Abstract
Postharvest fruit loss is caused by the absence of advanced handling and storage technologies and the quiescent presence of fungal pathogens. Therefore, there is a growing demand for sustainable decisions for the planet. This study focused on the use of two types of edible coatings: one was based on the essential oil of Origanum vulgare L. subsp. viridulum with Aloe arborescens Mill. gel (EC1), and the other was based on the hydrolate only (EC2). These treatments were applied to provide defense against fungal infections in papaya (Carica papaya L. cv Solo), and the storage time was 25 days (T5 ± 1 °C). Fruits coated with EC1 were more contaminated with fungal pathogens than both control (CTR) and EC2 fruit. EC2 showed a statistically lower decay index than CTR and EC1 and maintained its organoleptic characteristics better, showing a 15% loss of firmness after 25 days of storage. Furthermore, the lowest decay index (1.14 after 25 days) was found for the EC1 and CTR. These findings suggest that the use of hydrolate can be useful for extending the shelf life and maintaining the quality of papaya fruit, representing an alternative to the use of synthetic fungicides for food safety.
1. Introduction
One of the global challenges includes the implementation of safety along the food supply chain while maintaining the high nutritional value of fruit and vegetables that is necessary for a healthy diet [1]. Perishable fruits are subject to the risk of mechanical damage, forming lesions and micro-lesions that become entry points for bacteria and fungi, which can hinder food safety for consumers [2]. Amongst fruit products, papaya (Carica papaya L.) is very susceptible to quality decay due to its highly perishable nature [3]. Papaya is native to Costa Rica and southern Mexico and, in the last decade, has been introduced to Mediterranean-climate areas, particularly Sicily, in protected environments, such as greenhouses. Papaya and many other tropical species, such as mango [4], avocado [5], litchi [6], and papaya [7], appear to be viable alternatives to traditional crops [8] in this area of Italy. Papaya is a climacteric fruit and is characterized by a thin skin that is highly vulnerable to mechanical damage and postharvest injury [9]. For these reasons, special care must be taken during postharvest storage to avoid the contamination of the peel by fungal pathogens, especially fungi, which are responsible for many postharvest losses [10].
One of the most aggressive microorganisms in protected crops is Colletotrichum gloeosporioides, which infects most tropical fruits, such as mango, banana, avocado, guava, and dragon fruit [11,12,13,14]. In addition, several other postharvest pathogenic agents can be latent in the field and reactivate during the storage process. These include Asperisporium caricae, Cercospora papaye, and Phomopsis caricae-papaye, which are responsible for necrotic lesions on the leaves [15,16,17], and several Alternaria species, such as A. alternata and A. solani, which are responsible for brown spots on the aerial and fleshy organs [18].
Likewise, the presence of Phomopsis caricae-papaye, Lasiodiplodia theobromae, Botryodiplodia theobromae, Mycospherella spp., and Phytophthora palmivora may cause infection in the tissues, including those within the pedicel supporting the fruit [19], whilst the presence of Cladosporium spp., Fusarium spp., and Penicillium spp. leads to the internal deterioration of the tissues, as mycelium attacks them aggressively until they become dark and moist [15].
Among the various fungi that cause postharvest diseases are the Ascomycetes or mitosporic fungi, such as the genera Aspergillus, Rhyzopus, and Mucor. Of the Mucor genus, Botrytis in particular is responsible for contaminating the fruit in the field that has not reached full maturity. The fruit products that are affected by this pathogen have a water-soaked appearance, and they then tend to darken until the tissue deteriorates.
Botrytis tends to develop in damp cold environments and in temperatures below 0 degrees. Attention must be paid to Aspergilli, which is dangerous because, in addition to causing persistent food spoilage, they can produce mycotoxins, including ochratoxin and aflatoxin [20,21,22]. To date, the presence of fungal diseases in the postharvest environment is managed with the use of synthetic fungicides, which can also improve the appearance of the fruit but can lead to resistance phenomena [10,23,24] and resistant isolates [12,25,26]. Therefore, it is necessary to move toward environmentally friendly approaches to control pathogenic fungal diseases in the postharvest environment and safeguard human health [27].
In fact, to combat the disproportionate use of these chemicals, sustainable alternatives based on natural products are being developed. Amongst these sustainable alternatives, essential oils and their co-products, termed hydrolates (or aromatic waters), are increasingly attracting the attention of researchers [28,29,30]. Hydrolates are obtained as a by-product during the steam distillation process of essential oils, and them are separated at the end of the distillation [31,32,33,34,35]. These natural products have the potential to be supplemented with hydrocolloids or mucilage. Therefore, they can be used as eco-friendly film enhancers affixed onto the surface of whole or ready-to-eat fruit, as they are also edible and extend the shelf life of fruit. These films, also termed edible coatings, can be made of plant-derived materials, such as the gels from Aloe vera L. and A. arborescens Mill. These are endowed with chemical gels, and the physical characteristics of the gels are suitable for the preservation of fruit and vegetables [5,36,37].
Amongst these properties, appreciable, antioxidant, and antimicrobial activities are essential to counter fruit browning [31]. A. vera contains 20 of the 22 amino acids required by the human diet, as well as 7 of the 8 essential amino acids. Therefore, it is a good source of vitamins that act as antioxidants and neutralize free radicals [38,39].
In recent studies, Aloe vera gels have been applied to papaya [36], apple [5], and pear [37] in the form of edible coatings, showing excellent results in terms of decreased weight loss, the maintenance of firmness, a reduction in browning, and fungal alterations on the surface of the treated fruit.
Essential oils (EOs) are a complex set of volatile compounds present in aromatic plants [40], and they can be extracted from different parts of a plant through a distillation process. Due to their bacteriostatic, bactericidal, and fungistatic activities, EOs have been suggested for postharvest use. Furthermore, their lipophilic characteristics play important roles in the rupture of fungal membranes and the denaturation of intercellular proteins [41]. Many aromatic plants are known at present, and they have been studied for their ability and potential to produce essential oils that are effective in the control of postharvest diseases in fresh fruit [42,43,44,45].
Oregano (Origanum vulgare L.) is one of the most important plants used in this field, and it has been proven to be effective in the control of postharvest gray mold in table grapes [46]. Sicilian oregano, Origanum vulgare subsp. viridulum (Martrin-Donos) Nyman, is a native species growing wild in the Sicilian countryside. As a wild species, it is strongly suited to a semi-arid environment, which is characterized by high temperatures and low rainfall [47]. According to Kokkini [48], the oregano genotypes growing in Southern Mediterranean areas are the highest EO-yielding genotypes, whereas the genotypes found in Northern European environments have lower EOs yields. Research on oregano EOs has shown that its active component may exhibit a high intraspecific variability, allowing for the differentiation of three chemogroups: (a) the linalool, terpinen-4-ol, and sabinene hydrate group; (b) the carvacrol and/or thymol group; and (c) the sesquiterpenes group [47,49]. The prevalence of one chemotype over another mostly depends on the genetic makeup of the plants [40,50]. Amongst the components characterizing oregano EOs, thymol has been shown to increase inhibitory activity against certain fungi, including Aspergillus flavus, and carvacrol has also been shown to exhibit good antifungal activity [51].
Hydrolates, by-products of distillation, are a complex combination of water-soluble active compounds obtained together with the corresponding essential oils, and these by-products are characterized by having the same antimicrobial activities of EOs. However, they are characterized by up to a maximum of 1% of the terpene components present in the corresponding essential oil [31], although to a lower extent. The application of hydrolates to foods is beneficial, as they protect food from fast deterioration and are also nontoxic to human health [52,53]. There is a growing interest in the application of edible coatings to manage the postharvest decay of fresh fruit [54].
