Effects of Temperature on Growth and Isoprene Metabolism Pathway in Eucommia ulmoides Oliv
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Material
2.2. Methods
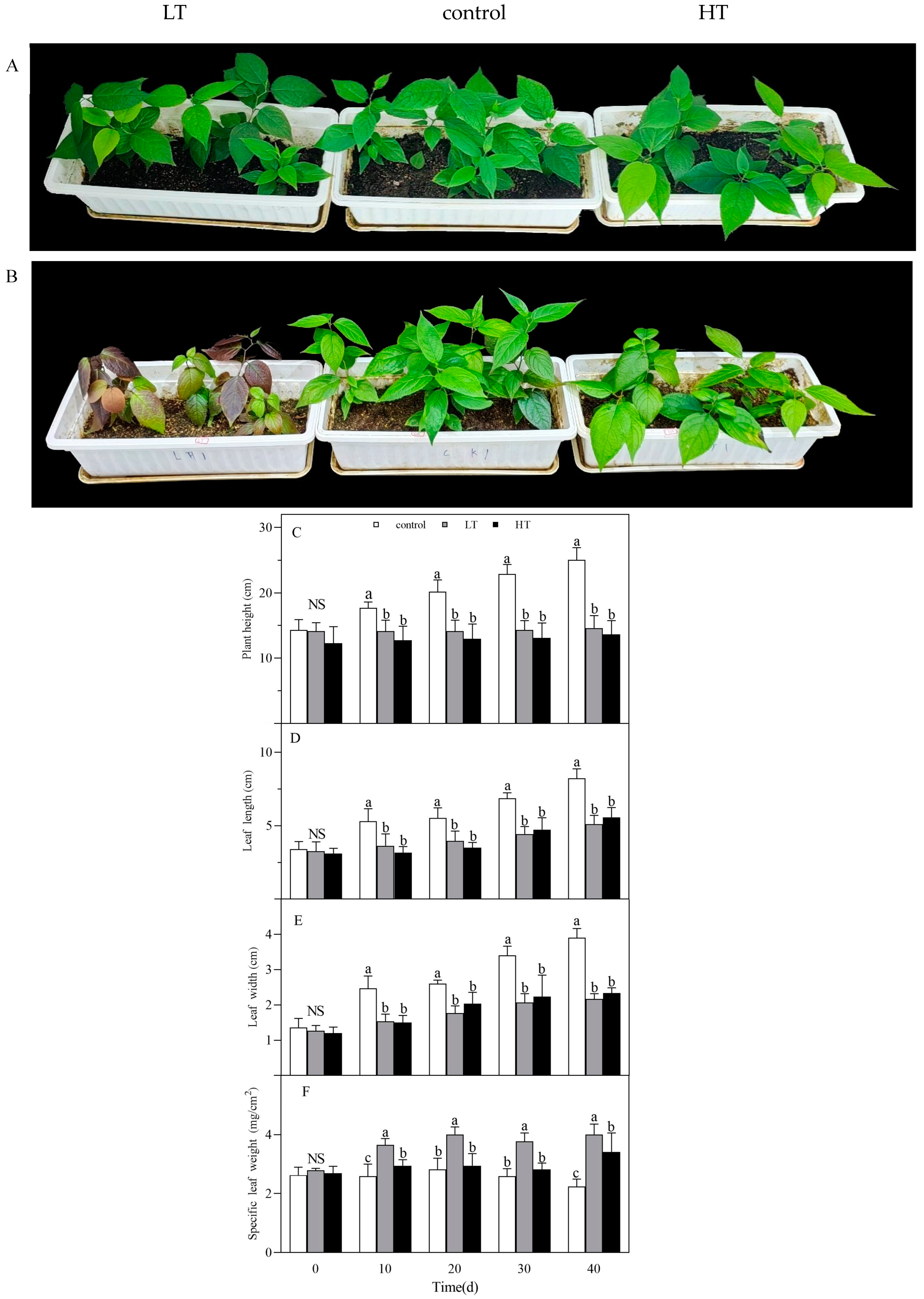
2.2.1. Analysis of Phenotypic Changes
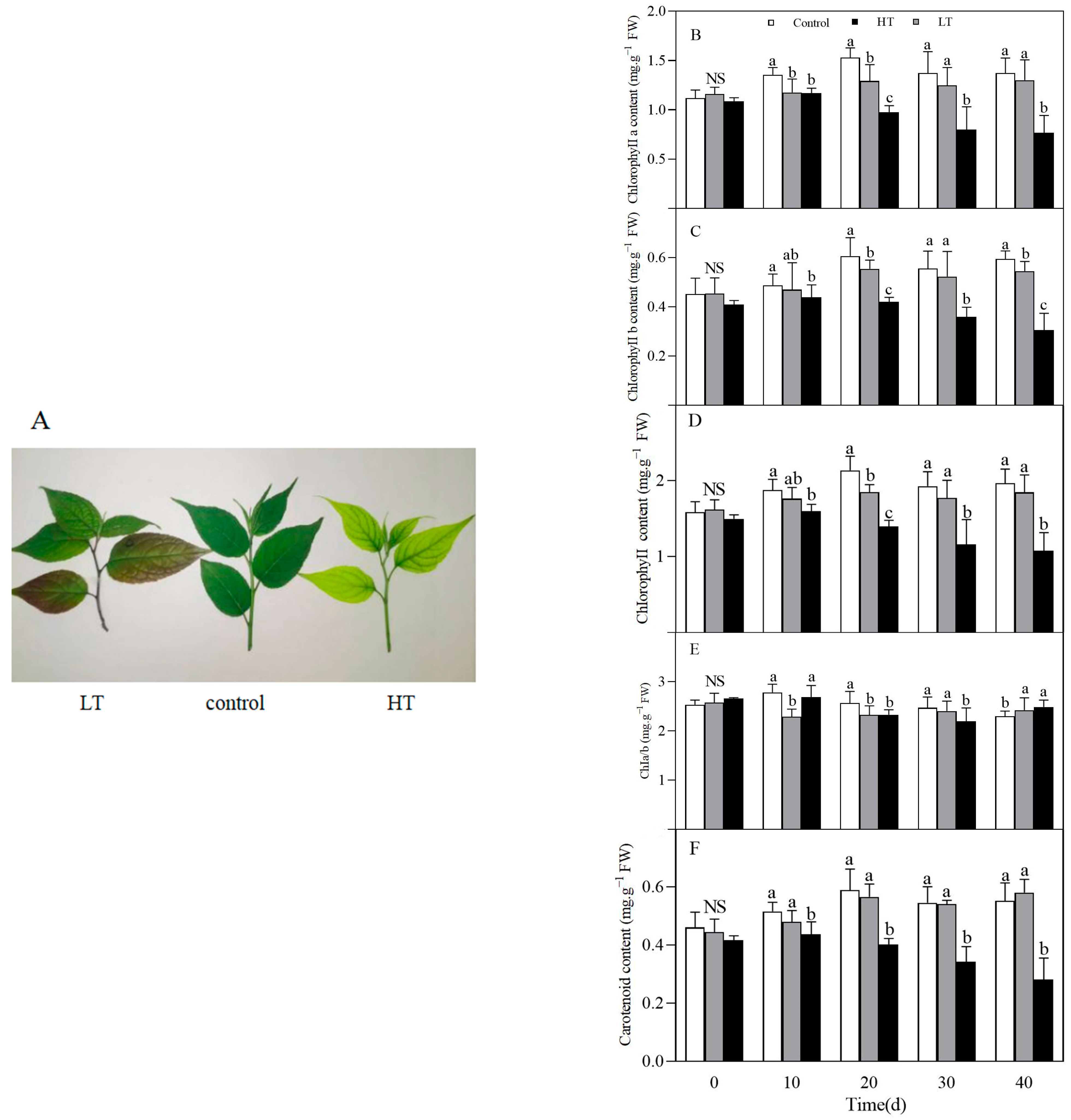
2.2.2. Determination of the Chlorophyll Content
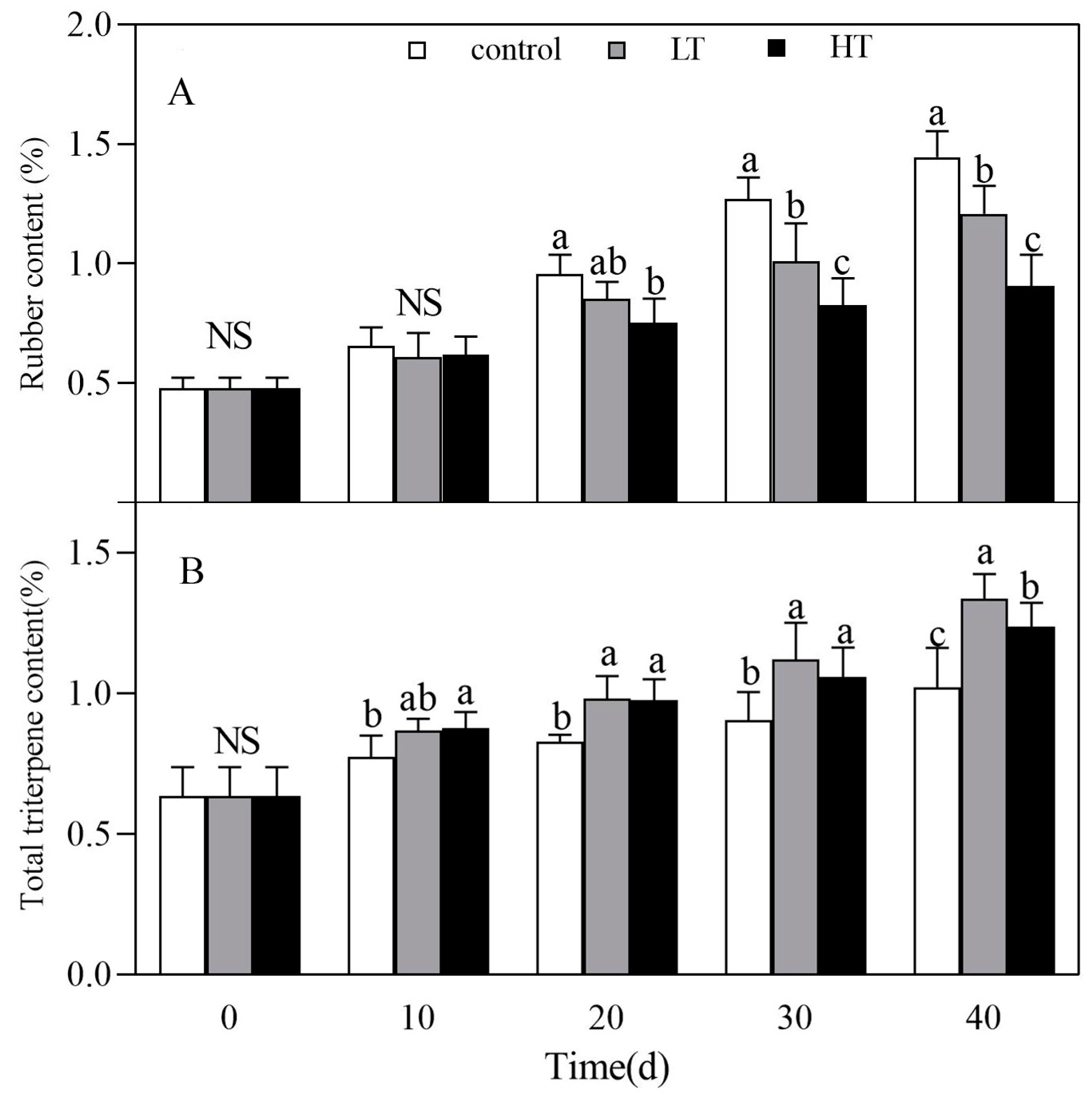
2.2.3. Determination of Total Triterpene Content
2.2.4. Determination of TPI Content
2.2.5. Total RNA Extraction, cDNA Library Construction, and Illumina Sequencing
2.2.6. Transcriptome Data Analysis
2.2.7. RT-qPCR Validation
2.2.8. Statistical Analysis of Data
3. Results
3.1. Effect of Temperature on Phenotype
3.2. Effect of Temperature on Chlorophyll and Carotenoid Contents
3.3. Effect of Temperature on Rubber and Total Triterpene Contents
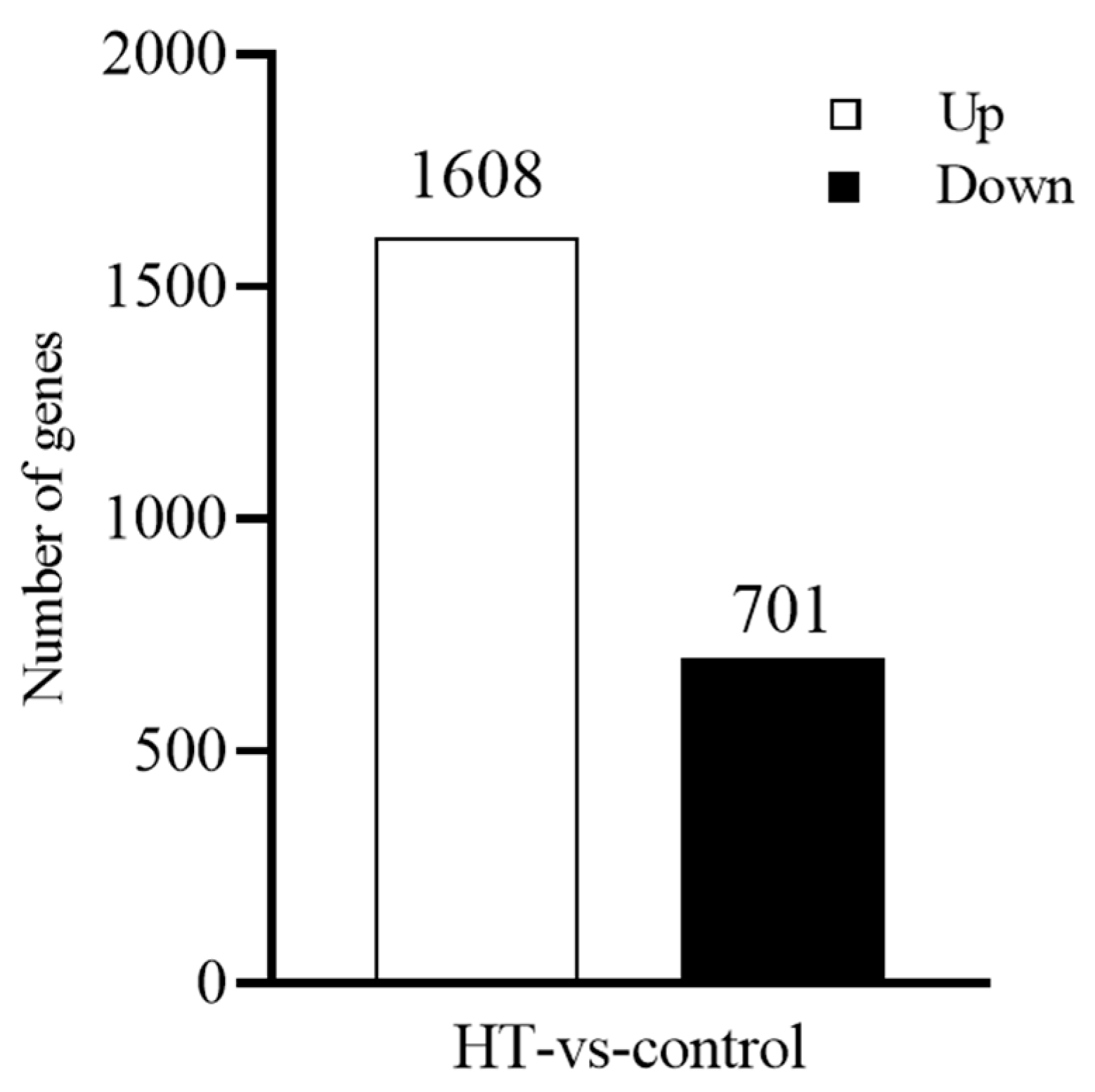
3.4. Transcriptome Data Analyses
3.4.1. Transcriptome Data Quality
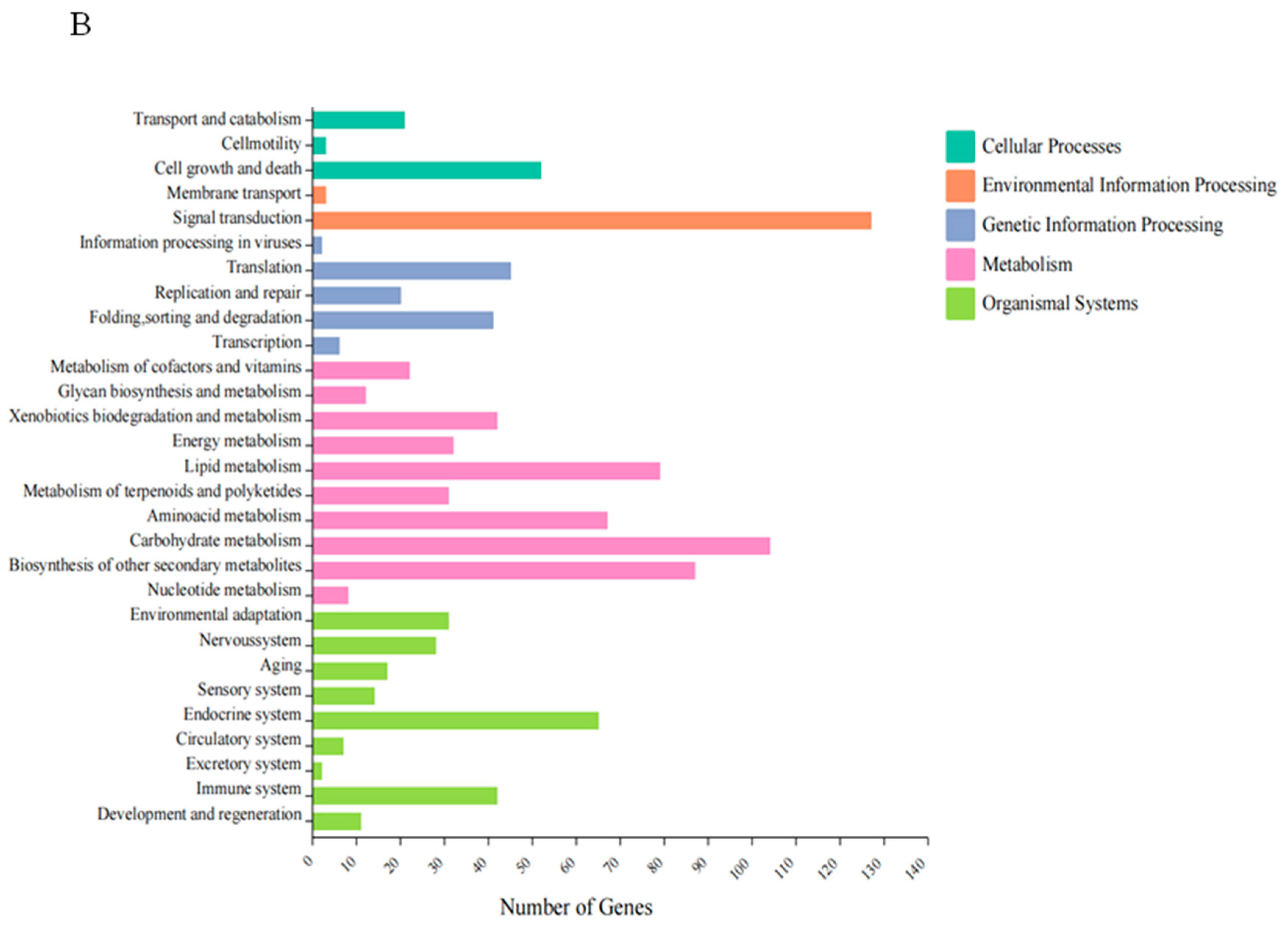
3.4.2. GO and KEGG Enrichment Analysis of DEGs
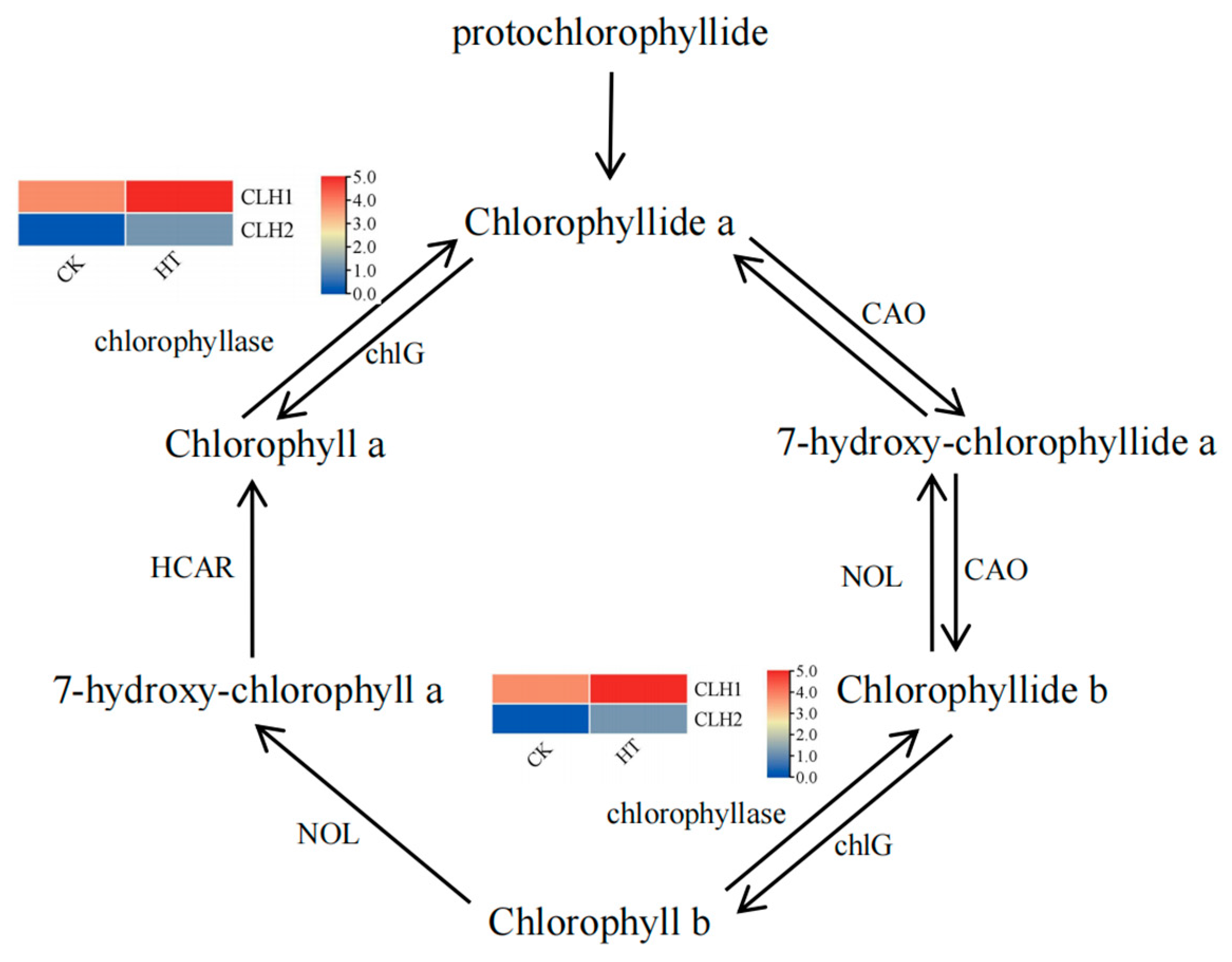
3.5. Effect of Temperature on Chlorophyll Metobolic Pathway
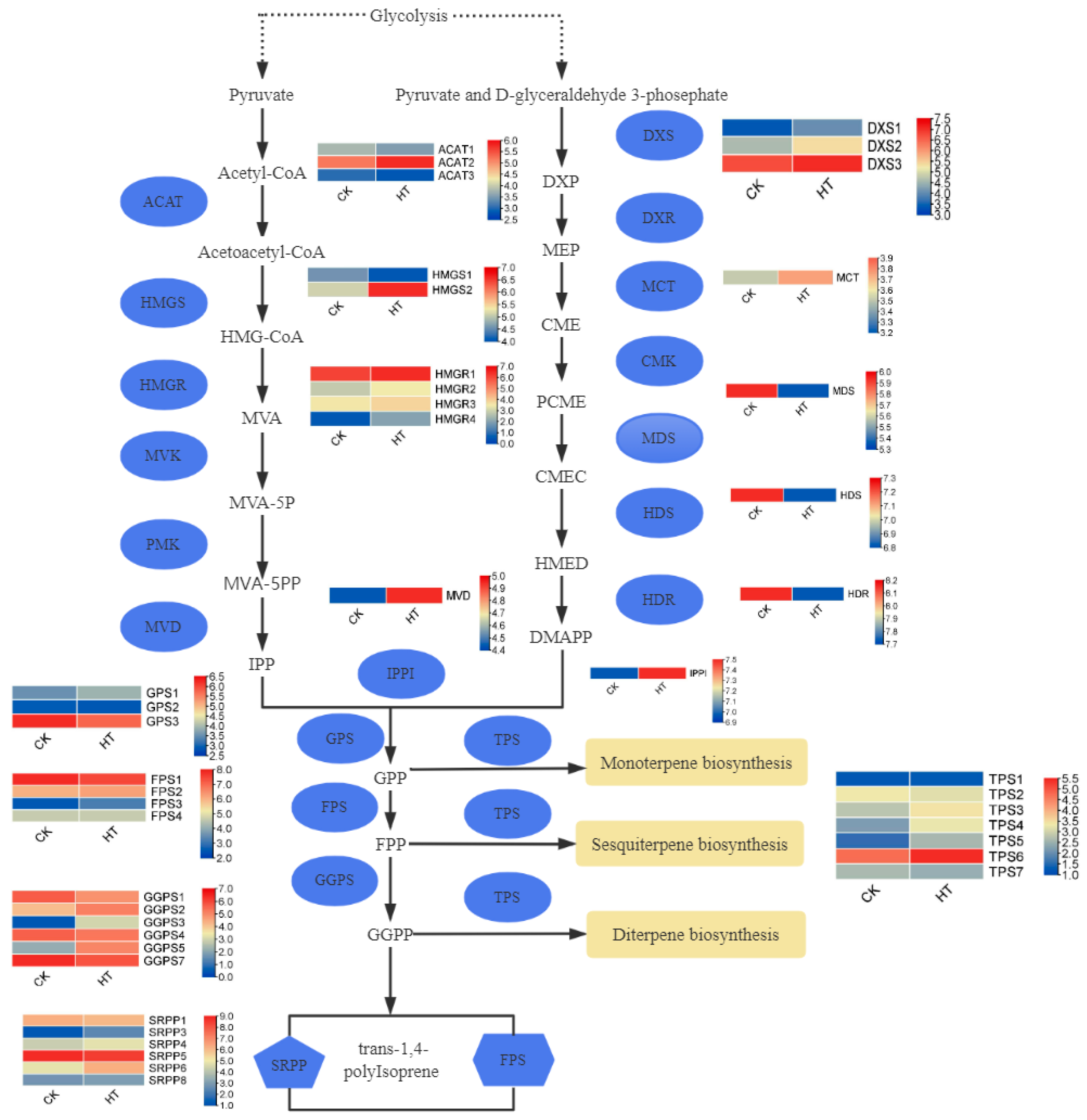
3.6. Effect of Temperature on Isoprene Metabolic Pathway
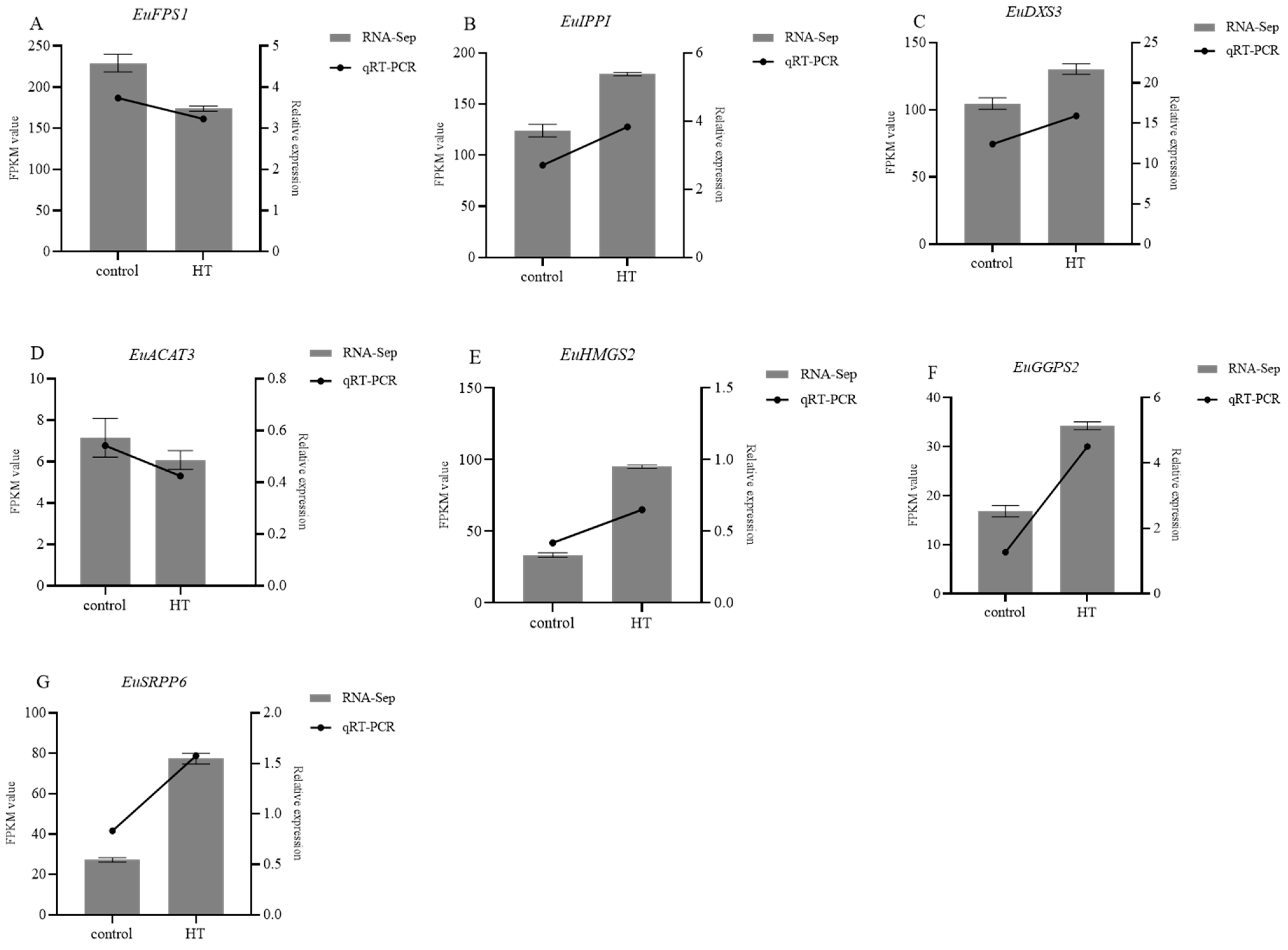
3.7. Validation of the Differential Gene Expression Level Using qRT-PCR
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vranová, E.; Coman, D.; Gruissem, W. Network analysis of the MVA and MEP pathways for isoprenoid synthesis. Annu. Rev. Plant Biol. 2013, 64, 665–700. [Google Scholar] [CrossRef] [PubMed]
- Simkin, A.J.; Guirimand, G.; Papon, N.; Courdavault, V.; Thabet, I.; Ginis, O.; Bouzid, S.; Giglioli-Guivarc, H.N.; Clastre, M. Peroxisomal localisation of the final steps of the mevalonic acid pathway in planta. Planta 2011, 234, 903–914. [Google Scholar] [CrossRef] [PubMed]
