Plant Growth Promoting Bacteria for Aquaponics as a New Strategy That Grants Quality and Nutrient Efficiency in Kohlrabi Cultivation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Mineral Content
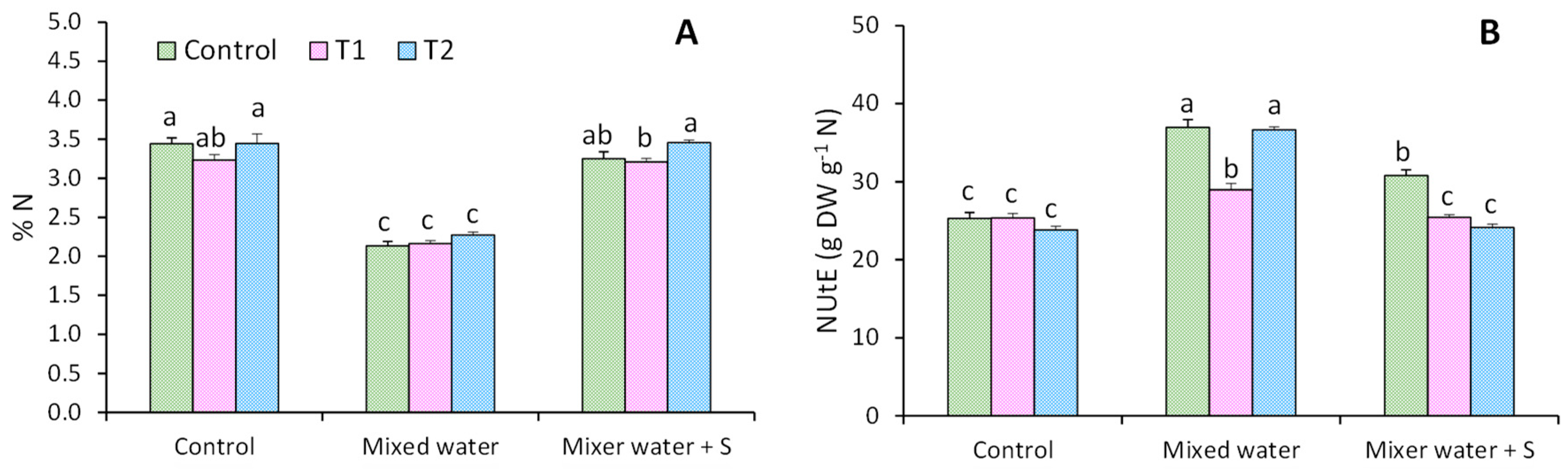
2.3. NUtE Calculation
2.4. Total Phenolic Compounds and Antioxidant Activity (ABTS+*)
2.5. Total Soluble Sugars
2.6. Statistical Analysis
3. Results
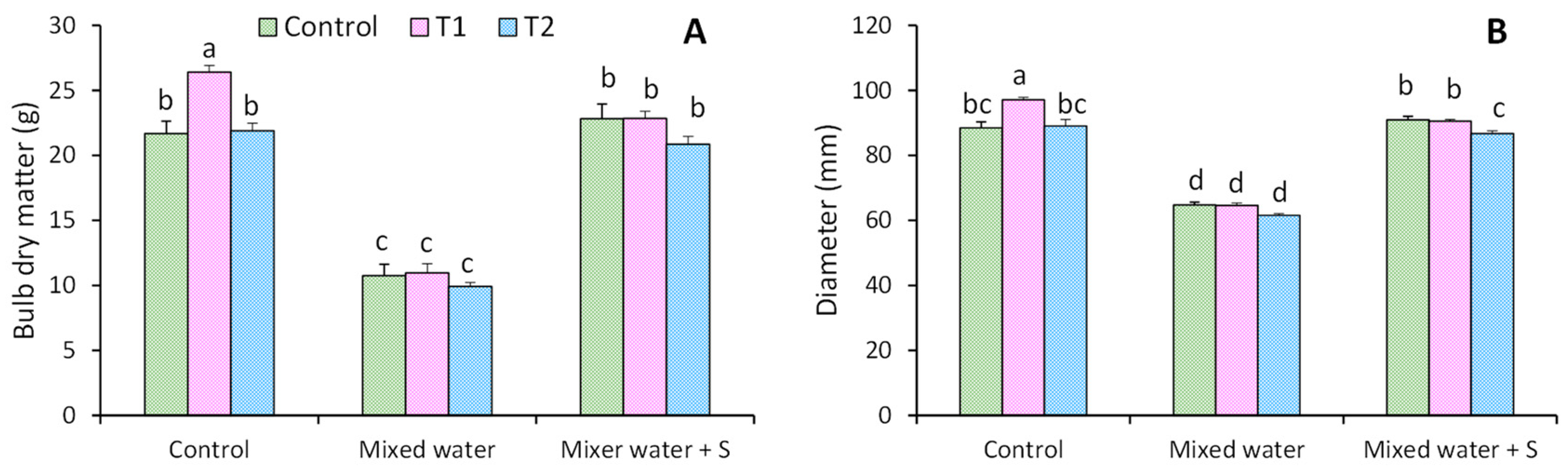
3.1. Physical Characterization of Kohlrabi Bulb
3.2. Mineral Content
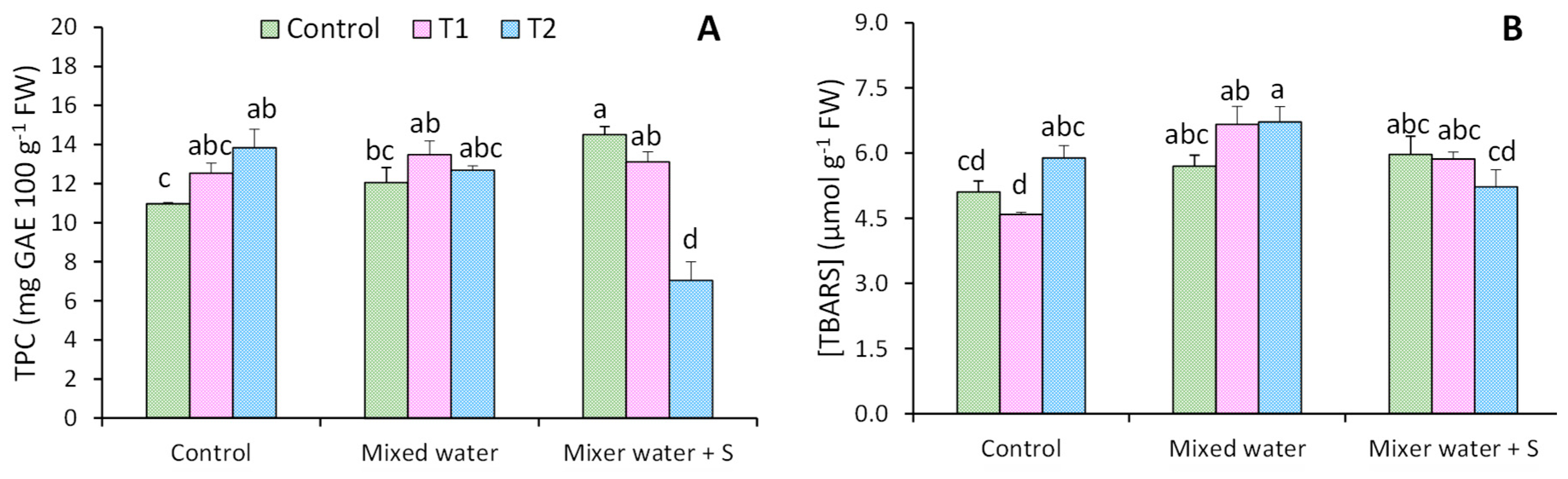
3.3. Total Phenolic Compounds and Antioxidant Activity (ABTS+*)
3.4. Total Soluble Sugars
4. Discussion
4.1. Physical Characterization of Kohlrabi Bulb
4.2. Mineral Content
4.3. Total Phenolic Compounds and Antioxidant Activity (ABTS+*)
4.4. Total Soluble Sugars
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soltan, H.A.H.; Osman, S.A.M.; Tantawy, I.A.A. The Role of New Transformants Phosphate Bio-Stimulates (PBS) Bacteria Inoculates on Growth, Yield and Quality of Kohlrabi Plants. J. Plant Sci. 2021, 12, 541–551. [Google Scholar] [CrossRef]
- Smychkovich, A.; Hashemi, M. Yield and Nutrient Concentrations of Kohlrabi Bulbs and Leaves as Affected by Spring Transplanting Dates. Agronomy 2022, 12, 770. [Google Scholar] [CrossRef]
- Iradukunda, M.; Read, P.E. Influence of Fertilizer Rate on Swollen Stem Formation (“Bulbing”) and Vitamin C Content in Different Kohlrabi Cultivars. Ph.D. Thesis, University of Nebraska, Lincoln, NE, USA, 2022. [Google Scholar]
- Petropoulos, S.A.; Fernandes, Â.; Calhelha, R.C.; Di Gioia, F.; Kolovou, P.; Barros, L.; Ferreira, F.R.I.C. Grown to be blue—Antioxidant properties and health effects of colored vegetables. Part I: Root vegetables. Antioxidants 2019, 8, 617. [Google Scholar] [CrossRef]
- Piñero, M.C.; Otálora, G.; Collado-González, J.; López-Marín, J.; Del Amor, F.M. Effects of Selenium on the Chlorophylls, Gas Exchange, Antioxidant Activity and Amino Acid Composition of Lettuce Grown under an Aquaponics System. Horticulturae 2022, 8, 30. [Google Scholar] [CrossRef]
- Goddek, S.; Alyssa, J.; Kotzen, B.; Burnell, G.M. Aquaponics Food Production Systems: Combined Aquaculture and Hydroponic Production Technologies for the Future; Springer: Berlin/Heidelberg, Germany, 2019; pp. 353–378. [Google Scholar]
