The Content of Heavy Metals in Medicinal Plants in Various Environmental Conditions: A Review
Abstract
:1. Introduction
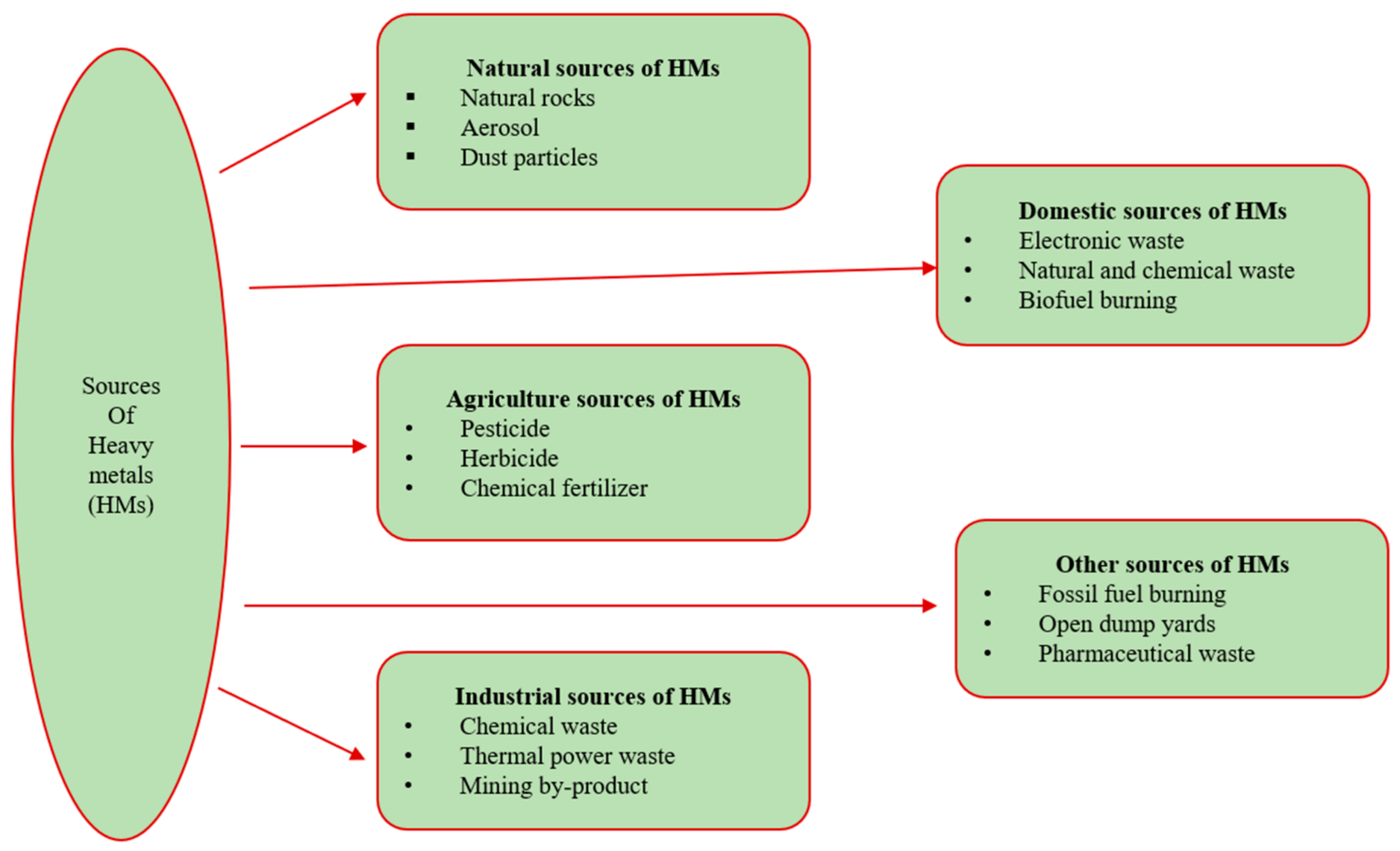
2. Heavy Metals, Medicinal Herbs, and Regulatory Documents
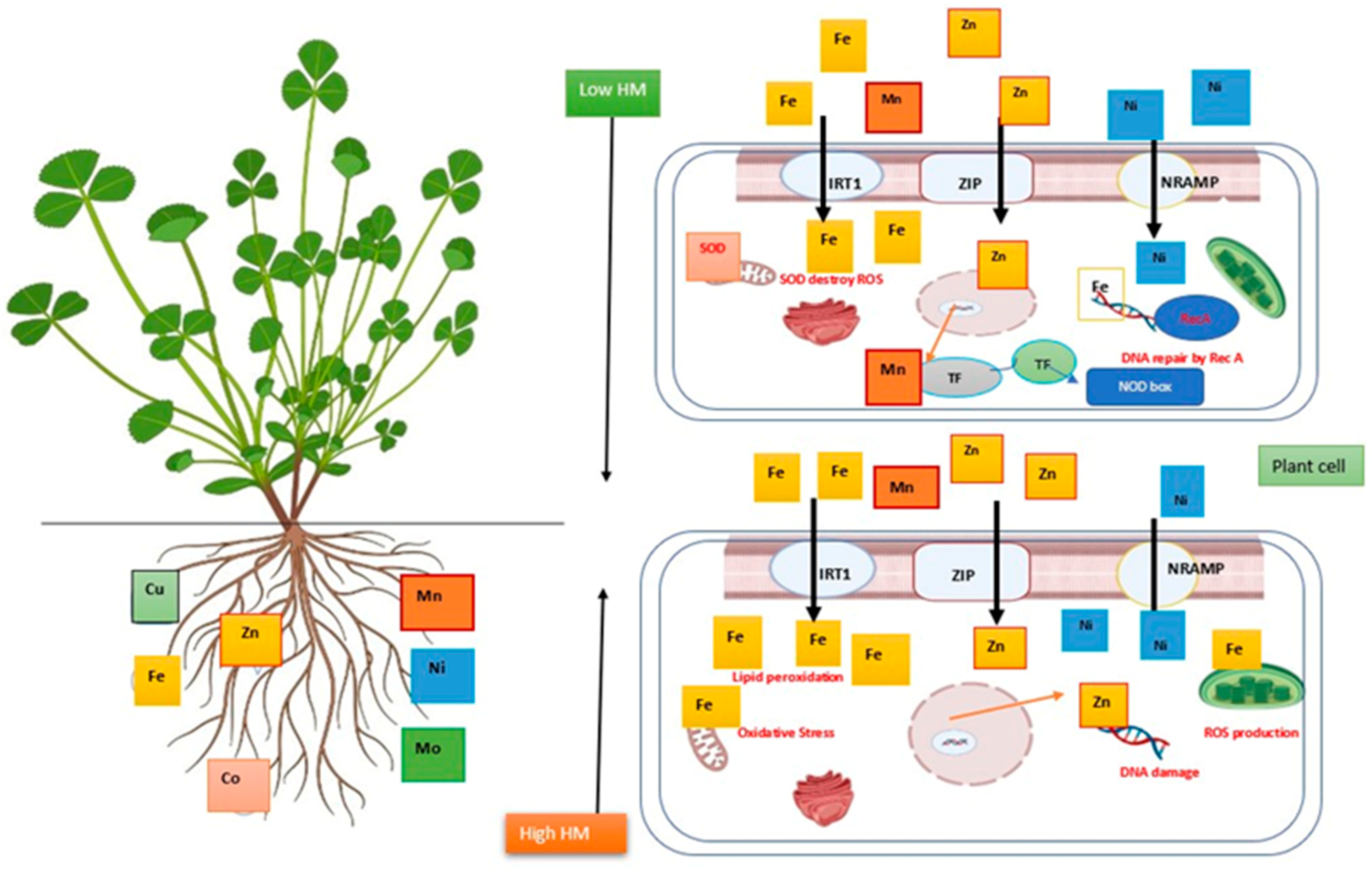
3. Impact of Heavy Metal on Herbal Plants
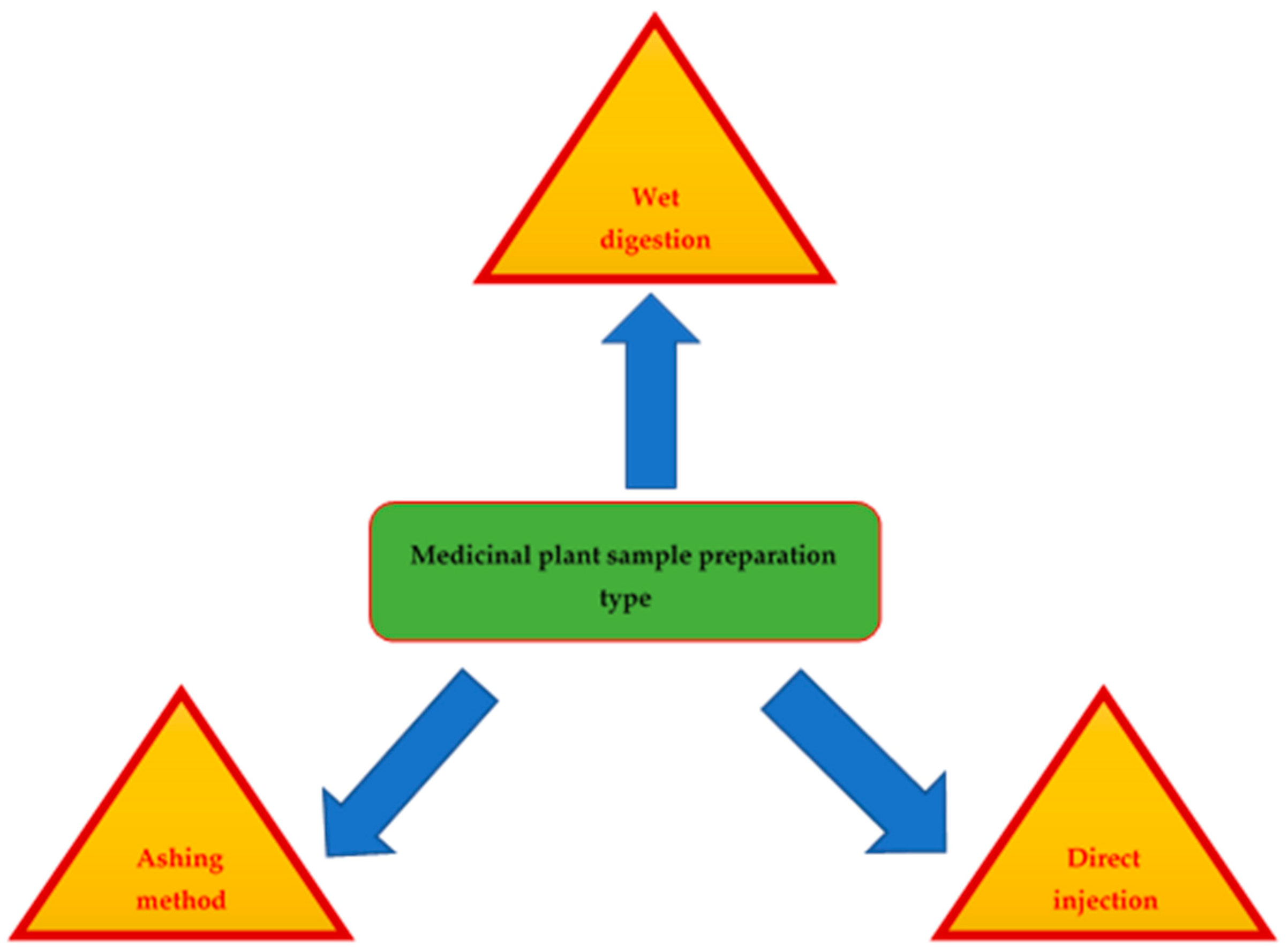
4. Herbal Sample Preparation
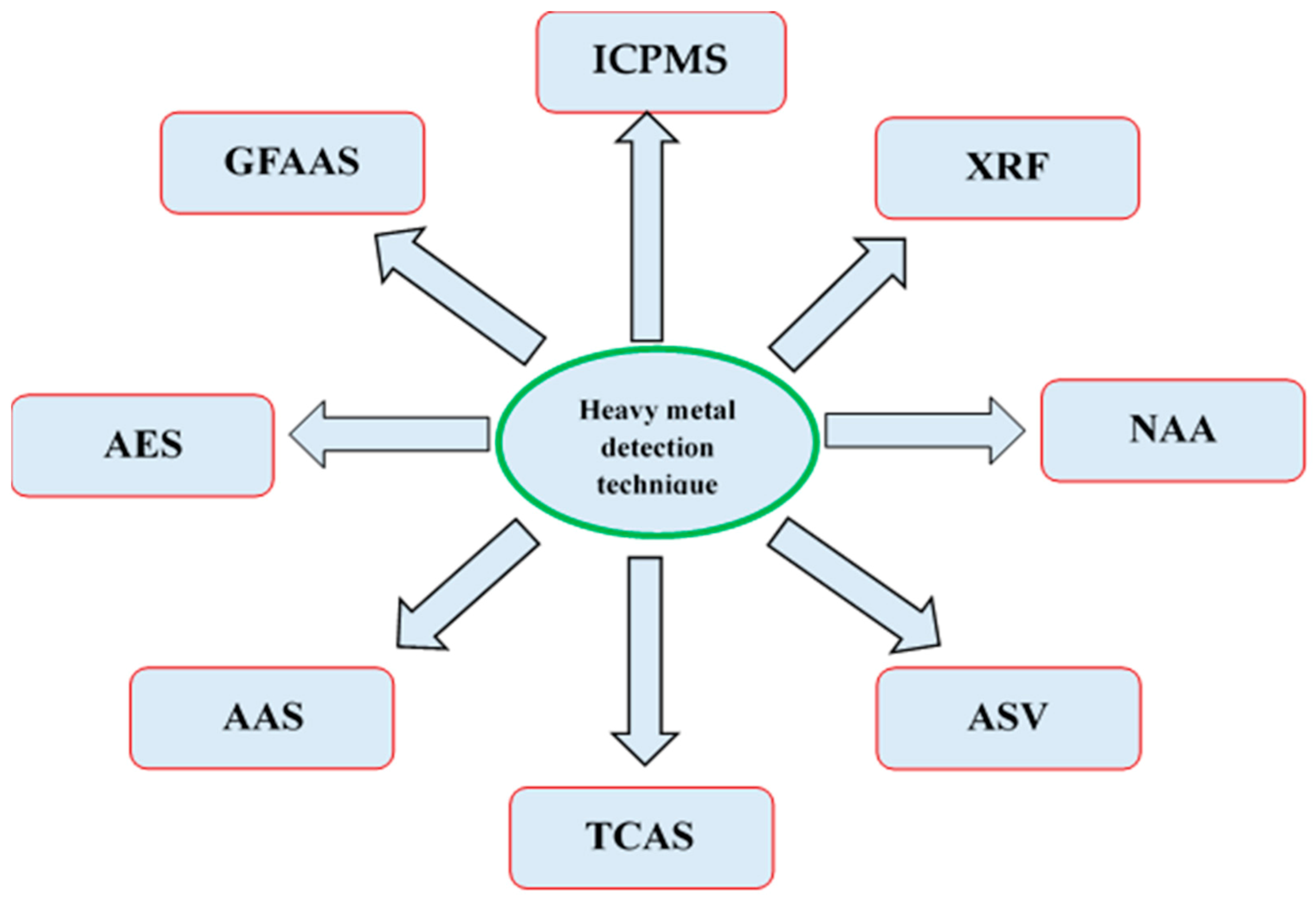
5. Detection Method of the Heavy Metals in Medicinal Plants
5.1. ICPMS
5.2. AES
5.3. XRF
5.4. NAA
5.5. ASV
5.6. TCAS
5.7. AAS
5.8. GFAAS
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mafimisebi, T.E.; Oguntade, A.E.; Ajibefun, I.A.; Mafimisebi, O.E.; Ikuemonisan, E.S. The expanding market for herbal, medicinal and aromatic plants in Nigeria and the international scene. Med. Aromat. Plants. 2013, 144, 2167–2412. [Google Scholar]
- Brinckmann, J.A. Geographical indications for medicinal plants: Globalization, climate change, quality and market implications for geo-authentic botanicals. World J. Tradit. Chin. Med. 2015, 1, 16–23. [Google Scholar] [CrossRef]
- Nikulin, A.; Potanina, O.; Alyussef, M.; Vasil’ev, V.; Abramovich, R.; Novikov, O.; Boyko, N.; Khromov, A.; Platonov, E. Development of a technique for determining cadmium, lead, arsenic with the etaas method in medicinal plant raw materials. Farmacia 2021, 69, 566–575. [Google Scholar] [CrossRef]
- Petrukhina, I.K.; Yagudina, R.I.; Ryazanova, T.K.; Kurkin, V.A.; Pervushkin, S.V.; Egorova, A.V.; Loginova, L.V.; Khusainova, A.I.; Blinkova1, P.R. Analysis of the implementation of the federal assurance program of supporting beneficiaries with indispensable medicinal preparations in the subjects of the Russian Federation. Farmatsiya Farmakol. 2021, 8, 273–284. [Google Scholar] [CrossRef]
- Fitzgerald, M.; Heinrich, M.; Booker, A. Medicinal plant analysis: A historical and regional discussion of emergent complex techniques. Front. Pharmacol. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- D’yakova, N.A.; Samylina, I.A.; Slivkin, A.I.; Gaponov, S.P.; Myndra, A.A. Estimated heavy-metal and Arsenic contents in medicinal plant raw materials of the Voronezh region. Pharm. Chem. J. 2018, 52, 220–223. [Google Scholar] [CrossRef]
