Influence of the Phytosanitary Status, Cultivar, and Harvest Time on the Phenolic, Chlorophyll, and Alkaloid Content of Rosa sp. Leaves
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Sampling
2.2. Chemicals and Reagents
2.3. Extraction Procedures and Biochemical Determinations
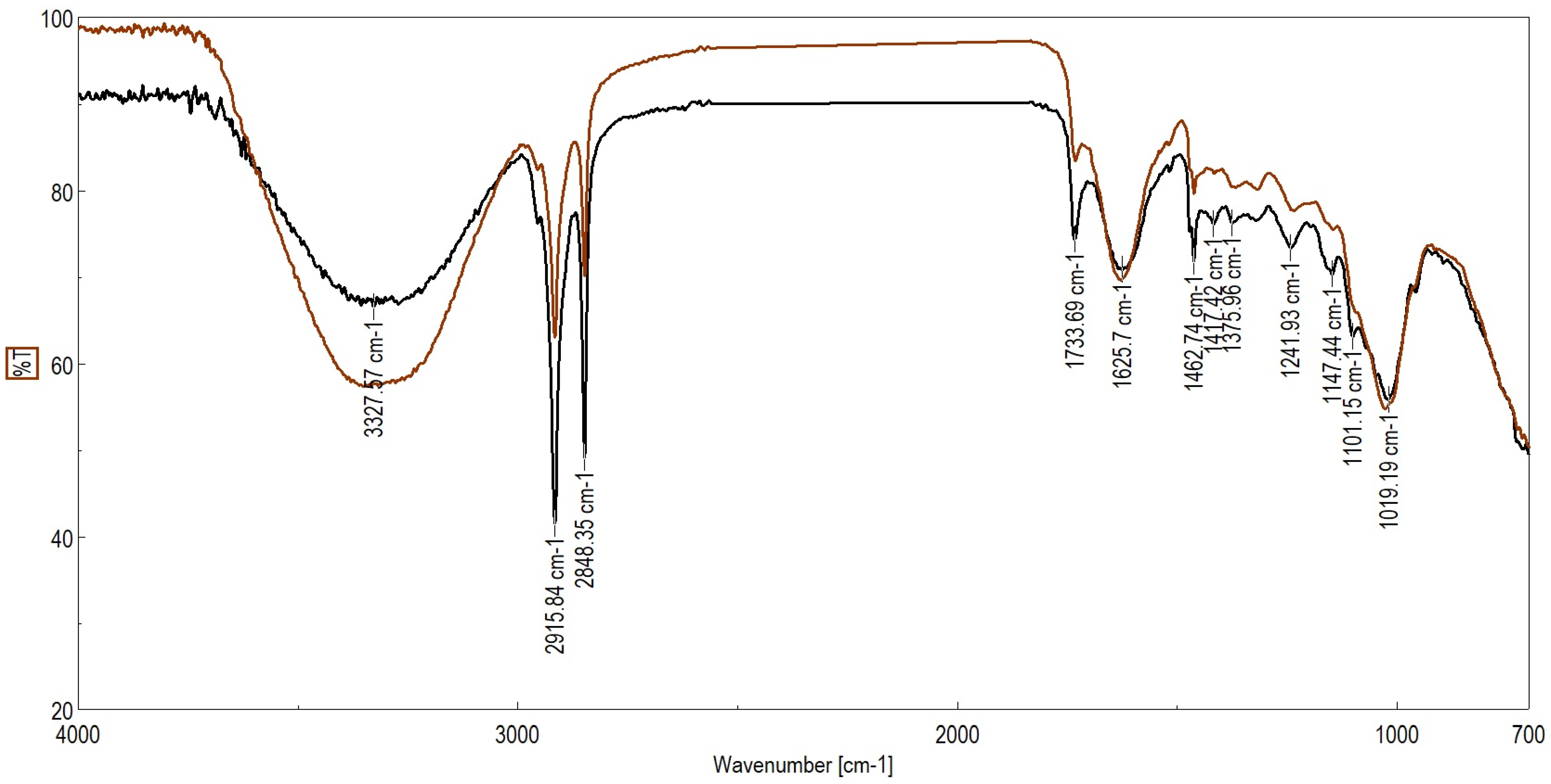
2.4. ATR-FTIR Analysis
2.5. Statistical Analysis
3. Results and Discussions
3.1. Attack Severity Analysis of Diplocarpon rosae Wolf on the Rose Leaves
3.2. Influence of the Phytosanitary Status, Cultivar, and Harvest Time on the Phenolic, Chlorophyll, and Alkaloid Content of Rose Leaves
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Khatun, S.; Bandyopadhyay, P.K.; Chatterjee, N.C. Phenols with their oxidizing enzymes in defence against black spot of rose (Rosa centifolia). Asian J. Exp. Sci. 2009, 23, 249–252. [Google Scholar]
- Zlesak, D.C.; Whitaker, V.M.; George, S.; Hokanson, S.C. Evaluation of roses from the Earth-Kind trials: Black spot (Diplocarpon rosae Wolf) resistance and ploidy. HortScience 2023, 45, 1779–1789. [Google Scholar] [CrossRef]
- Olech, M.; Pietrzak, W.; Nowak, R. Characterization of free and bound phenolic acids and flavonoid aglycones in Rosa rugosa Thunb. leaves and achenes using LC–ESI–MS/MS–MRM methods. Molecules 2020, 25, 1804. [Google Scholar] [CrossRef]
- dos Santos, A.M.P.; Silva, E.F.R.; dos Santos, W.N.L.; da Silva, E.G.P.; dos Santos, L.O.; da S. Santos, B.R.B.R.D.S.; da S. Sauthier, M.C.; dos Santos, W.P.C. Evaluation of minerals, toxic elements and bioactive compounds in rose petals (Rosa spp.) using chemometric tools and artificial neural networks. Microchem. J. 2018, 138, 98–108. [Google Scholar] [CrossRef]
- von Malek, B.; Debener, T. Genetic analysis of resistance to blackspot (Diplocarpon rosae) in tetraploid roses. Theor. Appl. Genet. 1998, 96, 228–231. [Google Scholar] [CrossRef]
- Whitaker, V.M.; Bradeen, J.M.; Debener, T.; Biber, A.; Hokanson, S.C. Rdr3, a novel locus conferring black spot disease resistance in tetraploid rose: Genetic analysis, LRR profiling, and SCAR marker development. Theor. Appl. Genet. 2010, 120, 573–585. [Google Scholar] [CrossRef]
- Drewes-Alvarez, R. Disease/black spot. In Encyclopedia of Rose Science; Roberts, A., Debener, T., Gudin, S., Eds.; Elsevier Academic Press: Amsterdam, The Netherlands, 2003; pp. 148–153. [Google Scholar]
- Debener, T. Strategies for the introduction of disease resistance genes from wild rose species into cultivated varieties. Hist. Rose J. 2000, 19, 29–33. [Google Scholar]
- Horst, R.K.; Cloyd, R.A. Rose Diseases. In Compendium of Rose Diseases and Pests, 2nd ed.; Lipa, J.J., Ed.; APS Press—The American Phytopathological Society: St. Paul, MN, USA, 2007; 83p. [Google Scholar]