The aim of this work was to develop an edible coating based on oregano essential oil and hydrolate for papaya. This study took into consideration the suitability of these edible coatings to provide protection against rapid deterioration in the postharvest period of fungal agents that could establish themselves, reduce the use of synthetic fungicides, and safeguard human health and improve food safety.
2. Materials and Methods
2.1. Vegetal Material
The fruits of Carica papaya cv. Solo were harvested at Farm Agropolis, located in Ragusa, Sicily (37°42′39.1″ N, 13°58′57.1″ E). The fruits were grown in a protected environment and collected at maturity stage 3 (MS3), according to the method described by Garillos-Manliguez and Chiang (2021). Fifty fruits without visible defects and injuries were collected from 5 different trees, and they were used in this study. Before treatment, every single fruit was sanified with NaClO 2% v/w for 20 min and left to dry and then washed with deionized water and air-dried.
Subsequently, the fruits were divided in two subsamples, and then they were treated with the two different coating formulations. The different applied treatments were stored in a refrigerated environment and at a temperature of 5 ± 1 °C with a 90–95% relative humidity for 25 days.
For the distillation and collection of both the oregano essential oil and hydrolate, twenty-seven kilograms of fresh biomass of O. vulgare L. subsp viridulum was sampled at the Filippone farm, located in Castellana Sicula, Sicily (37°42′39.1″ N, 13°58′57.1″ E) 971 m above sea level. To produce edible coatings, 1 kg of leaves of A. arborescens was collected from a catalogue field within the facilities of the University of Palermo. After harvesting and processing, the leaves were washed in running water and immersed in 100 µL-L−1 sodium hypochlorite for 5 min. They were then peeled by removing the lateral spines and the mesophyll was harvested, separated from the outer epidermis, using a stainless-steel knife. It was triturated using an ultra-Turrax T25 (Janke and Kunkle, IKa Labortechnik, Breisgau, Germany) for 5 min at 24,500 rpm to form a homogeneous substance and filtered to remove the fibrous elements. Five-hundred milliliters of extract was obtained [55].
2.2. Extraction of the Essential Oils and Hydrolates of O. vulgare subsp. viridulum
The hydrodistillation process took place after partially drying the biomass for a period of 3–4 days at 20 ± 1 °C to stabilize its moisture content to around 50–60%. For this purpose, the freshly harvested biomass was evenly spread out on sheets, protected from light to avoid altering the active ingredients [56,57,58]. EOs and hydrolates were obtained via hydrodistillation in a Clevenger-type apparatus (Albrigi Luigi Srl, Stallavena (VR), Italy) for 3 h [59]. The extraction yield (% v/w) was determined, and the essential oil and hydrolate were collected and separately stored at 4 ± 1 °C.
2.3. Essential Oil and Hydrolate Composition
Analyses were performed with an Agilent 7820A gas chromatograph interfaced to an Agilent 5977E mass spectrometer with single quadrupole electron ionization. The gas chromatograph method was as follows: Agilent DB-Wax UI polar capillary column, with a length of 60 mm, an internal diameter of 0.250 mm, and a film thickness of 0.5 mm; carrier gas Helium 5.5; a carrier flow of 1.2 mL/min; a starting temperature of 40 °C, 5 °C/min up to 200 °C, and 10 °C/min up to 240 °C; a spitless injection (1 min); an injected volume of 1 µL; and an injector temperature of 250 °C. The relative amount (percentages) of each volatile terpenoid is expressed as a percentage of the total volatile terpenoids [60,61].
2.4. Coating Formulations and Experimental Design
Edible coatings (ECs) were made entirely from natural sources, and two different treatments were undertaken:
- EC1: A. arborescens 25 mL + essential oil of O. vulgare subsp. viridulum 1 mL was added to 200 mL of distilled water.
- EC2: Hydrolate of O. vulgare subsp. viridulum 2 mL was added to 200 mL of distilled water.
EC1 and EC2 were then homogenized to create the coatings, and for them to be homogeneous in their coverage, an ultra-Turrax T25 (Janke and Kunkle, IKa Labortechnik, Breisgau, Germany) at 3000 rpm for 3 min was used. For each treatment, 20 fruits × 3 replications, homogeneous in shape and stage of ripeness (MS3), were used and stored in trays after treatment at 5 ± 1 °C for 25 days. The treated fruits were compared with an untreated sample (CTR). EC was obtained through the application of the following two different techniques:
- Spraying, using an N2-fed airbrush and 0.8 mm nozzles, achieving a film thickness on the fruit of about 3 mm;
- Brushing, using a food brush in top-down direction, thus covering the entire epicarp of the fruit and achieving a film thickness on the fruit of about 3 mm.
Pathological analyses were carried out during the storage to ascertain the whole observation period. Visual observations were carried out based on the presence or absence of mycelia and the level of contamination during the cold storage period. Once the presence of mycelia was ascertained, direct and indirect isolations were carried out to identify them.
On the last day of the trial, a physicochemical analysis was performed on the fresh fruits to assess their evolution compared to the fresh fruits at T0.
2.5. Daily Pathological Surveys
Surveys were carried out for a period of 25 days and consisted of the evaluation of symptoms (lesions) and signs (molds) on the fruits. The contamination level was assessed according to the classification suggested in [62]:
- No infection (N.I.), healthy fruit, level 0;
- Slight infection (S.I.), 1–4 lesions (spots), level 1;
- Moderate infection (M.I.), 5–10 lesions, level 2;
- High infection (H.I.), more than 10 lesions (fruit covered with spots), level 3.
2.6. Morphological Identification of External Fungal Contaminants
During the test, the fungal colonies developing on the epicarps of the fruits were observed and numbered, and their development was followed. The most frequent colonies were isolated directly and aseptically, on a universal sterile agar substrate (Potato Dextrose Agar, PDA, Oxoid), in 10 cm-diameter plates. The inoculated plates were incubated at 25 ± 1 °C and observed every 3 days. The growth colonies were first observed under a stereoscopic microscope (Zeiss, Oberkochen, Germany), and their microscopic characteristics (the shape, dimension, and color of the gamic and agamic structures, hyphal features, etc.) were taken into consideration to identify them. From the margin and the edge of each colony, small mycelial masses were aseptically taken with a needle and mounted onto slides with a drop of clear lactophenol (H2O 100 cc, glycerin 100 cc, lactic acid 100 cc, phenol crystals 100 g) or with lactophenol-methylene blue (0.01 p/v). The fungal structures were observed and measured with an Axioskop (Zeiss, Germany) microscope, and images were captured with an AxioCam MRc5 camera (Zeiss, Germany). The structure dimensions were observed with the software AxioVision 4.6. Finally, pure colonies were established for identification and morphological characterization [63,64,65].
2.7. Decay Index
The decay index was evaluated according to the methodology described by Morcia et al. (2012) [66,67]. The morphological damage caused by the infection of the contaminating fungi and the deterioration of the peel was evaluated visually and calculated using the following Equation (1):
where n is the number of fruits classified for each level of contamination, and N is the total number of fruits analyzed for each treatment in the time interval considered.
2.8. Physicochemical Analyses
A Minolta colorimeter (Chroma Meter CR-400, Konica Minolta Sensing Inc., Tokyo, Japan) was used to evaluate the color. Using the CIELAB L*a*b* system, the color space is shown as follows: brightness (L* value), red tendency (a*), and yellowness (b*). The saturation of the color, the chroma, (C* (2)), which represents the degree of color saturation (higher values indicate a brighter color and, consequently, a higher market value) was determined. These parameters were obtained from colorimetric unities as follows:
Prior to the analysis of the samples, the instrument was calibrated using a standard white plate.