- Chen, Y.G.; Huang, J.H.; Luo, R.; Ge, H.Z.; Wołowicz, A.; Wawrzkiewicz, M.; Gładysz-Płaska, A.; Li, B.; Yu, Q.X.; Kołodyńska, D.; et al. Impacts of heavy metals and medicinal crops on ecological systems, environmental pollution, cultivation, and production processes in China. Ecotoxicol. Environ. Safet. 2021, 219, 17. [Google Scholar] [CrossRef]
- Carrubba, A.; Scalenghe, R. The scent of Mare Nostrum: Medicinal and aromatic plants in Mediterranean soils. J. Sci. Food Agric. 2012, 92, 1150–1170. [Google Scholar] [CrossRef]
- Buettner, C.; Mukamal, K.J.; Gardiner, P.; Davis, R.B.; Phillips, R.S.; Mittleman, M.A. Herbal supplement use and blood lead levels of United States adults. J. Gen. Intern. Med. 2009, 24, 1175–1182. [Google Scholar] [CrossRef]
- Ernst, E. Risks of herbal medicinal products. Pharmacoepidemol. Drug Saf. 2004, 13, 767–771. [Google Scholar] [CrossRef]
- Liu, X.; Ju, Y.; Mandzhieva, S.; Pinskii, D.; Minkina, T.; Rajput, V.D.; Roane, T.; Huang, S.; Li, Y.; Ma, L.Q.; et al. Sporadic Pb accumulation by plants: Influence of soil biogeochemistry, microbial community and physiological mechanisms. J. Hazard. Mater 2023, 444, 130391. [Google Scholar] [CrossRef]
- Chaplygin, V.; Dudnikova, T.; Chernikova, N.; Fedorenko, A.; Mandzhieva, S.; Fedorenko, G.; Sushkova, S.; Nevidomskaya, D.; Minkina, T.; Sathishkumar, P.; et al. Phragmites australis cav. As a bioindicator of hydromorphic soils pollution with heavy metals and polyaromatic hydrocarbons. Chemosphere 2022, 308, 136409. [Google Scholar] [CrossRef]
- Bezuglova, O.S.; Gorbov, S.N.; Tischenko, S.A.; Aleksikova, A.S.; Tagiverdiev, S.S.; Sherstnev, A.K.; Dubinina, M.N. Accumulation and migration of heavy metals in soils of the Rostov region, south of Russia. J. Soils Sediments 2016, 16, 1203–1213. [Google Scholar] [CrossRef]
- Erofeeva, E.A. Hormesis in plants: Its common occurrence across stresses. Curr. Opin. Toxicol. 2022, 30, 100333. [Google Scholar] [CrossRef]
- State Pharmacopoeia of the Russian Federation, XIV ed.; Ministry of Health of the Russian Federation: Moscow, Russia, 2018; Volume II, p. 1449.
- European Directorate for the Quality of Medicines & HealthCare (EDQM); Council of Europe: Strasbourg, France, 2019; Volume 7.
- USP44–NF39; 561 Articles of Botanical Origin. United States Pharmacopeia: Rockville, MD, USA, 2020; p. 15.
- European Pharmacopoeia, 10th ed.; Council of Europe: Strasbourg, France, 2019; Volume 1, p. 4370.