- Saidulu, Y.; Tejaswini, P.; Upreti, K.K.; Sriram, S.; Seetharamu, G.K.; Devappa, V.; Mythili, J. B Biochemical characterization of defense responses in rose genotypes in response to artificial inoculation with black spot pathogen Diplocarpon rosae. J. Hortic. Sci. 2022, 17, 209–219. [Google Scholar] [CrossRef]
- Cahill, D.M.; McComb, J.A. A comparison of changes in phenylalanine ammonia-lyase activity, lignin and phenolic synthesis in the roots of Eucalyptus calophylla (field resistant) and E. marginata (susceptible) when infected with Phytophthora cinnamomi. Physiol. Mol. Plant Pathol. 1992, 40, 315–332. [Google Scholar] [CrossRef]
- Klessig, D.F.; Malamy, J. The salicylic acid signal in plants. Plant Mol Biol. 1994, 26, 1439–1458. [Google Scholar] [CrossRef]
- Munteanu, A.L.; Mitrea, R. Quantification of the attack produced by Sphaerotheca Pannosa (wallr.) lev. cousin. rosae Woron and Diplocarpon rosae Wolf in some rose varieties. Ann. Univ. Craiova Agric. Mont. Cadastre Ser. 2023, 52, 288–293. [Google Scholar] [CrossRef]
- Ami, D.; Natalello, A.; Doglia, S.M. Fourier transform infrared microspectroscopy of complex biological systems: From intact cells to whole organisms. Methods Mol. Biol. 2012, 895, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.M.; Dumas, P. Chemical imaging of biological tissue with synchrotron infrared light. Biochim. Biophys. Acta 2006, 1758, 846–857. [Google Scholar] [CrossRef]
- McCann, M.C.; Hammouri, M.; Wilson, R.; Belton, P.; Roberts, K. Fourier transform infrared microspectroscopy is a new way to look at plant cell walls. Plant Physiol. 1992, 100, 1940–1947. [Google Scholar] [CrossRef] [PubMed]
- Topală, C.M.; Tătaru, L.D.; Buciumeanu, E.C.; Guţă, I.C. FTIR spectra of grapevines (Vitis vinifera L.) in the presence of virus infections. Acta Hortic. 2017, 1188, 313. [Google Scholar] [CrossRef]
- Săvescu, A.; Rafailă, C. Prognoza în Protectia Plantelor; Ceres: București, Romania, 1978; p. 354. [Google Scholar]
- Elzinga, C.L.; Salzer, D.W.; Willoughby, J.W. Measuring and Monitoring Plant Populations; U.S. Bureau of Land Management Papers: Denver, CO, USA, 1998; pp. 61–90. [Google Scholar]
- Marin, A.M.; Butcaru, A.C.; Iacomi, B.M. Edible climbing rose diseases management in the organic system. Sci. Pap. Ser. B Hortic. 2022, LXVI, 109–114. [Google Scholar]
- Vijan, L.E.; Giura, S.; Mazilu, I.C.; Botu, M. Effect of Temperature and Storage Time on Some Biochemical Compounds from the Kernel of Some Walnut Cultivars Grown in Romania. Horticulturae 2023, 9, 544. [Google Scholar] [CrossRef]
- Giosanu, D.; Bărbuceanu, M.; Anghel, M.; Vîjan, L. The determination of the content of phenolic compounds from different Romanian wines using Folin-Ciocîlteu method. Curr. Trends Nat. Sci. 2018, 7, 155–159. [Google Scholar]
