Abstract
Cadmium (Cd) contamination is a growing concern, as exposure to the metal has been shown to inhibit plant growth and development. However, soil Cd pollution in China is typically mild, and thus its concentration often does not impede plant growth. On the other hand, it is unknown if increased plant growth impacts Cd uptake, movement, and accumulation. Here, we analyzed the relationship between Cd accumulation in 31 tomato cultivars and the impact on specific growth parameters in mild Cd contamination. The results showed that there are variations in the Cd distribution among the 31 tomato cultivars studied. There were higher Cd concentrations in shoots of the cultivar ‘SV3557’, whereas root Cd concentrations were the lowest. The roots of the cultivar ‘HF11’ recorded the lowest Cd content but had higher Cd content in the shoots. The Cd concentration in roots and shoots was not related to root length, plant height, and root weight. However, Cd accumulation in the shoots was markedly promoted by root length and plant height, and Cd accumulation in the roots was promoted by root weight. Subsequently, we imposed Cd on four selected tomato cultivars to ascertain their accumulation in the shoot tissues. The results revealed that, among the four tomato cultivars, Cd was highly accumulated in the leaves, followed by the stems, and the fruits (leaf > stem > fruit). When identifying significant loci associated with Cd accumulation in tomato plants, it is crucial to find a suitable indicator to assess the plant’s ability to accumulate Cd. Thus, Cd concentration in shoots can be used as a reliable proxy for evaluating tomato plants’ capacity for Cd accumulation. This study serves as a valuable reference in guiding the selection of such an index.
1. Introduction
The severity of heavy metal pollution has been on the rise as a consequence of various human activities, including mining, discharge of industrial wastewater, and excessive application of pesticides and fertilizers [1,2]. Cadmium (Cd) is a common heavy metal element that is highly toxic and not essential for plant growth [3]. Cadmium frequently engages in competition with Zn2+, Fe2+, Mn2+, and other divalent metal ions for transport channels, resulting in detrimental effects such as inadequate nutrient uptake, reduced photosynthetic activity, and oxidative stress, thereby impeding plant development [4,5,6]. Furthermore, the expeditious migration and protracted half-life of Cd culminate in its bioaccumulation within organisms subsequent to its introduction into the ecological food web, thereby causing a gradual onset of chronic toxicity in humans [7]. Although the impact of Cd concentration on plant growth and development is minimal, it is important to note that the accumulation of Cd in edible plant parts may surpass the optimal safety thresholds [8]. Based on the findings of a survey [9], it has been determined that the prevalence of soil Cd pollution in China stands at approximately 7%. Within this percentage, soil that is categorized as slightly polluted and lightly polluted constitutes 6% (for dry land with pH ≤ 7.5, the concentration of cadmium was between 0.3~0.9 mg/kg), whereas moderately and severely polluted soil represents a mere 1% [9]. Hence, it is imperative to study plants cultivated in soils mildly contaminated with Cd in order to ensure the safety of agricultural produce.
The inhibitory effect of a high concentration of Cd on the growth of tomato and other plants has been widely investigated [10,11,12]. However, it has been observed that mild Cd pollution does not impact plant growth. The root serves as the primary means by which plants uptake mineral elements, making it a crucial component of nutrient uptake. Additionally, the root functions as the initial line of defense against Cd accumulation. Cadmium stress resulted in the inhibition of root length and lateral root formation of melon and pea, as observed in studies by Chen et al. [13] and Fusconi et al. [14]. According to Kubo et al. [15], wheat cultivars exhibiting high Cd accumulation demonstrate substantial Cd distribution in roots compared to cultivars with low Cd accumulation. Hence, this study employed a total of 31 distinct tomato cultivars characterized by varying root lengths and root weights. The objective was to investigate the potential influence of root growth, specifically under low Cd concentration, processes of Cd absorption, transport, and accumulation. Similarly, overabundance of Cd has been found to impede both shoot biomass and plant height in ornamental plants, as demonstrated by Liu et al. [16] and Wu et al. [17]. It is noteworthy that studies in wheat and Leptoplax emarginata have found that the transpiration rate affects plant height and shoot biomass, subsequently influencing the absorption of Cd [18,19]. This study aimed to examine the potential correlation between plant height and Cd accumulation in a sample of 31 tomato cultivars, and also to determine whether plants could influence Cd accumulation through physiological processes such as transpiration rate or photosynthesis.