The juice extracted from the papaya by means of a centrifuge (Ariete, Florence, Italy) was used to determine the TSS, expressed as °Brix, using a digital refractometer (Atago Co, Ltd., Tokyo, Japan) and the TA, expressed as grams of citric acid per liter, using a pH meter titrator (Crison Instruments, SA, Barcelona, Spain). Titration was carried out to a pH point of 8.2, using 5 mL of juice diluted with 50 mL of distilled water. Fruit firmness was assessed by using a digital penetrometer (mod. 53205, Turoni, Forlì, Italia) with an 8 mm tip, and it is expressed in Newton (N). The texture was evaluated on the two equatorial sides of each fruit. The mature index (M.I.) was determined using the following Equation (3) described in [68]:
2.9. Sensorial Analysis
Ten judges with extensive experience in the sensory evaluation of food performed a hedonic liking test on the papaya fruit (EC and CTR) previously prepared for the sensory analysis. This method uses a 9-point hedonic scale (1 = ‘extremely disliked’, 5 = ‘neither liked nor disliked’, 9 = ‘extremely liked’) [5,8]. The panel test consisted of a total of 21 parameters: visual parameters (pulp color and the presence of filaments), olfactory parameters (sea odor, peach odor, exotic fruit odor, medicinal odor cheese odor, burnt oil odor, and oregano odor), texture parameters (sour, sweet, bitter, juiciness, and flouriness), and, finally, aroma-related parameters (sea flavor, peach flavor, exotic fruit flavor, medicinal flavor, cheese flavor, burnt oil flavor, and oregano flavor). At the end of each tasting, a glass of water, for rinsing the mouth, was provided to the judges. In addition, all descriptors were assessed from day 0 (as fresh product) to the last day of storage (d 25).
2.10. Statistical Analysis
All collected data, presented as mean ± standard deviation (SD), were subjected to statistical analyses according to an experimental factorial scheme with 3 repetitions, using the statistical package Minitab 17.1 (Minitab Inc., State College, PA, USA, 2013). For each experimental variable, an analysis of variance (ANOVA) was performed, and differences among the mean values were appreciated using Tukey’s test at p ≤ 0.05.
3. Results and Discussion
3.1. Quantitative and Qualitative Analyses of O. vulgare subsp. viridulum Essential Oil
The results obtained from the chromatographic analysis of the volatile compounds (Table 1) show that the EOs contains a high percentage of thymol (39.11%), confirming that the chemotype of Sicilian oregano is “high thymol” and “low carvacrol” (0.68%), as also reported by Napoli et al. (2020) [47].

Table 1.
Relative contents (%) of volatile terpenoids of Oregano vulgare L. essential oil. EOs %: essential oil; Hydr.%.: hydrolate; W.D.%: water distillation.
In addition, γ-terpinene (15.97%) and p-cymene (8.20%) are also part of the phytocomplex. These compounds have already been used in food preservation [69] and in the prevention of fungal pathogens [70]. Their antimicrobial action is determined by the presence of the -OH group, which binds to cell membranes and damages them [71]. The hydrolate obtained from the hydrodistillation of the essential oil contains a high percentage of thymol (87.6%), followed by p-cymene (2.18%) and carvacrol (2.22%).
3.2. Daily Pathological Survey and Decay Severity Index
Table 2 shows the evaluations of the pathological observations during the storage period. No significant differences between the treatments were recorded in the first 10 days of observation. Starting from the 15th day of observation, in the CTR fruit, lesions and spots progressively and increasingly appeared until the 24th day, when more than 70% of the fruit showed a high level of contamination, i.e., level 3 (Figure 1). EC1 fruit, which showed increasing contamination from the 16th day of observation. At the end of the storage period, appeared more damaged than both CTR and EC2 fruit. The EC1 samples also reached a contamination level of 3. Contrastingly, the contamination trend of the EC2-treated fruit was significantly different. In fact, lesions formed from day 15 onward up until day 25. These remained confined to the affected area less than/equal to 25% of the entire surface. In fact, the deterioration index of EC2 fruit was the lowest compared to CTR and EC2 fruit, with a value of less than 2.

Table 2.
Development over time of daily mean values of contamination levels in fruit of C. papaya L. treated with different extracts from O. vulgare subsp. viridulum L. The levels of contamination (C.L.) are described as follows: N.I. (no infection—level 0); S.I. (slight infection, 1–4 lesions liv.1); M.I. (moderate infection, 5–10 lesions, liv.2); and H.I. (high infection, >10 lesions, liv.3). Each value refers to the average of three repetitions ± S.E. CTR = control; EC1 = A. arborescens and essential oil; and EC2 = only hydrolate. The values with the same letter are not significantly different at p ≤ 0.05 (Tukey’s test). The lowercase letters in the apex are used to indicate differences in time for the same treatment; the uppercase letters indicate differences between treatments at the same time of storage. For each treatment, different letters are indicated significant differences between sampling data.

Figure 1.
Representative fruits assigned to an empirical scale (0–3) of symptoms caused by fungal infection to quantify the incidence and severity of the disease on the papaya fruit: 0 = no visible symptoms (healthy fruit); 1 = 1–25% of the surface is covered with light mycelium (mild infection); 2 = 26–50% of the surface is covered with mycelium (moderate infection); 3 = 51–75% of the sample is necrotic with masses of spores appearing (strong infection).
The data, in Table 2, from day 15 until the last day of observation are shown, as there were no statistically significant data before then.
From the obtained data, we can state that there was no inhibitory activity exerted by the oregano essential oil combined with A. arborescens. This was also confirmed by the evaluation of the decay index; in the EC1 thesis, out of a range from 0 to 4, a value of 3.53 was achieved (Figure 2). A possible explanation for the high contamination of the papayas could be since, as reported in other studies [72,73], Aloe gel can act as a substrate for microbial growth, as it is rich in soluble solids, proteins, and lipids. The CTR fruit were affected with a severity index of 3.11, whilst the most effective inhibitory activity was shown in the fruit treated with EC2, i.e., with the hydrolate. The ANOVA showed that all three of these showed significant differences in the decay index over time (p ≤ 0.05).
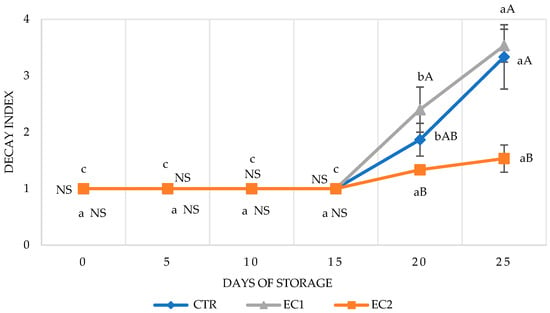
Figure 2.
Decay index (D.I.) rated from 1 to 4 on papaya fruit measured for 25 days post-treatment in cold storage 5 ± 1 °C. Values are expressed as mean ± SD. Means with the same letter are not significantly different at p ≤ 0.05 (Tukey’s test). CTR: control fruit; EC1: Aloe arborescens Mill. and essential oil; EC2: only hydrolate. Lowercase letters indicate differences between treatments at the same time of storage; uppercase letters indicate differences between treatments at the same time of storage. NS = non-significant (p ≤ 0.05).
The lowest decay index was found on the EC2 fruit (1.14), which exhibited mild circumscribed injuries. This inhibitory effect may be attributed to the presence of thymol and carvacrol [74]. These two compounds have been identified as antimicrobial [75] and antifungal agents due to their ability to influence and modify mycelium morphology, resulting in changes in the chitin and hyphae [76]. However, the biocidal effectiveness of the active components present in the oregano EOs was probably hampered by the presence of Aloe arborescens gel, rich in polysaccharides, lipids, sugars, and phenolic compounds [77], which contributed to the proliferation of the fungal agents, serving as a growth medium itself.