- Giura, S.; Botu, M.; Vulpe, M.; Vîjan, L.E.; Mitrea, R. Evolution of the polyphenols, flavonoids, and tannins content in walnut leaves and green walnut husk during growing season. Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 1264–1271. [Google Scholar] [CrossRef]
- Singh, D.K.; Srivastava, B.; Sahu, A. Spectrophotometric determination of Rauwolfia alkaloids: Estimation of reserpine in pharmaceuticals. Anal. Sci. 2004, 20, 571–573. [Google Scholar] [CrossRef]
- Vîjan, L.E.; Uleanu, F.; Vulpe, M.; Giosanu, D.; Ghiorghe, C. The effect of organic fertilizers on the biochemical parameters of tomato seedlings. Oltenia. Stud. Si Comun. Ser. Stiintele Nat. 2019, 35, 185–192. [Google Scholar]
- Yasin, N.A.; Ahmed, S.; Khan, W.U.; Ashraf, Y. Survey and pathogenicity of black spot disease of rose in Pakistan. J. Hortic. 2016, 3, 189. [Google Scholar] [CrossRef]
- Knight, C.; Wheeler, B.E.J. Evaluating the resistance of roses to blackspot. J. Phytopathol. 1978, 91, 218–229. [Google Scholar] [CrossRef]
- Dovbeshko, G.I.; Gridina, N.Y.; Kruglova, E.B.; Pashchuk, O.P. FTIR spectroscopy studies of nucleic acid damage. Talanta 2000, 53, 233–346. [Google Scholar] [CrossRef]
- Dovbeshko, G.I.; Chegel, V.I.; Gridina, N.Y.; Repnytska, O.P.; Shirshov, Y.M.; Tryndiak, V.P.; Todor, I.M.; Solyanik, G.I. Surface enhanced IR absorption of nucleic acids from tumor cells: FTIR reflectance study. Biopolymers 2002, 67, 470–486. [Google Scholar] [CrossRef]
- Yang, Y.; Sulé-Suso, J.; Sockalingum, G.D.; Kegelaer, G.; Manfait, M.; El Haj, A.J. Study of tumor cell invasion by Fourier transform infrared microspectroscopy. Biopolymers 2005, 78, 311–317. [Google Scholar] [CrossRef]
- Nowak, R.; Gawlik-Dziki, U. Polyphenols of Rosa L. leaves extracts and their radical scavenging activity. Z. Naturforsch. C. J. Biosci. 2007, 62, 32–38. [Google Scholar] [CrossRef]
- Cunja, V.; Mikulic-Petkovsek, M.; Stampar, F.; Schmitzer, V. Compound identification of selected Rose species and cultivars: An insight to petal and leaf phenolic profiles. J. Amer. Soc. Hortic. Sci. 2014, 139, 157–166. [Google Scholar] [CrossRef]
- D’Angiolillo, F.; Mammano, M.M.; Fascella, G. Pigments, polyphenols and antioxidant activity of leaf extracts from four wild rose species grown in Sicily. Not. Bot. Horti Agrobot. Cluj-Napoca 2018, 46, 402–409. [Google Scholar] [CrossRef]
- Ozsoy, N.; Kultur, S.; Melikoglu, G.; Can, A. Screening of the antioxidant potential of the leaves and flowers from Rosa horrida Fischer. J. Med. Plants Res. 2013, 7, 573–578. [Google Scholar]
- Sparinska, A.; Rostoks, N. Comparing ornamental and other quality traits of Rosa rugosa hybrids in Latvia. Acta Hortic. 2012, 953, 277–283. [Google Scholar] [CrossRef]
