Multiplication, Phenological Period and Growth Vigor of Thirty-One Grapevine Rootstocks and the Role of Parentage in Vigor Heredity
Abstract
1. Introduction
2. Materials and Methods
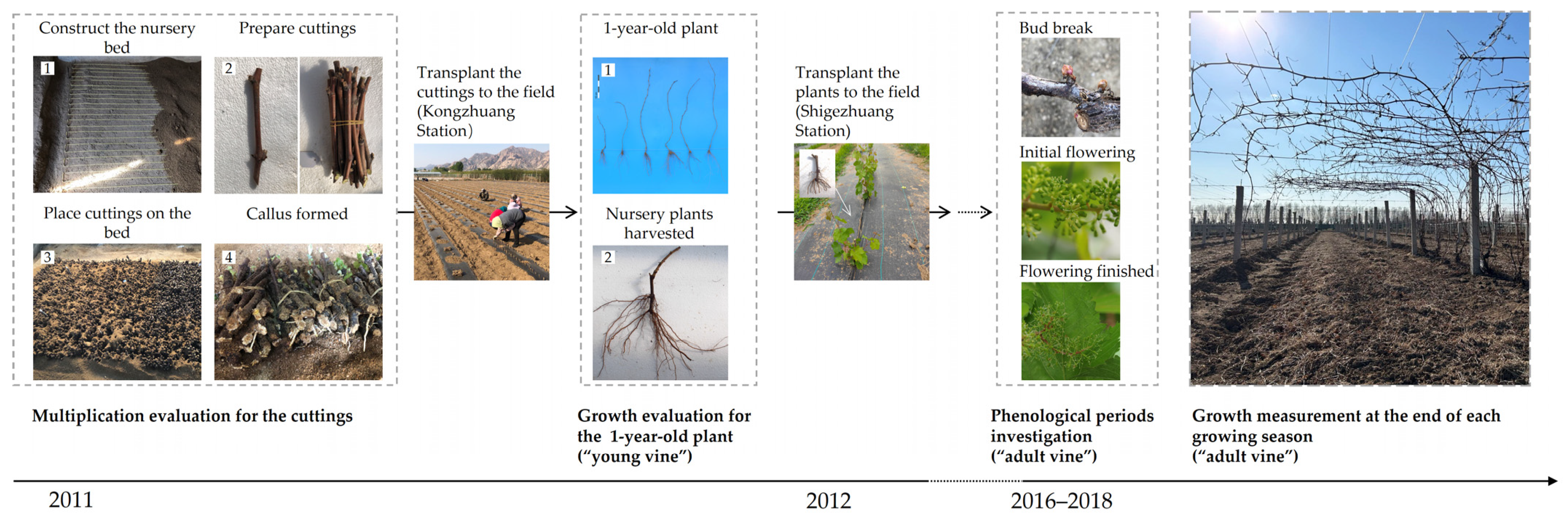
2.1. Multiplication Evaluation of the Plant Materials
2.2. Evaluation of Cutting Development
2.3. Growth Measurements for the Vines
2.4. Phenological Periods Investigation for the Vines
2.5. Statistical Analysis
3. Results
3.1. Multiplication Characteristics of the Cuttings
3.2. Growth of the One-Year-Old Vine in the Field
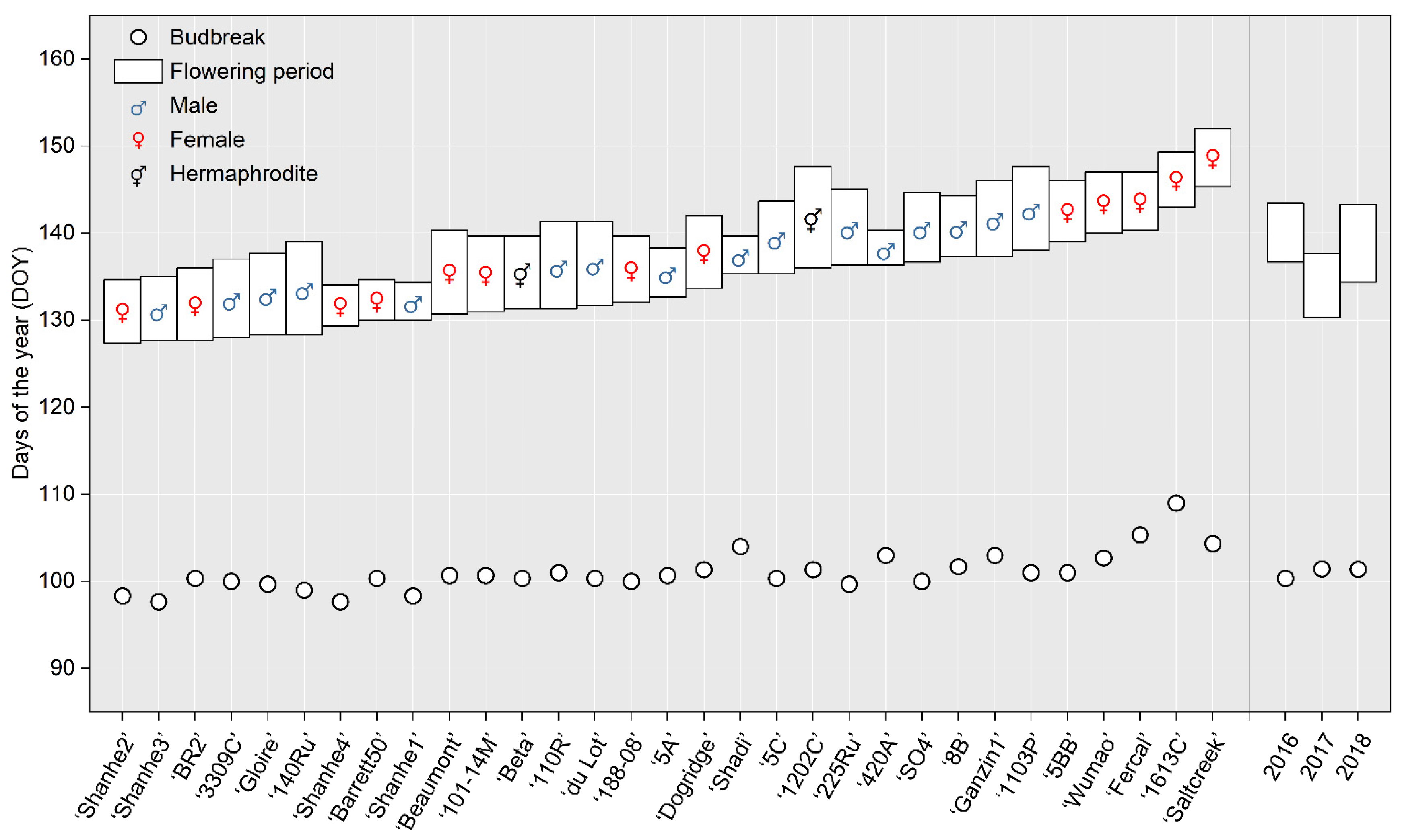
3.3. Phenological Periods of the Rootstocks
3.4. Growth of the Adult Vine
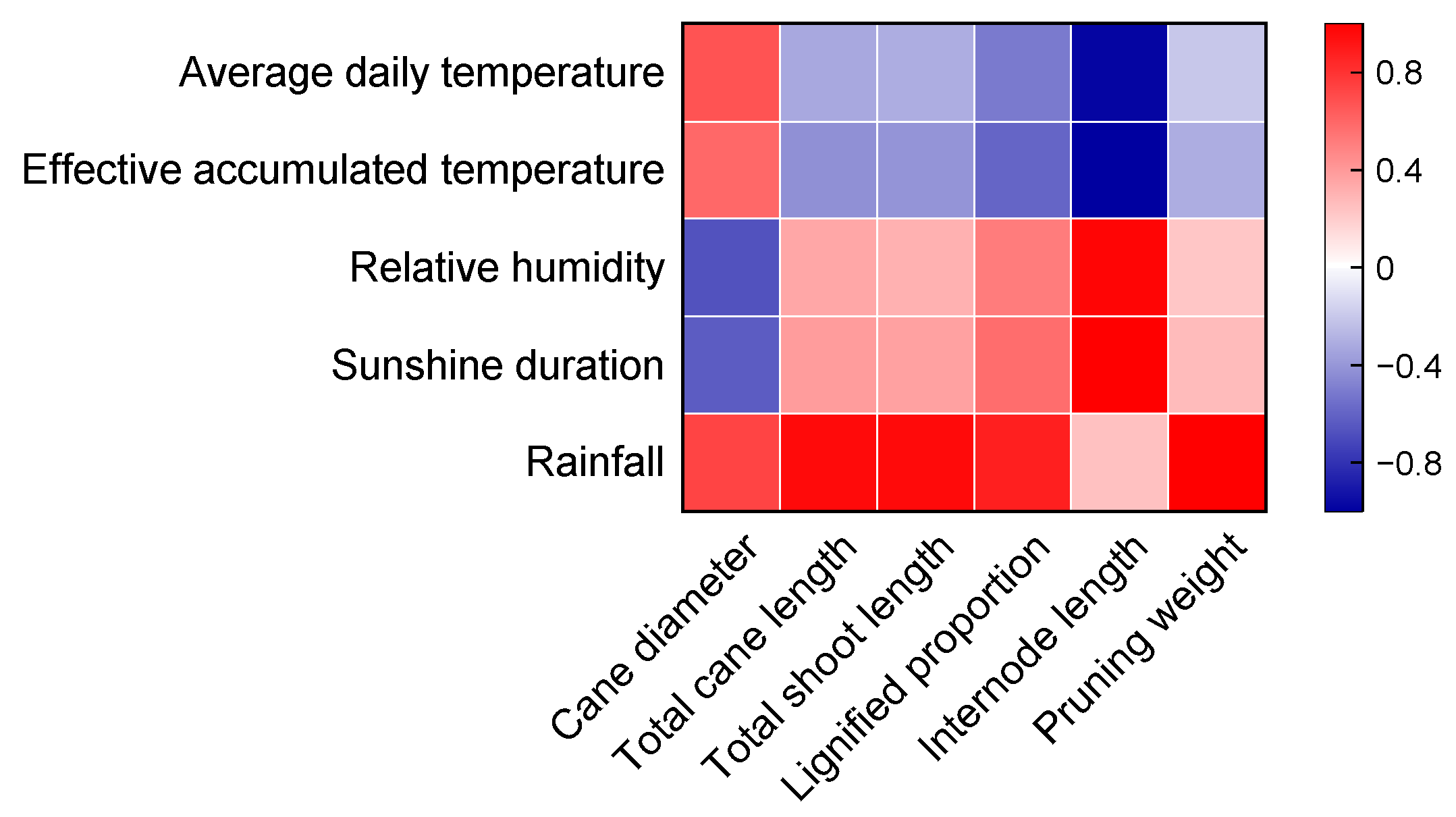
3.5. Correlations between Meteorological Data and Vine Growth
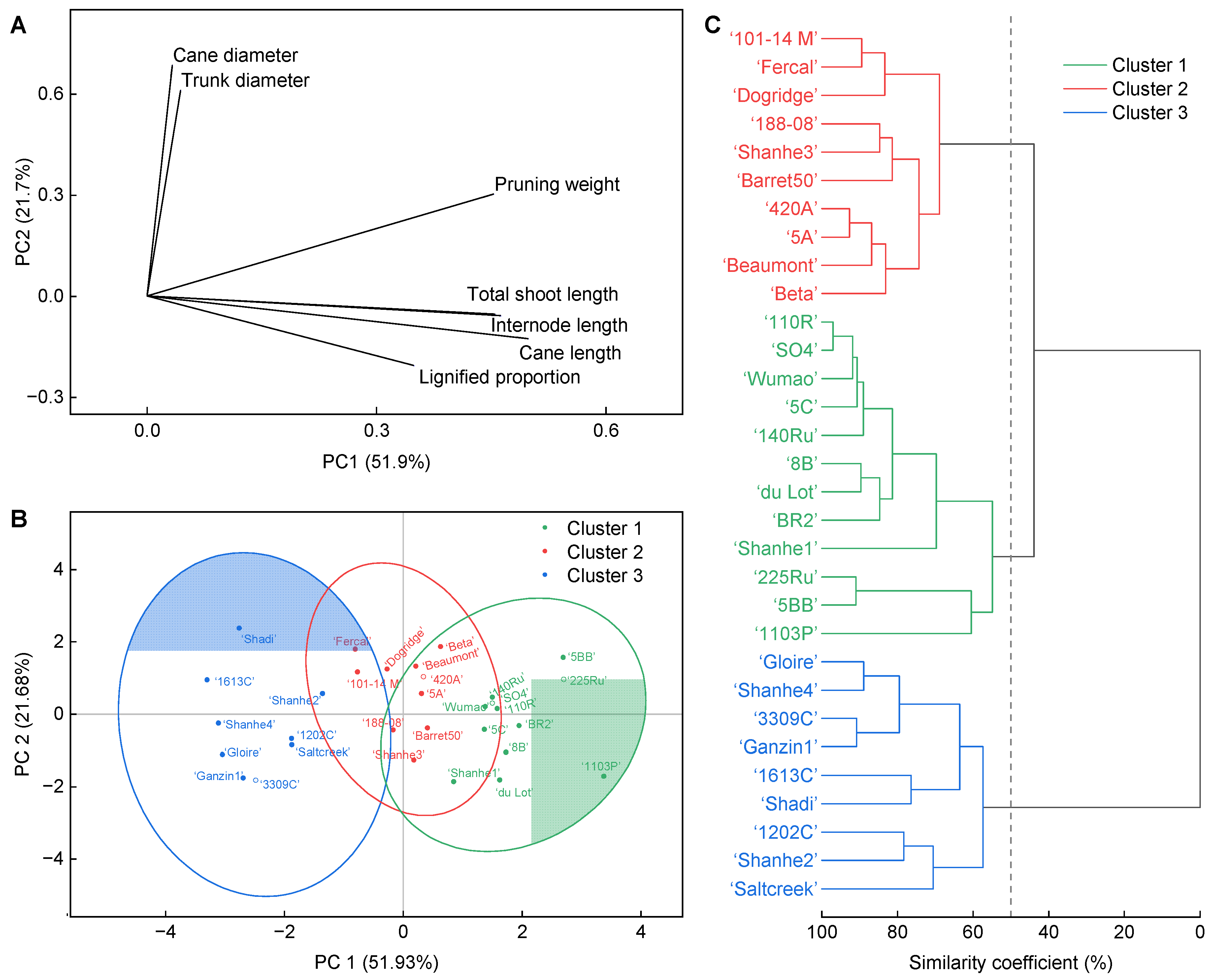
3.6. Principle Component Analysis (PCA) and Hierarchical Cluster Analysis (HCA) for the Rootstocks Based on the Growth Indicators
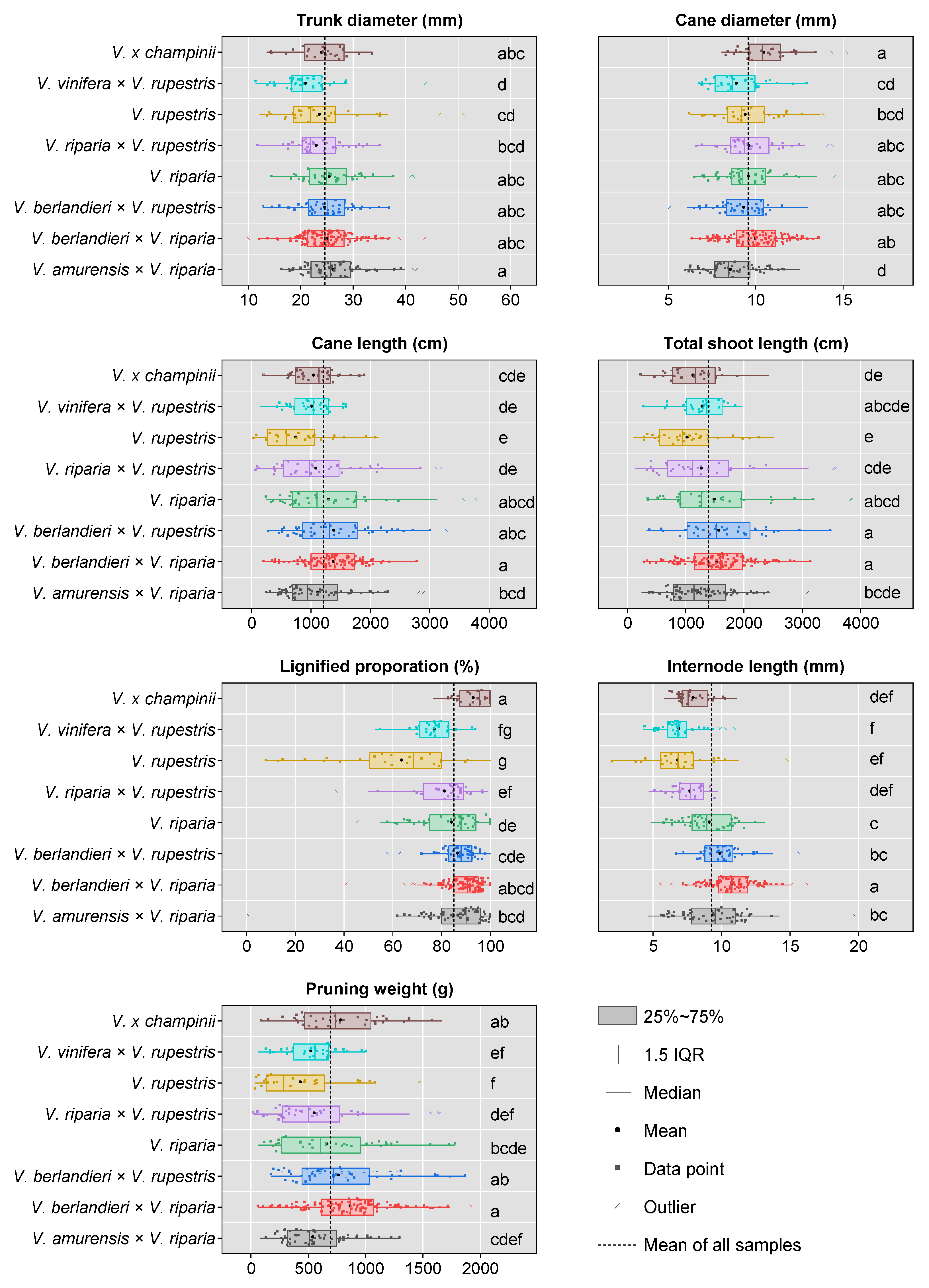
3.7. Genetic Background Effects on Tree Growth
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Corso, M.; Bonghi, C. Grapevine rootstock effects on abiotic stress tolerance. Plant Sci. Today 2014, 1, 108–113. [Google Scholar] [CrossRef]
- Walker, R.R.; Blackmore, D.H.; Clingeleffer, P.R. Impact of rootstock on yield and ion concentrations in petioles, juice and wine of Shiraz and Chardonnay in different viticultural environments with different irrigation water salinity. Aust. J. Grape Wine Res. 2010, 16, 243–257. [Google Scholar] [CrossRef]
- Ferris, H.; Zheng, L.; Walker, M.A. Resistance of grape rootstocks to plant-parasitic nematodes. J. Nematol. 2012, 44, 377–386. [Google Scholar] [PubMed]
- Pavloušek, P. Tolerance to lime-induced chlorosis and drought in grapevine rootstocks. In Abiotic Stress—Plant Responses and Applications in Agriculture; Vahdati, K., Leslie, C., Eds.; InTech: Vienna, Austria, 2013; pp. 277–305. [Google Scholar]
- Fort, K.; Fraga, J.; Grossi, D.; Walker, M.A. Early measures of drought tolerance in four grape rootstocks. J. Am. Soc. Hortic. Sci. 2017, 142, 36–46. [Google Scholar] [CrossRef]
- Cheng, J.; Li, H.; Wang, W.; Duan, C.; Wang, J.; He, F. The influence of rootstocks on the scions’ aromatic profiles of Vitis vinifera L. cv. Chardonnay. Sci. Hortic. 2020, 272, 109517. [Google Scholar] [CrossRef]
- Yin, Y.; Jia, N.; Li, M.; Liu, C.; Yuan, J.; Han, B.; Sun, Y.; Zhao, S.; Guo, Z. Rootstocks induce shifts in tree vigor, yield and berry quality of ‘Summer Black’ grapevines. Eur. J. Hortic. Sci. 2021, 86, 41–48. [Google Scholar] [CrossRef]
- Jones, T.H.; Cullis, B.R.; Clingeleffer, P.R.; Rühl, E.H. Effects of novel hybrid and traditional rootstocks on vigour and yield components of Shiraz grapevines. Aust. J. Grape Wine Res. 2009, 15, 284–292. [Google Scholar] [CrossRef]
- Zhang, L.; Marguerit, E.; Rossdeutsch, L.; Ollat, N.; Gambetta, G.A. The influence of grapevine rootstocks on scion growth and drought resistance. Theor. Exp. Plant Physiol. 2016, 28, 143–157. [Google Scholar] [CrossRef]
- Cochetel, N.; Escudie, F.; Cookson, S.J.; Dai, Z.; Vivin, P.; Bert, P.F.; Munoz, M.S.; Delrot, S.; Klopp, C.; Ollat, N.; et al. Root transcriptomic responses of grafted grapevines to heterogeneous nitrogen availability depend on rootstock genotype. J. Exp. Bot. 2017, 68, 4339–4355. [Google Scholar] [CrossRef]
- Lu, J.; Liu, C. Grapevine breeding in China. In Grapevine Breeding Programs for the Wine Industry; Reynolds, A., Ed.; Woodhead Publishing: Cambridge, UK, 2015; pp. 273–310. [Google Scholar]
- Duan, C.Q.; Liu, C.H.; Liu, F.Z.; Wang, Z.Y.; Liu, Y.L.; Xu, L.M. Fruit scientific research in New China in the past 70 years: Grape. J. Fruit Sci. 2019, 36, 1292–1301. [Google Scholar] [CrossRef]
- Liu, X.; Liu, C.; Fan, X.; Guo, D.; Zhang, G.; Sun, H. Construction of primary core collection of grape genetic resources. J. Plant Genet. Resour. 2012, 13, 72–76. [Google Scholar]
- van Leeuwen, C. Terroir: The effect of the physical environment on vine growth, grape ripening and wine sensory attributes. In Managing Wine Quality: Viticulture and Wine Quality; Reynolds, A.G., Ed.; Woodhead Publishing: Cambridge, UK, 2010; pp. 273–315. [Google Scholar]
- Khouni, S.; Laiadi, Z.; Bertazzon, N.; Angelini, E.; Migliaro, D. Preservation and sanitary status of Algerian grapevine germplasm: Management and improvement. S. Afr. J. Bot. 2023, 153, 346–356. [Google Scholar] [CrossRef]