- Piñero, M.C.; Otálora, G.; Collado-González, J.; López-Marín, J.; del Amor, F.M. Effects triggered by foliar selenium application on growth, enzyme activities, mineral nutrients and carbohydrates in lettuce under an aquaculture system. Plant Physiol. Biochem. 2022, 180, 1–8. [Google Scholar] [CrossRef]
- Del Amor, F.M.; Cuadra-Crespo, P. Plant growth-promoting bacteria as a tool to improve salinity tolerance in sweet pepper. Funct. Plant Biol. 2012, 39, 82–90. [Google Scholar] [CrossRef]
- Consentino, B.B.; Aprile, S.; Rouphael, Y.; Ntatsi, G.; De Pasquale, C.; Iapichino, G.; Alibrandi, P.; Sabatino, L. Application of PGPB Combined with Variable N Doses Affects Growth, Yield-Related Traits, N-Fertilizer Efficiency and Nutritional Status of Lettuce Grown under Controlled Condition. Agronomy 2022, 12, 236. [Google Scholar] [CrossRef]
- Lastochkina, O.; Aliniaeifard, S.; Seifikalhor, M.; Yuldashev, R.; Pusenkova, L.; Garipova, S. Plant Growth-Promoting Bacteria: Biotic Strategy to Cope with Abiotic Stresses in Wheat. In Wheat Production in Changing Environments; Springer: Berlin/Heidelberg, Germany, 2019; pp. 579–614. [Google Scholar]
- Naqqash, T.; Malik, K.A.; Imran, A.; Hameed, S.; Shahid, M.; Hanif, M.K.; Majeed, A.; Iqbal, M.J.; Qaisrani, M.M.; van Elsas, J.D. Inoculation With Azospirillum spp. Acts as the Liming Source for Improving Growth and Nitrogen Use Efficiency of Potato. Front. Plant Sci. 2022, 13, 929114. [Google Scholar] [CrossRef]
- Gupta, G.; Parihar, S.S.; Ahirwar, N.K.; Snehi, S.K.; Singh, V. Plant growth promoting rhizobacteria (PGPR): Current and future prospects for development of sustainable agriculture. J. Microb. Biochem. Technol. 2015, 7, 96–102. [Google Scholar] [CrossRef]
- Sharma, S.D.; Kumar, P.; Raj, H.; Bhardwaj, S.K. Isolation of arbuscular mycorrhizal fungi and Azotobacter chroococcum from local litchi orchards and evaluation of their activity in the air-layers system. Sci. Hortic. 2009, 123, 117–123. [Google Scholar] [CrossRef]
- Martins da Costa, E.; Azarias Guimarães, A.; Pereira Vicentin, R.; de Almeida Ribeiro, P.R.; Ribas Leão, A.C.; Balsanelli, E.; Lebbe, L.; Aerts, M.; Willems, A.; de Souza Moreira, F.M. Bradyrhizobium brasilense sp. nov., a symbiotic nitrogen-fixing bacterium isolated from Brazilian tropical soils. Arch. Microbiol. 2017, 199, 1211–1221. [Google Scholar] [CrossRef] [PubMed]