- Pharmacopoeia of the Eurasian Economic Union; Ministry of Health of the Russian Federation: Moscow, Russia, 2021; 568p.
- Pharmacopoeia of the People’s Republic of China; China Food and Drug Administration: Beijing, China, 2015; Volume 1, 2266p.
- World Health Organization (WHO). Quality Control Methods for Medicinal Plant Materials; World Health Organization: Geneva, Switzerland, 2005. [Google Scholar]
- Debnath, M.; Paul, N.; Bhattacharya, S.; Biswas, M.; Haldar, P.K. Formulation and assessment of microbial and heavy metal contents of Vidangadilouham: A classical Ayurvedic formulation. Int. J. Herb. Med. 2020, 8, 101–102. [Google Scholar]
- Vyas, P.; Vohora, D. Pharmaceutical regulations for complementary medicine. In Pharmaceutical Medicine and Translational Clinical Research; Vohora, D., Singh, G., Eds.; Academic Press: Cambridge, MA, USA, 2018; Chapter 13; pp. 233–264. [Google Scholar] [CrossRef]
- Shchukin, V.M.; Kuzmina, N.E.; Erina, A.A.; Yashkir, V.A.; Merkulov, V.A. Comparative analysis of the heavy metal, Aluminum, and Arsenic contents in brown algae of various origins. Pharm. Chem. J. 2018, 52, 627–634. [Google Scholar] [CrossRef]
- Chizzola, R.; Michitsch, H.; Franz, C. Monitoring of metallic micronutrients and heavy metals in herbs, spices and medicinal plants from Austria. Eur. Food Res. Technol. 2003, 216, 407–411. [Google Scholar] [CrossRef]
- Siromlya, T.I. Influence of traffic pollution on ecological state of Plantago major L. Contemp. Probl. Ecol. 2011, 4, 499–507. [Google Scholar] [CrossRef]
- Parveen, R.; Abbasi, A.M.; Shaheen, N.; Shah, M.H. Accumulation of selected metals in the fruits of medicinal plants grown in urban environment of Islamabad, Pakistan. Arab. J. Chem. 2017, 13, 308–317. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Yang, H.; Tang, Y. Five heavy metals accumulation and health risk in a traditional Chinese medicine cortex Moutan collected from different sites in China. Hum. Ecol. Risk Assess. 2018, 24, 2288–2298. [Google Scholar] [CrossRef]
- Kuzovkova, A.A.; Drebenkova, I.V.; Velentei, Y.N.; Pleshkova, A.A.; Bychok, G.E.; Chernik, D.V.; Maskalevich, N.V. Heavy metal contamination of wild and cultivated medicinal plants in the Republic of Belarus. Occup. Med. Human Ecol. 2020, 4, 112–117. [Google Scholar]
