Antisense Overexpression of Gγ Subunit CsGG3.1-2 Reduces Soluble Sugar Content and Chilling Tolerance in Cucumber
Abstract
1. Introduction
2. Materials and Methods
2.1. Vector Construction and Plant Transformation
2.2. Plant Materials and Cold Treatments
2.3. Leaf Chlorophyll Fluorescence Imaging
2.4. Biochemical Analysis Assays
2.5. Quantitative Real-Time PCR
2.6. Chilling Tolerance Assessment
2.7. Data Analyses
3. Results
3.1. Overexpression of Antisense CsGG3.1-2 Reduced the Chilling Tolerance of the Transgenic Cucumbers
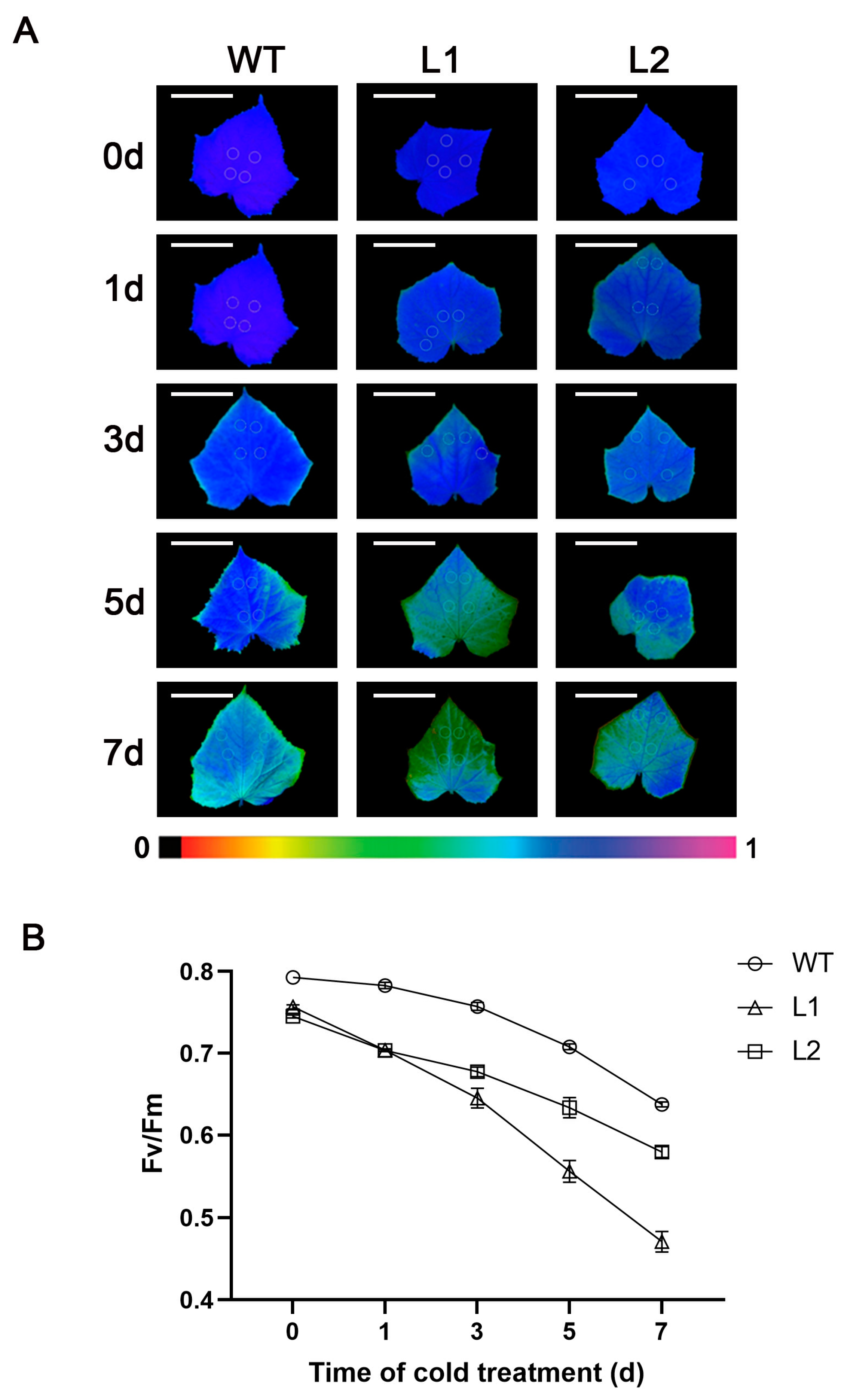
3.2. Overexpression of Antisense CsGG3.1-2 Reduces Chlorophyll Fluorescence Parameters (Fv/Fm)
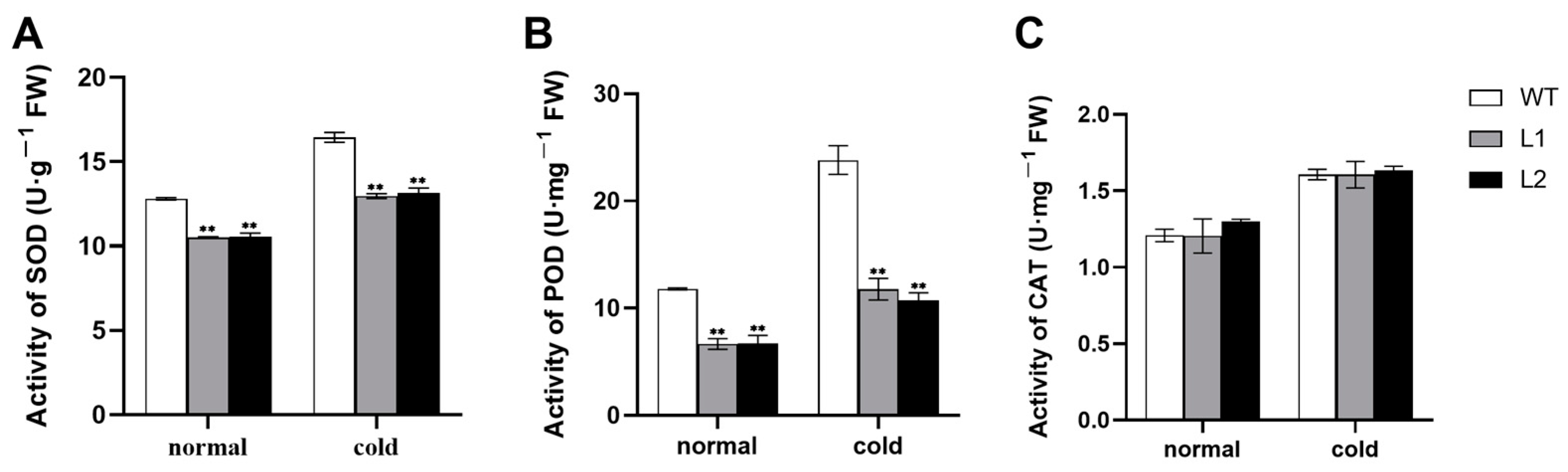
3.3. Activities of Antioxidant Enzymes and Accumulation of H2O2 and MDA under Cold Stress
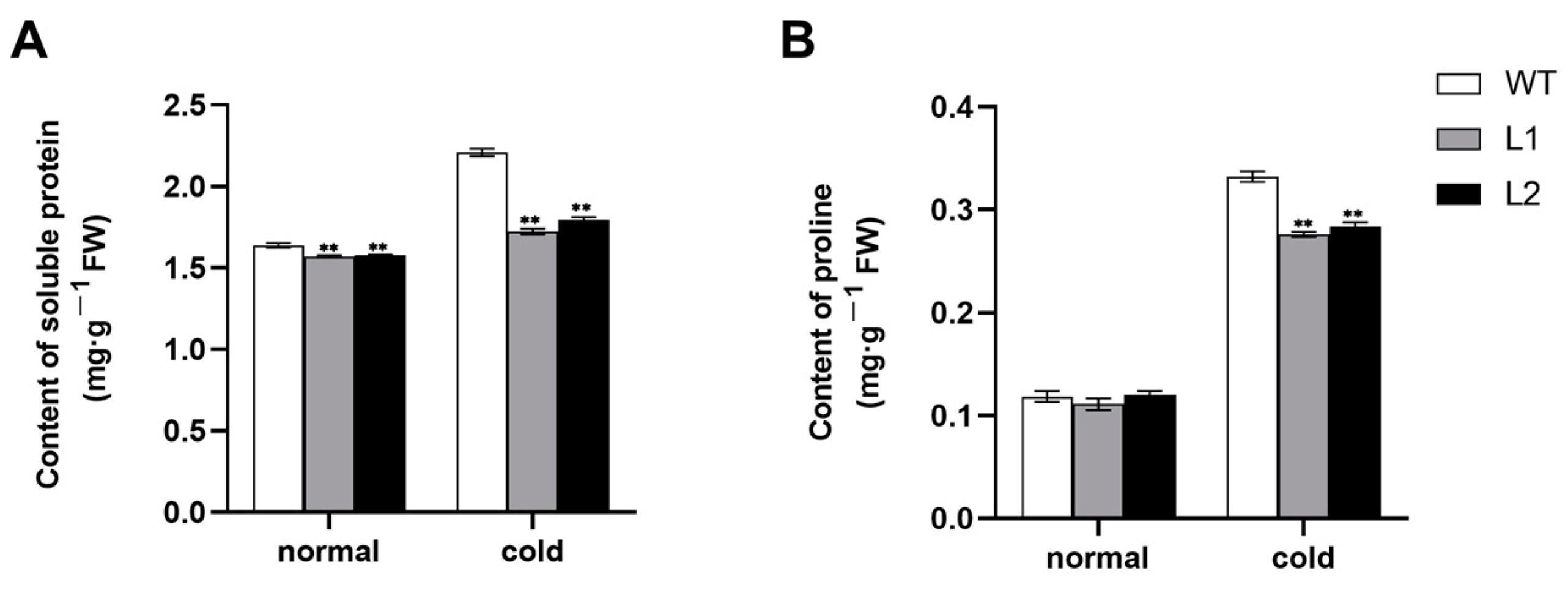
3.4. The Accumulation of Soluble Protein and Proline under Cold Stress
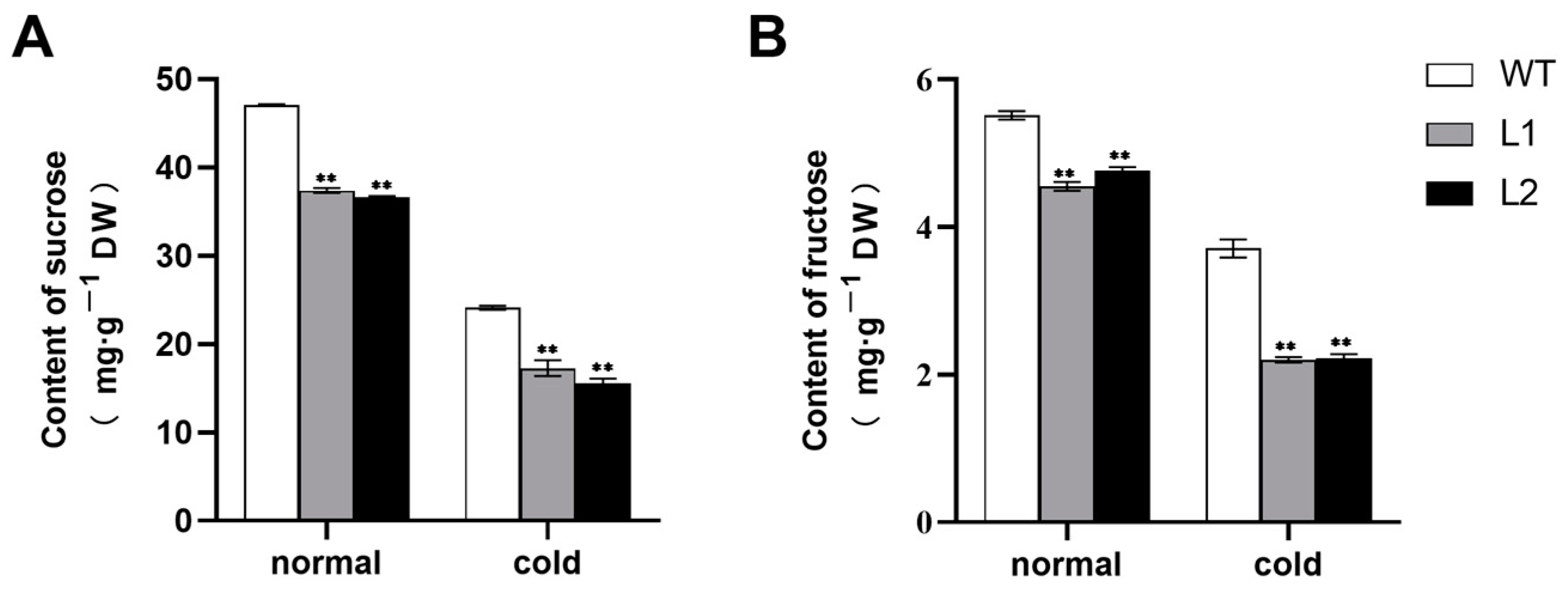
3.5. The Accumulation of Soluble Sugars under Cold Stress
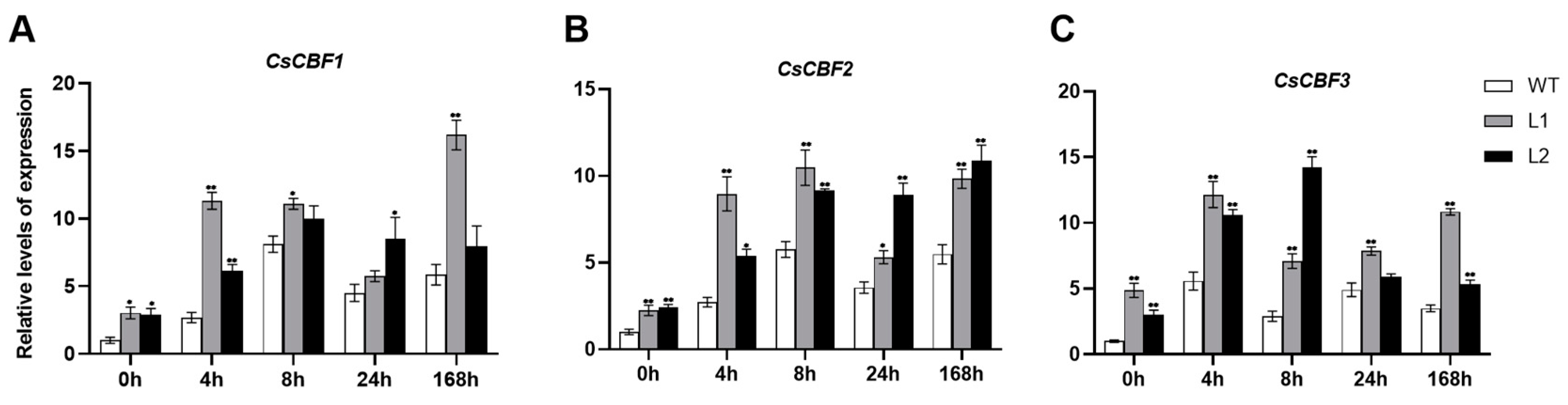
3.6. The Expression of CBF and COR Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Urano, D.; Chen, J.G.; Botella, J.R.; Jones, A.M. Heterotrimeric G protein signalling in the plant kingdom. Open Biol. 2013, 3, 120186. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Botella, J.R. Heterotrimeric G Protein Signaling in Abiotic Stress. Plants 2022, 11, 876. [Google Scholar] [CrossRef] [PubMed]
- Bisht, N.C.; Jez, J.M.; Pandey, S. An elaborate heterotrimeric G-protein family from soybean expands the diversity of plant G-protein networks. New Phytol. 2011, 190, 35–48. [Google Scholar] [CrossRef]
- Trusov, Y.; Chakravorty, D.; Botella, J.R. Diversity of heterotrimeric G-protein γ subunits in plants. BMC Res. Notes 2012, 5, 608. [Google Scholar] [CrossRef]