- Muthukumar, T.; Udaiyan, K. Growth of nursery-grown bamboo inoculated with arbuscular mycorrhizal fungi and plant growth promoting rhizobacteria in two tropical soil types with and without fertilizer application. New For. 2006, 31, 469–485. [Google Scholar] [CrossRef]
- Pinero, M.C.; Perez-Jimenez, M.; Lopez-Marin, J.; del Amor, F.M. Changes in the salinity tolerance of sweet pepper plants as affected by nitrogen form and high CO2 concentration. J. Plant Physiol. 2016, 200, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Albornoz, F.; Nario, A.; Saavedra, M.; Videla, X. Rootstock x Environment Interactions on Nitrogen-Use Efficiency in Grafted Tomato Plants at Different Phenological Stages. Agronomy 2020, 10, 350. [Google Scholar] [CrossRef]
- Kahkonen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Balibrea, M.E.; Cuartero, J.; Bolarín, M.C.; Pérez-Alfocea, F. Sucrolytic activities during fruit development of Lycopersicon genotypes differing in tolerance to salinity. Physiol. Plant. 2003, 118, 38–46. [Google Scholar] [CrossRef]
- Jamil, N. Growth and Yield of Kohlrabi as Influenced by Fertilizers and Mulches. Master’s Thesis, Agricultural University, Mymensingh, Bangladesh, 2017. [Google Scholar]
- Ghalib, W.K.; Ghassan, J.Z. Effect of Seedling Age and NPK Fertilization on Growth and Yield of Kohlrabi (Brassica oleracea var gongylodes) Planted in Gypsiferous Soil. IOP Conf. Ser. Earth Environ. Sci. 2023, 1158, 042028. [Google Scholar] [CrossRef]
- Ketskalo, V.; Voitovska, V.; Kononenko, L.; Kovtuniuk, Z.; Yevchuk, Y.; Poltoretska, N.; Vyshnevska, L. Peculiarities of the Chemical Composition of Kohlrabi Varieties Cultivated in Ukraine. Ecol. Eng. Environ. Technol. 2023, 24, 46–50. [Google Scholar] [CrossRef]
- Ferreira, C.M.H.; Soares, H.M.V.M.; Soares, E.V. Promising bacterial genera for agricultural practices: An insight on plant growth-promoting properties and microbial safety aspects. Sci. Total Environ. 2019, 682, 779–799. [Google Scholar] [CrossRef]
- de Sousa, S.M.; de Oliveira, C.A.; Andrade, D.L.; de Carvalho, C.G.; Ribeiro, V.P.; Pastina, M.M.; Marriel, I.E.; de Paula Lana, U.G.; Gomes, E.A. Tropical Bacillus Strains Inoculation Enhances Maize Root Surface Area, Dry Weight, Nutrient Uptake and Grain Yield. J. Plant Growth Regul. 2021, 40, 867–877. [Google Scholar] [CrossRef]