- Vinogradova, N.A.; Glukhov, A.Z. Ecological and phytochemical features of Crataegus fallacina Klokov under conditions of technogenic pollution. Contemp. Probl. Ecol. 2021, 14, 90–97. [Google Scholar] [CrossRef]
- Buskunova, G.G.; Khasanova, R.F.; Semenova, I.N.; Ilbulova, G.R. The heavy metals in the system “soil as a wild-growing medicinal plant” on the example of Tanacetum vulgare L. Ecol. Ind. Russ. 2020, 24, 37–41. [Google Scholar] [CrossRef]
- Zhang, Z.; Song, J.; Zhang, H.; Zheng, Z.; Li, T.; Wu, S.; He, B.; Mao, B.; Yu, Y.; Fang, H. Analysis method development and health risk assessment of pesticide and heavy metal residues in Dendrobium Candidum. RSC Adv. 2022, 1, 6869–6875. [Google Scholar] [CrossRef]
- Dylenova, E.P.; Zhigzhitzhapova, S.V.; Randalova, T.E.; Radnaeva, L.D.; Shiretorova, V.G.; Pavlov, I.A. Biophile elements and heavy metals in Artemisia frigida willd. and Artemisia jacutica drob. Khimiya Rastitel’nogo Syr’ya 2019, 4, 199–205. [Google Scholar] [CrossRef]
- Babkina, L.A.; Lukyanchikov, D.S.; Lukyanchikova, O.V. Features of the accumulation of heavy metals by plantain leaves. Samara Sci. Bull. 2018, 7, 19–24. [Google Scholar] [CrossRef]
- Luo, L.; Wang, B.; Jiang, J.; Fitzgerald, M.; Huang, Q.; Yu, Z.; Li, H.; Zhang, J.; Wei, J.; Yang, C.; et al. Heavy metal contaminations in herbal medicines: Determination, comprehensive risk assessments, and solutions. Front. Pharmacol. 2021, 11, 595335. [Google Scholar] [CrossRef]
- Dghaim, R.; Al-Khatib, S.; Rasool, H.; Ali Khan, M. Determination of heavy metals concentration in traditional herbs commonly consumed in the United Arab Emirates. J. Environ. Public Health 2015, 973878. [Google Scholar] [CrossRef]
- Kamanina, I.Z.; Kaplina, S.P.; Salikhova, F.S. The content of heavy metals in medicinal plants Scientific review. Biol. Sci. 2019, 1, 29–34. [Google Scholar]
- Srivastava, S.K.; Rai, V.; Srivastava, M.; Rawat, A.K.S.; Mehrotra, S. Estimation of heavy metals in different berberis species and Its market samples. Environ. Monit. Assess. 2006, 116, 315–320. [Google Scholar] [CrossRef]
- Rojas, P.; Ruiz-Sánchez, E.; Ríos, C.; Ruiz-Chow, A.; Resendiz-Albor, A.A. A health risk assessment of lead and other metals in pharmaceutical herbal products and dietary supplements containing Ginkgo biloba in the Mexico city metropolitan area. Int. J. Environ. Res. Public Health 2021, 18, 8285–8304. [Google Scholar] [CrossRef]
