Maternal Environment and Priming Agents Effect Germination and Seedling Quality in Pitaya under Salt Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Preparing Solutions and Performing Seed Priming
2.3. Experimental Layout
2.4. Germination-Related Traits
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Crane, J.H.; Balerdi, C.F. Pitaya Growing in the Florida home Landscape; IFAS Extension: Lake City, FL, USA, 2005. [Google Scholar]
- Wu, L.C.; Hsu, H.W.; Chen, Y.C.; Chiu, C.C.; Lin, Y.I.; Ho, J.A.A. Antioxidant and antiproliferative activities of red pitaya. Food Chem. 2006, 95, 319–327. [Google Scholar] [CrossRef]
- Esquivel, P.; Stintzing, F.C.; Carle, R. Comparison of morphological and chemical fruit traits from different pitaya genotypes (Hylocereus sp.) grown in Costa Rica. J. Appl. Bot. Food Qual. 2007, 81, 7–14. [Google Scholar]
- Cheok, A.; Xu, Y.; Zhang, Z.; Caton, P.W.; Rodriguez-Mateos, A. Betalain-rich dragon fruit (pitaya) consumption improves vascular function in men and women: A double-blind, randomized controlled crossover trial. Am. J. Clin. Nutr. 2022, 115, 1418–1431. [Google Scholar] [CrossRef]
- Nishikito, D.F.; Borges, A.C.A.; Laurindo, L.F.; Otoboni, A.M.B.; Direito, R.; Goulart, R.D.A.; Nicolau, C.C.T.; Fiorini, A.M.R.; Sinatora, S.M.; Barbalho, S.M. Anti-inflammatory, antioxidant, and other health effects of dragon fruit and potential delivery systems for ıts bioactive compounds. Pharmaceutics 2023, 15, 159. [Google Scholar] [CrossRef]
- Ariffin, A.A.; Bakar, J.; Tan, C.P.; Rahman, R.A.; Karim, R.; Loi, C.C. Essential fatty acids of pitaya (dragon fruit) seed oil. Food Chem. 2009, 114, 561–564. [Google Scholar] [CrossRef]
- Mahmud, M.H.; Raihan, M.T.; Shakhik, M.T.Z.; Khan, F.T.; Islam, M.T. Dragon fruit (Hylocereus polyrhizus): A green colorant for cotton fabric. Colorants 2023, 2, 230–244. [Google Scholar] [CrossRef]
- Mercado-Silva, E.M. Pitaya—Hylocereus undatus (haw). In Exotic Fruits; Academic Press: Cambridge, MA, USA, 2018; pp. 339–349. [Google Scholar]
- Tel-Zur, N. Pitahayas: Introduction, agrotechniques, and breeding. VII Int. Congr. Cactus Pear Cochineal 2010, 995, 109–115. [Google Scholar] [CrossRef]
- Evrenosoğlu, Y.; Mertoğlu, K.; Bilgin, N.A.; Misirli, A.; Özsoy, A.N. Inheritance pattern of fire blight resistance in pear. Sci. Hortic. 2019, 246, 887–892. [Google Scholar] [CrossRef]
- Suarez-Roman, R.S.; Caetano, C.M.; Ramírez, H.; Morales, J.G. Caracterización morfoanatómica y fisiológica de semilla sexual de pitahaya amarilla Selenicereus megalanthus (Haw.) Britt & Rose. Rev. De La Asoc. Colomb. De Cienc. Biológicas 2012, 24, 97–111. [Google Scholar]
- Carvalho, N.M.; Nakagawa, J. Sementes: Ciência, tecnologia e produção. In Seeds: Science, Technology and Production Funep, 5th ed.; Atena: Jaboticabal, Brazil, 2012. [Google Scholar]
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef]
- Mizrahi, Y.; Nerd, A.; Sitrit, Y. New Fruits for arid climates. In Trends in New Crops and New Uses; Janick, J., Whipkey, A., Eds.; ASHS Press: Alexandria, VA, USA, 2002. [Google Scholar]