- Trusov, Y.; Rookes, J.E.; Tilbrook, K.; Chakravorty, D.; Mason, M.G.; Anderson, D.; Chen, J.; Jones, A.M.; Botella, J.R. Heterotrimeric G protein gamma subunits provide functional selectivity in Gbetagamma dimer signaling in Arabidopsis. Plant Cell. 2007, 19, 1235–1250. [Google Scholar] [CrossRef]
- Botella, J.R. Can heterotrimeric G proteins help to feed the world? Trends Plant Sci. 2012, 17, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, Y.; Zheng, L.; Chen, L.; Li, N.; Corke, F.; Lu, Y.; Fu, X.; Zhu, Z.; Bevan, M.W.; et al. The plant-specific G protein γ subunit AGG3 influences organ size and shape in Arabidopsis thaliana. New Phytol. 2012, 194, 690–703. [Google Scholar] [CrossRef]
- Roy, C.S.; Riesselman, A.J.; Pandey, S. Constitutive or seed-specific overexpression of Arabidopsis G-protein γ subunit 3 (AGG3) results in increased seed and oil production and improved stress tolerance in Camelina sativa. Plant Biotechnol. J. 2014, 12, 49–59. [Google Scholar]
- Chinnusamy, V.; Zhu, J.; Zhu, J.K. Cold stress regulation of gene expression in plants. Trends Plant Sci. 2007, 12, 444–451. [Google Scholar] [CrossRef]
- Lee, D.H.; Lee, C.B. Chilling stress-induced changes of antioxidant enzymes in the leaves of cucumber: In gel enzyme activity assays. Plant Sci. 2000, 159, 75–85. [Google Scholar] [CrossRef]
- Tarkowski, Ł.P.; Van den Ende, W. Cold tolerance triggered by soluble sugars: A multifaceted countermeasure. Front. Plant Sci. 2015, 6, 203. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhu, J.K. The broad roles of CBF genes: From development to abiotic stress. Plant Signal. Behav. 2016, 11, e1215794. [Google Scholar] [CrossRef] [PubMed]
- Eremina, M.; Rozhon, W.; Poppenberger, B. Hormonal control of cold stress responses in plants. Cell. Mol. Life Sci. 2016, 73, 797–810. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Zhou, M.; Wei, D.; Chen, H.; You, C.; Lin, J. Transcriptome profiling reveals the negative regulation of multiple plant hormone signaling pathways elicited by overexpression of C-repeat binding factors. Front. Plant Sci. 2017, 8, 1647. [Google Scholar] [CrossRef]
