Study of Molecular Biodiversity and Population Structure of Vitis vinifera L. ssp. vinifera on the Volcanic Island of El Hierro (Canary Islands, Spain) by Using Microsatellite Markers
Abstract
:1. Introduction
2. Materials and Methods
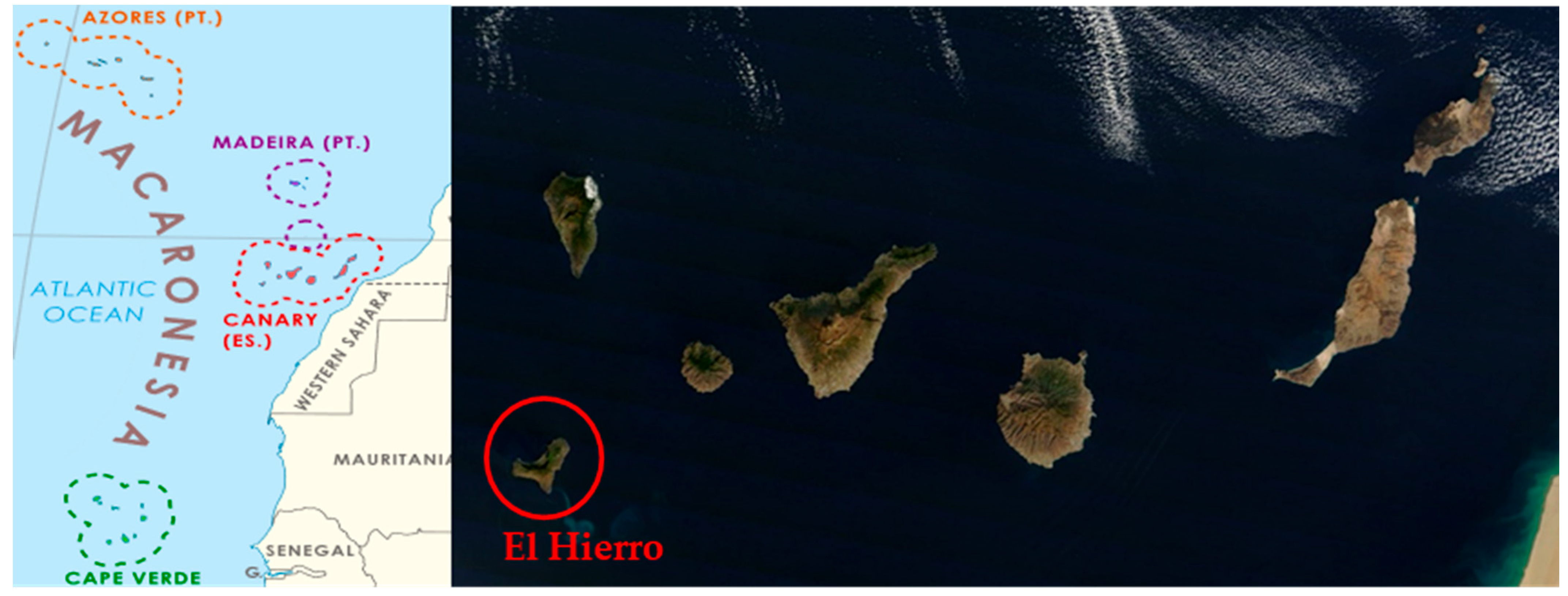
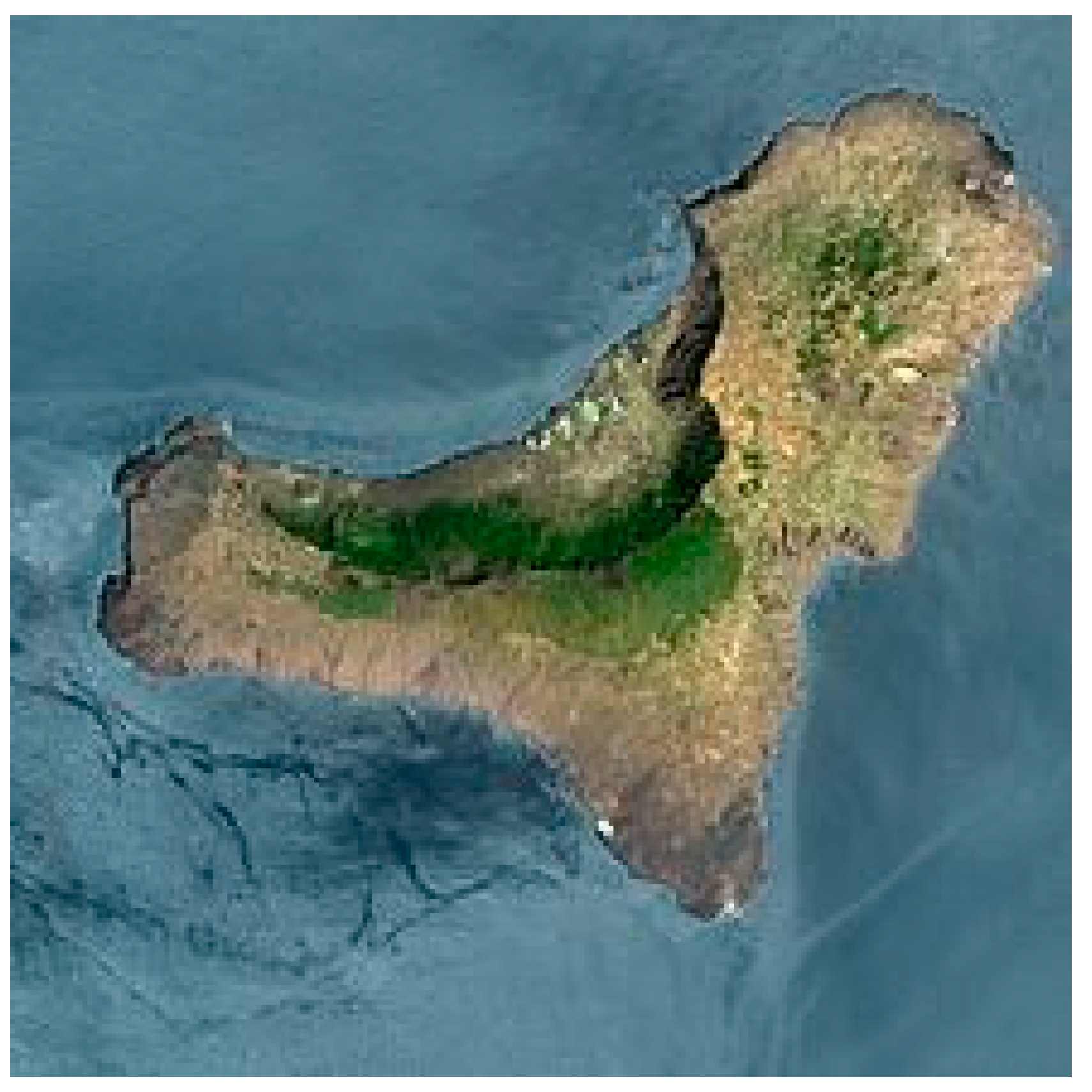
2.1. Plant Material
2.2. DNA Extraction and Purification
2.3. Simple Sequence Repeat (SSR) Markers
2.4. DNA Amplification and Polymerase Chain Reaction (PCR)
2.5. Amplified Fragments Length Measurement
2.6. Data Analysis
3. Results
3.1. SSR Polymorphism
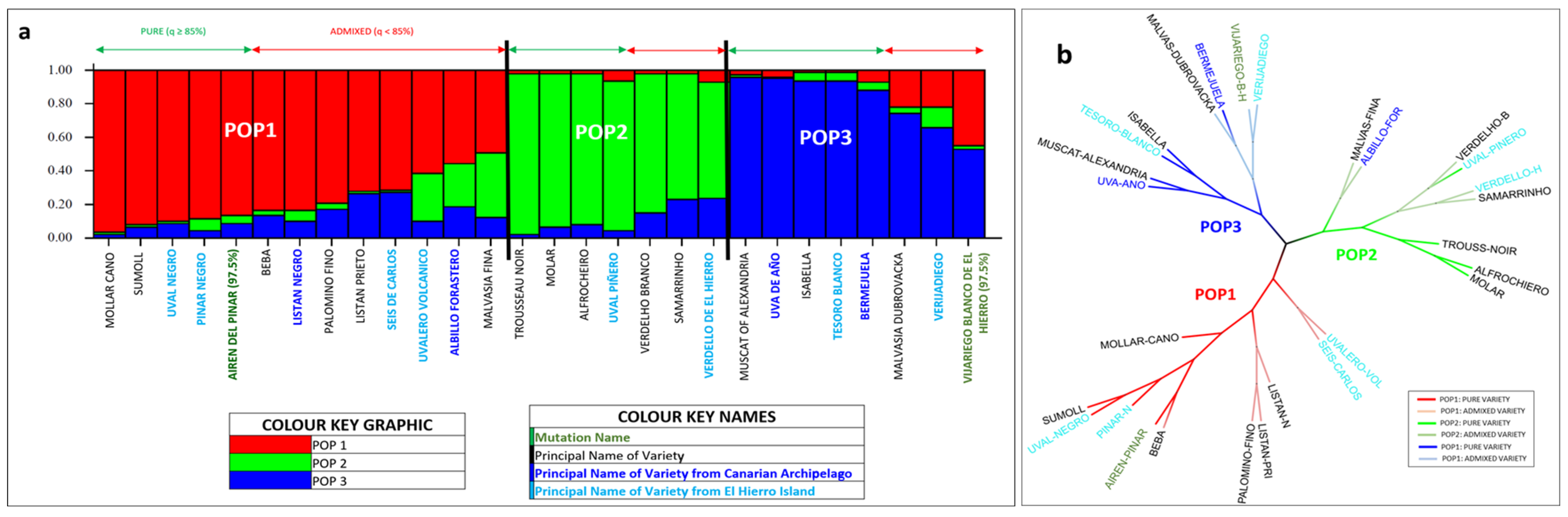
3.2. Grapevine Variety Analysis
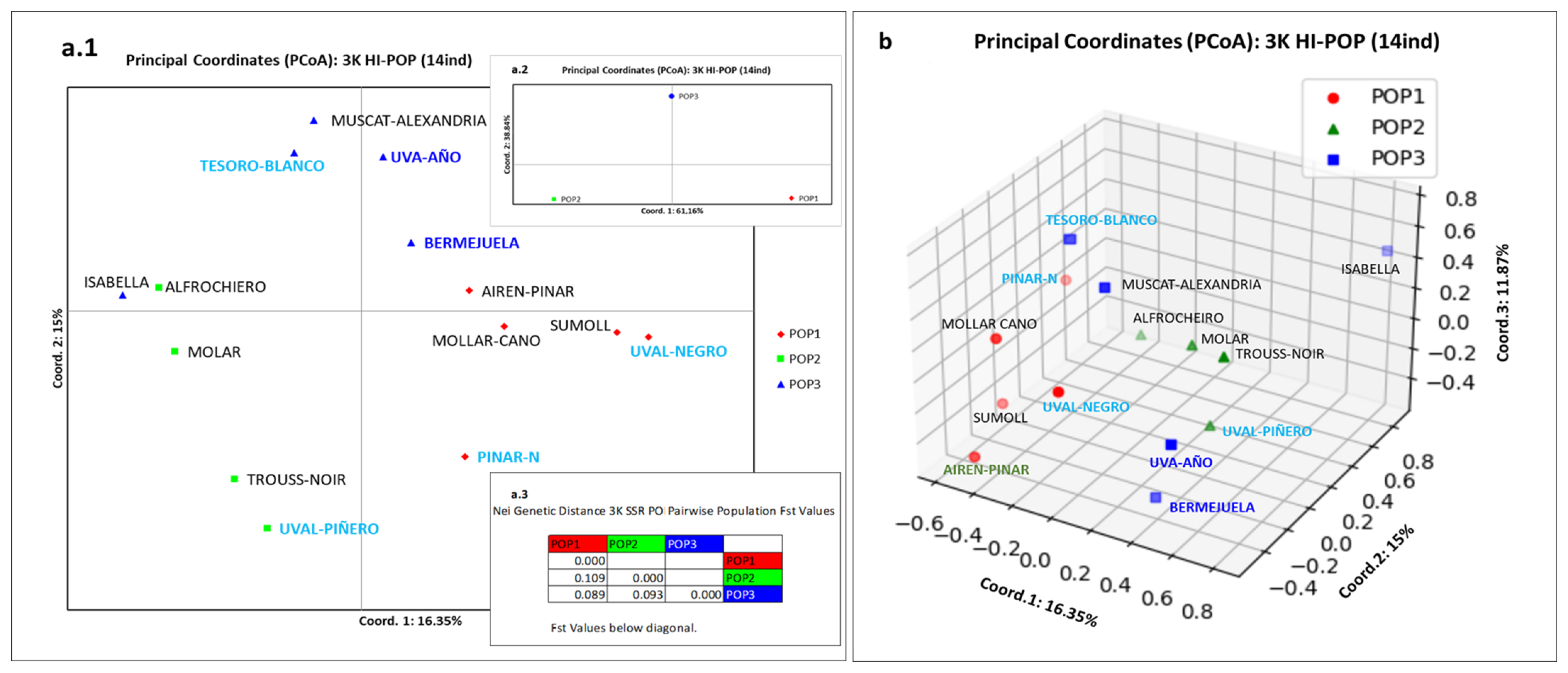