The tomato (Solanum lycopersicum) is an economically valuable crop that is cultivated globally [20,21]. The tomato plant has a shorter growth cycle, a readily observable growth phenotype, and a comparatively compact genome [22,23]. The tomato plant, belonging to the Solanaceae family, holds significance not only as a food security vegetable crop but also as a prominent model plant for biological studies [22,24]. The distribution of Cd in tissues varies among different species or cultivars, as reported by An et al. [25], Hu et al. [26], and Wang et al. [27]. To the best of our knowledge, there is limited research examining potential variations in the distribution of Cd within different tomato cultivars. Hence, the present study is undertaken to assess the distribution of Cd within the tissues of four distinct tomato cultivars subjected to varying concentrations of Cd. This study provides valuable insights into the key characteristics associated with the accumulation of Cd in tomato plants. Moreover, there is a lack of literature regarding the potential impact of plant growth on the processes of absorption, transport, and accumulation of Cd in soils with low Cd concentrations. This will enable us to more accurately assess the capacity of tomato plants to accumulate Cd. As an illustration, in cloning major loci associated with Cd accumulation in tomato, the utilization of a genome-wide association study (GWAS) typically involves the examination of numerous tomato accessions, each exhibiting distinct growth potential. It is imperative to evaluate the potential impact of tomato growth on its ability to accumulate Cd, as well as determine the most suitable index that can effectively mitigate the interference caused by growth.
2. Materials and Methods
2.1. Experimental Materials and Design
Two independent experiments were conducted in a greenhouse at the Huazhong Agricultural University in Wuhan, China. In the first experiment, 31 tomato cultivars were sown in a substrate with 1 mg/kg Cd to evaluate the accumulation pattern. Regarding the risk control standard for soil contamination of agricultural land [28] (China) (GB 15618-2018) and the standard level of pollution of soils in China, the concentration of Cd in the substrate was set to a light pollution level, 1 mg/kg. Cd was added as CdCl2·2.5H2O, mixed well with the cultivation substrate, and loaded into the planting groove. Thirty-one commercially available tomato cultivars were germinated at 25 °C and sown in the planting groove, and five individual plants were retained for each cultivar (Supplementary Table S1).
In the second experiment, the tissue distribution of Cd in ‘HX’, ‘HF12′, ‘HF15′, and ‘JNBL’ was investigated. Seeds of the four cultivars were planted in a greenhouse without Cd treatment at 25 °C for one month, and the seedlings with similar growth were selected and transplanted to the substrate with Cd concentration of 0, 0.5, 1.0, 1.5, 2.0, and 4.0 mg/kg. We watered the soil regularly to keep it moist and to ensure the growth of the plants. After the plant entered the vigorous growth period, we removed all the lateral branches, leaving only the main stems. The scheme of two independent experiments was presented (Supplementary Figure S1). Cadmium-contaminated substrates and tomato were treated professionally by the department of Hazardous Waste Recovery at Huazhong Agricultural University.
2.2. Determination of Growth Parameters
Thirty-one tomato cultivars were used in this study. Seedlings were harvested and divided into shoots and roots from the end of the stem. The plant height and root length were measured. The plants were then rinsed with deionized water, quickly dried with gauze, and their fresh weight was measured. Subsequently, the samples were dried at 110 °C for 15 min and then dried to a constant weight at 75 °C. The dry weights (DW) were recorded.
2.3. Determination of Cd Concentration
Tissue samples of 31 tomato cultivars were dried, ground, and passed through a 0.149 mm mesh nylon sieve for chemical analyses. Samples weighing 0.2 g were digested with 10 mL nitric acid at a gradient of temperatures spanning 120–180 °C for 1 h using a MARS6 microwave. After digestion, the samples were diluted to 25 mL with deionized water, and the content of Cd was determined by an Agilent graphite furnace (Agilent AA 240Z), with a detection limit of 0.019 μg/L. The Cd accumulation and the translocation factor (TF) were calculated as follows:
Cd accumulation (μg/plant) = Cd concentration × dry weight per plant [29]. The translocation factor (TF) is defined as the ration of Cd concentration in shoots and Cd concentration in roots [30].
2.4. Statistical Analyses
Statistical analyses were performed using IMB-SPSS statistical software (version 26.0). Data were analyzed using a one-way analysis of variance with the least significant difference test at a 5% significance level. The polar heatmaps with dendrogram were created using Origin software (version 2022; Figure 1). The scatter plots (Figure 2, Figure 3 and Figure 4) and curve (Figure 5) were created using the GraphPad Prism software (version 8).