- Lange, B.M.; Wildung, M.R.; McCaskill, D.; Croteau, R. A family of transketolases that directs isoprenoid biosynthesis via a mevalonateindependent pathway. Proc. Natl. Acad. Sci. USA 1998, 95, 2100–2104. [Google Scholar] [CrossRef] [PubMed]
- Lois, L.M.; Rodrigue-Concepcion, M.; Gallego, F.; Boronat, A. Carotenoid biosynthesis during tomato fruit development: Regulatory role of 1-deoxy-D-xylulose 5-phosphate synthase. Plant J. 2000, 22, 503–513. [Google Scholar] [CrossRef]
- Degenhardt, J.; Köllner, T.G.; Gershenzon, J. Monoterpene and sesquiterpene synthases and the origin of terpene skeletal diversity in plants. Phytochemistry 2009, 70, 1621–1637. [Google Scholar] [CrossRef]
- Hou, X.; Rivers, J.; León, P.; McQuinn, R.P.; Pogson, B.J. Synthesis and function of apocarotenoid signals in plants. Trends Plant Sci. 2016, 21, 792–803. [Google Scholar] [CrossRef]
- Sharkey, T.D.; Wiberley, A.E.; Donohue, A.R. Isoprene emission from plants: Why and how. Ann. Bot. 2008, 101, 5–18. [Google Scholar] [CrossRef]
- Duhl, T.R.; Helmig, D.; Guenther, A. Sesquiterpene emissions from vegetation: A review. Biogeosciences 2008, 5, 761–777. [Google Scholar] [CrossRef]
- Jia, Q.; Köllner, T.G.; Gershenzon, J.; Chen, F. MTPSLs: New terpene synthases in nonseed plants. Trends Plant Sci. 2018, 23, 121–128. [Google Scholar] [CrossRef]
- Jiang, S.Y.; Jin, J.; Sarojam, R.; Ramachandran, S. A comprehensive survey on the terpene synthase gene family provides new insight into its evolutionary patterns. Genome Biol. Evol. 2019, 11, 2078–2098. [Google Scholar] [CrossRef]