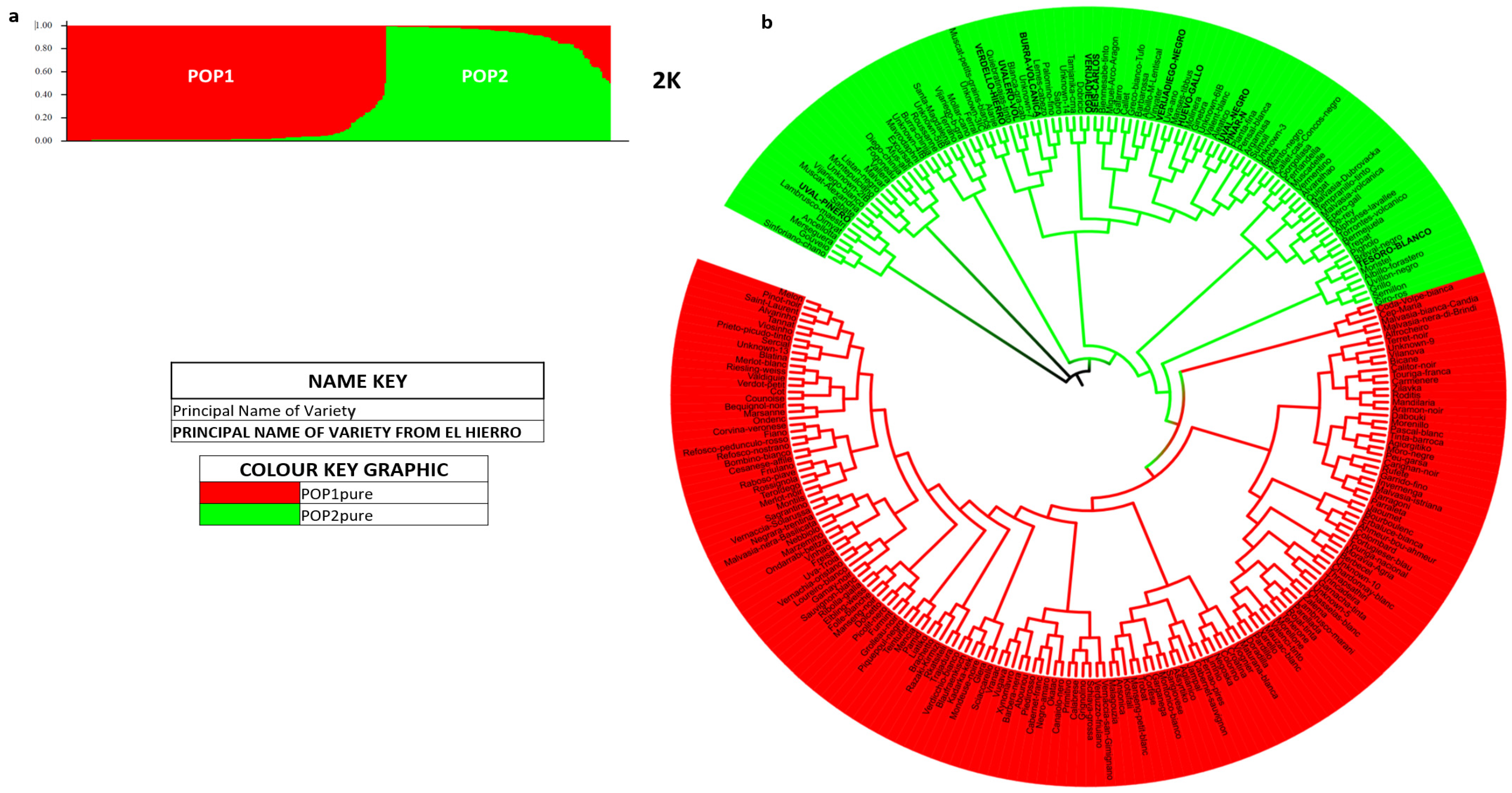
3.3. El Hierro Grapevine Population Genetic Structure
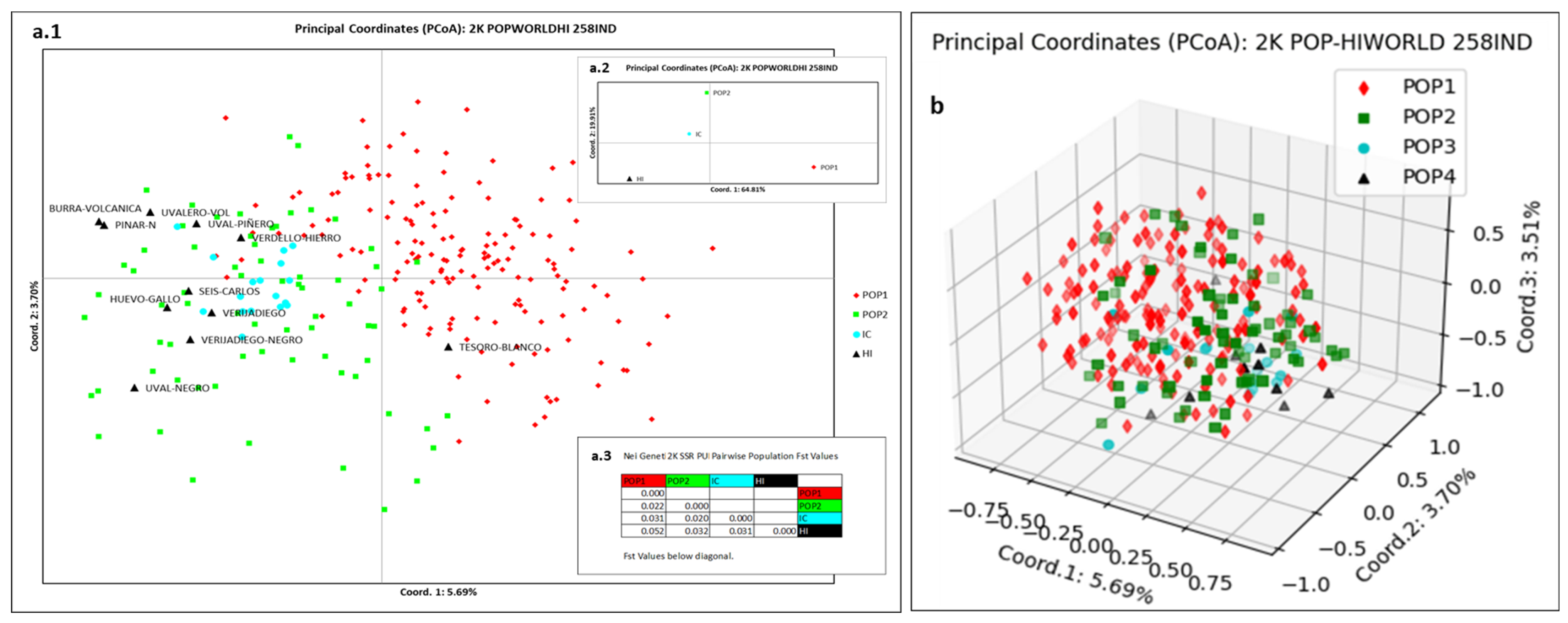
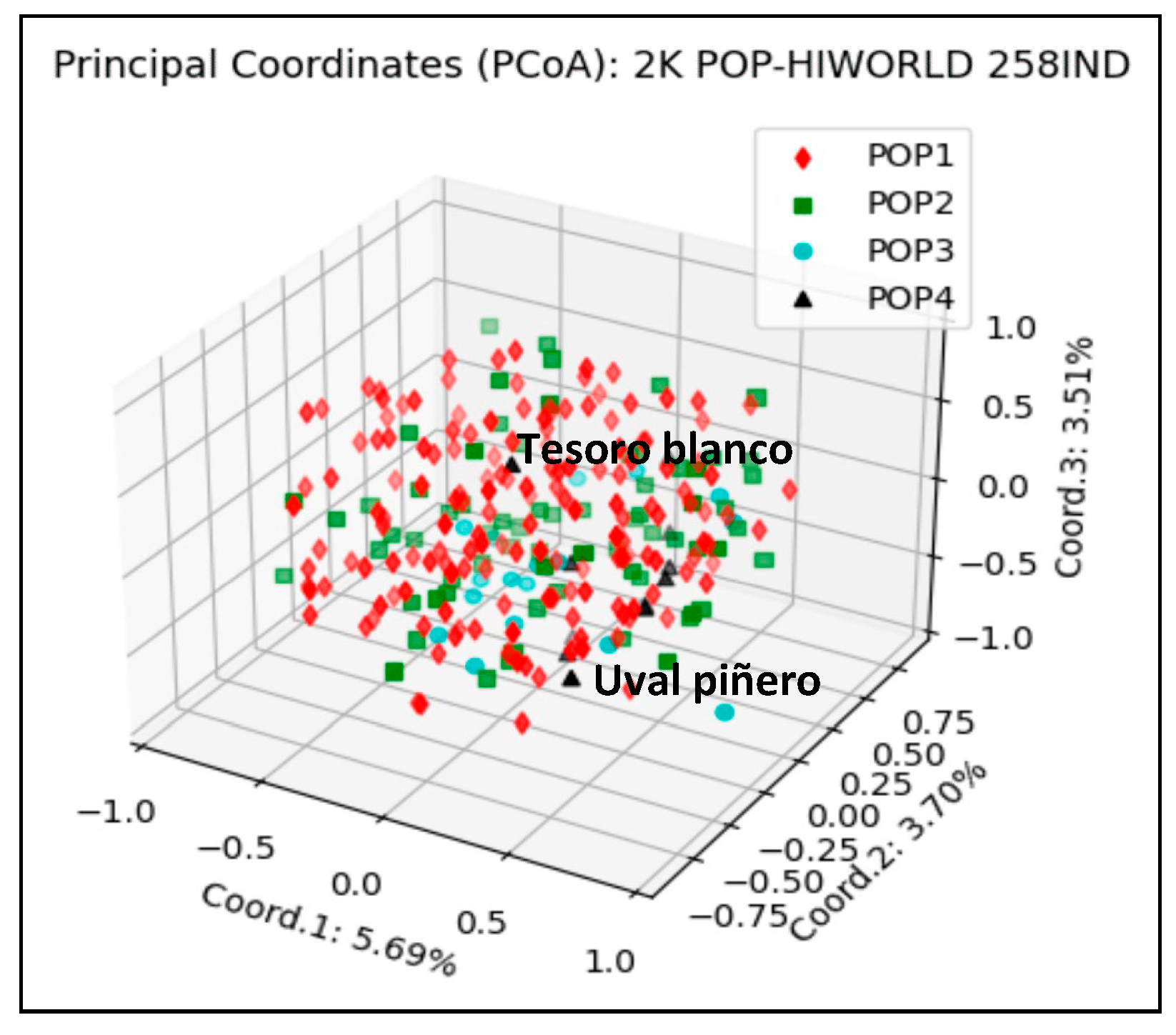
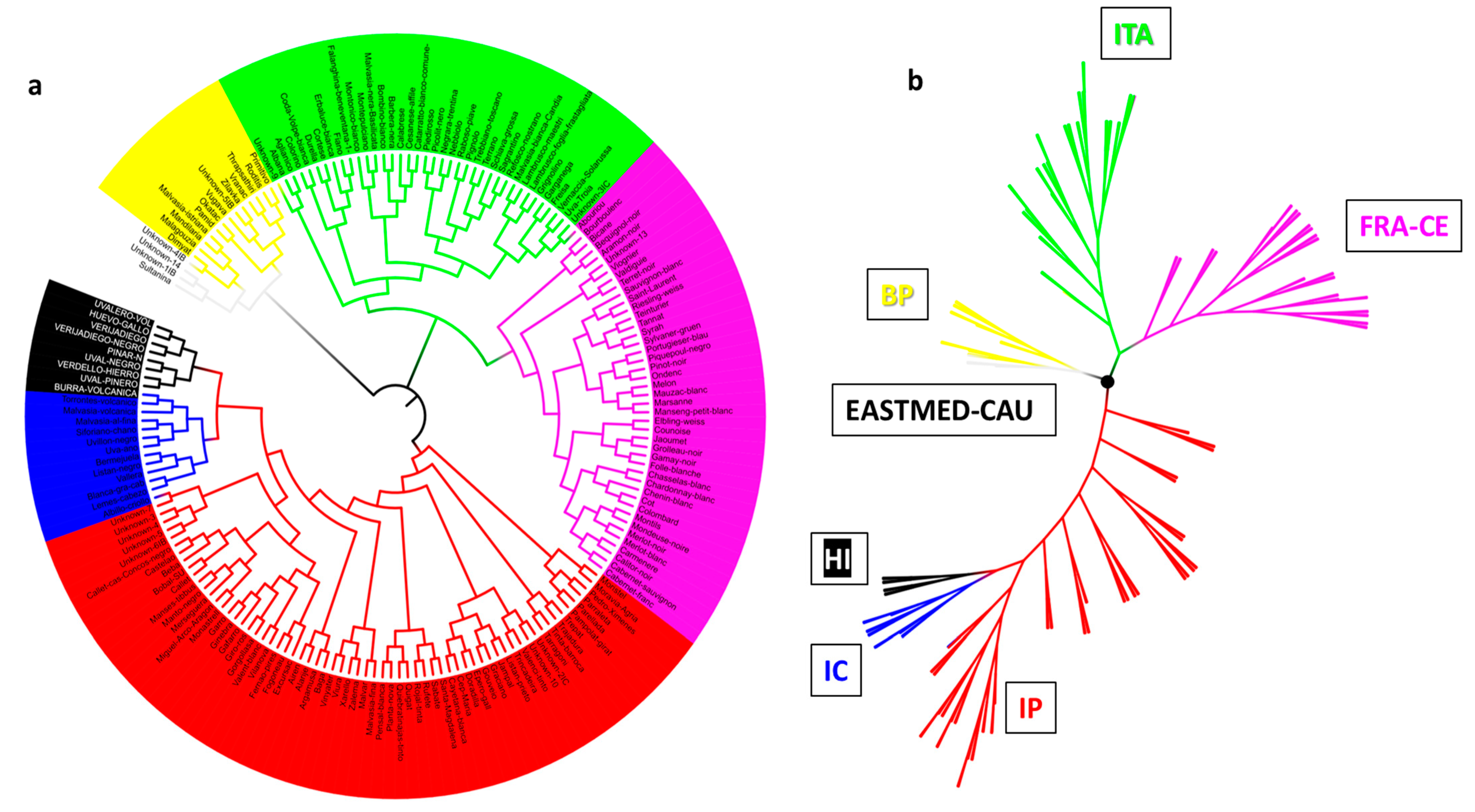
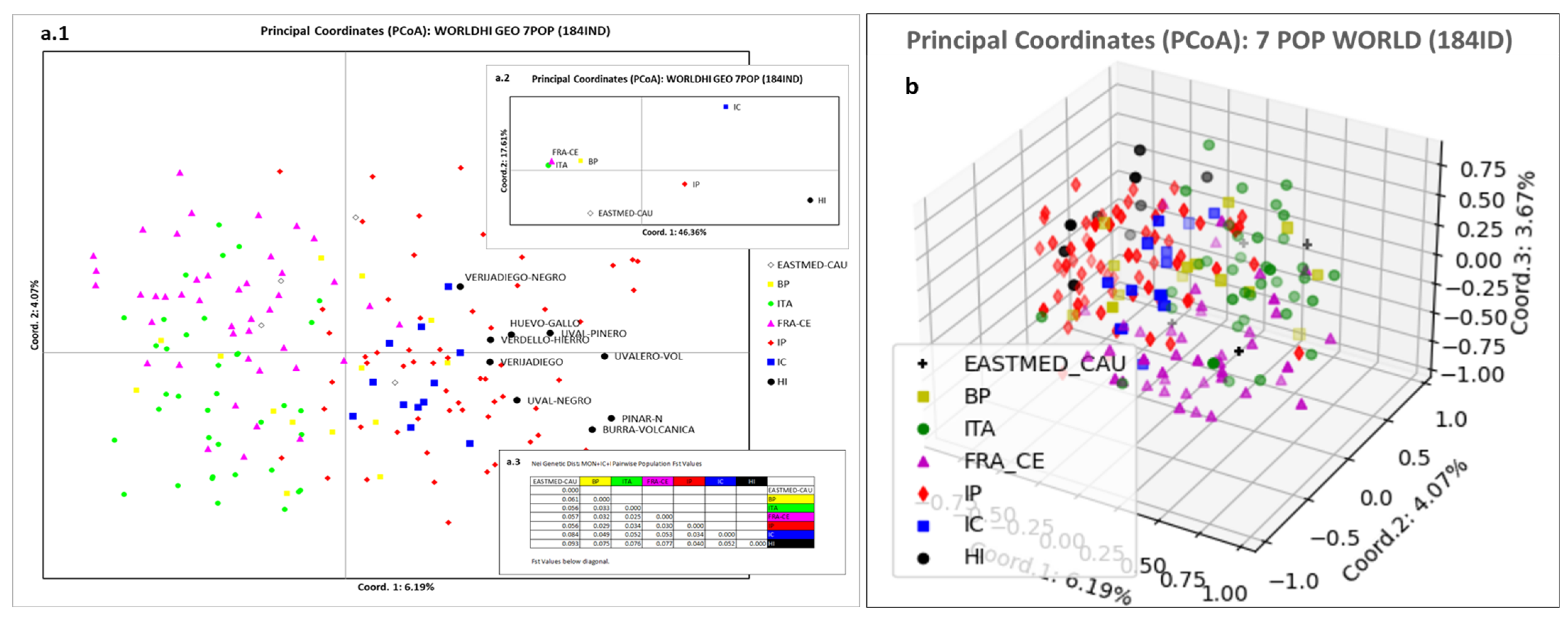
3.4. El Hierro Grapevine Population Genetic Structure Respect to the World Population
4. Discussion
4.1. SSR Polymorphism
4.2. Grapevine Varieties Analysis
4.3. El Hierro Grapevine Population Genetic Structure
4.4. El Hierro Grapevine Population Genetic Structure with Respect to the World Population
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- This, P.; Lacombe, T.; Thomas, M.R. Historical origins and genetic diversity of wine grapes. Trends Genet. 2006, 22, 511–519. [Google Scholar] [CrossRef]