3.3. Morphological Identification of External Fungal Agents
The macroscopic and microscopic analyses of the isolated fungal colonies allowed for the identification the following fungal taxa: Cladosporium (Figure 3(a1–a3)), Alternaria (Figure 3(b1–b3)), and Botrytis (sp. cinerea) (Figure 3(c1–c3)). These pathogens were found in all treatments and the control (CTR, EC1, and EC2), with the difference being that, in the EC2-treated fruit, the formed lesions did not extend over the entire epicarp surface, remaining circumscribed and topical. These fungal taxa have been described in papaya fruit during the postharvest phase [42,73,78,79,80] and, as reported in the literature, are active in the quiescent phase during fruit ripening [81]. The genus Cladosporium, which causes greenish-black spots on papayas, is usually found confined to the skin surface, resulting in the aesthetic degradation of the fruit, which lowers its commercial value [82]. The genus Alternaria insinuates itself into host tissues through wounds or natural openings [83]. Its presence at the postharvest stage is of utmost importance since this fungus is a producer of mycotoxins (Alternaria toxins) that are harmful to human health and responsible for mutations, chromosomal aberrations, and DNA damage [84]. Furthermore, Botrytis cinerea is one of the best known and most common recurring infecting fungi in fruit, both in the field and postharvest. The colonization of this fungus usually occurs quiescently before harvest, and as the ripening stage continues, the fungus moves from a latent state to the active necrotrophic stage, thus causing gray mold [82,83,85].

Figure 3.
Most recurrent fungal Taxa isolated from naturally infected papaya fruit, identified by their macroscopic and microscopic features. Cladosporium (a1–a3), Alternaria (b1–b3), and Botrytis cinerea (c1–c3).
3.4. Physicochemical Analyses
As shown in the results of the physicochemical analysis (Table 3), the difference in the flesh color between the CTR and the treated fruit was particularly pronounced. Increases in the values of the color indices C* could be seen on the last day of storage. The fruits from the EC1 and EC treatments showed smaller increases in the values compared to those on day 0, while the CTR fruit showed an increase in color saturation from about 28 to 45 for the C* value. This results in a maturation process of the papaya fruit ripening during the observation period, which was slowed down in the EC-treated fruit. This confirms what has already been reported in the literature by Dhall and Salehi [86,87], according to whom, ECs, by creating a barrier on the fruit surface, slow down the rate of respiration and, thus, the rate of ripening. Indeed, through the development of these two parameters, chroma and hue angle, it is possible to understand when the degradation of chlorophyll starts to give way to the synthesis of carotenoids [88,89]. A relationship linking the synthesis of carotenoids to the change in color intensity is perfectly described by the chroma parameter [90]. The Chroma parameter was different in the CTR fruit, which had a duller and darker colour, as it was subjected to the ripening process, and, thus, its organic components degraded. Other parameters considered for assessing the degradation of organic acids and, thus, the synthesis of aromatic and sugar components are Brix° degrees and titratable acids (TAs). At time T0, higher TA values were recorded for the Brix° degrees in the fresh fruit than in the CTR fruit. This was made possible by the continuation of the ripening process of the fruit, which was even more exposed to this development, probably because it was not coated by the coatings [91]. Fruit firmness, which is one of the most important quality parameters used to evaluate a fruit’s crunchiness, decreased after 25 days of storage but without significant differences between treatments. Although all treatments were not statistically different from the control, the EC2 treatment maintained a higher firmness at d 25, and this can be attributed to the applied coatings, which, as reported by several studies, limit fruit respiration and evapotranspiration, slowing cell degradation phenomena and ethylene production [5,55]. Furthermore, many researchers [92,93,94] have reported how the inclusion of essential oils or their derivatives, as they contain bioactive compounds, can maintain fruit firmness because they can reduce fruit respiration and, in particular, the activity of cell wall hydrolases, thus preventing transpiration and water loss. This makes it possible to preserve the crispness and freshness of a fruit product, an increasingly important requirement for today’s consumers [95].

Table 3.
Physicochemical characteristics of fresh C. papaya fruit (F.f.) at d 0 and on last day of storage (d25). Treatments (Tr): CTR = control; EC1 = A. arborescens with EOs of Origanum vulgare subsp. viridulum; EC2 = hydrolate- Lightness (L*), redness (a*), yellowness (b*), chroma (C*); titratable acidity (TA), total soluble solids (TSSs); maturity index (MI), firmness (FF). Values means ± S.E. The processed results are respectively mean ± standard error (S.E.) of three replicates. Values with the same letter are not significantly different at p ≤ 0.05 (Tukey’s test). Lowercase letters in the apex are used to indicate differences in time for the same treatment; uppercase letters indicate differences between treatments at the same time of storage.
3.5. Sensorial Analysis
The sensory evaluation showed that, after the entire storage period, juiciness and pulp color were the parameters most appreciated by consumers in all three treatments (Figure 4). In particular, the pulp color descriptor obtained a value of 5.83 in all three treatments, while the juiciness descriptor obtained high values in the EC2 treatment (6.33). The sweetness descriptor obtained higher scores in the CTR and EC1(respectively) treatments than in the EC2 treatment while concerning exotic fruit odor both treatments (EC1 and EC2) has shown higher values than CTR. For the negative descriptors, i.e., those related to the presence of filaments, sea odor, medicinal odor, cheese odor, burnt oil odor, oregano odor, acidity, bitterness, flouriness, and sea aroma, the values obtained were about 1 (absence of the descriptor) in both the CTR and EC-treated fruits. Hence, we can state that, although the epicarp of the fruit was damaged by the presence of pathogens, particularly in the CTR and EC1 treatments, this did not alter the sensory quality of the pulp.
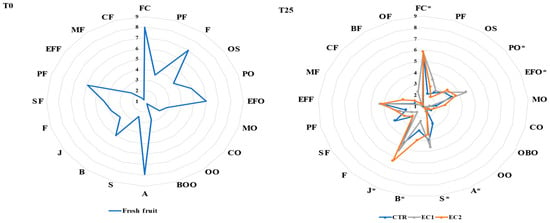
Figure 4.
Sensory analysis at T0 and T25 of C. papaya descriptor legend: flesh color (FC*), presence of filaments (PF), odor of sea (OS), peach odor (PO*), exotic fruit odor (EFO*), medicinal odor (MO), cheese odor (CO), odor of burnt oil (OBO), odor of oregano (OO), acid (A*), sweet (S*), bitter (B*), juiciness (J), flouriness (F), sea flavor (SF), peach flavor (PF), exotic fruit flavor (EFF), medicinal flavor (MF), cheese flavor (CF), burnt oil flavor (BF), oregano flavor (OF). The descriptors marked with an * were those that reported a statistically significant value of p ≤ 0.05.
4. Conclusions
This paper found that EC1 edible coatings, with A. arborescens and oregano essential oil, had no positive effect on retarding or reducing fungal growth. This suggests that the film may have acted as a growth substrate for external fungal agents. However, the antifungal and antimicrobial activities of O. vulgare subsp. viridulum were particularly evident in the hydrolate treatment because, although lesions and spots formed on the epicarp of the fruit, they remained well circumscribed from the beginning of the contamination until the end of the observation period. Furthermore, the EC2 treatment responded positively to both the spraying and brushing application methods. Both application methods equally ensured that the characteristics of the C. papaya fruit were not altered during the trial. Finally, the sensory analysis performed to evaluate the organoleptic characteristics of the fruit showed mostly positive parameters; an interesting oregano aroma was perceived in both of the EC1 and EC2 treatments. In conclusion, the treatment with hydrolate (EC2), which has always been regarded a waste product of the distillation process, can be considered a promising alternative to improve the safety of papaya fruit and to prolong its shelf life postharvest.