- Tel-Zur, N.; Abbo, S.; Bar-Zvi, D.; Mizrahi, Y. Genetic relationships among Hylocereus and Selenicereus vine cacti (Cactaceae): Evidence from hybridization and cytological studies. Ann. Bot. 2004, 94, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, S.M.C.; Paiva, E.P.D.; Torres, S.B.; Neta, M.L.D.S.; Leite, M.D.S.; Benedito, C.P.; Albuquerque, C.C.D.; Sá, F.V.D.S. Pre-germination treatments in pitaya (Hylocereus spp.) seeds to attenuate salt stress. Rev. Ciência Agronômica 2022, 53, e20218121. [Google Scholar] [CrossRef]
- Orozco, A.; Gardea, A.A.; Rascón-Chu, A.; Sánchez, A. Effect of salinity on seed germination, growth and metabolic activity of pitaya seedlings [Stenocereus thurberi (Engelm.) Buxb.]. J. Prof. Assoc. Cactus Dev. 2017, 19, 67–78. [Google Scholar] [CrossRef]
- Ortiz, T.A.; Gomes, G.R.; Takahashi, L.U.S.A.; Urbano, M.R.; Strapasson, E. Water and salt stress in germinating seeds of pitaya genotypes (Hylocereus spp.). Afr. J. Agric. Res. 2014, 9, 3610–3619. [Google Scholar]
- Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, S.M. Effect of salinity stress on plants and its tolerance strategies: A review. Environ. Sci. Pollut. Res. 2015, 22, 4056–4075. [Google Scholar] [CrossRef]
- Oliveira, A.B.; Alencar, N.L.M.; Gallão, M.I.; Gomes Filho, E. Avaliação citoquímica durante a germinação de sementes de sorgo envelhecidas artificialmente e osmocondicionadas, sob salinidade [Cytochemical evaluation during the germination of artificial aged and primed sorghum seeds under salinity]. Rev. Ciênc. Agron. 2011, 42, 223–231. [Google Scholar] [CrossRef]
- Singh, A.L.; Hsriprasanna, K.; Chaudhari, V. Differential nutrients absorption an important tool for screening and identification of soil salinity tolerant peanut genotypes. Indian J. Plant Physiol. 2016, 21, 83–92. [Google Scholar] [CrossRef]
- Isayenkov, S.V.; Maathuis, F.J. Plant salinity stress: Many unanswered questions remain. Front. Plant Sci. 2019, 10, 80. [Google Scholar] [CrossRef]
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef]
- Pereira, M.R.R.; Martins, C.C.; Souza, G.S.F.; Martins, D. Influência do estresse hídrico e salino na germinação de Urochloa decumbens e Urochloa ruziziensis [Influence of saline and water stress on germination of Urochloa decumbens and Urochloa ruziziensis]. Biosci. J. 2012, 28, 537–545. [Google Scholar]
- Hussain, M.; Farooq, S.; Hassan, W.; Ul-Allah, S.; Tanveer, M.; Farooq, M.; Nawaz, A. Drought stress in sunflower: Physiological effects and its management throughout breeding and agronomic alternatives. Agric. Water Manag. 2018, 201, 152–166. [Google Scholar] [CrossRef]
- Shaar-Moshe, L.; Blumwald, E.; Peleg, Z. Unique physiological and transcriptional shifts under combinations of salinity, drought, and heat. Plant Physiol. 2017, 174, 421–434. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.; Shivay, Y.S. Oxalic acid/oxalates in plants: From self-defence to phytoremediation. Curr. Sci. 2017, 112, 1665–1667. [Google Scholar] [CrossRef]