- Marsal, G. Caracterización e Identificación de 449 Accesiones de Vitis vinifera L. Procedentes de dos Colecciones Ampelográficas. Ph.D. Thesis, Universidad Rovira i Virgili, Tarragona, Spain, 2015. [Google Scholar]
- Wan, Y.; Schwaninger, H.; Baldo, A.M.; Labate, J.A.; Zhong, G.Y.; Simon, C.J. A phylogenetic analysis of the grape genus (Vitis L.) reveals broad reticulation and concurrent diversification during neogene and quaternary climate change. BMC Evol. Biol. 2013, 13, 141. [Google Scholar] [CrossRef]
- Dong, Y.; Duan, S.; Xia, Q.; Liang, Z.; Dong, X.; Margaryan, K.; Musayev, M.; Goryslavets, S.; Zdunić, G.; Bert, P.-F.; et al. Dual domestications and origin of traits in grapevine evolution. Science 2023, 379, 892–901. [Google Scholar] [CrossRef]
- Wolkovich, E.M.; García de Cortázar-Atauri, I.; Morales-Castilla, I.; Nicholas, K.A.; Lacombe, T. From Pinot to Xinomavro in the world’s future wine-growing regions. Nat. Clim. Chang. 2018, 8, 29–37. [Google Scholar] [CrossRef]
- Marsal, G.; Bota, J.; Martorell, A.; Canals, J.M.; Zamora, F.; Fort, F. Local cultivars of Vitis vinifera L. in Spanish Islands: Balearic Archipelago. Sci. Hortic. 2017, 226, 122–132. [Google Scholar] [CrossRef]
- Spence, C. Multisensory experiential wine marketing. Food Qual. Prefer. 2019, 71, 106–116. [Google Scholar] [CrossRef]