Author Contributions
Conceptualization, A.C. (Alessandra Culmone), I.T. and V.F.; methodology, V.F., L.T., I.T., A.C. (Alessandra Carrubba), M.G.B. and G.R.; formal analysis, A.C. (Alessandra Culmone), I.T., M.M. and G.M.; investigation, A.C. (Alessandra Culmone), I.T., M.M. and G.M.; data curation, A.C. (Alessandra Culmone), I.T., V.F., A.C. (Alessandra Carrubba) and L.T.; writing—original draft preparation, A.C. (Alessandra Culmone), I.T. and V.F.; writing—review and editing, V.F., L.T., I.T., M.M., G.R., M.G.B., A.C. (Alessandra Carrubba) and A.C. (Alessandra Culmone); supervision, V.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
A special acknowledgement is addressed to “Istituto per la Protezione Sostenibile delle Piante, Consiglio Nazionale delle Ricerche, Sesto Fiorentino, Italy” for the analysis of the essential oil and hydrolate. We would like to thank the companies Agropolis, l’Orto di Rosolino, and Filippone for kindly providing us with the plant material for the experiment; Roberta Passafiume, Vincenzo Guarino, Pasquale Roppolo, and Alessandro Ruggeri for collaborating and providing support throughout the testing phase; and Claire Roberson for her editing. The authors dedicate this paper to the memory of Dott. Salvatore Scianò.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gustafson, A.; Christian, J.W.; Lewis, S.; Moore, K.; Jilcott, S. Food Venue Choice, Consumer Food Environment, but Not Food Venue Availability within Daily Travel Patterns Are Associated with Dietary Intake among Adults, Lexington Kentucky 2011. Nutr. J. 2013, 12, 17. [Google Scholar] [CrossRef] [PubMed]
- Pessu, P.; Agoda, S.; Isong, I.; Ikotun, I. The Concepts and Problems of Post-harvest Food Losses in Perishable Crops. Afr. J. Food Sci. 2011, 5, 603–613. [Google Scholar]
- Prasad, K.; Paul, J.R. Post-harvest Losses of Papaya and Practice for Management. Food Sci. Rep. 2021, 2, 7. [Google Scholar]
- Farina, V.; Passafiume, R.; Tinebra, I.; Scuderi, D.; Saletta, F.; Gugliuzza, G.; Gallotta, A.; Sortino, G. Post-harvest Application of Aloe vera Gel-Based Edible Coating to Improve the Quality and Storage Stability of Fresh-Cut Papaya. J. Food Qual. 2020, 2020, 8303140. [Google Scholar] [CrossRef]
- Adiletta, G.; Di Matteo, M.; Albanese, D.; Farina, V.; Cinquanta, L.; Corona, O.; Magri, A.; Petriccione, M. Changes in physico-chemical traits and enzymes oxidative system during cold storage of ‘formosa’ papaya fresh cut fruit grown in the Mediterranean area (Sicily). Ital. J. Food Sci. 2020, 32, 3483–3492. [Google Scholar] [CrossRef]
- Migliore, G.; Farina, V.; Tinervia, S.; Matranga, G.; Schifani, G. Consumer Interest towards Tropical Fruit: Factors Affecting Avocado Fruit Consumption in Italy. Agric. Food Econ. 2017, 5, 24. [Google Scholar] [CrossRef]
- Sortino, G.; Caviglia, V.; Liguori, G.; De Pasquale, C.; Gianguzzi, G.; Farina, V. Quality Changes of Tropical and Subtropical Fresh-Cut Fruit Mix in Modified Atmosphere Packaging. Chem. Eng. 2017, 58, 397–402. [Google Scholar]
- Tinebra, I.; Sortino, G.; Inglese, P.; Fretto, S.; Farina, V. Effect of Different Modified Atmosphere Packaging on the Quality of Mulberry Fruit (Morus alba L. Cv Kokuso 21). Int. J. Food Sci. 2021, 2021, 8844502. [Google Scholar] [CrossRef]
- Sivakumar, D.; Tuna Gunes, N.; Romanazzi, G. A Comprehensive Review on the Impact of Edible Coatings, Essential Oils, and Their Nano Formulations on Post-harvest Decay Anthracnose of Avocados, Mangoes, and Papayas. Front. Microbiol. 2021, 12, 711092. [Google Scholar] [CrossRef]
- Alvarez, A.; Nishijima, W. Post-harvest Diseases of Papaya. Plant Dis. 1987, 71, 681–686. [Google Scholar] [CrossRef]
- Baños-Guevara, P.E.; Zavaleta Mejía, E.; Colinas-León, M.; Luna-Romero, I.; Gutiérrez-Alonso, J. Control Biológico de Colletotrichum gloeosporioides [(Penz.) Penz. y Sacc.] En Papaya Maradol Roja (Carica papaya L.) y Fisiología Postcosecha de Frutos Infectados. Rev. Mex. Fitopatol. 2004, 22, 198–205. [Google Scholar]
- Ventura, J.A.; Costa, H.; Tatagiba, J.d.S. Papaya Diseases and Integrated Control. In Diseases of Fruit and Vegetables: Volume II; Springer: Berlin/Heidelberg, Germany, 2004; pp. 201–268. [Google Scholar]
- Yon, R.M. Papaya: Fruit Development, Post-Harvest Physiology, Handling and Marketing in ASEAN; ASEAN Food Handling Bureau: Kuala Lumpur, Malaysia, 1994; ISBN 967-9932-22-2. [Google Scholar]
- Zakaria, L. Diversity of Colletotrichum Species Associated with Anthracnose Disease in Tropical Fruit Crops—A Review. Agriculture 2021, 11, 297. [Google Scholar] [CrossRef]
- Echerenwa, M.C.; Umechuruba, C. Post-Harvest Fungal Diseases of Pawpaw (Carica papaya L.) Fruit and Seeds in Nigeria. Glob. J. Pure Appl. Sci. 2004, 10, 69–73. [Google Scholar] [CrossRef]
- Kumaran, K. Unit-2 Papaya (Carica papaya Linn.); Indira Gandhi National Open University: New Delhi, India, 2021. [Google Scholar]
- Vivas, M.; Silveira, S.F.d.; Terra, C.E.P.d.S.; Pereira, M.G. Testers for Combining Ability and Selection of Papaya Hybrids Resistant to Fungal Diseases. Crop. Breed. Appl. Biotechnol. 2011, 11, 36–42. [Google Scholar] [CrossRef]
- Vawdrey, L.; Grice, K.; Westerhuis, D. Field and Laboratory Evaluations of Fungicides for the Control of Brown Spot (Corynespora cassiicola) and Black Spot (Asperisporium caricae) of Papaya in Far North Queensland, Australia. Australas. Plant Pathol. 2008, 37, 552–558. [Google Scholar] [CrossRef]
- Chau, K.; Alvarez, A.M. Role of Mycosphaerella ascospores in Stem-End Rot of Papaya Fruit. Phytopathology 1979, 69, 500–503. [Google Scholar] [CrossRef]
- Abarca, M.L.; Accensi, F.; Cano, J.; Cabañes, F.J. Taxonomy and Significance of Black Aspergilli. Antonie Van Leeuwenhoek 2004, 86, 33–49. [Google Scholar] [CrossRef]
- Abbas, H.K.; Shier, W.; Horn, B.; Weaver, M. Cultural Methods for Aflatoxin Detection. J. Toxicol. Toxin Rev. 2004, 23, 295–315. [Google Scholar] [CrossRef]