- Janda, T.; Szalai, G.; Pál, M. Salicylic acid signalling in plants. Int. J. Mol. Sci. 2020, 21, 2655. [Google Scholar] [CrossRef]
- War, A.R.; Paulraj, M.G.; War, M.Y.; Ignacimuthu, S. Jasmonic acid- mediated induced resistance in groundnut (Arachis hypogaea L.) against Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae). J. Plant. Growth. Regul. 2011, 30, 512–523. [Google Scholar] [CrossRef]
- Chia, L. What Are the Differences between Mepiquat Chloride and Chlormequat Chloride? Plant Hormones. 2018. Available online: https://www.plantgrowthhormones.com/info/ (accessed on 14 October 2023).
- Tian, S.; Wan, Y.; Qin, G.; Xu, Y. Induction of defense responses against Alternaria rot by different elicitors in harvested pear fruit. Appl. Microbiol. Biotechnol. 2006, 70, 729–734. [Google Scholar] [CrossRef]
- Pareek, S. Novel postharvest treatments of fresh produce. Innov. Postharvest. Technol. 2017, 68, 35–51. [Google Scholar]
- Sakouhi, L.; Kharbech, O.; Massoud, M.B.; Munemasa, S.; Murata, Y.; Chaoui, A. Oxalic acid mitigates cadmium toxicity in Cicer arietinum L. germinating seeds by maintaining the cellular redox homeostasis. J. Plant. Growth. Regul. 2022, 41, 697–709. [Google Scholar] [CrossRef]
- Srivastava, L.M. Gibberellins. In Plant Growth and Development; Srivastava, L.M., Ed.; Academic Press: New York, NY, USA, 2022; pp. 172–181. [Google Scholar]
- Siebert, J.D.; Stewart, A.M. Influence of plant density on cotton response to mepiquat chloride application. Agron. J. 2006, 98, 1634–1639. [Google Scholar] [CrossRef]
- Mahdavian, K.; Ghorbanli, M.; Kalantari, K.M. Role of salicylic acid in regulating ultraviolet radiation ınduced oxidative stress in pepper leaves. Russ. J. Plant Physiol. 2008, 55, 560–563. [Google Scholar] [CrossRef]
- Tong, R.; Zhou, B.; Cao, Y.; Ge, X.; Jiang, L. Metabolic profiles of moso bamboo in response to drought stress in a field investigation. Sci. Total Environ. 2020, 720, 137722. [Google Scholar] [CrossRef]
- Lehner, A.; Meimoun, P.; Errakhi, R.; Madiona, K.; Barakate, M.; Bouteau, F. Toxic and signaling effects of oxalic acid: Natural born killer or natural born protector? Plant Signal. Behav. 2008, 3, 746–748. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Lai, T.; Qin, G.; Tian, S. Response of jujube fruits to exogenous oxalic acid treatment based on proteomic analysis. Plant Cell Physiol. 2009, 50, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Hwang, B.K. An important role of the pepper phenylalanine ammonia-lyase gene (PAL1) in salicylic acid-dependent signalling of the defence response to microbial pathogens. J. Exp. Bot. 2014, 65, 2295–2306. [Google Scholar] [CrossRef]
- Zhang, Q.C.; Deng, X.X.; Wang, J.G. The effects of mepiquat chloride (DPC) on the soluble protein content and the activities of protective enzymes in cotton in response to aphid feeding and on the activities of detoxifying enzymes in aphids. BMC Plant Biol. 2022, 22, 213. [Google Scholar] [CrossRef] [PubMed]