- Li, H.; Ye, K.; Shi, Y.; Cheng, J.; Zhang, X.; Yang, S. BZR1 positively regulates freezing tolerance via CBF-dependent and CBF-independent pathways in Arabidopsis. Mol. Plant 2017, 10, 545–559. [Google Scholar] [CrossRef]
- Jeon, J.; Kim, J. Arabidopsis response Regulator1 and Arabidopsis histidine phosphotransfer Protein2 (AHP2), AHP3, and AHP5 function in cold signaling. Plant Physiol. 2013, 161, 408–424. [Google Scholar] [CrossRef]
- Miura, K.; Ohta, M. SIZ1, a small ubiquitin-related modifier ligase, controls cold signaling through regulation of salicylic acid accumulation. J. Plant Physiol. 2010, 167, 555–560. [Google Scholar] [CrossRef]
- Ma, Y.; Dai, X.; Xu, Y.; Luo, W.; Zheng, X.; Zeng, D.; Pan, Y.; Lin, X.; Liu, H.; Zhang, D.; et al. COLD1 confers chilling tolerance in rice. Cell 2015, 160, 1209–1221. [Google Scholar] [CrossRef]
- Yan, Y.; Sun, M.; Ma, S.; Feng, Q.; Wang, Y.; Di, Q.; Zhou, M.; He, C.; Li, Y.; Gao, L.; et al. Mechanism of CsGPA1 in regulating cold tolerance of cucumber. Hortic. Res. 2022, 9, uhac109. [Google Scholar] [CrossRef]
- Dong, W.; Gao, T.; Song, Y. A wheat GTP-binding protein like gene reduces tolerance to low temperature in Arabidopsis. Biochem. Biophys. Res. Commun. 2019, 509, 148–153. [Google Scholar] [CrossRef]
- Yadav, D.K.; Islam, S.M.; Tuteja, N. Rice heterotrimeric G-protein gamma subunits (RGG1 and RGG2) are differentially regulated under abiotic stress. Plant Signal. Behav. 2012, 7, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Liu, Y.; Mu, Y.; Anwar, A.; He, C.; Yan, Y.; Li, Y.; Yu, X. Heterotrimeric G-protein γ subunit CsGG3.2 positively regulates the expression of CBF genes and chilling tolerance in cucumber. Front. Plant Sci. 2018, 9, 488. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wu, Q.; Sprague, S.A.; Park, J.; Oh, M.; Rajashekar, C.B.; Koiwa, H.; Nakata, P.A.; Cheng, N.; Hirschi, K.D.; et al. Tomato expressing Arabidopsis glutaredoxin gene AtGRXS17 confers tolerance to chilling stress via modulating cold responsive components. Hortic. Res. 2015, 2, 15051. [Google Scholar] [CrossRef]