- Oliveri, C.; Torta, L.; Catara, V. A Polyphasic Approach to the Identification of Ochratoxin A-Producing Black Aspergillus Isolates from Vineyards in Sicily. Int. J. Food Microbiol. 2008, 127, 147–154. [Google Scholar] [CrossRef]
- Couey, H.; Alvarez, A.; Nelson, M. Comparison of Hot-Water Spray and Immersion Treatments for Control of Post-harvest Decay of Papaya. Plant Dis. 1984, 68, 436–437. [Google Scholar] [CrossRef]
- Couey, H. Control of Post-harvest Decay of Papaya. HortScience 1979, 14, 719–721. [Google Scholar] [CrossRef]
- da Silva Pereira, A.V.; Martins, R.B.; Michereff, S.J.; Da Silva, M.B.; Câmara, M.P.S. Sensitivity of Lasiodiplodia theobromae from Brazilian Papaya Orchards to MBC and DMI Fungicides. Eur. J. Plant Pathol. 2012, 132, 489–498. [Google Scholar] [CrossRef]
- Dembele, A.; Traore, S.K.; Kone, M.; Coulibaly, D.T. Export Papaya Post-Harvest Protection by Fungicides and the Problems of the Maximal Limit of Residues. Afr. J. Biotechnol. 2005, 4, 109–112. [Google Scholar]
- Wisniewski, M.; Droby, S.; Norelli, J.; Liu, J.; Schena, L. Alternative Management Technologies for Post-harvest Disease Control: The Journey from Simplicity to Complexity. Post-Harvest Biol. Technol. 2016, 122, 3–10. [Google Scholar] [CrossRef]
- Bosquez-Molina, E.; Ronquillo-de Jesús, E.; Bautista-Baños, S.; Verde-Calvo, J.; Morales-López, J. Inhibitory Effect of Essential Oils against Colletotrichum gloeosporioides and Rhizopus stolonifer in Stored Papaya Fruit and Their Possible Application in Coatings. Post-Harvest Biol. Technol. 2010, 57, 132–137. [Google Scholar] [CrossRef]
- Du Plooy, W.; Regnier, T.; Combrinck, S. Essential Oil Amended Coatings as Alternatives to Synthetic Fungicides in Citrus Post-harvest Management. Post-Harvest Biol. Technol. 2009, 53, 117–122. [Google Scholar] [CrossRef]
- Espitia, P.J.P.; Soares, N.d.F.F.; Botti, L.C.M.; Melo, N.R.d.; Pereira, O.L.; Silva, W.A.d. Assessment of the Efficiency of Essential Oils in the Preservation of Post-harvest Papaya in an Antimicrobial Packaging System. Braz. J. Food Technol. 2012, 15, 333–342. [Google Scholar] [CrossRef]
- Aćimović, M.G.; Tešević, V.V.; Smiljanić, K.T.; Cvetković, M.T.; Stanković, J.M.; Kiprovski, B.M.; Sikora, V.S. Hydrolates: By-Products of Essential Oil Distillation: Chemical Composition, Biological Activity and Potential Uses. Adv. Technol. 2020, 9, 54–70. [Google Scholar] [CrossRef]
- D’Amato, S.; Serio, A.; López, C.C.; Paparella, A. Hydrosols: Biological Activity and Potential as Antimicrobials for Food Applications. Food Control 2018, 86, 126–137. [Google Scholar] [CrossRef]
- Pellegrini, M.; Rossi, C.; Palmieri, S.; Maggio, F.; Chaves-López, C.; Lo Sterzo, C.; Paparella, A.; De Medici, D.; Ricci, A.; Serio, A. Salmonella Enterica Control in Stick Carrots through Incorporation of Coriander Seeds Essential Oil in Sustainable Washing Treatments. Front. Sustain. Food Syst. 2020, 4, 14. [Google Scholar] [CrossRef]
- Perito, M.A.; Chiodo, E.; Serio, A.; Paparella, A.; Fantini, A. Factors Influencing Consumers’ Attitude towards Biopreservatives. Sustainability 2020, 12, 10338. [Google Scholar] [CrossRef]
- Saǧdıç, O.; Özcan, M. Antibacterial Activity of Turkish Spice Hydrosols. Food Control 2003, 14, 141–143. [Google Scholar] [CrossRef]
- Farina, V.; Tinebra, I.; Perrone, A.; Sortino, G.; Palazzolo, E.; Mannino, G.; Gentile, C. Physicochemical, Nutraceutical and Sensory Traits of Six Papaya (Carica papaya L.) Cultivars Grown in Greenhouse Conditions in the Mediterranean Climate. Agronomy 2020, 10, 501. [Google Scholar] [CrossRef]
- Passafiume, R.; Gugliuzza, G.; Gaglio, R.; Busetta, G.; Tinebra, I.; Sortino, G.; Farina, V. Aloe-Based Edible Coating to Maintain Quality of Fresh-Cut Italian Pears (Pyrus communis L.) during Cold Storage. Horticulturae 2021, 7, 581. [Google Scholar] [CrossRef]
- Erdemli, M.E.; Akgül, H.; Ege, B.; Aksungur, Z.; Gozukara, H.; Selamoglu, Z. The Effects of Grapeseed Extract and Low Level Laser Therapy Administration on the Liver in Experimentally Fractured Mandible. J. Turgut Ozal Med Cent. 2017, 24, 127–133. [Google Scholar] [CrossRef]
- Selamoglu, Z.; Dusgun, C.; Akgul, H.; Gulhan, M.F. In-Vitro Antioxidant Activities of the Ethanolic Extracts of Some Contained-Allantoin Plants. Iran. J. Pharm. Res. IJPR 2017, 16, 92. [Google Scholar]
- Novak, J.; Lukas, B.; Franz, C. Temperature Influences Thymol and Carvacrol Differentially in Origanum Spp. (Lamiaceae). J. Essent. Oil Res. 2010, 22, 412–415. [Google Scholar] [CrossRef]
- Prakash, B.; Singh, P.; Kedia, A.; Dubey, N. Assessment of Some Essential Oils as Food Preservatives Based on Antifungal, Antiaflatoxin, Antioxidant Activities and in Vivo Efficacy in Food System. Food Res. Int. 2012, 49, 201–208. [Google Scholar] [CrossRef]
- Sivakumar, D.; Romanazzi, G. Use of Essential Oils to Improve Post-harvest Quality and Control Post-harvest Decay of Tropical, Subtropical, and Temperate Fruit. In Post-Harvest Pathology of Fresh Horticultural Produce; CRC Press: Boca Raton, FL, USA, 2019; pp. 659–676. [Google Scholar]
- Cindi, M.D.; Soundy, P.; Romanazzi, G.; Sivakumar, D. Different Defense Responses and Brown Rot Control in Two Prunus persica Cultivars to Essential Oil Vapours after Storage. Post-Harvest Biol. Technol. 2016, 119, 9–17. [Google Scholar] [CrossRef]
- Landi, L.; Peralta-Ruiz, Y.; Chaves-López, C.; Romanazzi, G. Chitosan Coating Enriched with Ruta graveolens L. Essential Oil Reduces Postharvest Anthracnose of Papaya (Carica papaya L.) and Modulates Defense-Related Gene Expression. Front. Plant Sci. 2021, 12, 765806. [Google Scholar] [CrossRef]
- Reichling, J.; Schnitzler, P.; Suschke, U.; Saller, R. Essential Oils of Aromatic Plants with Antibacterial, Antifungal, Antiviral, and Cytotoxic Properties–an Overview. Complement. Med. Res. 2009, 16, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Servili, A.; Feliziani, E.; Romanazzi, G. Exposure to Volatiles of Essential Oils Alone or under Hypobaric Treatment to Control Post-harvest Gray Mold of Table Grapes. Post-Harvest Biol. Technol. 2017, 133, 36–40. [Google Scholar] [CrossRef]