- Bahrabadi, E.; Tavakkol Afshari, R.; Mahallati, M.N.; Seyyedi, S.M. Abscisic, gibberellic, and salicylic acids effects on germination indices of corn under salinity and drought stresses. J. Crop Improv. 2022, 36, 73–89. [Google Scholar] [CrossRef]
- Zerpa-Catanho, D.; Hernández-Pridybailo, A.; Madrigal-Ortiz, V.; Zúñiga-Centeno, A.; Porras-Martínez, C.; Jiménez, V.M.; Barboza-Barquero, L. Seed germination of pitaya (Hylocereus spp.) as affected by seed extraction method, storage, germination conditions, germination assessment approach and water potential. J. Crop Improv. 2019, 33, 372–394. [Google Scholar] [CrossRef]
- ISTA. International Rules for Seed Testing; International Seed Testing Association: Wallisellen, Switzerland, 2003. [Google Scholar]
- Ergin, N.; Kulan, E.; Gözükara, M.; Kaya, M.; Çetin, Ş.; Kaya, M.D. Response of germination and seedling development of cotton to salinity under optimal and suboptimal temperatures. Kahramanmaraş Sütçü İmam Üniversitesi Tarım Doğa Derg. 2021, 24, 108–115. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis: Pearson New International Edition; Pearson Higher, Ed.; Pearson Higher: Harlow, UK, 2013. [Google Scholar]
- Demir, I.; Kuzucu, C.O.; Ermis, S.; Öktem, G. Radicle emergence as seed vigour test estimates seedling quality of hybrid cucumber (Cucumis sativus L.) cultivars in low temperature and salt stress conditions. Horticulturae 2022, 9, 3. [Google Scholar] [CrossRef]
- El-Keblawy, A.; Gairola, S.; Bhatt, A. Maternal salinity environment affects salt tolerance during germination in Anabasis setifera: A facultative desert halophyte. J. Arid. Land 2016, 8, 254–263. [Google Scholar] [CrossRef]
- Attar, Ş.H.; Gündeşli, M.A.; Urün, I.; Kafkas, S.; Kafkas, N.E.; Ercisli, S.; Ge, C.; Mlcek, J.; Adamkova, A. Nutritional analysis of red-purple and white-fleshed pitaya (Hylocereus) species. Molecules 2022, 27, 808. [Google Scholar] [CrossRef] [PubMed]
- Çolak, A.M.; Mertoğlu, K.; Alan, F.; Esatbeyoglu, T.; Bulduk, İ.; Akbel, E.; Kahramanoğlu, I. Screening of naturally grown european cranberrybush (Viburnum opulus L.) genotypes based on physico-chemical characteristics. Foods 2022, 11, 1614. [Google Scholar] [CrossRef]
- Kulan, E.G.; Arpacıoğlu, A.; Ergin, N.; Kaya, M.D. Evaluation of germination, emergence and physiological properties of sugar beet cultivars under salinity. Trak. Univ. J. Nat. Sci. 2021, 22, 263–274. [Google Scholar] [CrossRef]
- Ibrahim, E.A. Seed priming to alleviate salinity stress in germinating seeds. J. Plant Physiol. 2016, 192, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.C.; Coutinho, G. Nitrogênio no desenvolvimento inicial de mudas de pitaya vermelha. Glob. Sci. Technol. 2019, 12, 32–43. [Google Scholar]
- Läuchli, A.; Grattan, S.R. Plant growth and development under salinity stress advances. In Molecular Breeding toward Drought and Salt Tolerant Crops; Jenks, M.A., Hasegawa, P.M., Jain, S.M., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 285–315. [Google Scholar]
- McNeil, S.D.; Nuccie, M.L.; Hanson, A.D. Betaines and related osmoprotectants. Targets for metabolic engineering of stress resistance. Plant Physiol. 1999, 120, 945–949. [Google Scholar] [CrossRef]