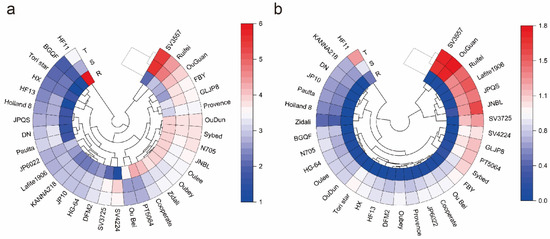
Figure 1.
Cd concentration and accumulation in 31 tomato cultivars. Cd concentration (mg/kg DW) in root, shoot, and total plant of 31 tomato cultivars (a). Cd accumulation (μg/plant) in root, shoot, and total plant of 31 tomato cultivars (b). The “R” in the figure represents root, “S” represents shoot, and ‘‘T’’ represents the total plant.

Figure 2.
Effect of root length on Cd accumulation in tomato plants. Correlation between root length and Cd concentration in roots (a), Cd accumulation in roots (b), Cd concentration in shoots (c), Cd accumulation in shoots (d), Cd concentration of whole plant (e), total Cd accumulation in plant (f), translocation factor (g), and shoot weight (h). R2, coefficient of determination. n.s, no significance.
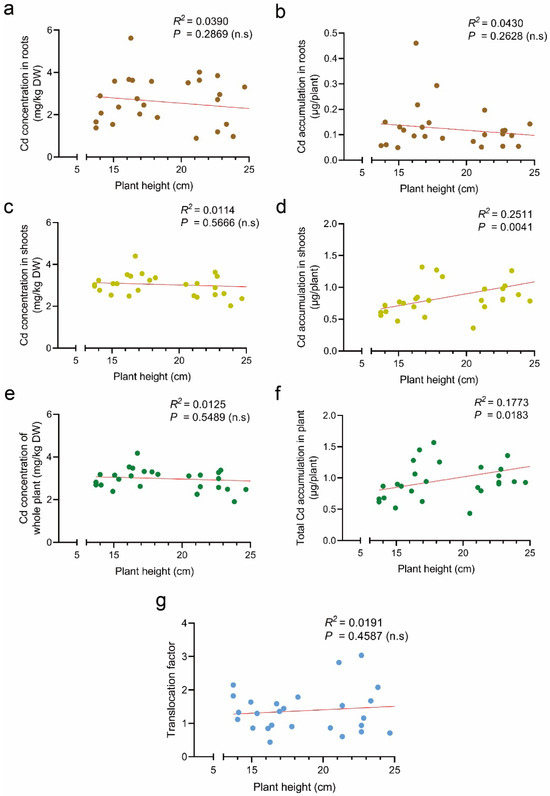
Figure 3.
Effect of plant height on Cd accumulation in tomato plants. Correlation between plant height and Cd concentration in roots (a), Cd accumulation in roots (b), Cd concentration in shoots (c), Cd accumulation in shoots (d), Cd concentration of whole plant (e), total Cd accumulation in plant (f), and translocation factor (g). R2, coefficient of determination. n.s, no significance.

Figure 4.
Effect of root weight on Cd accumulation in tomato plants. Correlation between root weight and Cd concentration in roots (a), Cd accumulation in roots (b), Cd concentration in shoots (c), Cd accumulation in shoots (d), Cd concentration of whole plant (e), total Cd accumulation in plant (f), and translocation factor (g). R2, coefficient of determination. n.s, no significance.
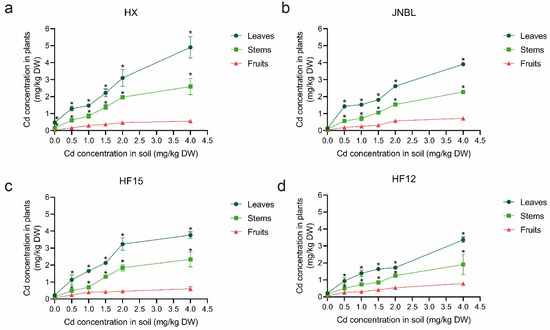
Figure 5.
Distribution of Cd in tomato tissues. Cd content in stems, leaves, and fruits of ‘HX’ (a), ‘JNBL’ (b), ‘HF15’ (c), and ‘HF12’ (d) under 0, 0.5, 1.0, 1.5, 2.0, and 4.0 mg/kg Cd treatments. ‘*’ means a significant difference with Cd concentration in fruits at the 5% significance level by one-way analysis of variance.