- Zi, J.; Mafu, S.; Peters, R.J. To gibberellins and beyond! surveying the evolution of (di)terpenoid metabolism. Annu. Rev. Plant Biol. 2014, 65, 259–286. [Google Scholar] [CrossRef] [PubMed]
- Swiezewska, E.; Danikiewicz, W. Polyisoprenoids: Structure, biosynthesis and function. Prog. Lipid Res. 2005, 44, 235–258. [Google Scholar] [CrossRef] [PubMed]
- Call, V.; Dilcher, D. The fossil record of Eucommia (Eucommiaceae) in North America. Am. J. Bot. 1997, 84, 798. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.R.; Zhu, M.Q.; Liang, J.; Su, Y.Q. Antioxidant and antimicrobial activities and component analysis of crude pyroligneous acids of Eucommia ulmoides branch made at different temperatures. J. Northwest For. Univ. 2016, 31, 220–226. [Google Scholar]
- Sun, Z.; Li, F.; Du, H.; Zhu, J.; Wang, Y.P. A novel silvicultural model for increasing biopolymer production from Eucommia ulmoides Oliver trees. Ind. Crops Prod. 2013, 42, 216–222. [Google Scholar] [CrossRef]
- K Kang, H.L.; Gong, M.; Xu, M.; Wang, H.Y.; Li, Y.S.; Fang, Q.H.; Zhang, L.Q. Fabricated biobased Eucommia ulmoides gum/polyolefin elastomer thermoplastic vulcanizates into a Shape memory material. Ind. Eng. Chem. Res. 2019, 58, 6375–6384. [Google Scholar] [CrossRef]
- Yang, R.F. Prospects and research progress on Eucommia ulmoides gum. Prog. Chem. 1995, 7, 65–71. [Google Scholar]
- Qi, X.; Zhao, X.; Li, Y.X.; Zhang, J.C.; Yue, D.M. A high toughness elastomer based on natural Eucommia ulmoides gum. J. Appl. Polym. Sci. 2020, 11, 50007. [Google Scholar] [CrossRef]
- Zhang, J.C.; Xue, Z.H. Study on under-water sound absorption properties of Eucommia ulmoides gum and its blends. Polym. Bull. 2011, 67, 511–525. [Google Scholar] [CrossRef]