- Napoli, E.; Giovino, A.; Carrubba, A.; How Yuen Siong, V.; Rinoldo, C.; Nina, O.; Ruberto, G. Variations of Essential Oil Constituents in Oregano (Origanum vulgare Subsp. viridulum (= O. Heracleoticum) over Cultivation Cycles. Plants 2020, 9, 1174. [Google Scholar] [CrossRef] [PubMed]
- Sivropoulou, A.; Papanikolaou, E.; Nikolaou, C.; Kokkini, S.; Lanaras, T.; Arsenakis, M. Antimicrobial and Cytotoxic Activities of Origanum Essential Oils. J. Agric. Food Chem. 1996, 44, 1202–1205. [Google Scholar] [CrossRef]
- Skoula, M.; Harborne, J. Oregano: The Genera Origanum and Lippia; Taxonomy and Chemistry of Origanum. Medicinal and Aromatic Plants Industrial Profiles; Kintzios, S.E., Ed.; CRC Press: Boca Raton, FL, USA, 2002; pp. 67–108. [Google Scholar]
- Crocoll, C.; Asbach, J.; Novak, J.; Gershenzon, J.; Degenhardt, J. Terpene Synthases of Oregano (Origanum vulgare L.) and Their Roles in the Pathway and Regulation of Terpene Biosynthesis. Plant Mol. Biol. 2010, 73, 587–603. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.K.; Singh, P.; Prakash, B.; Kedia, A.; Dubey, N.K.; Chanotiya, C. Assessing Essential Oil Components as Plant-Based Preservatives against Fungi That Deteriorate Herbal Raw Materials. Int. Biodeterior. Biodegrad. 2013, 80, 16–21. [Google Scholar] [CrossRef]
- Chorianopoulos, N.; Giaouris, E.; Skandamis, P.; Haroutounian, S.; Nychas, G. Disinfectant Test against Monoculture and Mixed-culture Biofilms Composed of Technological, Spoilage and Pathogenic Bacteria: Bactericidal Effect of Essential Oil and Hydrosol of Satureja thymbra and Comparison with Standard Acid–Base Sanitizers. J. Appl. Microbiol. 2008, 104, 1586–1596. [Google Scholar] [CrossRef]
- Garneau, F.-X.; Collin, G.; Gagnon, H. Chemical Composition and Stability of the Hydrosols Obtained during Essential Oil Production. I. The Case of Melissa officinalis L. and Asarum canadense L. Am. J. Essent. Oils Nat. Prod. 2014, 2, 54–62. [Google Scholar]
- Romanazzi, G.; Moumni, M. Chitosan and Other Edible Coatings to Extend Shelf Life, Manage Post-harvest Decay, and Reduce Loss and Waste of Fresh Fruit and Vegetables. Curr. Opin. Biotechnol. 2022, 78, 102834. [Google Scholar] [CrossRef]
- Passafiume, R.; Gaglio, R.; Sortino, G.; Farina, V. Effect of Three Different Aloe vera Gel-Based Edible Coatings on the Quality of Fresh-Cut “Hayward” Kiwifruit. Foods 2020, 9, 939. [Google Scholar] [CrossRef]
- Caputo, L.; Amato, G.; de Bartolomeis, P.; De Martino, L.; Manna, F.; Nazzaro, F.; De Feo, V.; Barba, A.A. Impact of Drying Methods on the Yield and Chemistry of Origanum vulgare L. Essential Oil. Sci. Rep. 2022, 12, 3845. [Google Scholar] [CrossRef] [PubMed]
- Rocha, R.; Melo, E.; Raduuml, L. Influence of Drying Process on the Quality of Medicinal Plants: A Review. J. Med. Plants Res. 2011, 5, 7076–7084. [Google Scholar] [CrossRef]
- Thamkaew, G.; Sjöholm, I.; Galindo, F.G. A Review of Drying Methods for Improving the Quality of Dried Herbs. Crit. Rev. Food Sci. Nutr. 2021, 61, 1763–1786. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.F.; Dwivedi, A.K. A Review on Techniques Available for the Extraction of Essential Oils from Various Plants. Int. Res. J. Eng. Technol. 2018, 5, 5–8. [Google Scholar]
- Di Vito, M.; Bellardi, M.G.; Mondello, F.; Modesto, M.; Michelozzi, M.; Bugli, F.; Sanguinetti, M.; Sclocchi, M.C.; Sebastiani, M.L.; Biffi, S. Monarda citriodora Hydrolate vs Essential Oil Comparison in Several Anti-Microbial Applications. Ind. Crops Prod. 2019, 128, 206–212. [Google Scholar] [CrossRef]
- Marriott, P.J.; Shellie, R.; Cornwell, C. Gas Chromatographic Technologies for the Analysis of Essential Oils. J. Chromatogr. A 2001, 936, 1–22. [Google Scholar] [CrossRef]
- Saleh, I.; Al-Thani, R. Fungal Food Spoilage of Supermarkets’ Displayed Fruit. Vet. World 2019, 12, 1877. [Google Scholar] [CrossRef]
- Mirabile, G.; Cirlincione, F.; Venturella, G.; Torta, L. Seed Vitality and Fungal Contamination in Abies nebrodensis. Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2022, 1–7. [Google Scholar] [CrossRef]
- Torres-Calzada, C.; Tapia-Tussell, R.; Higuera-Ciapara, I.; Perez-Brito, D. Morphological, Pathological and Genetic Diversity of Colletotrichum Species Responsible for Anthracnose in Papaya (Carica papaya L). Eur. J. Plant Pathol. 2013, 135, 67–79. [Google Scholar] [CrossRef]
- Torta, L.; Burruano, S.; Giambra, S.; Conigliaro, G.; Piazza, G.; Mirabile, G.; Pirrotta, M.; Calvo, R.; Bellissimo, G.; Calvo, S. Cultivable Fungal Endophytes in Roots, Rhizomes and Leaves of Posidonia Oceanica (L.) Delile along the Coast of Sicily, Italy. Plants 2022, 11, 1139. [Google Scholar] [CrossRef]
- Barrera Bello, E.; Gil Loaiza, M.; García Pajón, C.M.; Durango Restrepo, D.L.; Gil González, J.H. Empleo de Un Recubrimiento Formulado Con Propóleos Para El Manejo Poscosecha de Frutos de Papaya (Carica papaya L. Cv. Hawaiana). Rev. Fac. Nac. Agron. Medellín 2012, 65, 6497–6506. [Google Scholar]
- Perdones, A.; Sánchez-González, L.; Chiralt, A.; Vargas, M. Effect of Chitosan–Lemon Essential Oil Coatings on Storage-Keeping Quality of Strawberry. Post-Harvest Biol. Technol. 2012, 70, 32–41. [Google Scholar] [CrossRef]
- Peralta-Ruiz, Y.; Tovar, C.G.; Sinning-Mangonez, A.; Bermont, D.; Cordero, A.P.; Paparella, A.; Chaves-López, C. Colletotrichum gloesporioides Inhibition Using Chitosan-Ruta Graveolens L Essential Oil Coatings: Studies in Vitro and in Situ on Carica papaya Fruit. Int. J. Food Microbiol. 2020, 326, 108649. [Google Scholar] [CrossRef] [PubMed]
- Gaba, A.B.M.; Hassan, M.A.; Abd EL-Tawab, A.A.; Abdelmonem, M.A.; Morsy, M.K. Protective Impact of Chitosan Film Loaded Oregano and Thyme Essential Oil on the Microbial Profile and Quality Attributes of Beef Meat. Antibiotics 2022, 11, 583. [Google Scholar] [CrossRef]