- Welbaum, G.E.; Tissaoui, T.; Bradford, K.J. Water relations of seed development and germination in muskmelon (Cucumis melo L.) III. Sensitivity of germination to water potential and abscisic acid during development. Plant. Physiol. 1999, 92, 1029–1037. [Google Scholar] [CrossRef]
- Khajeh-Hosseini, M.; Powell, A.; Bingham, I. The interaction between salinity stress and seed vigour during germination of soyabean seeds. Seed Sci. Technol. 2003, 31, 715–725. [Google Scholar] [CrossRef]
- Freire, M.H.D.C.; Sousa, G.G.D.; de Souza, M.V.; de Ceita, E.D.; Fiusa, J.N.; Leite, K.N. Emergence and biomass accumulation in seedlings of rice cultivars irrigated with saline water. Rev. Bras. De Eng. Agrícola E Ambient. 2018, 22, 471–475. [Google Scholar] [CrossRef]
- Kaya, M.D.; Akdoğan, G.; Kulan, E.G.; Dağhan, H.; Sari, A. Salinity tolerance classification of sunflower (Helianthus annuus L.) and safflower (Carthamus tinctorius L.) by cluster and principal component analysis. Appl. Ecol. Environ. Res. 2019, 17, 3849–3857. [Google Scholar] [CrossRef]
- Escribano, J.; Pedreño, M.A.; García-Carmona, F.; Muñoz, R. Characterization of the antiradical activity of betalains from Beta vulgaris L. roots. Phytochem. Anal. Int. J. Plant Chem. Biochem. Tech. 1998, 9, 124–127. [Google Scholar] [CrossRef]
- Tamby Chik, C.; Bachok, S.; Baba, N.; Abdullah, A.; Abdullah, N. Quality characteristics and acceptability of three types of pitaya fruits in a consumer acceptance test. J. Tour. Hosp. Culin. Arts 2011, 3, 89–98. [Google Scholar]
- Nizamlıoğlu, N.M.; Ünver, A.; Kadakal, Ç. Mineral content of pitaya (Hylocereus polyrhizus and Hylocereus undatus) seeds grown in Turkey. Erwerbs-Obstbau 2021, 63, 209–213. [Google Scholar] [CrossRef]
- Paśko, P.; Galanty, A.; Zagrodzki, P.; Ku, Y.G.; Luksirikul, P.; Weisz, M.; Gorinstein, S. Bioactivity and cytotoxicity of different species of pitaya fruits—A comparative study with advanced chemometric analysis. Food Biosci. 2021, 40, 100888. [Google Scholar] [CrossRef]
- Guirra, K.S.; Torres, S.B.; Leite, M.D.S.; Guirra, B.S.; Nogueira Neto, F.A.; Rêgo, A.L. Phytohormones on the germination and initial growth of pumpkin seedlings under different types of water. Rev. Bras. De Eng. Agrícola E Ambient. 2020, 24, 827–833. [Google Scholar] [CrossRef]
- Kerchev, P.; van der Meer, T.; Sujeeth, N.; Verlee, A.; Stevens, C.V.; Van Breusegem, F.; Gechev, T. Molecular priming as an approach to induce tolerance against abiotic and oxidative stresses in crop plants. Biotechnol. Adv. 2020, 40, 107503. [Google Scholar] [CrossRef] [PubMed]
- Rubio, J.S.; García-Sánchez, F.; Rubio, F.; García, A.L.; Martínez, V. The importance of K+ in ameliorating the negative effects of salt stress on the growth of pepper plants. Eur. J. Hortic. Sci. 2010, 75, 33–41. [Google Scholar]
- Guo, S.H.; Niu, Y.J.; Zhai, H.; Han, N.; Du, Y.P. Effects of alkaline stress on organic acid metabolism in roots of grape hybrid rootstocks. Sci. Hortic. 2018, 227, 255–260. [Google Scholar] [CrossRef]
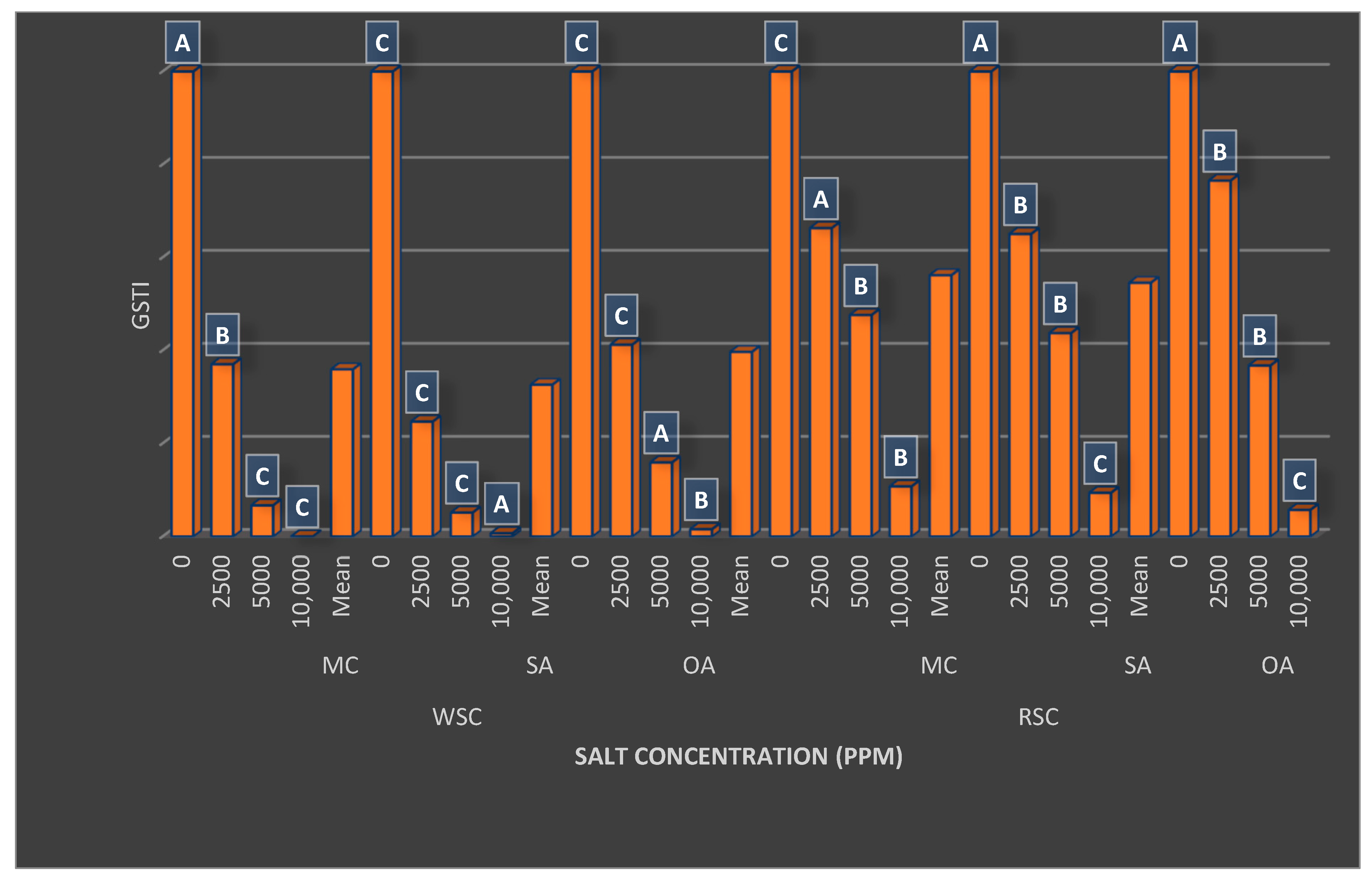