3. Results
3.1. Accumulation of Cd in Tomato
The distribution of Cd in different tomato cultivars was found to be different. For instance, the cultivar ‘SV3557′ recorded the highest Cd concentration in the shoot but the lowest Cd concentration in the root. The cultivar ‘HF11’ had a low Cd concentration in the shoot but higher Cd concentration in the root. Additionally, the Cd concentration in the shoots and roots of the cultivars ‘OuDun’, ‘Sybed’, ‘JP6022′ and most of the cultivars was similar (Figure 1a). To facilitate a more comprehensive assessment of Cd accumulation capacity of the 31 tomato cultivars, we computed the Cd accumulation per plant in the tomato cultivars. It is noteworthy that the roots of tomato cultivars exhibited limited capacity for Cd accumulation, a characteristic that can be attributed to their relatively low root biomass (Figure 1b).
3.2. Effect of Root Length on Cd Accumulation in Tomato
To examine the potential influence of growth phenotype of the tomato cultivars on Cd accumulation, we analyzed the relationship between these two variables (Supplementary Table S2). Initially, we observed no significant correlation between the length of roots and the concentration of Cd in the roots, shoots, and overall tomato. This finding suggests that the length of the root does not impact on Cd concentration in tomato plants (Figure 2a,c,e). Additionally, further analysis was performed on the relationship between the length of the roots and Cd accumulation in each individual tomato cultivar. It is noteworthy that no significant correlation was observed between the length of tomato roots and the Cd accumulation in the roots. However, a significant positive correlation was established between the length of tomato roots and Cd accumulation in the shoots (Figure 2b,d,f). This finding indicates that while the length of tomato roots had no significant impact on Cd concentration in tomato plants, it may contribute to an increased accumulation of Cd per plant by enhancing the biomass of tomato plants.
3.3. Effect of Plant Height on Cd Accumulation in Tomato
We investigated the correlation between tomato plant height and Cd accumulation. The results showed that there was no significant correlation between plant height and Cd concentration in the root, shoot, and overall tomato, indicating that plant height may not impact Cd concentration in tomato plants (Figure 3a,c,e). We also analyzed the correlation between plant height and Cd accumulation per tomato plant. There was no significant correlation between tomato plant height and Cd accumulation in the roots, but a significant positive correlation was found between plant height and Cd accumulation in shoots (Figure 3b,d,f). This is because plant height significantly affects the biomass of tomato and directly affects the calculation of Cd accumulation per plant.
3.4. Effect of Root Weight on Cd Accumulation in Tomato
Finally, we analyzed the correlation between tomato root weight and Cd accumulation. The results showed that there was no significant correlation between root weight and Cd concentration in root, shoot, and the whole plant of the tomato, indicating that root weight had no significant effect on Cd concentration in tomato plants (Figure 4a,c,e). We also analyzed the correlation between root weight and Cd accumulation per plant. There was no significant correlation between tomato root weight and Cd accumulation in shoots, but there was a significant positive correlation between tomato root weight and Cd accumulation in roots and the whole plant (Figure 4b,d,f). This finding demonstrates that the accumulation of Cd in tomato roots is a significant component of the overall Cd accumulation process.
3.5. Differences of Cd Accumulation in Tomato Tissues
To investigate the distribution of Cd in tomato tissues, four tomato cultivars ‘HX’, ‘HF12’, ‘HF15’, and ‘JNBL’, were planted in soils with Cd concentrations of 0, 0.5, 1.0, 1.5, 2.0, and 4.0 mg/kg. The findings of this study demonstrate a positive correlation between Cd concentration in soils and the corresponding Cd concentration in tissues of tomato plants. Furthermore, it was observed that the distribution of Cd concentration in the four tomato cultivars followed the pattern of leaf > stem > fruit (Figure 5). The present findings illustrate the spatial movement of Cd from the soil, through the root, and ultimately reaching the shoot. This information contributes to a deeper understanding of the intricate dynamics between Cd and plants.
4. Discussion
Differential accumulation of Cd was observed among different crop cultivars [26,31]. In this study, we investigated the root length, root weight, plant height, and other growth traits of 31 tomato cultivars, as well as their capacity for Cd accumulation in lightly polluted soil (Figure 1 and Table S1). We found that there were differences in the growth phenotype and Cd accumulation ability of the 31 tomato cultivars. The Cd concentration in the ‘HF11’ cultivar was high in roots, but low in shoots, whereas in the ‘SV3557’ cultivar, it was low in roots and high in shoots. This therefore suggests that Cd concentration in the roots may not accurately represent the Cd absorption capacity of plants. Once more, the findings suggest the presence of certain genes that may be selectively activated in vascular bundles to govern the distribution of Cd across different tissues. This disparity in Cd accumulation among the 31 cultivars examined in this study can likely be attributed to this underlying genetic regulation. In a recent study by Szwalec et al. [32], the accumulation of heavy metals in plants, specifically European Aspen and silver birch, was predominantly observed in the roots. However, the findings of this study indicate that the majority of the Cd accumulation was in the shoot, while the roots exhibited a minimal absolute accumulation of Cd (Figure 1b). Our study suggests that the primary function of the roots is to absorb and transport Cd, rather than serving as a storage site for this element. The observed disparity between our study and previous studies may potentially be attributed to genetic variations in the study materials (tomato).