- Marín, D.; Miranda, C.; Abad, F.J.; Urrestarazu, J.; Mayor, B.; Villa-Llop, A.; Santesteban, L.G. Agronomic evaluation of eight 41 B × 110 Richter grapevine genotypes as rootstock candidates for Mediterranean viticulture. Hortic. Plant J. 2022. [Google Scholar] [CrossRef]
- Provost, C.; Campbell, A.; Dumont, F. Rootstocks impact yield, fruit composition, nutrient deficiencies, and winter survival of hybrid cultivars in eastern Canada. Horticulturae 2021, 7, 237. [Google Scholar] [CrossRef]
- Bianchi, D.; Brancadoro, L. Water use efficiency and nutritional status of a new grapevine rootstock selection. Horticulturae 2021, 7, 503. [Google Scholar] [CrossRef]
- Yuan, J.; Li, M.; Jia, N.; Liu, C.; Han, B.; Yin, Y.; Sun, Y.; Guo, Z.; Zhao, S. Evaluation of salt stress tolerance in twenty-one grape rootstocks. Acta Agric. Boreali-Occident. Sin. 2019, 28, 602–606. [Google Scholar] [CrossRef]
- Xia, J.; Chen, C.; Liu, T.; Liu, C.; Liu, S.; Fang, J.; Shangguan, L. Germplasm resource evaluation and the underlying regulatory mechanisms of the differential copper stress tolerance among Vitis species. Environ. Exp. Bot. 2022, 206, 105198. [Google Scholar] [CrossRef]
- Gao, Z.; Li, J.; Zhu, H.; Sun, L.; Du, Y.; Zhai, H. Using differential thermal analysis to analyze cold hardiness in the roots of grape varieties. Sci. Hortic. 2014, 174, 155–163. [Google Scholar] [CrossRef]
- Li, Y.; Fu, Y.; Ma, Y.; Du, Y.; Zhai, H. Effects of waterlogging on characteristics of growth and photosynthesis in different grape rootstocks. Sci. Agric. Sin. 2013, 46, 995–1004. [Google Scholar] [CrossRef]
- Zhang, J.L.; Cao, Z.Y.; Ma, J.F. Screening of cold-resistant seedlings of a Chinese wild grape (Vitis piasezkii Maxim var. pagnucii) native to loess plateau of eastern Gansu province, China, as rootstocks. Sci. Hortic. 2009, 122, 125–128. [Google Scholar] [CrossRef]
- Zhang, P.; Leng, X.; Fan, X.; Liu, G.; Fang, J. Current status and research progress of grape rootstock germplasm resources. SINO-Overseas Grapevine Wine 2018, 219, 58–63. [Google Scholar] [CrossRef]
- Li, X.; Dong, W.; Lin, J.; Wang, Z.; Yang, Q.; Chang, Y.; Zhang, Z. Structural, physiological and biochemical responses of Pyrus calleryana offspring to salt stress. Eur. J. Hortic. Sci. 2015, 80, 306–315. [Google Scholar] [CrossRef]
- Coombe, B.G. Growth stages of the grapevine: Adoption of a system for identifying grapevine growth stages. Aust. J. Grape Wine Res. 1995, 1, 104–110. [Google Scholar] [CrossRef]
- Waite, H.; Whitelaw-Weckert, M.; Torley, P. Grapevine propagation: Principles and methods for the production of high-quality grapevine planting material. N. Z. J. Crop Hortic. Sci. 2014, 43, 144–161. [Google Scholar] [CrossRef]
- Hartmann, H.T.; Kester, D.E.; Davies, F.T.; Geneve, R.L. Plant Propagation: Principles and Practices, 5th ed.; Prentice-Hall Inc.: Englewood Cliffs, NJ, USA, 1990; p. 647. [Google Scholar]
- Köse, B.; Karabulut, B.; Ceylan, K. Effect of rootstock on grafted grapevine quality. Eur. J. Hortic. Sci. 2014, 79, 197–202. [Google Scholar]
- Rossdeutsch, L.; Schreiner, R.P.; Skinkis, P.A.; Deluc, L. Nitrate uptake and transport properties of two grapevine rootstocks with varying vigor. Front. Plant Sci. 2020, 11, 608813. [Google Scholar] [CrossRef]
- Lecourt, J.; Lauvergeat, V.; Ollat, N.; Vivin, P.; Cookson, S.J. Shoot and root ionome responses to nitrate supply in grafted grapevines are rootstock genotype dependent. Aust. J. Grape Wine Res. 2015, 21, 311–318. [Google Scholar] [CrossRef]
- Trought, M.C.T.; Dixon, R.; Mills, T.; Greven, M.; Agnew, R.; Mauk, J.L.; Praat, J.-P. The impact of differences in soil texture within a vineyard on vine vigour, vine earliness and juice composition. OENO One 2008, 42, 67–72. [Google Scholar] [CrossRef]
- van Leeuwen, C.; Friant, P.; Chone, X.; Tregoat, O.; Koundouras, S.; Dubourdieu, D. Influence of climate, soil, and cultivar on terroir. Am. J. Enol. Vitic. 2004, 55, 207–217. [Google Scholar] [CrossRef]
- Dodson Peterson, J.C.; Duncan, R.; Hirschfelt, D.; Ingels, C.; McGourty, G.; Smith, R.; Weber, E.; Wolpert, J.; Anderson, M.; Benz, J.; et al. Grape rootstock breeding and their performance based on the Wolpert trials in California. In The Grape Genome; Cantu, D., Walker, M.A., Eds.; Springer Nature: Cham, Switzerland, 2019; pp. 301–318. [Google Scholar]
- Hugalde, I.P.; Aguero, C.B.; Barrios-Masias, F.H.; Romero, N.; Viet Nguyen, A.; Riaz, S.; Piccoli, P.; McElrone, A.J.; Walker, M.A.; Vila, H.F. Modeling vegetative vigour in grapevine: Unraveling underlying mechanisms. Heliyon 2020, 6, e05708. [Google Scholar] [CrossRef]