- Yang, F.; Li, H.; Li, F.; Zhao, L.; Zheng, Y.; Hu, Q. Effect of nano-packing on preservation quality of fresh strawberry (Fragaria ananassa Duch. cv Fengxiang) during storage at 4 °C. J. Food Sci. 2010, 75, C236–C240. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Nguyen, H.T.T.; Bhowmik, S.D.; Long, H.; Cheng, Y.; Mundree, S.; Hoang, L.T.M. Rapid accumulation of proline enhances salinity tolerance in Australian wild rice Oryza australiensis Domin. Plants 2021, 10, 2044. [Google Scholar] [CrossRef]
- Amin, B.; Atif, M.J.; Wang, X.; Meng, H.; Ghani, M.I.; Ali, M.; Ding, Y.; Li, X.; Cheng, Z. Effect of low temperature and high humidity stress on physiology of cucumber at different leaf stages. Plant Biol. Stuttg 2021, 23, 785–796. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Rao, M.V.; Paliyath, G.; Ormrod, D.P. Ultraviolet-B- and ozone-induced biochemical changes in antioxidant enzymes of Arabidopsis thaliana. Plant Physiol. 1996, 110, 125–136. [Google Scholar] [CrossRef]
- Shen, J.; Wang, Y.; Shu, S.; Jahan, M.S.; Zhong, M.; Wu, J.; Sun, J.; Guo, S. Exogenous putrescine regulates leaf starch overaccumulation in cucumber under salt stress. Sci. Hortic. 2019, 253, 99–110. [Google Scholar] [CrossRef]
- Jaglo, K.R.; Kleff, S.; Amundsen, K.L.; Zhang, X.; Haake, V.; Zhang, J.Z.; Deits, T.; Thomashow, M.F. Components of the Arabidopsis C-repeat/dehydration-responsive element binding factor cold-response pathway are conserved in Brassica napus and other plant species. Plant Physiol. 2001, 127, 910–917. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Gorbe, E.; Calatayud, A. Applications of chlorophyll fluorescence imaging technique in horticultural research: A review. Sci. Hortic. 2012, 138, 24–35. [Google Scholar] [CrossRef]