As previously established [33,34,35], in environments with high Cd concentrations, reactive oxygen species (ROS) impede plant root growth. Conversely, in environments with low Cd concentrations, the root length of plants is typically unaffected [33,34,35]. Nevertheless, the relationship between plant root length and Cd accumulation in environments with low Cd concentration remains uncertain. The current study found no significant correlation between the length of tomato roots and Cd concentration in both the root and shoot (Figure 2a,c,e). It is striking that a notable positive correlation was observed between root length and Cd accumulation in the shoots of tomato plants. However, no significant correlation was found between root length and Cd accumulation in the roots of tomato plants (Figure 2b,d,f). Previous studies indicate a correlation between longer root length and enhanced Cd absorption, resulting in higher total Cd accumulation in plants [36,37]. It is noteworthy that a direct correlation exists between the accumulation of Cd and biomass. Therefore, we analyzed the correlation between root length and shoot biomass. The findings indicated a significant positive association between root length and shoot biomass (Figure 2h). Hence, it is possible to argue that the assertion that increased root length directly facilitates the uptake of Cd may not be entirely accurate. An increased root length in plants may lead to a corresponding increase in biomass, thereby resulting in higher overall levels of Cd accumulation. Ultimately, root length did not exhibit any significant alteration in response to varying concentrations of Cd in tomato plants.
Cadmium stress can inhibit plant height and biomass. However, the concentration of cadmium stress varies for different species, and even the appropriate concentration of cadmium treatment can promote plant height and biomass [38,39,40,41]. The inhibition of cadmium stress on plant growth is serious. However, there are few studies on whether plant height and biomass affect cadmium uptake in low-cadmium environments that do not affect plant growth. In this study of 31 tomato cultivars treated with mild cadmium pollution, we found that a different plant height and root weight did not cause the difference in cadmium concentration in tomato plants (Figure 3a,c,e and Figure 4,a,c,e). Plant height and root weight were significantly correlated with cadmium accumulation per tomato plant, which was caused by the calculation formula (Figure 3d,f and Figure 4b,f).
Cadmium is absorbed, transported, and distributed from soil to plants. Species vary in their absorptive capacities and the distribution of Cd in different tissues. Wang et al. [27] found that the concentration of Cd in apple organs following Cd treatment ranked in the order of root > leaf > stem. An et al. [25] demonstrated that Cd distribution in maize plants following Cd treatment exhibited a pattern of leaf > stem > fruit. The concentration of Cd in the feeder roots of sweet potato plants was higher compared to other tissues, while the concentration of Cd in the leaf was lower than in the stem [42].Given that the plant growth and development is genetically and integrally controlled [43], this study aimed to investigate potential variations in the distribution of Cd among different tomato cultivars. To achieve this, four cultivars were selected for analysis to determine their respective Cd accumulation capacities. The study found that the concentration of Cd in the stems, leaves, and fruits of the four tomato cultivars increased as the treatment concentration increased. Additionally, the distribution of Cd in the four tomato cultivars followed the pattern of leaf > stem > fruit (Figure 5). In contrast to a study on pepper by Hu et al. [26], their findings indicate notable variations in the distribution of Cd among different pepper varieties. Specifically, the Cd distribution pattern in the pepper variety ‘Luojiao 318’ was observed to be fruit > leaf > stem. The genetic background of the four tested tomato cultivars may not differ significantly, resulting in similar distribution of Cd. It is noteworthy that the Cd concentration in tomato fruits by fresh weight is much lower than the maximum permissible concentration of 0.05 mg/kg permitted in China.
5. Conclusions
This study revealed variations in the Cd accumulation capacity among different tomato cultivars. The study found that tomato roots primarily functioned in the absorption and transportation of Cd, rather than storage. The growth phenotypes, including root length, plant height, and root weight, did not have an impact on the concentration of Cd in tomato tissues. This implies that the Cd accumulation ability of tomato can be objectively assessed based on Cd concentration. The concentration of Cd in tomato was highest in the leaves, followed by the stems and then the fruit. This implies that leaves may serve as storage tissues for Cd, exhibiting higher Cd concentrations, while stems may act as transport tissues for Cd, displaying lower Cd concentrations. The low concentration of Cd in fruits may be attributed to the dilution caused by their high-water content.