- Sancho-Galán, P.; Amores-Arrocha, A.; Palacios, V.; Jiménez-Cantizano, A. Identification and Characterization of White Grape Varieties Autochthonous of a Warm Climate Region (Andalusia, Spain). Agronomy 2020, 10, 205. [Google Scholar] [CrossRef]
- GEVIC (Gran Enciclopedia Virtual Islas Canarias). 2007. Available online: https://www.gevic.net/info/contenidos/mostrar_contenidos.php?idcat=22&idcap=91&idcon=526 (accessed on 7 July 2023).
- Wikimedia Commons. 2014. Available online: https://commons.wikimedia.org/w/index.php?curid=33566121#file (accessed on 7 July 2023).
- NASA. 2011. Available online: https://www.flickr.com/photos/gsfc/6630087415/in/photostream/ (accessed on 7 July 2023).
- Macías, A.M. Colonización y viticultura. El caso de las Canarias, 1350–1550. Douro Estud. Doc. 2002, VII, 285–296. Available online: https://ojs.letras.up.pt/index.php/dou/article/view/12586 (accessed on 7 July 2023).
- Hidalgo, J.; Hidalgo, L. La Filoxera. In Tratado de Viticultura, 2nd ed.; Mundi-Prensa: Madrid, Spain, 2019; Volume 1. [Google Scholar]
- NASA World Wind. 2006. Available online: https://es.m.wikipedia.org/wiki/Canarias#/media/Archivo%3ASanta_Cruz_de_Tenerife_SPOT_1320.jpg (accessed on 7 July 2023).
- DOP Vinos de El Hierro. Available online: http://doelhierro.es/ (accessed on 7 July 2023).