- Patterson, B.D.; Payne, L.A.; Chen, Y.; Graham, D. An inhibitor of catalase induced by cold in chilling-sensitive plants. Plant Physiol. 1984, 76, 1014–1018. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, Q.; Li, Y.; Li, J.; Chen, J.; Liu, Z.; Huang, J.; AlHarbi, M.S.; Ali, E.F.; Eissa, M.A. Mechanisms of Nitric oxide in the regulation of chilling stress tolerance in Camellia sinensis. Horticulturae 2021, 7, 410. [Google Scholar] [CrossRef]
- Gao, Y.; Li, T.; Liu, Y.; Ren, C.; Zhao, Y.; Wang, M. Isolation and characterization of gene encoding G protein α subunit protein responsive to plant hormones and abiotic stresses in Brassica napus. Mol. Biol. Rep. 2010, 37, 3957–3965. [Google Scholar] [CrossRef]
- Suzuki, K.; Nagasuga, K.; Okada, M. The chilling injury induced by high root temperature in the leaves of rice seedlings. Plant Cell Physiol. 2008, 49, 433–442. [Google Scholar] [CrossRef]
- Aazami, M.A.; Asghari-Aruq, M.; Hassanpouraghdam, M.B.; Ercisli, S.; Baron, M.; Sochor, J. Low temperature stress mediates the antioxidants pool and chlorophyll fluorescence in Vitis vinifera L. Cultivars. Plants 2021, 10, 1877. [Google Scholar] [CrossRef]
- Kasote, D.M.; Katyare, S.S.; Hegde, M.V.; Bae, H. Significance of antioxidant potential of plants and its relevance to therapeutic applications. Int. J. Biol. Sci. 2015, 11, 982–991. [Google Scholar] [CrossRef]
- Liang, G.; He, H.; Nai, G.; Feng, L.; Li, Y.; Zhou, Q.; Ma, Z.; Yue, Y.; Chen, B.; Mao, J. Genome-wide identification of BAM genes in grapevine (Vitis vinifera L.) and ectopic expression of VvBAM1 modulating soluble sugar levels to improve low-temperature tolerance in tomato. BMC Plant Biol. 2021, 21, 156. [Google Scholar] [CrossRef]
- Sun, S.; Fang, J.; Lin, M.; Qi, X.; Chen, J.; Wang, R.; Li, Z.; Li, Y.; Muhannad, A. Freezing tolerance and expression of β-amylase gene in two Actinidia arguta cultivars with seasonal changes. Plants 2020, 9, 515. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.; Zhu, X.; Duan, N.; Liu, J. PtrBAM1, a β-amylase-coding gene of Poncirus trifoliata, is a CBF regulon member with function in cold tolerance by modulating soluble sugar levels. Plant Cell Environ. 2014, 37, 2754–2767. [Google Scholar] [CrossRef] [PubMed]