- Santos, M.S.; Nogueira, M.A.; Hungria, M. Microbial inoculants: Reviewing the past, discussing the present and previewing an outstanding future for the use of beneficial bacteria in agriculture. AMB Express 2019, 9, 205. [Google Scholar] [CrossRef] [PubMed]
- Masood, S.; Zhao, X.Q.; Shen, R.F. Bacillus pumilus promotes the growth and nitrogen uptake of tomato plants under nitrogen fertilization. Sci. Hortic. 2020, 272, 109581. [Google Scholar] [CrossRef]
- Kocheva, K.; Kartseva, T.; Nenova, V.; Georgiev, G.; Brestič, M.; Misheva, S. Nitrogen assimilation and photosynthetic capacity of wheat genotypes under optimal and deficient nitrogen supply. Physiol. Mol. Biol. Plants 2020, 26, 2139–2149. [Google Scholar] [CrossRef] [PubMed]
- Qin, K.; Dong, X.; Leskovar, D.I. Improving tomato nitrogen use efficiency with lignite-derived humic substances. Sci. Hortic. 2023, 321, 112243. [Google Scholar] [CrossRef]
- Jung, H.A.; Karki, S.; Ehom, N.Y.; Yoon, M.H.; Kim, E.J.; Choi, J.S. Anti-diabetic and anti-inflammatory effects of green and red kohlrabi cultivars (Brassica oleracea var. gongylodes). Prev. Nutr. Food Sci. 2014, 19, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Ramírez, M.I.; Feregrino-Pérez, A.A.; Aguirre Becerra, H.; Parra-Pacheco, B.; Oviedo-Olvera, M.V.; García-Trejo, J.F. Response of Phenolic Compounds in Lippia graveolens Kunth Irrigated with Aquaculture Wastewater and Steiner Solution. Int. J. Plant Biol. 2023, 14, 483–492. [Google Scholar] [CrossRef]
- Razmjooei, Z.; Etemadi, M.; Eshghi, S.; Ramezanian, A.; Abarghuei, F.M.; Alizargar, J. Potential Role of Foliar Application of Azotobacter on Growth, Nutritional Value and Quality of Lettuce under Different Nitrogen Levels. Plants 2022, 11, 406. [Google Scholar] [CrossRef]
- Shams, A.S. Effect of mineral, organic and bio-fertilizers on growth, yield, quality and sensory evaluation of Kohlrabi. Res. J. Agric. Biol. Sci. 2012, 8, 305–314. [Google Scholar]
- Di Benedetto, N.A.; Corbo, M.R.; Campaniello, D.; Cataldi, M.P.; Bevilacqua, A.; Sinigaglia, M.; Flagella, Z. The role of plant growth promoting bacteria in improving nitrogen use efficiency for sustainable crop production: A focus on wheat. AIMS Microbiol. 2017, 3, 413–434. [Google Scholar] [CrossRef]