In future studies, we will assess the Cd accumulation capacity of 506 tomato accessions by measuring their Cd concentrations. Subsequently, we will employ GWAS to identify and isolate the primary loci responsible for Cd accumulation in tomato.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae9121343/s1, Figure S1: The experimental schemes; Table S1: Names and sources of 31 tomato varieties; Table S2: Growth phenotype of 31 tomato varieties.
Author Contributions
Conceptualization, Y.Z. and X.Z.; methodology, X.Z.; formal analysis, X.Z.; investigation, C.Z.; resources, Y.Z.; data curation, C.Z.; writing—original draft preparation, X.Z.; writing—review and editing, Y.Z.; supervision, Y.Z.; project administration, Y.Z.; funding acquisition, Y.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the National Key Research & Development Plan (2022YFD1200502; 2021YFD1200201); National Natural Science Foundation of China (32372696; 31991182); Wuhan Biological Breeding Major Project (2022021302024852); Funds for High Quality Development of Hubei Seed Industry (HBZY2023B004); HZAU-AGIS Cooperation Fund (SZYJY2023022); Hubei Key Research & Development Plan (2022BBA0066; 2022BBA0062); Fundamental Research Funds for the Central Universities (2662022YLPY001).
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
The authors thank Limei Zhang and Tingyan Zhang from the College of Resources & Environment of Huazhong Agricultural University for technical assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, 26. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.M.; Wang, X.Y.; Liu, X.P. Detection of heavy metal ions by ratiometric photoelectric sensor. J. Agric. Food Chem. 2022, 70, 11468–11480. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Castro-Guerrero, N.; Mendoza-Cozatl, D.G. Moving toward a precise nutrition: Preferential loading of seeds with essential nutrients over non-essential toxic elements. Front. Plant Sci. 2014, 5, 7. [Google Scholar] [CrossRef]
- Koleli, N.; Eker, S.; Cakmak, I. Effect of zinc fertilization on cadmium toxicity in durum and bread wheat grown in zinc-deficient soil. Environ. Pollut. 2004, 131, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, J.C.; Yan, C.L.; Du, D.L.; Lu, H.L. The alleviation effect of iron on cadmium phytotoxicity in mangrove A. marina. Alleviation effect of iron on cadmium phytotoxicity in for mangrove Avicennia marina (Forsk.) Vierh. Chemosphere 2019, 226, 413–420. [Google Scholar]
- Topperwien, S.; Behra, R.; Sigg, L. Competition among zinc, manganese, and cadmium uptake in the freshwater alga Scenedesmus vacuolatus. Environ. Toxicol. Chem. 2007, 26, 483–490. [Google Scholar] [CrossRef]
- Pozgajova, M.; Navratilova, A.; Kovar, M. Curative potential of substances with bioactive properties to alleviate cd toxicity: A review. Int. J. Environ. Res. Public Health. 2022, 19, 42. [Google Scholar] [CrossRef]
- Liu, J.; Su, J.Y.; Wang, J.; Song, X.; Wang, H.W. A case study: Arsenic, cadmium and copper distribution in the soil-rice system in two main rice-producing provinces in China. Sustainability 2022, 14, 14355. [Google Scholar] [CrossRef]
- The Ministry of Environmental Protection. The Ministry of Land and Resources Report on the National Soil Contamination Survey; The Ministry of Environmental Protection: Beijing, China, 2014.