- Siromlya, T.I.; Zagurskaya, Y.V.; Bayandina, I.I. The elemental composition of Hypericum Perforatum plants sampled in environmentally different habitats by the example of West Siberia. Bot. Pac. 2020, 9, 127–132. [Google Scholar] [CrossRef]
- Shikov, A.N.; Shikova, V.A.; Whaley, A.O.; Burakova, M.A.; Flisyuk, E.V.; Whaley, A.K.; Terninko, I.I.; Generalova, Y.E.; Gravel, I.V.; Pozharitskaya, O.N. The ability of acid-based natural deep eutectic solvents to co-extract elements from the roots of Glycyrrhiza glabra L. and associated health risks. Molecules 2022, 27, 7690. [Google Scholar] [CrossRef]
- Yang, C.M.; Chien, M.Y.; Chao, P.C.; Huang, C.M.; Chen, C.H. Investigation of toxic heavy metals content and estimation of potential health risks in Chinese herbal medicine. J. Hazard Mater 2021, 412, 125142. [Google Scholar] [CrossRef]
- Alloway, B.J. Heavy metals and metalloids as micronutrients for plants and animals. In Heavy Metals in Soils; Springer: Dordrecht, The Netherlands, 2013; pp. 195–209. [Google Scholar]
- Peralta-Videa, J.R.; Lopez, M.L.; Narayan, M.; Saupe, G.; Gardea-Torresdey, J. The biochemistry of environmental heavy metal uptake by plants: Implications for the food chain. Int. J. Biochem. Cell Biol. 2009, 41, 1665–1677. [Google Scholar] [CrossRef]
- Hossain, M.A.; Piyatida, P.; da Silva, J.A.T.; Fujita, M. Molecular mechanism of heavy metal toxicity and tolerance in plants: Central role of glutathione in detoxification of reactive oxygen species and methylglyoxal and in heavy metal chelation. J. Bot. 2012, 37. [Google Scholar] [CrossRef]
- Hell, R.; Stephan, U.W. Iron uptake, trafficking and homeostasis in plants. Planta 2003, 216, 541–551. [Google Scholar] [CrossRef]
- Blaylock, M.J.; Huang, J.W. Phytoextraction of metals. In Phytoremediation of Toxic Metals: Using Plants to Clean Up the Environment; Wiley: New York, NY, USA, 2000; pp. 53–70. [Google Scholar]
- Rout, G.R.; Das, P. Effect of metal toxicity on plant growth and metabolism: I. Zinc. In Sustainable agriculture; Lichtfouse, E., Navarrete, M., Debaeke, P., Véronique, S., Alberola, C., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 873–884. [Google Scholar] [CrossRef]
- Arora, A.; Sairam, R.K.; Srivastava, G.C. Oxidative stress and antioxidative system in plants. Curr. Sci. 2002, 82, 1227–1238. [Google Scholar]