- Marsal, G.; Mendez, J.J.; Mateo-Sanz, J.M.; Ferrer, S.; Canals, J.M.; Zamora, F.; Fort, F. Molecular characterization of Vitis vinifera L. local cultivars from volcanic areas (the Canary Islands and Madeira) using SSR markers. Oeno One 2019, 4, 667–680. [Google Scholar] [CrossRef]
- Zerolo, J.; Cabello, F.; Espino, A.; Borrego, J.; Ibañez, J.; Rodriguez-Torres, I.; Muñoz-Organero, G.; Rubio, C.; Hernández, M. Variedades de Vid de Cultivo Tradicional en Canarias; Instituto Canario de Calidad Agroalimentaria. Gobierno de Canarias: Santa Cruz de Tenerife, Spain, 2006. [Google Scholar]
- Rodriguez-Torres, I. Variedades de vid Cultivadas en Canarias. Descriptores Morfológicos. Caracterización Morfológica, Molecular, Agronómica y Enológica; Instituto Canario de Investigaciones Agrarias. Gobierno de Canarias: Santa Cruz de Tenerife, Spain, 2018. [Google Scholar]
- Marsal, G.; Baiges, I.; Canals, J.M.; Zamora, F.; Fort, F. A fast, efficient method for extracting DNA from leaves, stems, and seeds of Vitis vinifera L. Am. J. Enol. Vitic. 2011, 62, 376–381. [Google Scholar] [CrossRef]
- Marsal, G.; Boronat, N.; Canals, J.M.; Zamora, F.; Fort, F. Comparison of the efficiency of some of the most usual DNA extraction methods for woody plants in different tissues of Vitis vinifera L. J. Int. Sci. Vigne Vin 2013, 47, 227–237. [Google Scholar] [CrossRef]
- Fort, F.; Hayoun, L.; Valls, J.; Canals, J.M.; Arola, L.; Zamora, F. A new and simple method for rapid extraction and isolation of high-quality RNA from grape (Vitis vinifera) berries. J. Sci. Food Agric. 2008, 88, 179–184. [Google Scholar] [CrossRef]
- Thomas, M.R.; Scott, N.S. Microsatellite repeats in grapevine reveal DNA polymorphisms when analyzed as sequence-tagged sites (STSs). Theor. Appl. Genet. 1993, 86, 985–990. [Google Scholar] [CrossRef] [PubMed]
- Bowers, J.E.; Dangl, G.S.; Vignani, R.; Meredith, C.P. Isolation and characterization of new polymorphic simple sequence repeat loci in grape (Vitis vinifera L.). Genome 1996, 39, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Bowers, J.E.; Dangl, G.S.; Meredith, C.P. Development and characterization of additional microsatellite DNA markers for grape. Am. J. Enol. Vitic. 1999, 50, 243–246. [Google Scholar] [CrossRef]
- Sefc, K.M.; Regner, F.; Turetschek, E.; Glössl, J.; Steinkellner, H. Identification of microsatellite sequences in Vitis riparia and their applicability for genotyping of different Vitis species. Genome 1999, 42, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Scott, K.D.; Eggler, P.; Seaton, G.; Rosseto, M.; Abblet, E.M.; Lee, L.S.; Henry, R.J. Analysis of SSRs derived from grape ESTs. Theor. Appl. Genet. 2000, 100, 723–726. [Google Scholar] [CrossRef]
- Lefort, F.; Kyvelos, C.; Zervou, M.; Edwards, K.; Roubelakis-Angelakis, K. Characterization of new microsatellite loci from Vitis vinifera and their conservation in some Vitis species and hybrids. Mol. Ecol. Resour. 2002, 2, 20–21. [Google Scholar] [CrossRef]
- Cipriani, G.; Spadotto, A.; Jurman, I.; Di Gaspero, G.; Crespan, M.; Meneghetti, S.; Frare, E.; Vignani, R.; Cresti, M.; Morgante, M.; et al. The SSR-based molecular profile of 1005 grapevine (Vitis vinifera L.) accessions uncovers new synonymy and parentages and reveals a large admixture amongst varieties of different geographic origins. Theor. Appl. Genet. 2010, 121, 1569–1585. [Google Scholar] [CrossRef]
- Dalbó, M.A.; Ye, G.N.; Weeden, N.F.; Steinkellner, H.; Sefc, K.M.; Reisch, B.I. A gene-controlling sex in grapevines is placed on a molecular marker-based genetic map. Genome 2000, 43, 333–340. [Google Scholar] [CrossRef]
- Maul, E.; Röckel, R. Vitis International Variety Catalogue (VIVC): A cultivar database referenced by genetic profiles and morphology. BIO Web Conf. 2015, 5, 01009. [Google Scholar] [CrossRef]
- This, P.; Jung, A.; Boccacci, P.; Borrego, J.; Botta, R.; Costantini, L.; Crespan, M.; Dangl, G.S.; Eisenheld, C.; Ferreira-Monteiro, F.; et al. Development of a standard set of microsatellite reference alleles for the identification of grape cultivars. Theor. Appl. Genet. 2004, 109, 1448–1458. [Google Scholar] [CrossRef] [PubMed]
- Paetkau, D.; Calvert, W.; Stirling, I.; Strobeck, C. Microsatellite analysis of population structure in Canadian polar bears. Mol. Ecol. 1995, 4, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research-an update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef] [PubMed]
- Paetkau, D.; Slade, R.; Burden, M.; Estoup, A. Genetic assignment methods for the direct, real-time estimation of migration rate: A simulation-based exploration of accuracy and power. Mol. Ecol. 2004, 13, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef] [PubMed]
- Falush, D.; Stephens, M.; Pritchard, J.K. Inference of population structure using multilocus genotype data: Linked loci and correlated allele frequencies. Genetics 2003, 164, 1567–1587. [Google Scholar] [CrossRef] [PubMed]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software structure: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic threes. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Three-Dimensional Plotting in Matplotlib (Python Data Science Handbook). Available online: https://jakevdp.github.io/PythonDataScienceHandbook/04.12-three-dimensional-plotting.html (accessed on 8 August 2023).
- Marsal, G.; Mateo, J.M.; Canals, J.M.; Zamora, F.; Fort, F. SSR analysis of 338 accessions planted in Penedes (Spain) reveals 28 unreported molecular profiles of Vitis vinifera L. Am. J. Enol. Vitic. 2016, 67, 466–470. [Google Scholar] [CrossRef]
- Fort, F.; Marsal, G.; Mateo-Sanz, J.M.; Pena, V.; Canals, J.M.; Zamora, F. Molecular characterisation of the current cultivars of Vitis vinifera L. in Lanzarote (Canary Islands, Spain) reveals nine individuals which correspond to eight new varieties and two new sports. Oeno One 2022, 56, 281–295. [Google Scholar] [CrossRef]
- Maul, E.; Röckel, F. Vitis International Variety Catalogue. 2015. Available online: http://www.vivc.de (accessed on 7 July 2023).