- Liu, W.T.; Zhou, Q.X.; Sun, Y.B.; Liu, R. Identification of Chinese cabbage genotypes with low cadmium accumulation for food safety. Environ. Pollut. 2009, 157, 1961–1967. [Google Scholar] [CrossRef]
- Selvam, A.; Wong, J.W.C. Cadmium uptake potential of Brassica napus cocropped with Brassica parachinensis and Zea mays. J. Hazard. Mater. 2009, 167, 170–178. [Google Scholar] [CrossRef]
- Delperee, C.; Lutts, S. Growth inhibition occurs independently of cell mortality in tomato (Solanum lycopersicum) exposed to high cadmium concentrations. J. Integr. Plant Biol. 2008, 50, 300–310. [Google Scholar] [CrossRef]
- Chen, X.M.; Shi, X.Y.; Ai, Q.; Han, J.Y.; Wang, H.S.; Fu, Q.S. Transcriptomic and metabolomic analyses reveal that exogenous strigolactones alleviate the response of melon root to cadmium stress. Hortic. Plant J. 2022, 8, 637–649. [Google Scholar] [CrossRef]
- Fusconi, A.; Repetto, O.; Bona, E.; Massa, N.; Gallo, C.; Dumas-Gaudot, E.; Berta, G. Effects of cadmium on meristem activity and nucleus ploidy in roots of Pisum sativum L. cv. Frisson seedlings. Environ. Exp. Bot. 2006, 58, 253–260. [Google Scholar] [CrossRef]
- Kubo, K.; Watanabe, Y.; Matsunaka, H.; Seki, M.; Fujita, M.; Kawada, N.; Hatta, K.; Nakajima, T. Differences in cadmium accumulation and root morphology in seedlings of japanese wheat varieties with distinctive grain cadmium concentration. Plant Prod. Sci. 2011, 14, 148–155. [Google Scholar] [CrossRef]
- Liu, Z.L.; Chen, M.D.; Lin, M.S.; Chen, Q.L.; Lu, Q.X.; Yao, J.; He, X.Y. Cadmium uptake and growth responses of seven urban flowering plants: Hyperaccumulator or bioindicator? Sustainability 2022, 14, 12. [Google Scholar] [CrossRef]
- Wu, M.X.; Luo, Q.; Liu, S.L.; Zhao, Y.; Long, Y.; Pan, Y.Z. Screening ornamental plants to identify potential Cd hyperaccumulators for bioremediation. Ecotoxicol. Environ. Saf. 2018, 162, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Bartoli, F.; Coinchelin, D.; Robin, C.; Echevarria, G. Impact of active transport and transpiration on nickel and cadmium accumulation in the leaves of the Ni-hyperaccumulator Leptoplax emarginata: A biophysical approach. Plant Soil 2012, 350, 99–115. [Google Scholar] [CrossRef]
- Van der Vliet, L.; Peterson, C.; Hale, B. Cd accumulation in roots and shoots of durum wheat: The roles of transpiration rate and apoplastic bypass. J. Exp. Bot. 2007, 58, 2939–2947. [Google Scholar] [CrossRef]
- Quinet, M.; Angosto, T.; Yuste-Lisbona, F.J.; Blanchard-Gros, R.; Bigot, S.; Martinez, J.P.; Lutts, S. Tomato fruit development and metabolism. Front. Plant Sci. 2019, 10, 23. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, X.C.; Jiang, J.B.; Zhao, T.T.; Xu, X.Y.; Yang, H.H.; Li, J.F. Virus-induced gene silencing of SlPYL4 decreases the drought tolerance of tomato. Hortic. Plant J. 2022, 8, 361–368. [Google Scholar] [CrossRef]
- Tomato Genome Consortium. The tomato genome sequence provides insights into fleshy fruit evolution. Nature 2012, 485, 635–641. [Google Scholar] [CrossRef]
- Zhou, Z.; Yuan, Y.Q.; Wang, K.T.; Wang, H.J.; Huang, J.Q.; Yu, H.; Cui, X. Rootstock-scion interactions affect fruit flavor in grafted tomato. Hortic. Plant J. 2022, 8, 499–510. [Google Scholar] [CrossRef]
- Zhao, T.T.; Pei, T.; Jiang, J.B.; Yang, H.H.; Zhang, H.; Li, J.F.; Xu, X.Y. Understanding the mechanisms of resistance to tomato leaf mold: A review. Hortic. Plant J. 2022, 8, 667–675. [Google Scholar] [CrossRef]
- An, L.Y.; Pan, Y.H.; Wang, Z.B.; Zhu, C. Heavy metal absorption status of five plant species in monoculture and intercropping. Plant Soil 2011, 345, 237–245. [Google Scholar] [CrossRef]
- Hu, X.T.; Li, T.; Xu, W.H.; Chai, Y.R. Distribution of cadmium in subcellular fraction and expression difference of its transport genes among three cultivars of pepper. Ecotoxicol. Environ. Saf. 2021, 216, 10. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, D.; Niu, D.S.; Deng, J.; Ma, F.W.; Liu, C.H. Overexpression of auxin response gene MdIAA24 enhanced cadmium tolerance in apple (Malus domestica). Ecotoxicol. Environ. Saf. 2021, 225, 8. [Google Scholar] [CrossRef]
- Ministry of Ecology and Environment of the People’s Republic of China. Soil Environmental Quality—Risk Control Standard for Soil Contamination of Agricultural Land; Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2018.