- Fang, Q.H.; Jin, X.; Yang, F.; Ma, C.; Gao, Y.; Wang, N. Preparation and characterizations of Eucommia ulmoides gum/polypropylene blend. Polym. Bull. 2016, 73, 357–367. [Google Scholar] [CrossRef]
- Sando, T.; Takaoka, C.; Mukai, Y.; Yamashita, A.; Hattori, M.; Ogasawara, N.; Fukusaki, E.; Kobayashi, A. Cloning and characterization of mevalonate pathway genes in a natural rubber producing plant, Hevea brasiliensis. Biosci. Biotechnol. Biochem. 2008, 72, 2049–2060. [Google Scholar] [CrossRef] [PubMed]
- Stermer, B.A.; Bostock, R.M. Involvement of 3-hydroxy-3-methylglutaryl coenzyme A reductase in the regulation of sesquiterpenoid phytoalexin synthesis in potato. Plant Physiol. 1987, 84, 404–408. [Google Scholar] [CrossRef]
- Morshedloo, M.R.; Craker, L.E.; Salami, A.; Nazeri, V.; Sang, H.; Maggi, F. Effect of prolonged water stress on essential oil content, compositions and gene expression patterns of mono- and sesquiterpene synthesis in two oregano (Origanum vulgare L.) subspecies. Plant Physiol. Biochem. 2017, 111, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Lee, O.R.; Oh, J.Y.; Jang, M.G.; Yang, D.C. Functional analysis of 3-hydroxy-3-methylglutaryl coenzyme A reductase encoding genes in triterpene saponin-producing ginseng. Plant Physiol. 2014, 165, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, A.; Ullah, S.; Dar, A.A.; Sardar, M.F.; Mehmood, T.; Tufail, M.A.; Shakoor, A.; Haris, M. Nexus on climate change: Agriculture and possible solution to cope future climate change stresses. Environ. Sci. Pollut. Res. 2021, 28, 14211–14232. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.M.; Li, H.X.; Chong, K. Crop improvement through temperature resilience. Annu. Rev. Plant Biol. 2019, 70, 753–780. [Google Scholar] [CrossRef] [PubMed]