- Oves, M.; Khan, S.; Qari, H.; Felemban, N.; Almeelbi, T. Heavy Metals: Biological importance and detoxification strategies. J. Bioremed. Biodegrad. 2016, 7, 334. [Google Scholar]
- Shenker, M.; Plessner, O.E.; Tel-Or, E. Manganese nutrition effects on tomato growth, chlorophyll concentration, and superoxide dismutase activity. J. Plant Physiol. 2004, 161, 197–202. [Google Scholar] [CrossRef]
- Moller, I.M.; Jensen, P.E.; Hansson, A. Oxidative modifications to cellular components in plants. Annu. Rev. Plant Biol. 2007, 58, 459–481. [Google Scholar] [CrossRef] [PubMed]
- Anjum, N.A.; Duarte, A.C.; Pereira, E.; Ahmad, I. Plant-beneficial elements status assessment in soil-plant system in the vicinity of a chemical industry complex: Shedding light on forage grass safety issues. Environ. Sci. Pollut. Res. Int. 2015, 22, 2239–2246. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Jucoski, G.; Cambraia, J.; Ribeiro, C.; de Oliveira, J.A.; de Paula, S.O.; Oliva, M.A. Impact of iron toxicity on oxidative metabolism in young Eugenia uniflora L. plants. Acta Physiol. Plant 2013, 35, 1645–1657. [Google Scholar] [CrossRef]
- Dehno, M.A.; Harami, S.R.M.; Noora, M.R. Environmental geochemistry of heavy metals in coral reefs and sediments of Chabahar Bay. Results Eng. 2022, 13, 100346. [Google Scholar] [CrossRef]
- Bharti, R.; Sharma, R. Effect of heavy metals: An overview. Mater. Today Proc. 2022, 51, 880–885. [Google Scholar]
- Rahman, M.M.; Hossain, M.K.F.B.; Afrin, S.; Saito, T.; Kurasaki, M. Effects of Metals on Human Health and Ecosystem; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–39. [Google Scholar]
- Pirhadi, M.; Shariatifar, N.; Bahmani, M.; Manouchehri, A. Heavy metals in wheat grain and its impact on human health: A mini-review. J. Chem. Health Risks 2022, 12, 421–426. [Google Scholar]
- Guo, C.; Lv, L.; Liu, Y.; Ji, M.; Zang, E.; Liu, Q.; Zhang, M.; Li, M. Applied analytical methods for detecting heavy metals in medicinal plants. Crit. Rev. Anal. Chem. 2023, 53, 339–359. [Google Scholar] [CrossRef]
- Wilschefski, S.C.; Baxter, M.R. Inductively coupled plasma mass spectrometry: Introduction to analytical aspects. Clin. Biochem. Rev. 2019, 40, 15. [Google Scholar] [CrossRef]