- Zhang, X.F.; Xia, H.P.; Li, Z.A.; Zhuang, P.; Gao, B. Potential of four forage grasses in remediation of Cd and Zn contaminated soils. Bioresour. Technol. 2010, 101, 2063–2066. [Google Scholar] [CrossRef]
- Rastmanesh, F.; Moore, F.; Keshavarzi, B. Speciation and phytoavailability of heavy metals in contaminated soils in sarcheshmeh area, Kerman Province, Iran. Bull. Environ. Contam. Toxicol. 2010, 85, 515–519. [Google Scholar] [CrossRef]
- Kubo, K.; Kobayashi, H.; Fujita, M.; Ota, T.; Minamiyama, Y.; Watanabe, Y.; Nakajima, T.; Shinano, T. Varietal differences in the absorption and partitioning of cadmium in common wheat (Triticum aestivum L.). Environ. Exp. Bot. 2016, 124, 79–88. [Google Scholar] [CrossRef]
- Szwalec, A.; Mundala, P.; Kedzior, R. Suitability of selected plant species for phytoremediation: A case study of a coal combustion ash landfill. Sustainability 2022, 14, 15. [Google Scholar] [CrossRef]
- Waheed, S.; Ahmad, R.; Irshad, M.; Khan, S.A.; Mahmood, Q.; Shahzad, M. Ca2SiO4 chemigation reduces cadmium localization in the subcellular leaf fractions of spinach (Spinacia oleracea L.) under cadmium stress. Ecotoxicol. Environ. Saf. 2021, 207, 10. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.T.; Wu, M.Y.; Yu, F.; Song, Q.; Zhao, Z.H.; Liao, L.; Tong, J.L. Enhanced cadmium phytoremediation capacity of poplar is associated with increased biomass and Cd accumulation under nitrogen deposition conditions. Ecotoxicol. Environ. Saf. 2022, 246, 11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.N.; Sa, G.; Zhang, Y.; Hou, S.Y.; Wu, X.; Zhao, N.; Zhang, Y.H.; Deng, S.R.; Deng, C.; Deng, J.Y.; et al. Populus euphratica annexin1 facilitates cadmium enrichment in transgenic Arabidopsis. J. Hazard. Mater. 2021, 405, 12. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.G.; Xia, S.L.; Liu, C.F.; Zhang, Z.; Shi, G.R. Variations in root morphology among 18 herbaceous species and their relationship with cadmium accumulation. Environ. Sci. Pollut. Res. 2017, 24, 4731–4740. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.Z.; Zhou, H.; Shao, L.L.; Wang, H.R.; Zhang, Y.B.; Zhu, T.; Ma, L.T.; Ding, Q.; Ma, L.J. Root characteristics critical for cadmium tolerance and reduced accumulation in wheat (Triticum aestivum L.). J. Environ. Manag. 2022, 305, 11. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.Y.; Hu, C.M.; Jia, X.W.; Ren, Y.F.; Su, D.M.; He, J.Y. Physiological and biochemical bases of spermidine-induced alleviation of cadmium and lead combined stress in rice. Plant Physiol. Biochem. 2022, 189, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.D.; Yang, H.L.; Guo, W.L.; Li, X.Z.; Chen, B.H. Defense response of pumpkin rootstock to cadmium. Sci. Hortic. 2023, 308, 11. [Google Scholar] [CrossRef]
- Wang, F.J.; Tan, H.F.; Huang, L.H.; Cai, C.; Ding, Y.F.; Bao, H.; Chen, Z.X.; Zhu, C. Application of exogenous salicylic acid reduces Cd toxicity and Cd accumulation in rice. Ecotoxicol. Environ. Saf. 2021, 207, 9. [Google Scholar] [CrossRef]
- Wu, F.B.; Dong, J.; Cai, Y.; Chen, F.; Zhang, G.P. Differences in Mn uptake and subcellular distribution in different barley genotypes as a response to Cd toxicity. Sci. Total Environ. 2007, 385, 228–234. [Google Scholar] [CrossRef]
- Zhang, D.W.; Dong, F.; Zhang, Y.; Huang, Y.L.; Zhang, C.F. Mechanisms of low cadmium accumulation in storage root of sweetpotato (Ipomoea batatas L.). J. Plant Physiol. 2020, 254, 8. [Google Scholar] [CrossRef]
- Zhang, T.Y.; Wang, Y.; Munir, S.; Wang, T.T.; Ye, Z.B.; Zhang, J.H.; Zhang, Y.Y. Cyclin gene SlCycB1;2 alters plant architecture in association with histone H3.2 in tomato. Hortic. Plant J. 2022, 8, 341–350. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).