- Shuogang, A.; Yagang, G. Extrapolation of the high yield physiological regulation of Hevea brasiliensis in Xishuangbanna. Proceeding of the IRRDB Symposium on Physiology and Exploitation of Hevea brasiliensis, Kunming, China, 6–7 October 1990; The International Rubber Research and Development Board: Kuala Lumpur, Malaysia, 1990; pp. 83–92. [Google Scholar]
- Liu, S.X.; Huang, Y.Z.; Luo, Z.J.; Huang, Y.C.; Yang, X.W. Effects of exogenous melatonin on accumulation and chemical form of Cd in rice. J. Appl. Ecol. 2017, 28, 1588–1594. [Google Scholar]
- Tao, W.Y.; Xu, H.Y.; Ao, Z.H.; Xu, Z.H. Quantitative analysis of triterpenoids from Antrodia camphorata in submerged culture. Chin. Trad. Patent Med. 2008, 30, 402–405. [Google Scholar]
- Lu, Z.K.; Xie, B.X.; Du, H.Y. Study on extraction method of Eucommia rubber. J. Fujian Coll. 2004, 24, 353–356. [Google Scholar]
- Al-Deeb, T.; Abo Gamar, M.; Khaleel, S.; Al-Ghzawi, A.L.; Al Khateeb, W.; Jawarneh, M.; Jahmani, M.Y.; Al-Zoubi, O.; Habeeb, T. Individual and interactive ecophysiological effect of temperature, watering regime and abscisic acid on the growth and development of tomato seedlings. Agronomy 2023, 13, 930. [Google Scholar] [CrossRef]
- Kim, S.; Hwang, G.; Lee, S.; Zhu, J.Y.; Paik, I.; Nguyen, T.T.; Kim, J.; Oh, E. High ambient temperature represses anthocyanin biosynthesis through degradation of HY5. Front. Plant Sci. 2017, 8, 1787. [Google Scholar] [CrossRef] [PubMed]
- Maulana, F.; Tesso, T.T. Cold temperature episode at seedling and flowering stages reduces growth and yield components in sorghum. Crop Sci. 2013, 53, 355–734. [Google Scholar] [CrossRef]