- Bacilieri, R.; Lacombe, T.; Cunff, L.L.; Di Vecchi-Staraz, M.; Laucou, V.; Genna, B.; Perós, J.P.; This, P.; Boursiquot, J.M. Genetic structure in cultivated grapevines is linked to geography and human selection. BMC Plant Biol. 2013, 13, 25. [Google Scholar] [CrossRef] [PubMed]
- Arroyo-García, R.; Ruiz-García, L.; Bolling, L.; Ocete, R.; López, M.A.; Arnold, C.; Ergul, A.; Söylemezo˝lu, G.; Uzun, H.I.; Cabello, F.; et al. Multiple origins of cultivated grapevine (Vitis vinifera L. ssp. sativa) based on chloroplast DNA polymorphisms. Mol. Ecol. 2006, 15, 3707–3714. [Google Scholar] [CrossRef] [PubMed]
- Aliquo, G.; Torres, R.; Lacombe, T.; Boursiquot, J.M.; Laucou, V.; Gualpa, J.; Fanzone, M.; Sari, S.; Pérez-Peña, J.; Prieto, J.A. Identity and parentage of some South American grapevine cultivars present in Argentina. Aust. J. Grape Wine Res. 2017, 23, 452–460. [Google Scholar] [CrossRef]
- Moita, A.; Santos, R.; Catarina, A. Unraveling the origin of Vitis vinifera L. Verdelho. Aust. J. Grape Wine Res. 2018, 24, 450–460. [Google Scholar] [CrossRef]
- Arslan, N.; Yılmaz Baydu, F.; Hazrati, N.; Yüksel Özmen, C.; Ergönül, O.; Uysal, T.; Yaşasın, A.S.; Özer, C.; Boz, Y.; Kuleyin, Y.S.; et al. Genetic Diversity and Population Structure Analysis of Anatolian Kara Grapevine (Vitis vinifera L.) Germplasm Using Simple Sequence Repeats. Horticulturae 2023, 9, 743. [Google Scholar] [CrossRef]
- Žulj Mihaljević, M.; Maletić, E.; Preiner, D.; Zdunić, G.; Bubola, M.; Zyprian, E.; Pejić, I. Genetic Diversity, Population Structure, and Parentage Analysis of Croatian Grapevine Germplasm. Genes 2020, 11, 737. [Google Scholar] [CrossRef]
- Jiménez-Cantizano, A.; Puig-Pujol, A.; Arroyo-García, R. Identification of Vitis vinifera L. Local Cultivars Recovered in Andalusia (Spain) by Using Microsatellite Markers. Horticulturae 2023, 9, 316. [Google Scholar] [CrossRef]
- Ibañez, J.; De Andrés, M.T.; Molino, A.; Borrego, J. Genetic study of key Spanish grapevine varieties using microsatellite analysis. Am. J. Enol. Vitic. 2003, 54, 22–30. [Google Scholar] [CrossRef]
- Vélez, M.D.; Ibáñez, J. Evaluation of the uniformity and stability of Microsatellite markers in grapevine. Acta Hortic. 2009, 827, 163–168. [Google Scholar] [CrossRef]
- Cabezas, A.; Ibañez, J.; Lijavetzky, D.; Vélez, D.; Bravo, G.; Rodríguez, V.; Carreño, I.; Jermakow, A.M.; Carreño, J.; Ruiz-García, L.; et al. A 48 SNP set for grapevine cultivar identification. BMC Plant Biol. 2011, 11, 153. [Google Scholar] [CrossRef] [PubMed]
- Riaz, S.; Garrison, K.E.; Dangl, G.S.; Boursiquot, J.M.; Meredith, C.P. Genetic divergence and chimerism within ancient asexually propagated wine grape cultivars. J. Am. Soc. Hort. Sci. 2002, 127, 508–514. [Google Scholar] [CrossRef]
- Grigoriou, A.; Tsaniklidis, G.; Hagidimitriou, M.; Nikoloudakis, N. The Cypriot Indigenous Grapevine Germplasm Is a Multi-Clonal Varietal Mixture. Plants 2020, 9, 1034. [Google Scholar] [CrossRef]
- Casanova, J.; Mozas, P.; Ortiz, J.M. Ampelography and microsatellite DNA analysis of autochthonous and endangered grapevine cultivars in the province of Huesca (Spain). Span. J. Agric. Res. 2011, 9, 790–800. [Google Scholar] [CrossRef]
- Macías, A. El paisaje vitícola de Canarias. Cinco siglos de historia. Ería 2005, 68, 351–364. [Google Scholar]
- Emanuelli, F.; Lorenzi, S.; Grzeskowiak, L.; Catalano, V.; Stefanini, M.; Troggio, M.; Myles, S.; Martínez-Zapater, J.M.; Zyprian, E.; Moreira, F.M.; et al. Genetic diversity and population structure assessed by SSR and SNP markers in large germplasm collection of grape. BMC Plant Biol. 2013, 13, 39. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fort, F.; Lin-Yang, Q.; Suárez-Abreu, L.R.; Sancho-Galán, P.; Canals, J.M.; Zamora, F. Study of Molecular Biodiversity and Population Structure of Vitis vinifera L. ssp. vinifera on the Volcanic Island of El Hierro (Canary Islands, Spain) by Using Microsatellite Markers. Horticulturae 2023, 9, 1297. https://doi.org/10.3390/horticulturae9121297
Fort F, Lin-Yang Q, Suárez-Abreu LR, Sancho-Galán P, Canals JM, Zamora F. Study of Molecular Biodiversity and Population Structure of Vitis vinifera L. ssp. vinifera on the Volcanic Island of El Hierro (Canary Islands, Spain) by Using Microsatellite Markers. Horticulturae. 2023; 9(12):1297. https://doi.org/10.3390/horticulturae9121297
Chicago/Turabian StyleFort, Francesca, Qiying Lin-Yang, Luis Ricardo Suárez-Abreu, Pau Sancho-Galán, Joan Miquel Canals, and Fernando Zamora. 2023. "Study of Molecular Biodiversity and Population Structure of Vitis vinifera L. ssp. vinifera on the Volcanic Island of El Hierro (Canary Islands, Spain) by Using Microsatellite Markers" Horticulturae 9, no. 12: 1297. https://doi.org/10.3390/horticulturae9121297
APA StyleFort, F., Lin-Yang, Q., Suárez-Abreu, L. R., Sancho-Galán, P., Canals, J. M., & Zamora, F. (2023). Study of Molecular Biodiversity and Population Structure of Vitis vinifera L. ssp. vinifera on the Volcanic Island of El Hierro (Canary Islands, Spain) by Using Microsatellite Markers. Horticulturae, 9(12), 1297. https://doi.org/10.3390/horticulturae9121297