- Klemens, P.A.; Patzke, K.; Deitmer, J.; Spinner, L.; Hir, R.L.; Bellini, C.; Bedu, M.; Chardon, F.; Krapp, A.; Neuhaus, H.E. Overexpression of the vacuolar sugar carrier AtSWEET16 modifies germination, growth, and stress tolerance in Arabidopsis. Plant Physiol. 2013, 163, 1338–1352. [Google Scholar] [CrossRef] [PubMed]
- Patzke, K.; Prananingrum, P.; Klemens, P.A.W.; Trentmann, O.; Rodrigues, C.M.; Keller, I.; Fernie, A.R.; Geigenberger, P.; Bölter, B.; Lehmann, M.; et al. The plastidic sugar transporter pSuT influences flowering and affects cold responses. Plant Physiol. 2019, 179, 569–587. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Song, Y.; Su, L. Freezing sensitivity in the gigantea mutant of Arabidopsis is associated with sugar deficiency. Biol. Plant. 2007, 51, 359–362. [Google Scholar] [CrossRef]
- Brandoli, C.; Petri, C.; Egea-Cortines, M.; Weiss, J. Gigantea: Uncovering new functions in flower development. Genes 2020, 11, 1142. [Google Scholar] [CrossRef]
- Hussain, H.A.; Hussain, S.; Khaliq, A.; Ashraf, U.; Anjum, A.S.; Men, S.; Wang, L. Chilling and drought stresses in crop plants: Implications, cross talk, and potential management opportunities. Front. Plant Sci. 2018, 9, 393. [Google Scholar] [CrossRef]
- Strauss, G.; Hauser, H. Stabilization of lipid bilayer vesicles by sucrose during freezing. Proc. Natl. Acad. Sci. USA 1986, 83, 2422–2426. [Google Scholar] [CrossRef]
- Vereyken, I.J.; Chupin, V.; Demel, R.A.; Smeekens, S.C.; Kruijff, B.D. Fructans insert between the headgroups of phospholipids. Biochim. Biophys. Acta 2001, 1510, 307–320. [Google Scholar] [CrossRef]