- Azoulay, S.T.; Harpaz, S.; Belausov, E.; Lovat, N.; Krokhin, O.; Spicer, V.; Standing, K.G.; Goldschmidt, E.E.; Eyal, Y. Citrus chlorophyllase dynamics at ethylene-induced fruit color-break: A study of chlorophyllase expression, posttranslational processing kinetics, and in situ intracellular localization. Plant Physiol. 2008, 148, 108–118. [Google Scholar] [CrossRef]
- Hu, X.; Makita, S.; Schelbert, S.; Sano, S.; Ochiai, M.; Tsuchiya, T.; Hasegawa, S.F.; Hörtensteiner, S.; Tanaka, A.; Tanaka, R. Reexamination of chlorophyllase function implies its involvement in defense against chewing herbivores. Plant Physiol. 2015, 167, 660–670. [Google Scholar] [CrossRef]
- Kariola, T.; Brader, G.; Li, J.; Palva, E.T. Chlorophyllase 1, a damage control enzyme, affects the balance between defense pathways in plants. Plant Cell 2005, 17, 282–294. [Google Scholar] [CrossRef] [PubMed]
- Chye, M.L.; Tan, C.T.; Chua, N.H. Three genes encode 3-hydroxy-3-methylglutaryl-coenzyme A reductase in Hevea brasiliensis: HMG1 and HMG3 are differentially expressed. Plant Mol. Biol. 1992, 19, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.L.; Wong, W.S.; Jang, I.C.; Chua, N.H. Co-expression of peppermint geranyl diphosphate synthase small subunit enhances monoterpene production in transgenic tobacco plants. New Phytol. 2017, 213, 1133–1144. [Google Scholar] [CrossRef]
- Hua, W.; Song, J.; Li, C.; Wang, Z. Molecular cloning and characterization of the promoter of SmGGPPs and its expression pattern in Salvia miltiorrhiza. Mol. Biol. Rep. 2012, 39, 5775–5783. [Google Scholar] [CrossRef]
- Deng, X.M.; Guo, D.; Yang, S.J.; Shi, M.J.; Chao, J.Q.; Li, H.L.; Peng, S.H.; Tian, W.M. Jasmonate signalling in the regulation of rubber biosynthesis in laticifer cells of rubber tree, Hevea brasiliensis. J. Exp. Bot. 2018, 69, 3559–3571. [Google Scholar] [CrossRef]