- Mueller-Riebau, F.; Berger, B.; Yegen, O. Chemical Composition and Fungitoxic Properties to Phytopathogenic Fungi of Essential Oils of Selected Aromatic Plants Growing Wild in Turkey. J. Agric. Food Chem. 1995, 43, 2262–2266. [Google Scholar] [CrossRef]
- Lv, F.; Liang, H.; Yuan, Q.; Li, C. In Vitro Antimicrobial Effects and Mechanism of Action of Selected Plant Essential Oil Combinations against Four Food-Related Microorganisms. Food Res. Int. 2011, 44, 3057–3064. [Google Scholar] [CrossRef]
- Bautista-Baños, S.; Sivakumar, D.; Bello-Pérez, A.; Villanueva-Arce, R.; Hernández-López, M. A Review of the Management Alternatives for Controlling Fungi on Papaya Fruit during the Post-harvest Supply Chain. Crop Prot. 2013, 49, 8–20. [Google Scholar] [CrossRef]
- Paull, R.E.; Nishijima, W.; Reyes, M.; Cavaletto, C. Post-harvest Handling and Losses during Marketing of Papaya (Carica papaya L.). Post-Harvest Biol. Technol. 1997, 11, 165–179. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, S.; Du, S.; Chen, S.; Sun, H. Antifungal Activity of Thymol and Carvacrol against Post-harvest Pathogens Botrytis cinerea. J. Food Sci. Technol. 2019, 56, 2611–2620. [Google Scholar] [CrossRef]
- Lambert, R.; Skandamis, P.N.; Coote, P.J.; Nychas, G. A Study of the Minimum Inhibitory Concentration and Mode of Action of Oregano Essential Oil, Thymol and Carvacrol. J. Appl. Microbiol. 2001, 91, 453–462. [Google Scholar] [CrossRef]
- Morcia, C.; Malnati, M.; Terzi, V. In Vitro Antifungal Activity of Terpinen-4-Ol, Eugenol, Carvone, 1, 8-Cineole (Eucalyptol) and Thymol against Mycotoxigenic Plant Pathogens. Food Addit. Contam. Part A 2012, 29, 415–422. [Google Scholar]
- Olennikov, D.; Ibragimov, T.; Nazarova, A.; Rokhin, A.; Zilfikarov, I. Chemical Composition of Aloe arborescens and Its Change by Biostimulation. Chem. Nat. Compd. 2009, 45, 478–482. [Google Scholar] [CrossRef]
- Bautista-Baños, S.; Hernández-López, M.; Bosquez-Molina, E.; Wilson, C.L. Effects of Chitosan and Plant Extracts on Growth of Colletotrichum gloeosporioides, Anthracnose Levels and Quality of Papaya Fruit. Crop Prot. 2003, 22, 1087–1092. [Google Scholar] [CrossRef]
- Bensch, K.; Groenewald, J.; Braun, U.; Dijksterhuis, J.; de Jesús Yáñez-Morales, M.; Crous, P.W. Common but Different: The Expanding Realm of Cladosporium. Stud. Mycol. 2015, 82, 23–74. [Google Scholar] [CrossRef]
- Fatima, N.; Batool, H.; Sultana, V.; Ara, J.; Ehteshamul-Haque, S. Prevalence of Post-Harvest Rot of Vegetables and Fruit in Karachi, Pakistan. Pak. J. Bot 2009, 41, 3185–3190. [Google Scholar]
- Amaral, D.D.; Monteiro, A.L.R.; Silva, E.I.D.; Lins, S.R.D.O.; Oliveira, S. Frequency of Quiescent Fungi and Post-Harvest Alternative Management of Stem End Rot in Papaya. Rev. Caatinga 2017, 30, 786–793. [Google Scholar] [CrossRef]
- Silveira, N.; Mariano, R.; Michereff, S.; Maia, L.; Oliveira, S. Hongos Fitopatógenos Asociados a Frutos Comercializados En Recife, Pernambuco (Brasil). Boletín Micológico 2001, 16. [Google Scholar] [CrossRef]
- Pearson, R.; Hall, D.H. Factors Affecting the Occurence and Severity of Blackmold of Ripe Tomato Fruit Caused by Alternaria alternata. Phytopathology 1975, 65, 1352–1359. [Google Scholar]
- Escrivá, L.; Oueslati, S.; Font, G.; Manyes, L. Alternaria Mycotoxins in Food and Feed: An Overview. J. Food Qual. 2017, 2017, 1569748. [Google Scholar] [CrossRef]
- Bautista-Baños, S. Post-Harvest Decay: Control Strategies; Elsevier: Amsterdam, The Netherlands, 2014; ISBN 0-12-411568-3. [Google Scholar]
- Salehi, F. Edible Coating of Fruit and Vegetables Using Natural Gums: A Review. Int. J. Fruit Sci. 2020, 20, S570–S589. [Google Scholar] [CrossRef]
- Dhall, R. Advances in Edible Coatings for Fresh Fruit and Vegetables: A Review. Crit. Rev. Food Sci. Nutr. 2013, 53, 435–450. [Google Scholar] [CrossRef]
- Elmer, P.; Reglinski, T. Biosuppression of Botrytis cinerea in Grapes. Plant Pathol. 2006, 55, 155–177. [Google Scholar] [CrossRef]
- Schweiggert, R.M.; Steingass, C.B.; Mora, E.; Esquivel, P.; Carle, R. Carotenogenesis and Physico-Chemical Characteristics during Maturation of Red Fleshed Papaya Fruit (Carica papaya L.). Food Res. Int. 2011, 44, 1373–1380. [Google Scholar] [CrossRef]
- Talcott, S.T.; Moore, J.P.; Lounds-Singleton, A.J.; Percival, S.S. Ripening Associated Phytochemical Changes in Mangos (Mangifera indica) Following Thermal Quarantine and Low-temperature Storage. J. Food Sci. 2005, 70, C337–C341. [Google Scholar] [CrossRef]
- Hashemi, S.M.B.; Khaneghah, A.M.; Ghahfarrokhi, M.G.; Eş, I. Basil-Seed Gum Containing Origanum vulgare Subsp. Viride Essential Oil as Edible Coating for Fresh Cut Apricots. Post-Harvest Biol. Technol. 2017, 125, 26–34. [Google Scholar] [CrossRef]
- de Souza, E.L.; Lundgren, G.A.; de Oliveira, K.Á.; Berger, L.R.; Magnani, M. An Analysis of the Published Literature on the Effects of Edible Coatings Formed by Polysaccharides and Essential Oils on Post-harvest Microbial Control and Overall Quality of Fruit. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1947–1967. [Google Scholar] [CrossRef]
- Ju, J.; Xie, Y.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Application of Edible Coating with Essential Oil in Food Preservation. Crit. Rev. Food Sci. Nutr. 2019, 59, 2467–2480. [Google Scholar] [CrossRef]
- Pirozzi, A.; Del Grosso, V.; Ferrari, G.; Donsì, F. Edible Coatings Containing Oregano Essential Oil Nanoemulsion for Improving Post-harvest Quality and Shelf Life of Tomatoes. Foods 2020, 9, 1605. [Google Scholar] [CrossRef]
- Choi, W.S.; Singh, S.; Lee, Y.S. Characterization of Edible Film Containing Essential Oils in Hydroxypropyl Methylcellulose and Its Effect on Quality Attributes of ‘Formosa’ Plum (Prunus salicina L.). LWT 2016, 70, 213–222. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).