- Xie, Y.; Tan, H.; Ma, Z.; Huang, J. DELLA proteins promote anthocyanin biosynthesis via sequestering MYBL1 and JAZ suppressors of the MYB/bHLH/WD40 complex in Arabidopsis thaliana. Mol. Plant 2016, 9, 711–721. [Google Scholar] [CrossRef]
- Peshev, D.; Vergauwen, R.; Moglia, A.; Hideg, E.; Van den Ende, W. Towards understanding vacuolar antioxidant mechanisms: A role for fructans? J. Exp. Bot. 2013, 64, 1025–1038. [Google Scholar] [CrossRef] [PubMed]
- Peukert, M.; Thiel, J.; Peshev, D.; Weschke, W.; Van den Ende, W.; Mock, H.P.; Matros, A. Spatio-temporal dynamics of fructan metabolism in developing barley grains. Plant Cell 2014, 26, 3728–3744. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, C.; Doherty, C.J.; Gilmour, S.J.; Kim, Y.; Thomashow, M.F. Regulation of the Arabidopsis CBF regulon by a complex low-temperature regulatory network. Plant J. 2015, 82, 193–207. [Google Scholar] [CrossRef] [PubMed]
- Chu, M.; Li, J.; Zhang, J.; Shen, S.; Li, C.; Gao, Y.; Zhang, S. AtCaM4 interacts with a Sec14-like protein, PATL1, to regulate freezing tolerance in Arabidopsis in a CBF-independent manner. J. Exp. Bot. 2018, 69, 5241–5253. [Google Scholar] [CrossRef]
- Guo, X.; Li, J.; Zhang, L.; Zhang, Z.; He, P.; Wang, W.; Wang, M.; Wang, A.; Zhu, J. Heterotrimeric G-protein α subunit (LeGPA1) confers cold stress tolerance to processing tomato plants (Lycopersicon esculentum Mill). BMC Plant Biol. 2020, 20, 394. [Google Scholar] [CrossRef]
- Swain, D.M.; Sahoo, R.K.; Chandan, R.K.; Ghosh, S.; Kumar, R.; Jha, G.; Tuteja, N. Concurrent overexpression of rice G-protein β and γ subunits provide enhanced tolerance to sheath blight disease and abiotic stress in rice. Planta 2019, 250, 1505–1520. [Google Scholar] [CrossRef]
- Yadav, D.K.; Shukla, D.; Tuteja, N. Isolation, in silico characterization, localization and expression analysis of abiotic stress-responsive rice G-protein β subunit (RGB1). Plant Signal. Behav. 2014, 9, e28890. [Google Scholar] [CrossRef]