- Galet, P.; Smith, J. Grape Varieties and Rootstock Varieties; Oenoplurimédia: Chaintré, France, 1998. [Google Scholar]
- Bettiga, L.J. Wine Grape Varieties in California; University of California, Agriculture and Natural Resources: Oakland, CA, USA, 2003. [Google Scholar]
- Keller, M. Taxonomy and anatomy. In The Science of Grapevines, 3rd ed.; Academic Press: New York, NY, USA, 2020; pp. 1–60. [Google Scholar]
- Gautier, A.; Cookson, S.J.; Lagalle, L.; Ollat, N.; Marguerit, E. Influence of the three main genetic backgrounds of grapevine rootstocks on petiolar nutrient concentrations of the scion, with a focus on phosphorus. OENO One 2020, 54, 1–13. [Google Scholar] [CrossRef]
| Rootstock | Abbreviation | Parentage |
|---|---|---|
| ‘Millardet et de Grasset 101-14’ | ‘101-14M’ | V. riparia × V. rupestris |
| ‘Paulsen 1103’ | ‘1103P’ | V. berlandieri × V. rupestris |
| ‘Richter 110’ | ‘110R’ | V. berlandieri × V. rupestris |
| ‘Couderc 1202’ | ‘1202C’ | V. vinifera × V. rupestris |
| ‘Ruggeri 140’ | ‘140Ru’ | V. berlandieri × V. rupestris |
| ‘Couderc 1613’ | ‘1613C’ | (V. riparia × V. longii) × ‘Othello’ |
| ‘Castel 188-08’ | ‘188-08’ | V. monticola × V. riparia |
| ‘Ruggeri 225’ | ‘225Ru’ | V. berlandieri × V. riparia |
| ‘Couderc 3309’ | ‘3309C’ | V. riparia × V. rupestris |
| ‘Millardet et de Grasse 420A’ | ‘420A’ | V. berlandieri × V. riparia |
| ‘Téléki 5 A’ | ‘5A’ | V. berlandieri × V. riparia |
| ‘Kober–Téléki 5BB’ | ‘5BB’ | V. berlandieri × V. riparia |
| ‘Téléki 5C’ | ‘5C’ | V. berlandieri × V. riparia |
| ‘Téléki 8B’ | ‘8B’ | V. berlandieri × V. riparia |
| ‘Berlandieri Rességuier No. 2’ | ‘BR2’ | V. berlandieri |
| ‘Riparia Barrett 50’ | ‘Barrett50’ | V. riparia |
| ‘Riparia Beaumont’ | ‘Beaumont’ | V. riparia |
| ‘Beta’ | ‘Beta’ | V. riparia × [(V. labrusca × V. vinifera) × V. labrusca] |
| ‘Dog ridge’ | ‘Dogridge’ | V. × champinii (V. rupestris × V. candicans) |
| ‘Fercal INRA Bordeaux’ | ‘Fercal’ | (V. berlandieri × V. vinifera) × (V. berlandieri × V. longii) |
| ‘Ganzin 1’ (‘AxR 1’) | ‘Ganzin1’ | V. vinifera × V. rupestris |
| ‘Riparia Gloire de Montpellier’ | ‘Gloire’ | V. riparia |
| ‘Rupestris du Lot’ | ‘du Lot’ | V. rupestris |
| ‘Saltcreek’ (‘Ramsey’) | ‘Saltcreek’ | V. × champinii (V. rupestris × V. candicans) |
| ‘Rupestris Scheele’ | ‘Shadi’ | V. rupestris |
| ‘Shanhe 1’ | ‘Shanhe1’ | V. amurensis × V. riparia |
| ‘Shanhe 2’ | ‘Shanhe2’ | V. amurensis × V. riparia |
| ‘Shanhe 3’ | ‘Shanhe3’ | V. amurensis × V. riparia |
| ‘Shanhe 4’ | ‘Shanhe4’ | V. amurensis × V. riparia |
| ‘Téléki-Fuhr Selektion Oppenheim No.4’ | ‘SO4’ | V. berlandieri × V. riparia |
| ‘Wumao’ | ‘Wumao’ | V. berlandieri × V. riparia |
| Rootstock | CFI | Rooting Rate (%) | Root Number | Budbreak Rate (%) | Bud Length (cm) |
|---|---|---|---|---|---|
| ‘101-14M’ | 0.94 ± 0.06 a–d | 62.5 ± 15.0 d–g | 1.3 ± 0.1 no | 85.0 ± 10.0 b–f | 1.05 ± 0.07 k–o |
| ‘1103P’ | 0.54 ± 0.01 m | 83.0 ± 17.0 abc | 4.2 ± 0.3 de | 92.8 ± 4.9 a–d | 1.17 ± 0.08 jkl |
| ‘110R’ | 0.72 ± 0.07 i–l | 87.5 ± 12.6 ab | 3.7 ± 0.4 ef | 82.5 ± 9.6 c–g | 1.08 ± 0.05 klm |
| ‘1202C’ | 0.92 ± 0.04 a–d | 90.0 ± 8.2 ab | 5.8 ± 0.4 b | 72.5 ± 9.6 ghi | 0.95 ± 0.03 mno |
| ‘140Ru’ | 0.87 ± 0.05 b–g | 87.5 ± 5.0 ab | 3.5 ± 0.3 fg | 92.5 ± 9.6 a–d | 1.35 ± 0.05 j |
| ‘1613C’ | 0.76 ± 0.04 h–k | 83.3 ± 6.1 abc | 2.6 ± 0.1 h–k | 71.8 ± 5.6 ghi | 1.86 ± 0.16 d–g |
| ‘188-08’ | 0.28 ± 0.07 n | 61.3 ± 13.7 d–g | 1.6 ± 0.2 l–o | 97.3 ± 5.5 ab | 1.84 ± 0.15 e–h |
| ‘225Ru’ | 0.88 ± 0.05 a–e | 90.0 ± 8.2 ab | 4.5 ± 0.4 d | 37.5 ± 9.6 l | 0.45 ± 0.03 r |
| ‘3309C’ | 0.94 ± 0.03 a–d | 62.5 ± 9.6 d–g | 2.3 ± 0.2 i–l | 92.5 ± 5.0 a–d | 1.68 ± 0.09 ghi |
| ‘420A’ | 0.78 ± 0.08 f–j | 5.0 ± 5.8 h | 0.2 ± 0.3 q | 67.5 ± 5.0 ij | 0.74 ± 0.06 pq |
| ‘5A’ | 0.70 ± 0.04 jkl | 100 ± 0 a | 10.2 ± 0.7 a | 95.0 ± 5.8 abc | 1.94 ± 0.14 def |
| ‘5BB’ | 0.92 ± 0.04 a–d | 67.5 ± 5.6 c–f | 2.9 ± 0.2 ghi | 85.3 ± 5.0 b–f | 1.24 ± 0.12 jk |
| ‘5C’ | 0.97 ± 0.03 a | 67.5 ± 5.0 c–f | 2.2 ± 0.1 i–l | 90.0 ± 8.2 a–e | 1.22 ± 0.11 jk |
| ‘8B’ | 0.93 ± 0.02 a–d | 80.0 ± 14.1 bcd | 5.7 ± 1.9 b | 60.0 ± 0 jk | 0.63 ± 0.03 q |
| ‘BR2’ | 0.77 ± 0.11 g–j | 48.0 ± 22.2 g | 0.5 ± 0.2 pq | 85.5 ± 17.1 b–f | 0.87 ± 0.09 nop |
| ‘Barrett50’ | 0.86 ± 0.06 c–g | 91.8 ± 8.4 ab | 5.3 ± 0.7 bc | 92.8 ± 5.3 a–d | 2.05 ± 0.25 cd |
| ‘Beaumont’ | 0.85 ± 0.05 d–h | 55.0 ± 10.0 fg | 2.6 ± 0.2 h–k | 85.0 ± 5.8 b–f | 1.05 ± 0.09 k–o |
| ‘Beta’ | 0.68 ± 0.06 kl | 77.5 ± 9.6 bcd | 3.7 ± 0.5 ef | 100 ± 0 a | 3.58 ± 0.26 a |