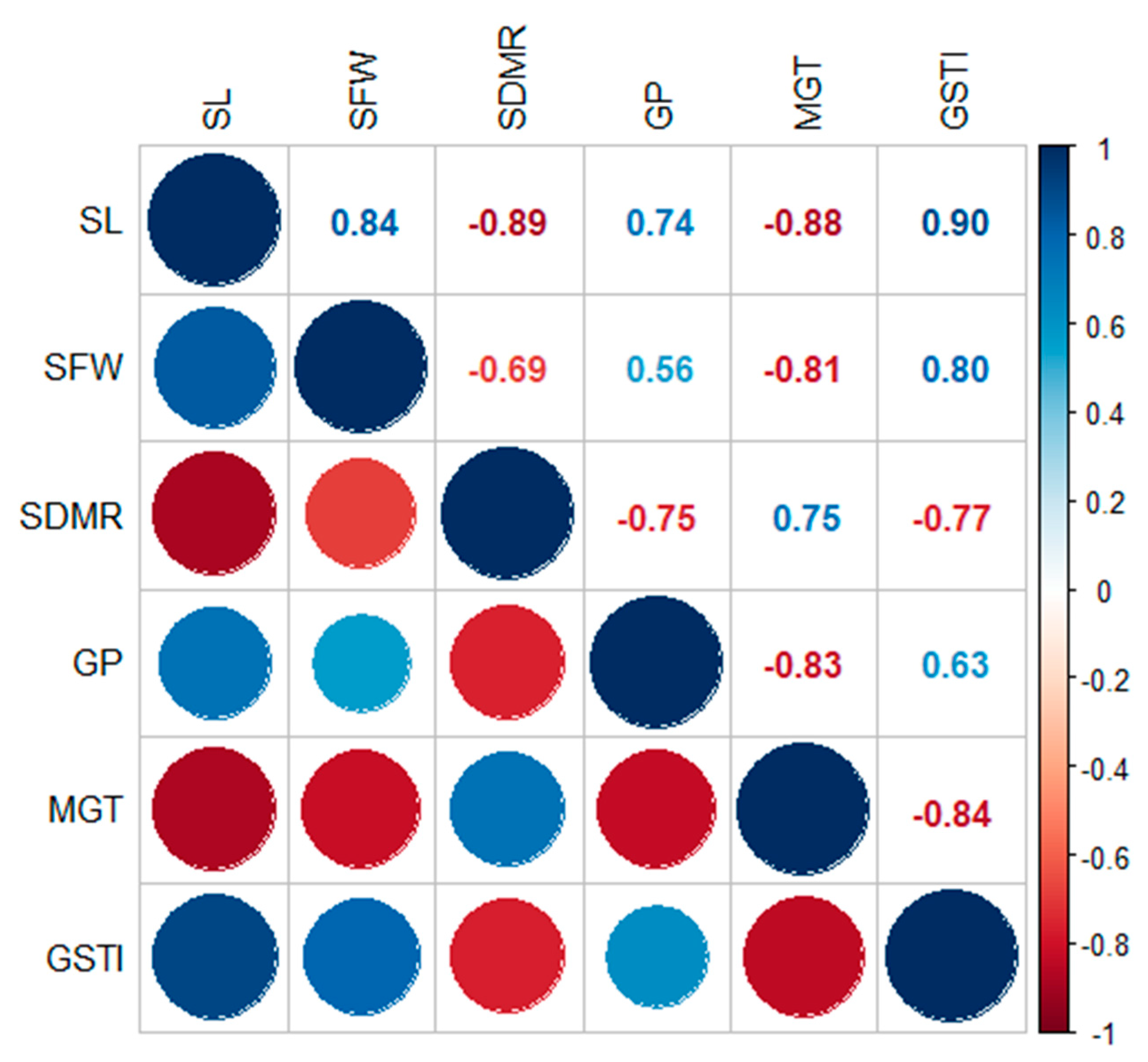
| Flesh Color (FC) | Germination (%) | MGT (Day) | Shoot Length (cm) | Fresh Seedling Weight (mg) | Seedling Dry Matter Ratio (%) | GSTI (%) |
|---|---|---|---|---|---|---|
| White | 81.17 ± 24.49 B | 11.24 ± 5.38 A | 27.90 ± 9.87 B | 12.29 ± 3.84 B | 4.44 ± 1.54 B | 36.08 ± 39.44 B |
| Red | 85.33 ± 13.21 A | 7.33 ± 3.02 B | 31.49 ± 10.69 A | 21.23 ± 7.54 A | 4.65 ± 1.99 A | 54.91 ± 34.08 A |
| Salt Concentrations (SC) | ||||||
| Control | 98.44 ± 1.27 A | 4.74 ± 0.58 D | 41.56 ± 3.01 A | 22.85 ± 6.16 A | 2.87 ± 0.15 D | 100.0 ± 0.00 A |
| 2500 ppm | 90.00 ± 6.45 B | 7.06 ± 1.40 C | 35.78 ± 3.71 B | 20.76 ± 6.65 B | 3.54 ± 0.31 C | 51.32 ± 18.66 B |
| 5000 ppm | 89.50 ± 8.23 B | 9.80 ± 2.40 B | 26.18 ± 2.65 C | 14.64 ± 4.06 C | 4.45 ± 0.41 B | 25.97 ± 17.67 C |
| 10,000 ppm | 55.06 ± 18.28 C | 15.55 ± 4.22 A | 15.26 ± 1.85 D | 8.79 ± 1.99 D | 7.33 ± 0.84 A | 4.68 ± 4.34 D |
| Plant Growth Regulators (PGRs) | ||||||
| MC | 83.54 ± 19.59 | 9.16 ± 4.78 B | 30.09 ± 10.59 | 16.96 ± 7.73 A | 4.46 ± 1.70 | 46.05 ± 37.90 B |
| SA | 82.21 ± 22.04 | 9.62 ± 5.07 A | 29.37 ± 10.69 | 16.28 ± 7.25 B | 4.58 ± 1.77 | 43.22 ± 38.44 C |
| OA | 84.00 ± 17.66 | 9.08 ± 4.52 B | 29.63 ± 10.16 | 17.04 ± 7.51 A | 4.60 ± 1.88 | 47.21 ± 38.12 A |
| ANOVA Significance levels | ||||||
| FC | ** | *** | *** | *** | * | *** |
| SC | *** | *** | *** | *** | *** | *** |
| PGR | ns | * | ns | ** | ns | *** |
| FC*SC | *** | *** | ns | *** | *** | ** |
| FC*PGR | ns | ns | ns | * | *** | *** |
| SC*PGR | * | ns | ns | ** | *** | *** |
| FC*SC*PGR | ns | ns | ns | ** | ** | *** |
| Germination Percentage (%) | |||||
| Flesh Color | Salt Concentrations | Hormones | |||
| MC | SA | OA | Mean | ||
| White | 0 | 97.67 ± 1.36 | 98.33 ± 1.63 | 98.00 ± 1.26 | 98.00 ± 1.37 A |
| 2500 | 94.67 ± 3.27 | 89.33 ± 5.46 | 93.33 ± 5.47 | 92.44 ± 5.11 A | |
| 5000 | 94.67 ± 3.26 | 92.33 ± 3.20 | 93.33 ± 4.84 | 93.44 ± 3.75 A | |
| 10,000 | 39.33 ± 6.89 | 34.27 ± 14.96 | 48.67 ± 8.55 | 40.76 ± 11.79 B | |
| Mean | 81.58 ± 25.25 | 78.57 ± 27.41 | 83.33 ± 21.20 | 81.16 ± 24.47 B | |
| Red | 0 | 98.67 ± 1.03 | 99.33 ± 1.03 | 98.67 ± 1.03 | 98.89 ± 1.02 A |
| 2500 | 83.33 ± 7.34 | 88.67 ± 6.89 | 90.67 ± 4.84 | 87.56 ± 6.84 B | |
| 5000 | 88.00 ± 5.66 | 89.33 ± 8.26 | 79.33 ± 11.98 | 85.56 ± 9.61 B | |
| 10,000 | 72.00 ± 10.43 | 66.00 ± 12.33 | 69.92 ± 10.79 | 69.31 ± 10.84 C | |
| Mean | 85.50 ± 11.75 | 85.83 ± 14.62 | 84.65 ± 13.66 | 85.33 ± 13.22 A | |
| Means of Flesh Colors | 0 | 98.17 ± 1.27 A | 98.83 ± 1.40 A | 98.33 ± 1.15 A | 98.44 ± 1.27 A |
| 2500 | 89.00 ± 8.02 A | 89.00 ± 5.94 A | 92.00 ± 5.12 A | 90.00 ± 6.45 B | |
| 5000 | 91.33 ± 5.61 A | 90.83 ± 6.18 A | 86.33 ± 11.37 A | 89.50 ± 8.23 B | |
| 10,000 | 55.67 ± 19.03 AB | 50.14 ± 21.10 B | 59.29 ± 14.47 A | 55.03 ± 18.28 C | |
| Mean | 83.54 ± 19.59 | 82.20 ± 22.04 | 83.99 ± 17.66 | 83.24 ± 19.71 | |
| Mean germination time (day) | |||||
| Flesh Color | Salt Concentrations | Hormones | |||
| MC | SA | OA | Mean | ||
