanol (QuPPe-Method); Version 9.2.; CVUA: Stuttgart, Germany, 2015. [Google Scholar]
- de Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research. R Package Version 1.3-3. Available online: https://CRAN.R-project.org/package=agricolae (accessed on 15 August 2022).
- Kim, H.Y. Statistical notes for clinical researchers: Assessing normal distribution (2) using skewness and kurtosis. Restor. Dent. Endod. 2013, 38, 52–54. [Google Scholar] [CrossRef]
- Bewick, V.; Cheek, L.; Ball, J. Statistics review 9: One-way analysis of variance. Crit. Care 2004, 8, 130. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).