| ‘Dogridge’ | 0.76 ± 0.02 h–k | 75.0 ± 12.9 b–e | 1.9 ± 0.1 k–n | 90.0 ± 8.2 a–e | 1.10 ± 0.03 klm |
| ‘Fercal’ | 0.66 ± 0.03 l | 100 ± 0 a | 5.9 ± 0.3 b | 75.0 ± 5.8 f–i | 2.28 ± 0.29 b |
| ‘Ganzin1’ | 0.80 ± 0.08 e–i | 77.5 ± 12.6 bcd | 3.3 ± 0.4 fgh | 95.0 ± 5.8 abc | 1.64 ± 0.12 hi |
| ‘Gloire’ | 0.96 ± 0.03 ab | 80.0 ± 8.2 bcd | 3.7 ± 0.3 ef | 92.5 ± 5.0 a–d | 1.79 ± 0.09 f–i |
| ‘du Lot’ | 0.87 ± 0.09 a–f | 85.0 ± 12.9 abc | 4.9 ± 0.4 cd | 70.0 ± 8.2 hij | 0.87 ± 0.11 nop |
| ‘Saltcreek’ | 0.94 ± 0.05 a–d | 85.0 ± 5.8 abc | 4.9 ± 0.5 cd | 52.5 ± 5.0 k | 0.85 ± 0.11 op |
| ‘Shadi’ | 0.93 ± 0.09 a–d | 57.5 ± 15.0 efg | 1.4 ± 0.1 mno | 80.0 ± 14.1 d–h | 1.07 ± 0.10 k–n |
| ‘Shanhe1’ | 0.91 ± 0.06 a–d | 77.5 ± 9.6 bcd | 2.6 ± 0.3 h–k | 87.5 ± 5.0 a–e | 1.63 ± 0.11 i |
| ‘Shanhe2’ | 0.85 ± 0.07 d–h | 75.0 ± 10.0 b–e | 1.9 ± 0.2 k–n | 95.0 ± 5.8 abc | 1.90 ± 0.17 def |
| ‘Shanhe3’ | 0.78 ± 0.08 f–j | 90.0 ± 0 ab | 2.8 ± 0.3 g–j | 92.5 ± 5.0 a–d | 2.02 ± 0.18 cde |
| ‘Shanhe4’ | 0.95 ± 0.05 abc | 55.0 ± 12.9 fg | 1.0 ± 0.1 op | 90.0 ± 11.5 a–e | 2.16 ± 0.18 bc |
| ‘SO4’ | 0.94 ± 0.05 a–d | 62.5 ± 22.2 d–g | 2.1 ± 0.1 j–m | 77.5 ± 5.0 e–i | 0.98 ± 0.08 l–o |
| ‘Wumao’ | 0.90 ± 0.05 a–e | 72.5 ± 15.4 b–f | 4.9 ± 0.4 cd | 40.0 ± 3.3 l | 0.42 ± 0.03 r |
| Rootstock | Shoot Basal Diameter (mm) | Cane Length (cm) | Internode Length (cm) | Shoot Length (cm) | Lignified Proportion (%) |
|---|---|---|---|---|---|
| ‘101-14M’ | 6.9 ± 0.9 b–f | 125.8 ± 25.8 abc | 6.5 ± 1.4 bcd | 126.2 ± 25.5 a–f | 99.6 ± 1.1 a |
| ‘1103P’ | 6.4 ± 0.8 c–f | 112.6 ± 29.9 b–g | 6.6 ± 1.5 bc | 112.6 ± 29.9 c–g | 100 ± 0 a |
| ‘110R’ | 7.1 ± 1.4 b–e | 148.6 ± 18.7 a | 7.8 ± 1.0 a | 150.3 ± 18.2 a | 98.9 ± 2.6 a |
| ‘1202C’ | 7.4 ± 1.3 bcd | 124.8 ± 22.8 a–d | 5.2 ± 0.7 efg | 143.0 ± 27.2 ab | 89.0 ± 16.5 abc |
| ‘140Ru’ | 6.5 ± 0.8 c–f | 120.3 ± 31.7 a–e | 5.4 ± 0.8 efg | 126.1 ± 29.0 a–f | 95.3 ± 10.0 ab |
| ‘1613C’ | 5.9 ± 1.2 ef | 72.7 ± 25.4 jkl | 3.7 ± 0.9 ij | 72.7 ± 25.4 i | 100 ± 0 a |
| ‘188-08’ | 6.7 ± 0.6 c–f | 91.8 ± 24.4 e–k | 4.8 ± 0.6 fgh | 100.2 ± 25.0 fgh | 91.6 ± 13.1 abc |
| ‘225Ru’ | 10.1 ± 2.6 a | 96.0 ± 23.9 d–j | 6.1 ± 1.3 b–e | 111.0 ± 31.1 c–g | 87.9 ± 11.8 abc |
| ‘3309C’ | 6.9 ± 1.7 b–f | 85.1 ± 26.7 g–l | 3.8 ± 0.9 ij | 113.2 ± 24.3 c–g | 74.7 ± 12.8 d |
| ‘420A’ | 6.7 ± 1.2 c–f | 135.8 ± 30.6 ab | 5.2 ± 0.7 efg | 137.3 ± 31.5 abc | 99.1 ± 3.0 a |
| ‘5A’ | 7.4 ± 1.2 bcd | 119.8 ± 24.3 a–e | 6.0 ± 0.8 b–e | 125.8 ± 24.7 a–f | 95.5 ± 6.7 ab |
| ‘5BB’ | 7.3 ± 1.3 bcd | 101.9 ± 30.4 c–i | 6.3 ± 1.0 b–e | 106.8 ± 33.2 d–g | 96.4 ± 8.4 ab |
| ‘5C’ | 6.4 ± 1.1 c–f | 80.7 ± 23.2 h–l | 4.7 ± 1.2 f–i | 100.5 ± 24.6 fgh | 81.9 ± 21.4 cd |
| ‘8B’ | 6.9 ± 1.1 b–f | 97.9 ± 16.8 c–j | 5.4 ± 0.8 efg | 121.0 ± 15.5 b–f | 82.1 ± 17.0 cd |
| ‘BR2’ | 6.5 ± 1.7 c–f | 110.3 ± 42.6 b–g | 6.0 ± 1.3 b–e | 115.5 ± 32.7 b–g | 92.5 ± 15.0 abc |
| ‘Barrett50’ | 6.8 ± 1.1 b–f | 127.4 ± 21.9 abc | 5.4 ± 1.1 d–g | 135.5 ± 14.2 a–d | 93.6 ± 8.7 abc |
| ‘Beaumont’ | 6.2 ± 1.1 def | 78.7 ± 32.4 i–l | 3.8 ± 0.7 ij | 105.5 ± 28.7 e–h | 73.5 ± 15.7 d |
| ‘Beta’ | 7.0 ± 0.6 b–e | 134.4 ± 14.9 ab | 6.1 ± 0.4 b–e | 136.3 ± 15.8 abc | 98.7 ± 2.7 a |
| ‘Dogridge’ | 7.8 ± 1.5 bc | 116.3 ± 28.4 b–f | 4.6 ± 0.8 ghi | 116.3 ± 28.4 b–g | 100 ± 0 a |
| ‘Fercal’ | 6.1 ± 1.6 def | 87.2 ± 30.2 g–k | 4.1 ± 1.2 hij | 98.1 ± 32.1 f–i | 90.7 ± 16.9 abc |
| ‘Ganzin1’ | 6.5 ± 1.1 c–f | 107.8 ± 37.5 b–h | 5.5 ± 1.5 d–g | 116.9 ± 28.7 b–g | 89.7 ± 15.8 abc |
| ‘Gloire’ | 7.2 ± 2.0 b–e | 57.8 ± 27.0 l | 3.7 ± 1.2 ij | 78.6 ± 29.2 hi | 73.0 ± 16.0 d |
| ‘du Lot’ | 6.3 ± 0.5 def | 86.7 ± 21.0 g–k | 6.1 ± 1.6 b–e | 88.4 ± 22.5 ghi | 98.5 ± 4.1 a |
| ‘Saltcreek’ | 5.6 ± 0.5 f | 88.8 ± 31.5 f–k | 6.2 ± 0.7 b–e | 88.8 ± 31.5 ghi | 100 ± 0 a |
| ‘Shadi’ | 6.8 ± 0.9 b–f | 63.7 ± 31.9 kl | 3.2 ± 0.7 j | 78.7 ± 28.4 hi | 83.9 ± 26.6 bcd |
| ‘Shanhe1’ | 7.0 ± 1.2 b–e | 116.2 ± 19.6 b–f | 7.0 ± 0.9 ab | 122.2 ± 17.4 a–f | 95.4 ± 10.3 ab |
| ‘Shanhe2’ | 6.1 ± 1.1 def | 132.6 ± 18.1 ab | 6.9 ± 0.7 ab | 132.6 ± 18.1 a–e | 100 ± 0 a |
| ‘Shanhe3’ | 7.2 ± 0.6 b–e | 116.7 ± 18.0 b–f | 5.7 ± 0.8 c–f | 118.1 ± 18.1 b–f | 98.9 ± 2.7 a |
| ‘Shanhe4’ | 8.1 ± 1.0 b | 121.3 ± 40.0 a–e | 5.2 ± 1.1 efg | 125.9 ± 39.0 a–f | 95.8 ± 4.9 ab |
| ‘SO4’ | 6.5 ± 0.6 c–f | 118.3 ± 11.4 b–e | 5.5 ± 0.6 d–g | 124.6 ± 11.9 a–f | 95.2 ± 6.8 ab |
| ‘Wumao’ | 6.2 ± 1.0 def | 137.2 ± 13.7 ab | 6.9 ± 0.9 ab | 142.7 ± 12.6 ab | 96.2 ± 5.4 ab |
| Rootstock | Number of Roots per Cutting | Thick Root Number | Thick Roots Proportion (%) | Average Root Length (cm) | Total Root Length (cm) |