| White | 0 | 5.31 ± 0.19 | 5.25 ± 0.19 | 5.30 ± 0.21 | 5.29 ± 0.19 D |
| 2500 | 8.20 ± 0.55 | 8.79 ± 0.56 | 7.96 ± 0.20 | 8.35 ± 0.56 C | |
| 5000 | 11.78 ± 0.72 | 12.32 ± 0.89 | 11.90 ± 1.49 | 12.00 ± 1.05 B | |
| 10,000 | 19.51 ± 1.86 | 20.10 ± 1.67 | 18.39 ± 1.34 | 19.33 ± 1.70 A | |
| Mean | 11.22 ± 5.50 | 11.61 ± 5.69 | 10.89 ± 5.12 | 11.24 ± 5.38 A | |
| Red | 0 | 4.20 ± 0.23 | 4.25 ± 0.21 | 4.15 ± 0.10 | 4.20 ± 0.18 D |
| 2500 | 5.73 ± 0.43 | 6.11 ± 0.27 | 5.46 ± 0.42 | 5.77 ± 0.46 C | |
| 5000 | 7.18 ± 0.54 | 7.54 ± 0.65 | 8.06 ± 0.54 | 7.59 ± 0.66 B | |
| 10,000 | 11.28 ± 0.57 | 12.56 ± 2.88 | 11.46 ± 1.43 | 11.77 ± 1.87 A | |
| Mean | 7.10 ± 2.72 | 7.62 ± 3.44 | 7.28 ± 2.95 | 7.33 ± 3.02 B | |
| Means of Flesh Colors | 0 | 4.75 ± 0.61 | 4.75 ± 0.56 | 4.72 ± 0.62 | 4.74 ± 0.58 D |
| 2500 | 7.02 ± 1.42 | 7.45 ± 1.46 | 6.71 ± 1.34 | 7.06 ± 1.40 C | |
| 5000 | 9.48 ± 2.48 | 9.93 ± 2.60 | 9.98 ± 2.27 | 9.78 ± 2.40 B | |
| 10,000 | 15.40 ± 4.49 | 16.33 ± 4.53 | 14.92 ± 3.85 | 15.55 ± 4.22 A | |
| Mean | 9.16 ± 4.77 B | 9.62 ± 5.07 A | 9.08 ± 4.51 B | 9.29 ± 4.77 | |
| Shoot Length (mm) | |||||
| Flesh Color | Salt Concentrations | Plant Growth Regulator | |||
| MC | SA | OA | Mean | ||
| White | 0 | 39.83 ± 3.31 | 38.83 ± 2.56 | 41.00 ± 2.00 | 39.89 ± 2.68 A |
| 2500 | 33.00 ± 1.05 | 31.92 ± 1.53 | 33.92 ± 1.20 | 32.94 ± 1.46 B | |
| 5000 | 25.00 ± 1.26 | 25.17 ± 2.06 | 24.00 ± 2.09 | 24.72 ± 1.82 C | |
| 10,000 | 14.67 ± 1.17 | 13.33 ± 1.57 | 14.17 ± 1.13 | 14.06 ± 1.35 D | |
| Mean | 28.12 ± 9.75 | 27.31 ± 9.78 | 28.27 ± 10.47 | 27.90 ± 9.87 B | |
| Red | 0 | 43.67 ± 2.50 | 43.83 ± 2.64 | 42.17 ± 1.94 | 43.22 ± 2.37 A |
| 2500 | 39.75 ± 1.44 | 39.75 ± 2.16 | 36.33 ± 3.92 | 38.61 ± 3.04 B | |
| 5000 | 29.08 ± 2.31 | 25.50 ± 2.49 | 28.33 ± 1.54 | 27.64 ± 2.57 C | |
| 10,000 | 15.75 ± 2.02 | 16.58 ± 0.74 | 17.08 ± 1.24 | 16.47 ± 1.46 D | |
| Mean | 32.06 ± 11.23 | 31.42 ± 11.35 | 30.98 ± 9.87 | 31.48 ± 10.69 A | |
| Means of Flesh Colors | 0 | 41.75 ± 3.44 | 41.33 ± 3.60 | 41.58 ± 1.97 | 41.56 ± 3.01 A |
| 2500 | 36.37 ± 3.72 | 35.83 ± 4.46 | 35.12 ± 3.04 | 35.78 ± 3.71 B | |
| 5000 | 27.04 ± 2.78 | 25.33 ± 2.19 | 26.17 ± 2.86 | 26.18 ± 2.65 C | |
| 10,000 | 15.21 ± 1.67 | 14.96 ± 2.06 | 15.62 ± 1.89 | 15.26 ± 1.85 D | |
| Mean | 30.09 ± 10.59 | 29.36 ± 10.68 | 29.62 ± 10.16 | 29.69 ± 10.41 | |
| Fresh seedling weight (mg) | |||||
| Flesh Color | Salt Concentrations | Plant Growth Regulator | |||
| MC | SA | OA | Mean | ||
| White | 0 | 16.89 ± 1.01 BC | 16.50 ± 0.95 BC | 17.06 ± 0.74 BC | 16.82 ± 0.89 A |
| 2500 | 14.71 ± 1.13 C | 14.18 ± 0.08 C | 14.48 ± 1.07 C | 14.45 ± 0.87 B | |
| 5000 | 11.39 ± 0.45 CD | 10.97 ± 1.06 CD | 10.51 ± 0.93 CD | 10.96 ± 0.89 C | |
| 10,000 | 7.07 ± 0.25 D | 6.71 ± 0.31 D | 6.97 ± 0.22 D | 6.92 ± 0.29 D | |
| Mean | 12.51 ± 3.86 A | 12.09 ± 3.81 A | 12.25 ± 3.99 A | 12.29 ± 3.83 B | |
| Red | 0 | 28.94 ± 0.55 A | 29.03 ± 0.54 A | 28.69 ± 0.40 A | 28.89 ± 0.49 A |
| 2500 | 28.28 ± 0.23 A | 25.58 ± 3.14 AB | 27.36 ± 2.41 A | 27.07 ± 2.44 B | |
| 5000 | 18.89 ± 1.41 B | 16.14 ± 1.43 B | 19.92 ± 1.38 B | 18.32 ± 2.11 C | |