- Del Cappellari, L.R.; Chiappero, J.; Santoro, M.V.; Giordano, W.; Banchio, E. Inducing phenolic production and volatile organic compounds emission by inoculating Mentha piperita with plant growth-promoting rhizobacteria. Sci. Hortic. 2017, 220, 193–198. [Google Scholar] [CrossRef]
- Ottaiano, L.; Di Mola, I.; Cozzolino, E.; El-Nakhel, C.; Rouphael, Y.; Mori, M. Biostimulant application under different nitrogen fertilization levels: Assessment of yield, leaf quality, and nitrogen metabolism of tunnel-grown lettuce. Agronomy 2021, 11, 1613. [Google Scholar] [CrossRef]
- Collado-González, J.; Piñero, M.C.; Otálora, G.; López-Marín, J.; Del Amor, F.M. Effects of different nitrogen forms and exogenous application of putrescine on heat stress of cauliflower: Photosynthetic gas exchange, mineral concentration and lipid peroxidation. Plants 2021, 10, 152. [Google Scholar] [CrossRef] [PubMed]
- Chea, L.; Meijide, A.; Meinen, C.; Pawelzik, E.; Naumann, M. Cultivar-Dependent Responses in Plant Growth, Leaf Physiology, Phosphorus Use Efficiency, and Tuber Quality of Potatoes Under Limited Phosphorus Availability Conditions. Front. Plant Sci. 2021, 12, 723862. [Google Scholar] [CrossRef]
- Dong, S.; Beckles, D.M. Dynamic changes in the starch-sugar interconversion within plant source and sink tissues promote a better abiotic stress response. J. Plant Physiol. 2019, 234, 80–93. [Google Scholar] [CrossRef]
- Collado-González, J.; Piñero, C.; Otálora, G.; López-Marín, J.; Del Amor, F.M. Merging Heat Stress Tolerance and Health-Promoting Properties: The Effects of Exogenous Arginine in Cauliflower (Brassica oleracea var. botrytis L.). Foods 2021, 10, 30. [Google Scholar] [CrossRef]
| Nutrient (mg L−1) | Control | Mixed Water | Mixed Water + S |
|---|---|---|---|
| NO3− | 606.70 ± 54.19 | 249.74 ± 38.27 | 550.22 ± 36.31 |
| PO43− | 367.73 ± 10.50 | 290.54 ± 8.09 | 445.75 ± 7.97 |
| SO42− | 380.46 ± 4.24 | 338.76 ± 2.19 | 392.04 ± 4.48 |
| K+ | 458.44 ± 12.32 | 193.32 ± 7.59 | 429.89 ± 7.15 |
| Ca2+ | 76.70 ± 3.07 | 80.61 ± 2.86 | 81.06 ± 2.84 |
| Mg2+ | 65.68 ± 0.97 | 66.20 ± 0.96 | 68.60 ± 1.29 |
| Irrigation | PGPR | Treatments |
|---|---|---|
| Control | 0 | Control |
| B1 | Control + B1 | |
| B2 | Control + B2 | |
| Mixed water | 0 | Mixed water |
| B1 | Mixed water + B1 | |