- Rehan, I.; Gondal, M.A.; Aldakheel, R.K.; Almessiere, M.A.; Rehan, K.; Khan, S.; Sultana, S.; Khan, M.Z. Determination of nutritional and toxic metals in black tea leaves using calibration free LIBS and ICP: AES technique. Arab. J. Sci. Eng. 2022, 47, 7531–7539. [Google Scholar] [CrossRef]
- Nguyen, H.M.; Huynh, N.T.K.; Nguyen, N.T.Y.; Ha, L.T.; Pham, T.T. Evaluating the content of some metal elements in soil and their effects on the total phenolic and flavonoid contents of some medicinal plants using X-ray fluorescence (XRF) Method. Res. Sq. 2022, 1, 1–24. [Google Scholar] [CrossRef]
- Garg, A.N.; Singh, R.; Maharia, R.S.; Dutta, R.K.; Datta, A. Quantification of minor, trace and toxic elements in stems of Santalum album (L.), Mangiferra indica (L.) and Tinospora cordifolia by instrumental neutron activation analysis. J. Plant Sci. Phytopathol. 2022, 6, 8–14. [Google Scholar] [CrossRef]
- Khamcharoen, W.; Duchda, P.; Songsrirote, K.; Ratanawimarnwong, N.; Limchoowong, N.; Jittangprasert, P.; Mantim, T.; Siangproh, W. An application of miniaturized electrochemical sensing for determination of arsenic in herbal medicines. Anal. Methods 2022, 14, 3087–3093. [Google Scholar] [CrossRef]
- Papadopoulos, A.; Assimomytis, N.; Varvaresou, A. Sample preparation of cosmetic products for the determination of heavy Metals. Cosmetics 2022, 9, 21. [Google Scholar] [CrossRef]
- Lawi, D.J.; Abdulwhaab, W.S.; Abojassim, A.A. Health risk study of heavy metals from consumption of drugs (solid and liquid) samples derived from medicinal plants in Iraq. Biol. Trace Elem. Res. 2022, 9, 1–13. [Google Scholar] [CrossRef]
- Hyder, Z.; Rizwani, G.H.; Ahmed, I.; Shareef, H.; Azhar, I.; Aqeel, E. Determination of Heavy metals content, Lead (Pb), Mercury (Hg), Cadmium (Cd), Nickel (Ni), and Copper (Cu) with risk assessment to human consumption as a food and medicine in herbal species through Atomic Absorption Spectroscopy. Res. Sq. 2022, 1, 1–16. [Google Scholar] [CrossRef]
- Alinia-Ahandani, E.; Nazem, H.; Malekirad, A.A.; Fazilati, M. The safety evaluation of toxic elements in medicinal plants: A Systematic Review. J. Hum. Environ. Health Promot. 2022, 8, 62–68. [Google Scholar] [CrossRef]
| Food Items and Medicinal Plant Materials | Regulatory Document | Permissible Concentrations (mg/kg) | |||
|---|---|---|---|---|---|
| Pb | Cd | Hg | As | ||