|---|---|---|---|---|---|
| ‘101-14M’ | 8.2 ± 2.66 k | 3.4 ± 1.8 c–g | 41.9 ± 16.3 bc | 12.6 ± 3.8 m | 99.3 ± 34.1 k |
| ‘1103P’ | 13.3 ± 3.84 e–j | 3.0 ± 1.8 e–h | 24.9 ± 19.1 d–j | 28.1 ± 6.8 b–h | 381.4 ± 156.1 d–h |
| ‘110R’ | 13.9 ± 4.38 d–j | 5.3 ± 1.7 ab | 39.9 ± 12.5 bcd | 12.8 ± 2.1 m | 178.5 ± 65.1 jk |
| ‘1202C’ | 10.0 ± 2.49 ijk | 3.3 ± 1.6 d–g | 33.6 ± 13.3 b–g | 23.9 ± 4.5 f–j | 241.8 ± 84.6 hij |
| ‘140Ru’ | 14.7 ± 5.38 c–i | 3.3 ± 0.8 d–g | 26.5 ± 13.2 d–j | 35.1 ± 7.3 ab | 492.6 ± 142.4 a–d |
| ‘1613C’ | 15.6 ± 7.37 a–h | 1.6 ± 1.3 gh | 15.6 ± 17.0 ijk | 21.8 ± 9.8 h–l | 297.3 ± 160.1 f–j |
| ‘188-08’ | 20.3 ± 4.52 ab | 2.8 ± 1.4 e–h | 14.6 ± 8.9 jk | 19.0 ± 2.8 j–m | 381.7 ± 87.8 d–h |
| ‘225Ru’ | 19.6 ± 5.13 abc | 3.7 ± 1.4 b–f | 21.1 ± 11.3 g–k | 30.8 ± 7.0 a–f | 585.3 ± 148.6 a |
| ‘3309C’ | 17.6 ± 3.44 a–e | 1.4 ± 1.3 h | 8.2 ± 6.8 k | 31.8 ± 7.4 a–e | 558.9 ± 181.5 abc |
| ‘420A’ | 13.3 ± 3.13 e–j | 4.1 ± 1.4 b–f | 30.5 ± 5.6 b–i | 24.4 ± 10.1 f–j | 334.1 ± 208.4 e–i |
| ‘5A’ | 16.3 ± 4.27 a–g | 5.2 ± 1.5 a–d | 34.2 ± 15.0 b–g | 34.9 ± 10.2 ab | 547.8 ± 125.4 abc |
| ‘5BB’ | 20.7 ± 7.92 a | 4.0 ± 2.8 b–f | 20.8 ± 12.5 g–k | 24.8 ± 4.8 e–j | 509.6 ± 206.4 a–d |
| ‘5C’ | 18.7 ± 1.63 a–d | 4.2 ± 1.2 b–f | 22.7 ± 7.9 f–k | 26.9 ± 4.2 c–i | 500.8 ± 76.7 a–d |
| ‘8B’ | 16.4 ± 3.95 a–f | 4.0 ± 1.8 b–f | 24.7 ± 12.5 e–j | 12.2 ± 2.2 m | 198.8 ± 58.0 ijk |
| ‘BR2’ | 8.3 ± 2.63 k | 5.3 ± 2.6 abc | 61.3 ± 10.6 a | 31.6 ± 16.5 a–e | 271.8 ± 173.4 g–j |
| ‘Barrett50’ | 17.8 ± 4.49 a–e | 4.3 ± 1.6 b–f | 25.8 ± 11.8 d–j | 25.6 ± 4.2 e–j | 444.3 ± 87.6 a–f |
| ‘Beaumont’ | 16.1 ± 4.26 a–g | 2.7 ± 1.0 e–h | 18.0 ± 7.8 h–k | 16.1 ± 3.1 lm | 258.7 ± 74.6 g–j |
| ‘Beta’ | 11.5 ± 3.34 f–k | 3.6 ± 1.3 b–f | 32.3 ± 11.0 b–h | 33.8 ± 6.8 abc | 386.9 ± 128.8 d–h |
| ‘Dogridge’ | 14.4 ± 4.38 d–j | 4.0 ± 1.9 b–f | 28.6 ± 13.5 b–j | 17.1 ± 4.6 klm | 257.9 ± 136.0 g–j |
| ‘Fercal’ | 11.9 ± 6.17 f–k | 4.3 ± 2.4 b–f | 39.6 ± 23.4 b–e | 28.6 ± 4.4 b–h | 338.3 ± 168.0 e–i |
| ‘Ganzin1’ | 11.2 ± 5.51 g–k | 2.9 ± 1.9 e–h | 30.4 ± 17.1 b–i | 29.9 ± 10.0 b–g | 331.7 ± 185.2 e–j |
| ‘Gloire’ | 15.2 ± 2.39 b–h | 3.3 ± 1.7 d–g | 22.0 ± 11.3 g–k | 20.3 ± 3.0 i–l | 306.9 ± 59.0 f–j |
| ‘du Lot’ | 17.9 ± 5.14 a–e | 3.6 ± 2.1 b–f | 22.0 ± 16.7 g–k | 22.6 ± 6.3 h–l | 424.3 ± 215.0 b–f |
| ‘Saltcreek’ | 9.6 ± 2.72 jk | 2.4 ± 1.0 fgh | 27.9 ± 14.8 c–j | 28.2 ± 7.0 b–h | 262.8 ± 74.1 g–j |
| ‘Shadi’ | 15.1 ± 3.81 c–h | 3.7 ± 1.4 b–f | 26.1 ± 11.4 d–j | 15.8 ± 2.0 lm | 239.4 ± 67.5 hij |
| ‘Shanhe1’ | 17.3 ± 4.24 a–e | 4.4 ± 2.1 b–e | 26.3 ± 11.4 d–j | 25.0 ± 6.7 e–j | 431.9 ± 153.3 b–f |
| ‘Shanhe2’ | 15.3 ± 4.64 b–h | 6.2 ± 2.3 a | 43.0 ± 14.5 b | 37.6 ± 6.0 a | 570.0 ± 192.4 ab |
| ‘Shanhe3’ | 15.7 ± 3.43 a–g | 3.6 ± 0.7 b–f | 23.6 ± 4.9 f–j | 25.9 ± 6.3 d–j | 408.7 ± 136.0 c–g |
| ‘Shanhe4’ | 18.4 ± 5.99 a–e | 3.3 ± 1.7 d–g | 19.7 ± 13.5 g–k | 26.8 ± 4.3 d–i | 479.9 ± 133.0 a–e |
| ‘SO4’ | 16.1 ± 4.72 a–g | 3.9 ± 1.2 b–f | 26.1 ± 10.1 d–j | 32.7 ± 6.6 a–d | 525.6 ± 200.4 a–d |
| ‘Wumao’ | 10.6 ± 3.41 h–k | 3.7 ± 1.2 b–f | 37.8 ± 16.3 b–f | 23.5 ± 4.8 g–k | 248.2 ± 85.7 hij |
| Season | Rootstock | Trunk Diameter (mm) | Cane Diameter (mm) | Total Cane Length (cm) | Total Shoot Length (cm) | Lignified Proportion (%) | Internode Length (cm) | Pruning Weight (g) |
|---|---|---|---|---|---|---|---|---|
| 2016 | ‘101-14M’ | 24.98 ± 4.55 a–d | 11.42 ± 1.72 ab | 1901.8 ± 863.0 a–e | 2169.2 ± 940.1 abc | 86.3 ± 6.6 b–e | 8.62 ± 0.85 g–l | 1051.0 ± 427.5 a–f |
| ‘1103P’ | 21.76 ± 2.17 bcd | 8.79 ± 1.05 e–i | 2768.3 ± 696.0 a | 2836.0 ± 761.7 a | 98.4 ± 2.1 a | 10.84 ± 0.90 b–f | 1033.1 ± 203.8 a–f | |
| ‘110R’ | 21.76 ± 4.50 bcd | 9.87 ± 1.20 b–i | 1881.1 ± 784.2 a–e | 2087.6 ± 861.8 abc | 89.3 ± 4.1 a–e | 10.18 ± 1.10 b–h | 1189.0 ± 471.6 a–d | |
| ‘1202C’ | 21.11 ± 2.98 bcd | 9.54 ± 0.72 b–i | 1202.0 ± 327.0 b–g | 1416.4 ± 332.3 bcd | 84.0 ± 8.1 cde | 5.49 ± 0.87 m | 711.0 ± 172.5 b–i | |
| ‘140Ru’ | 25.69 ± 2.10 abc | 10.89 ± 0.63 a–f | 2102.3 ± 534.3 abc | 2262.9 ± 563.7 ab | 92.4 ± 3.4 a–d | 10.32 ± 0.47 b–h | 1116.0 ± 291.0 a–e | |
| ‘1613C’ | 19.76 ± 0.98 cd | 10.29 ± 1.00 a–i | 496.0 ± 41.6 g | 953.0 ± 75.6 d | 51.3 ± 1.5 g | 7.57 ± 0.11 j–m | 433.3 ± 20.2 ghi | |
| ‘188-08’ | 19.18 ± 2.06 cd | 9.09 ± 0.90 c–i | 1416.1 ± 361.2 b–g | 1511.7 ± 400.7 bcd | 93.9 ± 4.1 abc | 8.80 ± 0.77 f–l | 634.4 ± 170.9 d–i | |
| ‘225Ru’ | 23.62 ± 2.16 bcd | 10.41 ± 0.93 a–i | 1584.2 ± 397.2 b–f | 1828.9 ± 342.5 a–d | 86.6 ± 7.3 b–e | 10.16 ± 0.96 b–h | 1228.0 ± 320.9 abc | |