- Liang, J.; Wang, D.; Li, X.; Huang, W.; Xie, C.; Fu, M.; Zhang, H.; Meng, Q. In silico genome-wide mining and analysis of terpene synthase gene family in Hevea brasiliensis. Biochem. Genet. 2023, 61, 1185–1209. [Google Scholar] [CrossRef]
- Yan, Y.; Li, M.; Zhang, X.; Kong, W.; Bendahmane, M.; Bao, M.; Fu, X. Tissue-specific expression of the terpene synthase family genes in Rosa chinensis and effect of abiotic stress conditions. Genes 2022, 13, 547. [Google Scholar] [CrossRef] [PubMed]
- Blanch, J.S.; Peñuelas, J.; Sardans, J.; Lusia, J. Drought, warming and soil fertilization effects on leaf volatile terpene concentrations in Pinus halepensis and Quercus ilex. Acta Physiol. Plant. 2009, 31, 207–218. [Google Scholar] [CrossRef]
- Copolovici, L.; Kännaste, A.; Pazouki, L.; Niinemets, U. Emissions of green leaf volatiles and terpenoids from Solanum lycopersicum are quantitatively related to the severity of cold and heat shock treatments. J. Plant Physiol. 2012, 169, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Omokhafe, K.O.O.; Emuedo, O.A.E. Evaluation of influence of five weather characters on latex yield in Hevea brasiliensis. Int. J. Agric. Res. 2006, 1, 234–239. [Google Scholar] [CrossRef]
- Chow, K.S.; Mat-Isa, M.N.; Bahari, A.; Ghazali, A.K.; Alias, H.; Mohd-Zainuddin, Z.; Hoh, C.C.; Wan, K.L. Metabolic routes affecting rubber biosynthesis in Hevea brasiliensis latex. J. Exp. Bot. 2012, 63, 1863–1871. [Google Scholar] [CrossRef]

| Gene Name | Primer Sequence (5′→3′) | Sequence Length |
|---|---|---|
| HMGS2 | F:GGAGAGCAGTTCGTGGGATGG R:GCAGCAGCTCCACCAGTCG | 99 |
| IPPI | F:ACTCGGTCCAGCTTTCGTCTTC R:AGAATCAGCGACGGCGGTATC | 81 |
| DXS3 | F:AGCAGGGCATAGTTCCACAAGC R:CATGGCTCCATCTCCGATCACC | 109 |