- Li, S.; Liu, W.; Zhang, X.; Liu, Y.; Li, N.; Li, Y. Roles of the Arabidopsis G protein γ subunit AGG3 and its rice homologs GS3 and DEP1 in seed and organ size control. Plant Signal. Behav. 2012, 7, 1357–1359. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, M.; Zhou, Y.; Wang, Y.; Shen, J.; Chen, H.; Zhang, L.; Lü, B.; Liang, G.; Liang, J. The rice G protein γ subunit DEP1/qPE9-1 positively regulates grain-filling process by increasing auxin and cytokinin content in rice grains. Rice 2019, 12, 91. [Google Scholar] [CrossRef]
- Wang, K.; Xu, F.; Yuan, W.; Sun, L.; Wang, S.; Aslam, M.M.; Zhang, J.; Xu, W. G protein γ subunit qPE9-1 is involved in rice adaptation under elevated CO2 concentration by regulating leaf photosynthesis. Rice 2021, 14, 67. [Google Scholar] [CrossRef]
- Wanner, L.A.; Junttila, O. Cold-induced freezing tolerance in Arabidopsis. Plant Physiol. 1999, 120, 391–400. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, L.; Zhu, H.; Shi, Y.; Li, Y.; Miao, Y.; Yu, X.; Zhang, Y.; Li, Y. Antisense Overexpression of Gγ Subunit CsGG3.1-2 Reduces Soluble Sugar Content and Chilling Tolerance in Cucumber. Horticulturae 2023, 9, 240. https://doi.org/10.3390/horticulturae9020240
Bai L, Zhu H, Shi Y, Li Y, Miao Y, Yu X, Zhang Y, Li Y. Antisense Overexpression of Gγ Subunit CsGG3.1-2 Reduces Soluble Sugar Content and Chilling Tolerance in Cucumber. Horticulturae. 2023; 9(2):240. https://doi.org/10.3390/horticulturae9020240
Chicago/Turabian StyleBai, Longqiang, Huixin Zhu, Yu Shi, Yaling Li, Yanxiu Miao, Xianchang Yu, Yi Zhang, and Yansu Li. 2023. "Antisense Overexpression of Gγ Subunit CsGG3.1-2 Reduces Soluble Sugar Content and Chilling Tolerance in Cucumber" Horticulturae 9, no. 2: 240. https://doi.org/10.3390/horticulturae9020240
APA StyleBai, L., Zhu, H., Shi, Y., Li, Y., Miao, Y., Yu, X., Zhang, Y., & Li, Y. (2023). Antisense Overexpression of Gγ Subunit CsGG3.1-2 Reduces Soluble Sugar Content and Chilling Tolerance in Cucumber. Horticulturae, 9(2), 240. https://doi.org/10.3390/horticulturae9020240