| ‘3309C’ | 20.86 ± 4.02 bcd | 8.53 ± 0.80 ghi | 1694.9 ± 369.6 b–f | 1953.1 ± 370.9 a–d | 86.1 ± 8.4 b–e | 7.02 ± 0.69 lm | 670.0 ± 126.0 c–i | |
| ‘420A’ | 23.96 ± 6.35 bcd | 10.53 ± 0.71 a–g | 1659.2 ± 694.8 b–f | 1859.4 ± 711.6 a–d | 88.9 ± 9.4 a–e | 9.81 ± 1.22 c–i | 977.0 ± 403.0 a–h | |
| ‘5A’ | 22.14 ± 3.09 bcd | 10.74 ± 1.82 a–g | 1533.3 ± 730.9 b–f | 1644.7 ± 736.2 bcd | 90.5 ± 8.3 a–d | 10.76 ± 1.48 b–g | 1000.0 ± 461.4 a–g | |
| ‘5BB’ | 25.74 ± 4.56 abc | 11.25 ± 1.32 abc | 1502.6 ± 477.8 b–f | 1734.7 ± 511.9 bcd | 86.5 ± 4.3 b–e | 12.17 ± 1.07 ab | 1027.0 ± 303.8 a–f | |
| ‘5C’ | 21.27 ± 3.38 bcd | 9.68 ± 0.70 b–i | 1724.8 ± 185.3 b–f | 1798.2 ± 214.4 a–d | 95.8 ± 2.9 ab | 10.28 ± 0.81 b–h | 961.0 ± 261.5 a–h | |
| ‘8B’ | 21.44 ± 3.72 bcd | 9.33 ± 1.01 b–i | 1567.7 ± 540.5 b–f | 1698.9 ± 553.2 bcd | 92.0 ± 4.6 a–d | 11.09 ± 0.80 b–e | 859.0 ± 280.2 a–i | |
| ‘BR2’ | 21.97 ± 2.03 bcd | 9.52 ± 0.61 b–i | 2184.5 ± 497.4 ab | 2265.2 ± 534.0 ab | 96.6 ± 2.0 ab | 11.79 ± 0.66 abc | 1364.0 ± 279.1 a | |
| ‘Barrett50’ | 21.53 ± 0.59 bcd | 10.93 ± 0.75 a–e | 1331.4 ± 527.4 b–g | 1559.4 ± 681.6 bcd | 87.2 ± 4.0 a–e | 11.28 ± 0.71 a–e | 906.0 ± 398.4 a–i | |
| ‘Beaumont’ | 25.00 ± 5.02 a–d | 10.75 ± 2.07 a–g | 1774.3 ± 728.0 a–f | 2154.2 ± 738.0 abc | 78.0 ± 15.2 ef | 9.29 ± 1.82 e–k | 1238.0 ± 482.0 abc | |
| ‘Beta’ | 25.75 ± 2.62 abc | 11.16 ± 1.84 a–d | 1552.7 ± 533.3 b–f | 1695.2 ± 554.3 bcd | 91.5 ± 5.6 a–d | 9.71 ± 2.61 c–j | 1018.0 ± 424.7 a–f | |
| ‘Dogridge’ | 27.32 ± 2.67 ab | 10.39 ± 1.44 a–i | 1419.2 ± 284.1 b–g | 1715.8 ± 362.1 bcd | 83.2 ± 3.6 cde | 7.12 ± 0.52 klm | 1028.0 ± 186.0 a–f | |
| ‘Fercal’ | 23.31 ± 5.36 bcd | 10.53 ± 2.92 a–g | 1183.1 ± 709.9 c–g | 1359.0 ± 806.6 bcd | 88.3 ± 6.3 a–e | 9.48 ± 1.63 d–j | 514.0 ± 381.1 f–i | |
| ‘Ganzin1’ | 19.20 ± 4.74 cd | 9.01 ± 1.22 c–i | 1293.7 ± 159.9 b–g | 1635.9 ± 192.8 bcd | 78.3 ± 3.0 ef | 6.85 ± 0.53 lm | 553.0 ± 69.8 e–i | |
| ‘Gloire’ | 19.43 ± 4.06 cd | 8.21 ± 0.82 hi | 982.4 ± 436.2 d–g | 1183.6 ± 493.2 cd | 81.8 ± 5.8 def | 7.84 ± 0.71 i–l | 368.0 ± 153.3 i | |
| ‘du Lot’ | 18.23 ± 2.96 d | 8.94 ± 1.13 d–i | 1570.3 ± 659.8 b–f | 1697.1 ± 709.7 bcd | 92.3 ± 4.4 a–d | 11.03 ± 1.66 b–e | 814.0 ± 363.4 a–i | |
| ‘Saltcreek’ | 18.05 ± 3.23 d | 10.49 ± 0.73 a–h | 811.2 ± 606.0 fg | 910.6 ± 686.2 d | 89.4 ± 4.8 a–e | 8.41 ± 0.87 h–l | 404.0 ± 342.5 hi | |
| ‘Shadi’ | 31.46 ± 5.90 a | 12.47 ± 1.25 a | 1172.0 ± 339.7 c–g | 1561.4 ± 354.4 bcd | 72.8 ± 8.4 f | 7.09 ± 0.74 lm | 920.0 ± 202.5 a–i | |
| ‘Shanhe1’ | 22.50 ± 2.67 bcd | 8.16 ± 0.46 i | 1977.2 ± 703.2 a–d | 2099.3 ± 774.5 abc | 93.8 ± 4.3 abc | 11.61 ± 0.37 a–d | 867.0 ± 322.8 a–i | |
| ‘Shanhe2’ | 24.42 ± 4.18 a–d | 10.26 ± 0.59 a–i | 1620.8 ± 285.6 b–f | 1715.4 ± 305.7 bcd | 94.6 ± 3.1 abc | 9.96 ± 0.96 c–i | 779.0 ± 133.0 b–i | |
| ‘Shanhe3’ | 23.90 ± 2.69 bcd | 8.62 ± 0.86 f–i | 1762.5 ± 376.1 b–f | 1881.4 ± 383.2 a–d | 92.7 ± 4.8 a–d | 11.15 ± 1.37 b–e | 779.0 ± 172.3 b–i | |
| ‘Shanhe4’ | 24.47 ± 7.31 a–d | 9.88 ± 0.69 b–i | 956.9 ± 353.5 efg | 1137.1 ± 365.6 cd | 83.2 ± 6.5 cde | 8.73 ± 2.14 f–l | 433.3 ± 155.3 ghi | |
| ‘SO4’ | 22.27 ± 2.90 bcd | 10.31 ± 1.04 a–i | 1903.7 ± 500.7 a–e | 2151.4 ± 567.7 abc | 87.9 ± 3.8 a–e | 9.97 ± 0.91 b–i | 1267.0 ± 319.1 ab | |
| ‘Wumao’ | 23.65 ± 2.48 bcd | 11.52 ± 1.15 ab | 1722.0 ± 470.3 b–f | 1874.4 ± 563.3 a–d | 93.0 ± 6.9 a–d | 13.39 ± 1.35 a | 1091.0 ± 363.4 a–e | |
| 2017 | ‘101-14M’ | 23.00 ± 5.29 ab | 10.10 ± 1.91 a–g | 610.0 ± 417.5 cd | 799.0 ± 493.3 cd | 72.2 ± 14.2 e | 7.78 ± 1.38 f–k | 374.5 ± 274.1 cd |
| ‘1103P’ | 21.75 ± 3.22 ab | 7.81 ± 1.72 ghi | 1523.8 ± 730.3 ab | 1673.8 ± 785.3 abc | 91.9 ± 4.9 abc | 10.21 ± 1.39 a–f | 775.6 ± 480.4 a–d | |
| ‘110R’ | 24.94 ± 4.48 ab | 10.73 ± 1.34 a–f | 1079.7 ± 340.7 a–d | 1373.9 ± 483.9 a–d | 81.5 ± 8.8 b–e | 9.88 ± 1.03 a–g | 683.0 ± 244.7 a–d | |
| ‘1202C’ | 25.51 ± 3.21 ab | 10.85 ± 1.08 a–e | 1077.0 ± 312.1 a–d | 1283.0 ± 399.6 a–d | 85.0 ± 5.5 b–e | 7.03 ± 0.34 h–k | 655.0 ± 187.0 a–d | |
| ‘140Ru’ | 26.94 ± 6.26 ab | 9.02 ± 0.80 c–i | 1254.0 ± 397.8 a–d | 1517.0 ± 474.7 a–d | 79.9 ± 9.0 cde | 8.89 ± 1.08 a–j | 595.0 ± 183.3 a–d | |
| ‘1613C’ | 25.23 ± 4.50 ab | 10.54 ± 0.06 a–f | 1115.0 ± 233.3 a–d | 1275.0 ± 176.8 a–d | 90.5 ± 10.6 abc | 7.29 ± 0.74 g–k | 620.0 ± 141.4 a–d | |
| ‘188-08’ | 29.18 ± 5.99 a | 10.83 ± 1.17 a–e | 1338.2 ± 511.5 abc | 1486.0 ± 575.0 a–d | 91.4 ± 3.2 abc | 8.47 ± 0.56 b–k | 612.8 ± 244.6 a–d | |