| Medicinal plant materials and herbal medicinal products | Russian pharmacopoeia [15] | 6.0 | 1.0 | 0.1 | 0.5 (Laminaria 90) |
| Herbal medicines, medicinal herbs | European pharmacopoeia [16] | 5.0 | 1.0 | 0.1 | There are no general regulations (Laminaria 90) |
| Herbal medicines | United States pharmacopeia [17] | 5.0 | 0.5 | 1.0 (total) methyl mercury 0.2 | Non-organic 2.0 |
| Medicinal plant materials and herbal medicinal products | Eurasian Economic Union pharmacopeia [18,19] | 6.0 | 1.0 | 0.1 | 0.5 |
| Medicinal plant materials (underground organs) | Pharmacopoeia of the People’s Republic of China [20] | 5.0 | 0.3 | 0.2 | 2.0 |
| Herbs consumed by humans | World Health Organization [21] | 10.0 | 0.3 | – | 1.0 |
| Traditional Chinese herbal medicines | ISO international standards [7] | 10.0 | 2.0 | 3.0 | 4.0 |
| Medicinal herbal preparations | Ayurvedic pharmacopoeia [22] | 10.0 | 0.3 | 1.0 | 3.0 |
| Medicinal herbal preparations | Thai pharmacopoeia [7] | 10.0 | 0.3 | – | 4.0 |
| Medicinal plant materials | Korean pharmacopoeia [7] | 5.0 | 0.3 | 0.2 | 3.0 |
| Traditional medicine products | Singapore Health Sciences Authority [23] | 20.0 | – | 0.5 | 5.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vinogradova, N.; Glukhov, A.; Chaplygin, V.; Kumar, P.; Mandzhieva, S.; Minkina, T.; Rajput, V.D. The Content of Heavy Metals in Medicinal Plants in Various Environmental Conditions: A Review. Horticulturae 2023, 9, 239. https://doi.org/10.3390/horticulturae9020239
Vinogradova N, Glukhov A, Chaplygin V, Kumar P, Mandzhieva S, Minkina T, Rajput VD. The Content of Heavy Metals in Medicinal Plants in Various Environmental Conditions: A Review. Horticulturae. 2023; 9(2):239. https://doi.org/10.3390/horticulturae9020239
Chicago/Turabian StyleVinogradova, Natalya, Alexander Glukhov, Victor Chaplygin, Pradeep Kumar, Saglara Mandzhieva, Tatiana Minkina, and Vishnu D. Rajput. 2023. "The Content of Heavy Metals in Medicinal Plants in Various Environmental Conditions: A Review" Horticulturae 9, no. 2: 239. https://doi.org/10.3390/horticulturae9020239
APA StyleVinogradova, N., Glukhov, A., Chaplygin, V., Kumar, P., Mandzhieva, S., Minkina, T., & Rajput, V. D. (2023). The Content of Heavy Metals in Medicinal Plants in Various Environmental Conditions: A Review. Horticulturae, 9(2), 239. https://doi.org/10.3390/horticulturae9020239