| B2 | Mixed water + B2 | |
| Mixed water + S | 0 | Mixed water + S |
| B1 | Mixed water + S + B1 | |
| B2 | Mixed water + S + B2 |
| Irrigation | PGPR | K | Ca | Mg | P | Fe | Cu | Mn | Zn | B |
|---|---|---|---|---|---|---|---|---|---|---|
| g Kg−1 DW | mg Kg−1 DW | |||||||||
| Control | Control | 48.9 ± 1.4 a | 2.51 ± 0.1 de | 2.49 ± 0.1 bcd | 6.7 ± 0.1 b | 46.9 ± 4.2 a | 3.7 ± 0.1 a | 24.16 ± 1.1 a | 27.7 ± 1.0 bcd | 24.92 ± 1.0 a |
| B1 | 42.6 ± 1.1 b | 2.20 ± 0.1 e | 2.16 ± 0.1 d | 6.3 ± 0.1 c | 34.9 ± 1.0 b | 3.0 ± 0.1 bc | 21.42 ± 0.8 ab | 26.6 ± 1.3 cd | 23.05 ± 1.3 cd | |
| B2 | 40.0 ± 0.5 b | 2.21 ± 0.1 e | 2.15 ± 0.1 d | 5.9 ± 0.0 d | 34.6 ± 1.1 b | 2.5 ± 0.2 c | 19.30 ± 1.1 b | 25.5 ± 1.0 d | 21.20 ± 1.0 d | |
| Mixed water | Control | 40.1 ± 1.0 b | 2.97 ± 0.1 abc | 2.72 ± 0.1 abc | 6.0 ± 0.1 cd | 31.6 ± 0.5 bc | 3.0 ± 0.2 bc | 22.42 ± 0.5 a | 28.1 ± 0.8 bcd | 23.40 ± 0.8 bcd |
| B1 | 42.5 ± 1.4 b | 3.29 ± 0.1 a | 2.95 ± 0.1 a | 6.0 ± 0.1 cd | 32.4 ± 1.6 bc | 3.4 ± 0.2 ab | 23.00 ± 0.9 a | 26.9 ± 0.9 cd | 24.55 ± 0.9 ab | |
| B2 | 41.2 ± 1.1 b | 2.79 ± 0.1 bcd | 2.55 ± 0.1 bc | 6.1 ± 0.1 cd | 28.9 ± 1.2 c | 3.4 ± 0.2 ab | 21.37 ± 0.4 ab | 26.1 ± 1.0 cd | 22.30 ± 1.0 cd | |
| Mixed water + S | Control | 42.7 ± 1.4 b | 2.66 ± 0.2 bc | 2.45 ± 0.2 cd | 6.3 ± 0.1 c | 34.9 ± 1.0 b | 2.8 ± 0.1 c | 22.53 ± 0.7 a | 29.0 ± 0.7 bc | 23.65 ± 0.7 abc |
| B1 | 48.2 ± 0.4 a | 3.14 ± 0.2 a | 2.84 ± 0.1 ab | 6.9 ± 0.1 ab | 35.8 ± 0.7 b | 2.6 ± 0.1 c | 22.42 ± 1.3 a | 30.1 ± 0.1 ab | 24.86 ± 0.1 a | |
| B2 | 47.8 ± 0.8 a | 3.09 ± 0.1 ab | 2.68 ± 0.1 abc | 7.2 ± 0.2 ab | 30.5 ± 1.4 bc | 1.9 ± 0.2 d | 21.87 ± 0.6 ab | 32.6 ± 0.8 a | 24.78 ± 0.8 a | |
| Irrigation | PGPB | Chloride | Nitrate | Phosphate | Sulfate |
|---|---|---|---|---|---|
| Control | Control | 3.55 ± 0.28 bc | 16.39 ± 0.81 a | 21.02 ± 0.60 abc | 11.32 ± 0.79 bcd |
| B1 | 3.55 ± 0.41 bc | 16.08 ± 1.87 a | 21.31 ± 0.34 ab | 10.35 ± 0.63 cd | |
| B2 | 3.20 ± 0.17 c | 17.52 ± 1.28 a | 21.89 ± 0.41 a | 10.18 ± 0.41 cd | |
| Mixed water | Control | 4.74 ± 0.29 a | 4.68 ± 0.55 b | 18.78 ± 0.62 d | 11.32 ± 0.29 bc |
| B1 | 5.15 ± 0.33 a | 5.18 ± 0.80 b | 19.89 ± 0.49 bcd | 13.10 ± 0.52 a | |
| B2 | 4.34 ± 0.25 ab | 5.23 ± 0.81 b | 20.14 ± 0.61 abcd | 11.94 ± 0.24 ab | |
| Mixed water + S | Control | 2.94 ± 0.31 c | 17.47 ± 1.92 a | 21.37 ± 0.56 ab | 9.56 ± 0.43 d |