| ‘225Ru’ | 26.11 ± 3.80 ab | 9.34 ± 0.90 a–h | 1568.0 ± 524.3 ab | 1862.2 ± 593.7 ab | 85.1 ± 3.7 b–e | 10.77 ± 1.11 a–d | 992.5 ± 384.4 ab | |
| ‘3309C’ | 20.48 ± 2.80 ab | 8.65 ± 0.60 e–h | 461.5 ± 203.2 d | 616.5 ± 266.1 d | 75.7 ± 8.6 de | 6.85 ± 1.22 ijk | 215.0 ± 92.7 d | |
| ‘420A’ | 27.07 ± 8.83 ab | 11.58 ± 1.64 ab | 942.1 ± 423.0 a–d | 1037.8 ± 463.3 bcd | 91.4 ± 5.9 abc | 8.63 ± 0.99 b–j | 746.5 ± 399.0 a–d | |
| ‘5A’ | 23.43 ± 4.60 ab | 10.90 ± 1.65 a–e | 943.3 ± 519.1 a–d | 1013.3 ± 570.6 bcd | 95.0 ± 5.3 ab | 10.49 ± 0.59 a–e | 590.8 ± 275.8 a–d | |
| ‘5BB’ | 27.12 ± 3.48 ab | 11.32 ± 1.05 a–d | 1634.2 ± 476.5 a | 2020.9 ± 498.2 a | 81.3 ± 6.0 b–e | 11.52 ± 1.29 a | 1096.1 ± 473.2 a | |
| ‘5C’ | 23.48 ± 2.94 ab | 10.76 ± 1.24 a–e | 1380.0 ± 330.3 abc | 1512.0 ± 413.1 a–d | 92.6 ± 5.3 abc | 10.78 ± 1.33 a–d | 802.5 ± 215.7 abc | |
| ‘8B’ | 24.24 ± 4.35 ab | 8.75 ± 1.50 e–i | 1275.7 ± 516.3 abc | 1400.0 ± 558.9 a–d | 91.7 ± 4.7 abc | 11.15 ± 2.60 ab | 685.0 ± 357.6 a–d | |
| ‘BR2’ | 24.23 ± 4.79 ab | 9.13 ± 1.45 b–i | 837.7 ± 353.1 bcd | 983.8 ± 428.3 bcd | 83.3 ± 7.7 b–e | 9.50 ± 2.05 a–i | 485.7 ± 221.3 bdc | |
| ‘Barrett50’ | - | - | - | - | - | - | - | |
| ‘Beaumont’ | 28.94 ± 6.12 a | 10.61 ± 1.50 a–f | 1112.4 ± 375.6 a–d | 1264.0 ± 390.9 a–d | 87.5 ± 6.2 a–d | 7.95 ± 0.67 e–k | 638.5 ± 336.2 a–d | |
| ‘Beta’ | 30.04 ± 4.37 a | 11.74 ± 1.28 a | 895.7 ± 595.9 a–d | 941.4 ± 620.3 cd | 95.1 ± 5.3 ab | 9.14 ± 1.03 a–i | 750.0 ± 476.6 a–d | |
| ‘Dogridge’ | 25.97 ± 2.84 ab | 11.08 ± 1.38 a–e | 1029.4 ± 373.6 a–d | 1095.4 ± 414.0 bcd | 94.5 ± 5.1 ab | 8.13 ± 1.48 d–k | 833.0 ± 421.3 abc | |
| ‘Fercal’ | 29.33 ± 7.31 a | 11.66 ± 1.92 a | 912.4 ± 375.1 a–d | 1113.7 ± 415.1 bcd | 79.3 ± 6.4 cde | 7.77 ± 1.52 f–k | 735.5 ± 476.3 a–d | |
| ‘Ganzin1’ | 21.10 ± 8.74 ab | 8.91 ± 1.26 d–i | 943.9 ± 372.4 a–d | 1252.9 ± 435.0 a–d | 72.0 ± 9.1 e | 5.95 ± 0.66 k | 450.0 ± 232.8 bdc | |
| ‘Gloire’ | 22.86 ± 6.08 ab | 9.69 ± 1.17 a–h | 927.2 ± 574.1 a–d | 1120.2 ± 666.8 a–d | 82.1 ± 6.3 b–e | 6.81 ± 1.16 ijk | 387.5 ± 236.9 cd | |
| ‘du Lot’ | 21.60 ± 3.68 ab | 9.17 ± 0.55 b–i | 1516.6 ± 495.5 ab | 1648.4 ± 506.7 abc | 91.4 ± 5.7 abc | 9.12 ± 1.41 a–i | 724.6 ± 299.7 a–d | |
| ‘Saltcreek’ | 17.65 ± 3.37 b | 11.48 ± 2.08 abc | 816.7 ± 279.3 bcd | 816.7 ± 279.3 cd | 100.0 ± 0.0 a | 8.29 ± 0.84 c–k | 790.0 ± 236.4 a–d | |
| ‘Shadi’ | 26.19 ± 8.32 ab | 10.70 ± 1.28 a–f | 1143.0 ± 694.4 a–d | 1452.5 ± 741.3 a–d | 75.5 ± 6.5 de | 7.21 ± 1.28 g–k | 862.5 ± 411.2 abc | |
| ‘Shanhe1’ | 24.70 ± 4.98 ab | 6.73 ± 0.44 i | 832.7 ± 363.2 bcd | 1022.1 ± 495.4 bcd | 83.0 ± 9.7 b–e | 8.87 ± 1.41 a–j | 370.6 ± 158.0 cd | |
| ‘Shanhe2’ | 25.23 ± 4.94 ab | 10.46 ± 1.21 a–f | 703.7 ± 230.1 cd | 860.2 ± 323.3 cd | 84.7 ± 5.9 b–e | 9.54 ± 0.86 a–h | 432.0 ± 170.9 bdc | |
| ‘Shanhe3’ | 26.78 ± 4.04 ab | 7.55 ± 1.06 hi | 1059.0 ± 301.6 a–d | 1222.6 ± 339.8 a–d | 87.5 ± 11.0 a–d | 10.94 ± 3.56 abc | 474.5 ± 162.6 bdc | |
| ‘Shanhe4’ | 27.92 ± 8.98 a | 8.26 ± 0.73 f–i | 783.8 ± 320.2 bcd | 944.5 ± 356.3 cd | 84.0 ± 7.6 b–e | 6.39 ± 1.39 jk | 400.0 ± 203.6 cd | |
| ‘SO4’ | 23.37 ± 6.71 ab | 9.40 ± 1.28 a–h | 959.0 ± 372.7 a–d | 1175.0 ± 512.1 a–d | 82.4 ± 8.6 b–e | 9.89 ± 1.28 a–g | 615.5 ± 196.7 a–d | |
| ‘Wumao’ | 24.02 ± 3.49 ab | 11.10 ± 1.44 a–e | 998.2 ± 184.8 a–d | 1086.4 ± 208.9 bcd | 91.6 ± 7.7 abc | 10.32 ± 1.41 a–f | 749.5 ± 181.3 a–d | |
| 2018 | ‘101-14M’ | 28.40 ± 4.90 a–d | 10.54 ± 1.16 ab | 1035.7 ± 558.2 abc | 1258.0 ± 564.9 ab | 78.2 ± 16.5 c–i | 8.28 ± 1.15 e–h | 642.0 ± 413.0 abc |
| ‘1103P’ | 27.45 ± 6.41 a–d | 8.72 ± 1.91 b–h | 1101.5 ± 699.2 abc | 1237.5 ± 751.3 ab | 64.0 ± 5.9 ijk | 12.12 ± 2.01 ab | 712.5 ± 410.4 abc | |
| ‘110R’ | 24.80 ± 5.13 a–d | 9.18 ± 1.30 a–h | 1282.6 ± 585.4 ab | 1444.2 ± 637.6 a | 89.1 ± 5.9 a–g | 10.80 ± 0.95 a–e | 864.0 ± 394.4 ab | |
| ‘1202C’ | 19.31 ± 3.54 cd | 7.38 ± 0.54 h | 833.7 ± 288.7 abc | 1205.5 ± 403.6 ab | 68.9 ± 6.5 hij | 8.53 ± 1.48 d–h | 490.0 ± 174.6 abc | |
| ‘140Ru’ | 29.33 ± 2.68 abc | 9.31 ± 1.06 a–h | 1216.5 ± 468.1 abc | 1351.5 ± 486.3 ab | 89.6 ± 5.2 a–f | 10.23 ± 2.21 a–f | 580.5 ± 254.2 abc | |
| ‘1613C’ | 29.25 ± 5.11 abc | 9.29 ± 0.71 a-h | 615.0 ± 49.5 bc | 1115.0 ± 162.6 ab | 56.5 ± 10.6 jk | 6.77 ± 1.59 h | 405.0 ± 106.1 bc | |
| ‘188-08’ | 27.66 ± 4.45 a–d | 8.34 ± 1.30 c–h | 1140.3 ± 441.3 abc | 1273.0 ± 518.6 ab | 90.1 ± 6.8 a–e | 8.56 ± 1.09 d–h | 484.0 ± 283.6 abc | |
| ‘225Ru’ | 31.43 ± 2.88 a | 9.82 ± 1.17 a–g | 1382.0 ± 338.7 ab | 1593.0 ± 427.8 a | 87.7 ± 4.4 a–g | 12.64 ± 0.93 a | 979.5 ± 326.2 a | |