| 10,000 | 9.53 ± 0.45 CD | 11.11 ± 0.09 CD | 11.31 ± 0.22 CD | 10.65 ± 0.86 D | |
| Mean | 21.41 ± 8.13 A | 20.46 ± 7.50 B | 21.82 ± 7.19 A | 21.23 ± 7.54 A | |
| Means of Flesh Colors | 0 | 22.92 ± 6.34 A | 22.76 ± 6.58 A | 22.87 ± 6.10 A | 22.85 ± 6.16 A |
| 2500 | 21.50 ± 7.13 A | 19.88 ± 6.32 B | 20.92 ± 6.96 A | 20.76 ± 6.65 B | |
| 5000 | 15.14 ± 4.04 C | 13.56 ± 2.95 BC | 15.21 ± 5.04 C | 14.64 ± 4.06 C | |
| 10,000 | 8.30 ± 1.33 D | 8.91 ± 2.31 D | 9.14 ± 2.27 D | 8.78 ± 1.99 D | |
| Mean | 16.96 ± 7.74 A | 16.28 ± 7.25 B | 17.04 ± 7.52 A | 16.76 ± 7.46 | |
| Seedling dry matter ratio (%) | |||||
| Flesh Color | Salt Concentrations | Plant Growth Regulator | |||
| MC | SA | OA | Mean | ||
| White | 0 | 2.99 ± 0.06 C | 3.01 ± 0.05 C | 2.97 ± 0.04 C | 2.99 ± 0.05 D |
| 2500 | 3.46 ± 0.15 BC | 3.66 ± 0.15 BC | 3.78 ± 0.07 BC | 3.64 ± 0.18 C | |
| 5000 | 4.51 ± 0.07 B | 4.39 ± 0.19 B | 3.90 ± 0.48 BC | 4.27 ± 0.39 B | |
| 10,000 | 7.06 ± 0.17 A | 7.37 ± 0.36 A | 6.19 ± 0.87 A | 6.88 ± 0.73 A | |
| Mean | 4.51 ± 1.61 A | 4.61 ± 1.71 A | 4.21 ± 1.31 B | 4.44 ± 1.54 B | |
| Red | 0 | 2.76 ± 0.12 C | 2.77 ± 0.13 C | 2.69 ± 0.09 C | 2.74 ± 0.11 D |
| 2500 | 3.10 ± 0.11 BC | 3.48 ± 0.35 BC | 3.76 ± 0.28 BC | 3.45 ± 0.38 C | |
| 5000 | 4.90 ± 0.30 B | 4.54 ± 0.43 B | 4.46 ± 0.08 B | 4.63 ± 0.35 B | |
| 10,000 | 8.00 ± 0.70 A | 7.43 ± 0.78 A | 7.92 ± 0.54 A | 7.78 ± 0.69 A | |
| Mean | 4.69 ± 2.15 A | 4.56 ± 1.87 A | 4.71 ± 2.02 A | 4.65 ± 1.99 A | |
| Means of Flesh Colors | 0 | 2.88 ± 0.15 B | 2.89 ± 0.16 B | 2.83 ± 0.16 B | 2.87 ± 0.15 D |
| 2500 | 3.28 ± 0.23 B | 3.57 ± 0.27 AB | 3.77 ± 0.19 AB | 3.54 ± 0.31 C | |
| 5000 | 4.71 ± 0.29 AB | 4.47 ± 0.33 AB | 4.18 ± 0.44 AB | 4.45 ± 0.41 B | |
| 10,000 | 7.53 ± 0.69 A | 7.40 ± 0.58 A | 7.06 ± 1.14 A | 7.34 ± 0.84 A | |
| Mean | 4.46 ± 1.70 | 4.58 ± 1.77 | 4.60 ± 1.88 | 4.55 ± 1.78 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kenanoğlu, B.B.; Mertoğlu, K.; Sülüşoğlu Durul, M.; Korkmaz, N.; Çolak, A.M. Maternal Environment and Priming Agents Effect Germination and Seedling Quality in Pitaya under Salt Stress. Horticulturae 2023, 9, 1170. https://doi.org/10.3390/horticulturae9111170
Kenanoğlu BB, Mertoğlu K, Sülüşoğlu Durul M, Korkmaz N, Çolak AM. Maternal Environment and Priming Agents Effect Germination and Seedling Quality in Pitaya under Salt Stress. Horticulturae. 2023; 9(11):1170. https://doi.org/10.3390/horticulturae9111170
Chicago/Turabian StyleKenanoğlu, Burcu Begüm, Kerem Mertoğlu, Melekber Sülüşoğlu Durul, Nazan Korkmaz, and Ayşen Melda Çolak. 2023. "Maternal Environment and Priming Agents Effect Germination and Seedling Quality in Pitaya under Salt Stress" Horticulturae 9, no. 11: 1170. https://doi.org/10.3390/horticulturae9111170
APA StyleKenanoğlu, B. B., Mertoğlu, K., Sülüşoğlu Durul, M., Korkmaz, N., & Çolak, A. M. (2023). Maternal Environment and Priming Agents Effect Germination and Seedling Quality in Pitaya under Salt Stress. Horticulturae, 9(11), 1170. https://doi.org/10.3390/horticulturae9111170