| B1 | 3.60 ± 0.20 bc | 18.77 ± 0.78 a | 21.73 ± 0.39 a | 10.80 ± 0.29 bcd | |
| B2 | 3.37 ± 0.16 c | 18.42 ± 0.76 ab | 19.52 ± 0.87 cd | 10.50 ± 0.27 bcd |
| Irrigation | PGPB | Glucose | Fructose | Sucrose | Total Free Sugars |
|---|---|---|---|---|---|
| Control | Control | 790.4 ± 53.8 a | 280.6 ± 25.7 ab | 39.4 ± 6.3 bc | 1110.4 ± 77.3 abc |
| B1 | 817.8 ± 42.1 a | 271.4 ± 8.6 ab | 42.3 ± 6.2 bc | 1131.5 ± 46.1 ab | |
| B2 | 628.2 ± 17.8 b | 215.3 ± 11.3 c | 21.5 ± 1.9 d | 864.9 ± 28.1 de | |
| Mixed water | Control | 633.6 ± 20.7 b | 245.1 ± 7.3 bc | 83.5 ± 4.6 a | 961.1 ± 25.5 de |
| B1 | 675.1 ± 13.0 b | 282.9 ± 20.2 ab | 46.0 ± 4.3 a | 1004.0 ± 27.6 bcd | |
| B2 | 816.8 ± 42.7 a | 316.2 ± 24.0 a | 74.6 ± 3.4 b | 1207.6 ± 64.8 a | |
| Mixed water + S | Control | 611.4 ± 25.9 b | 195.7 ± 8.5 c | 23.1 ± 2.7 d | 830.3.0 ± 33.4 e |
| B1 | 648.7 ± 42.7 b | 207.2 ± 14.4 c | 32.0 ± 3.0 cd | 888.0 ± 55.7 de | |
| B2 | 629.8 ± 18.4 b | 316.3 ± 7.9 a | 21.4 ± 0.8 d | 967.5 ± 18.1 cde |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piñero, M.C.; Collado-González, J.; Otálora, G.; López-Marín, J.; del Amor, F.M. Plant Growth Promoting Bacteria for Aquaponics as a New Strategy That Grants Quality and Nutrient Efficiency in Kohlrabi Cultivation. Horticulturae 2023, 9, 1299. https://doi.org/10.3390/horticulturae9121299
Piñero MC, Collado-González J, Otálora G, López-Marín J, del Amor FM. Plant Growth Promoting Bacteria for Aquaponics as a New Strategy That Grants Quality and Nutrient Efficiency in Kohlrabi Cultivation. Horticulturae. 2023; 9(12):1299. https://doi.org/10.3390/horticulturae9121299
Chicago/Turabian StylePiñero, María Carmen, Jacinta Collado-González, Ginés Otálora, Josefa López-Marín, and Francisco M. del Amor. 2023. "Plant Growth Promoting Bacteria for Aquaponics as a New Strategy That Grants Quality and Nutrient Efficiency in Kohlrabi Cultivation" Horticulturae 9, no. 12: 1299. https://doi.org/10.3390/horticulturae9121299
APA StylePiñero, M. C., Collado-González, J., Otálora, G., López-Marín, J., & del Amor, F. M. (2023). Plant Growth Promoting Bacteria for Aquaponics as a New Strategy That Grants Quality and Nutrient Efficiency in Kohlrabi Cultivation. Horticulturae, 9(12), 1299. https://doi.org/10.3390/horticulturae9121299