| ‘3309C’ | 20.54 ± 3.30 bcd | 8.82 ± 0.91 a–h | 821.5 ± 284.9 abc | 906.0 ± 296.2 b | 89.6 ± 5.9 a–f | 8.01 ± 1.10 fgh | 375.5 ± 129.6 bc | |
| ‘420A’ | 29.80 ± 7.75 ab | 8.97 ± 0.98 a–h | 1063.0 ± 507.0 abc | 1106.0 ± 509.7 ab | 95.5 ± 5.7 a | 9.25 ± 1.05 c–h | 658.5 ± 248.3 abc | |
| ‘5A’ | 26.74 ± 3.74 a–d | 9.83 ± 1.89 a–f | 1004.0 ± 544.2 abc | 1246.8 ± 567.8 ab | 76.6 ± 22.0 e–i | 10.16 ± 1.92 a–f | 750.0 ± 416.1 abc | |
| ‘5BB’ | 27.39 ± 6.47 a–d | 10.24 ± 1.16 a–d | 1317.0 ± 431.1 ab | 1408.0 ± 457.2 ab | 93.5 ± 2.3 ab | 12.29 ± 0.88 ab | 883.0 ± 335.9 ab | |
| ‘5C’ | 27.61 ± 4.86 a–d | 8.39 ± 0.93 b–h | 1199.2 ± 524.7 abc | 1457.3 ± 603.0 a | 80.9 ± 6.5 b–h | 11.20 ± 3.10 a–d | 441.0 ± 318.2 abc | |
| ‘8B’ | 23.68 ± 2.88 a–d | 9.12 ± 1.08 a–h | 1449.7 ± 467.3 a | 1608.8 ± 521.4 a | 91.1 ± 4.4 a–d | 11.69 ± 0.99 abc | 781.7 ± 285.3 abc | |
| ‘BR2’ | 27.85 ± 4.72 a–d | 9.21 ± 0.93 a–h | 1286.3 ± 492.3 ab | 1322.3 ± 503.3 ab | 97.6 ± 2.4 a | 11.21 ± 1.38 a–d | 858.5 ± 345.4 ab | |
| ‘Barrett50’ | 24.27 ± 3.88 a–d | 9.36 ± 1.38 a–h | 1119.5 ± 514.2 abc | 1150.5 ± 533.1 ab | 96.9 ± 4.0 a | 10.63 ± 1.64 a–f | 574.5 ± 323.1 abc | |
| ‘Beaumont’ | 29.43 ± 7.68 abc | 9.41 ± 1.42 a–h | 974.0 ± 494.4 abc | 1141.0 ± 491.0 ab | 84.4 ± 11.3 a–g | 8.36 ± 0.99 e–h | 499.5 ± 318.0 abc | |
| ‘Beta’ | 26.92 ± 3.41 a–d | 10.92 ± 1.07 a | 1234.1 ± 355.2 abc | 1275.1 ± 380.7 ab | 97.5 ± 1.8 a | 9.30 ± 1.23 c–h | 758.5 ± 216.5 abc | |
| ‘Dogridge’ | 26.62 ± 5.68 a–d | 9.49 ± 0.92 a–h | 1068.5 ± 328.1 abc | 1143.5 ± 398.2 ab | 96.3 ± 5.1 a | 7.34 ± 0.52 gh | 716.0 ± 300.5 abc | |
| ‘Fercal’ | 33.69 ± 7.54 a | 10.36 ± 1.41 abc | 1154.5 ± 558.6 abc | 1272.0 ± 689.8 ab | 92.2 ± 5.8 abc | 9.07 ± 1.29 c–h | 668.0 ± 526.4 abc | |
| ‘Ganzin1’ | 19.48 ± 3.47 cd | 7.96 ± 0.84 e–h | 747.9 ± 237.7 abc | 968.9 ± 303.7 ab | 75.7 ± 4.5 f–i | 7.95 ± 1.64 fgh | 282.8 ± 209.0 c | |
| ‘Gloire’ | 27.95 ± 2.04 a–d | 8.07 ± 0.89 d–h | 721.2 ± 177.8 abc | 1112.5 ± 243.2 ab | 88.0 ± 4.1 a–g | 9.53 ± 1.66 b–h | 249.0 ± 85.8 c | |
| ‘du Lot’ | - | - | - | - | - | - | - | |
| ‘Saltcreek’ | 18.42 ± 4.33 d | 9.63 ± 1.16 a–g | 810.0 ± 213.4 abc | 822.0 ± 224.3 b | 98.2 ± 2.7 a | 9.79 ± 1.09 b–g | 624.0 ± 424.0 abc | |
| ‘Shadi’ | 31.96 ± 13.92 a | 8.33 ± 1.31 c–h | 459.0 ± 243.0 c | 852.9 ± 398.2 b | 52.6 ± 15.9 k | 8.00 ± 3.13 fgh | 240.7 ± 150.2 c | |
| ‘Shanhe1’ | 29.90 ± 7.31 ab | 7.82 ± 1.05 fgh | 1242.8 ± 486.2 abc | 1277.8 ± 493.1 ab | 96.7 ± 4.3 a | 11.01 ± 0.83 a–e | 598.3 ± 275.0 abc | |
| ‘Shanhe2’ | 28.98 ± 4.77 abc | 9.35 ± 1.47 a–h | 724.5 ± 280.0 abc | 927.1 ± 346.6 ab | 77.9 ± 8.6 d–i | 6.92 ± 1.08 h | 509.8 ± 225.1 abc | |
| ‘Shanhe3’ | 29.52 ± 4.19 abc | 7.84 ± 1.13 fgh | 873.0 ± 402.5 abc | 885.1 ± 405.9 b | 98.0 ± 3.3 a | 10.10 ± 0.91 a–g | 447.5 ± 243.8 abc | |
| ‘Shanhe4’ | 28.07 ± 8.25 a–d | 8.07 ± 0.76 d–h | 747.9 ± 210.7 abc | 998.3 ± 289.2 ab | 75.0 ± 7.0 ghi | 6.94 ± 1.01 h | 355.6 ± 109.8 bc | |
| ‘SO4’ | 27.74 ± 2.38 a–d | 10.02 ± 1.39 a–e | 1361.0 ± 457.0 ab | 1454.0 ± 440.1 a | 93.0 ± 6.8 ab | 10.09 ± 0.83 a–g | 901.0 ± 340.7 ab | |
| ‘Wumao’ | 25.20 ± 3.09 a–d | 7.65 ± 0.49 gh | 1219.1 ± 334.3 abc | 1383.5 ± 360.7 ab | 86.3 ± 8.3 a–g | 11.46 ± 1.62 abc | 645.0 ± 213.7 abc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, Y.; Han, B.; Li, M.; Jia, N.; Liu, C.; Sun, Y.; Wang, Y.; Gao, Q.; Guo, Z. Multiplication, Phenological Period and Growth Vigor of Thirty-One Grapevine Rootstocks and the Role of Parentage in Vigor Heredity. Horticulturae 2023, 9, 241. https://doi.org/10.3390/horticulturae9020241
Yin Y, Han B, Li M, Jia N, Liu C, Sun Y, Wang Y, Gao Q, Guo Z. Multiplication, Phenological Period and Growth Vigor of Thirty-One Grapevine Rootstocks and the Role of Parentage in Vigor Heredity. Horticulturae. 2023; 9(2):241. https://doi.org/10.3390/horticulturae9020241
Chicago/Turabian StyleYin, Yonggang, Bin Han, Minmin Li, Nan Jia, Changjiang Liu, Yan Sun, Yingjie Wang, Qian Gao, and Zijuan Guo. 2023. "Multiplication, Phenological Period and Growth Vigor of Thirty-One Grapevine Rootstocks and the Role of Parentage in Vigor Heredity" Horticulturae 9, no. 2: 241. https://doi.org/10.3390/horticulturae9020241
APA StyleYin, Y., Han, B., Li, M., Jia, N., Liu, C., Sun, Y., Wang, Y., Gao, Q., & Guo, Z. (2023). Multiplication, Phenological Period and Growth Vigor of Thirty-One Grapevine Rootstocks and the Role of Parentage in Vigor Heredity. Horticulturae, 9(2), 241. https://doi.org/10.3390/horticulturae9020241