| SRPP6 | F:CGTCCGCTATGTCCGATCCATG R:GCGAACAGAGTCCAGGCAAGAG | 117 |
| ACAT3 | F:TCCAGCAGCAGCAACAGAGTG R:CCATTGGTGTTCGTGCAACTCC | 80 |
| GGPS2 | F:ACGACGACCTCCCCTGTATGG R:CCGTGGTGGAAGTGGCGATG | 144 |
| FPS1 | F:GGTTGGTGCATTGAGTGGCTTC R:TTGACCTCGGCGTGTGTGTG | 81 |
| Actin | F:TTGTTAGCAACTGGGATGATATGG R:CAGGGTGTTCTTCAGGAGCAA |
| Sample Name | Raw Reads | Clean Reads | Q20 (%) | Q30 (%) | Mapped Read (%) | GC Content (%) |
|---|---|---|---|---|---|---|
| control-1 | 40,885,940 | 40,082,724 | 96.46 | 90.87 | 95.28 | 47.16 |
| control-2 | 41,794,830 | 41,135,962 | 96.80 | 91.52 | 95.55 | 46.97 |
| control-3 | 41,940,464 | 41,265,186 | 97.34 | 92.71 | 95.93 | 47.23 |
| HT-1 | 43,679,536 | 43,233,386 | 97.38 | 92.70 | 95.65 | 47.12 |
| HT-2 | 42,241,678 | 43,233,386 | 97.46 | 92.89 | 95.75 | 47.05 |
| HT-3 | 43,511,804 | 43,079,342 | 97.59 | 93.19 | 95.81 | 47.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, W.; Zhao, D. Effects of Temperature on Growth and Isoprene Metabolism Pathway in Eucommia ulmoides Oliv. Horticulturae 2023, 9, 1298. https://doi.org/10.3390/horticulturae9121298
Yao W, Zhao D. Effects of Temperature on Growth and Isoprene Metabolism Pathway in Eucommia ulmoides Oliv. Horticulturae. 2023; 9(12):1298. https://doi.org/10.3390/horticulturae9121298
Chicago/Turabian StyleYao, Wenqin, and Degang Zhao. 2023. "Effects of Temperature on Growth and Isoprene Metabolism Pathway in Eucommia ulmoides Oliv" Horticulturae 9, no. 12: 1298. https://doi.org/10.3390/horticulturae9121298
APA StyleYao, W., & Zhao, D. (2023). Effects of Temperature on Growth and Isoprene Metabolism Pathway in Eucommia ulmoides Oliv. Horticulturae, 9(12), 1298. https://doi.org/10.3390/horticulturae9121298