- Taha, H.S.; El-Bahr, M.K.; Seif-El-Nasr, M.M. In vitro studies on Egyptian Catharanthus roseus (L.). Ii. Effect of biotic and abiotic stress on indole alkaloids production. J. Appl. Sci. Res. 2009, 5, 1826–1831. [Google Scholar]
- Amiri, S.; Fotovat, R.; Panahi, B.; Tarinezhad, A.; Mohammadi, S.A. Review of abiotic and biotic elicitors’ roles in secondary metabolites biosynthesis of periwinkle (Catharanthus roseus (Linn.) G. Don). J. Med. Plants 2020, 19, 1–24. [Google Scholar] [CrossRef]
- Höft, M.; Verpoorte, R.; Beck, E. Growth and alkaloid contents in leaves of Tabernaemontana pachysiphon Stapf (Apocynaceae) as influenced by light intensity, water and nutrient supply. Oecologia 1996, 107, 160–169. [Google Scholar] [CrossRef]
- Almagro, L.; Fernández-Pérez, F.; Pedreño, M.A. Indole alkaloids from Catharanthus roseus: Bioproduction and their effect on human health. Molecules 2015, 20, 2973–3000. [Google Scholar] [CrossRef]
- Hanafy, M.S.; Matter, M.A.; Asker, M.S.; Rady, M.R. Production of indole alkaloids in hairy root cultures of Catharanthus roseus L. and their antimicrobial activity. S. Afr. J. Bot. 2016, 105, 9–18. [Google Scholar] [CrossRef]
- Monnerat, C.S.; Freitas, M.S.M.; Vieira, I.J.C.; Martins, M.A.; de Carvalho, A.J.C.; dos Santos, P.C.; Lima, T.C. Ajmalicine bioproduction in Catharanthus roseus (L) G. don inoculated with arbuscular Mycorrhiza and fertilized with Nitrogen. Rev. Bras. Ciênc. Solo 2018, 42, e0170057. [Google Scholar] [CrossRef]



| Cultivar | Phytosanitary Status | Length (cm) | Width (cm) | Attack Degree (%) | Resistance Class |
|---|---|---|---|---|---|
| ‘Orient Express’ | Unattacked | 7.2 ± 1.1 | 4.8 ± 0.7 | 30 | Very sensitive (FS) |
| Attacked | 5.7 ± 1.2 | 4.2 ± 1.1 | |||
| Diminished appropriation (%) | 20.83 | 12.5 | |||
| ‘Paul McCartney’ | Unattacked | 6.2 ± 1.2 | 4.1 ± 1.3 | 15 | Very sensitive (FS) |
| Attacked | 6.0 ± 1.6 | 3.7 ± 1.8 | |||
| Diminished appropriation (%) | 3.22 | 9.76 | |||
| ‘Terracotta’ | Unattacked | 7.6 ± 1.6 | 4.9 ± 1.2 | 20 | Very sensitive (FS) |
| Attacked | 5.2 ± 1.4 | 4.2 ± 1.4 | |||
| Diminished appropriation (%) | 31.58 | 14.28 | |||
| ‘Glorious’ | Unattacked | 5.9 ± 1.5 | 4.1 ± 0.4 | 35 | Very sensitive (FS) |
| Attacked | 5.1 ± 0.9 | 3.9 ± 1.2 | |||
| Diminished appropriation (%) | 13.55 | 4.88 | |||
| ‘Mythos’ | Unattacked | 8.20 ±1.8 | 5.1 ± 1.2 | 35 | Very sensitive (FS) |
| Attacked | 7.15 ± 1.1 | 4.3 ± 1.0 | |||
| Diminished appropriation (%) | 12.80 | 15.69 | |||
| ‘Holstein Perle’ | Unattacked | 6.6 ± 1.9 | 4.2 ± 1.3 | 20 | Very sensitive (FS) |
| Attacked | 6.0 ± 1.2 | 3.6 ± 1.1 | |||
| Diminished appropriation (%) | 9.1 | 14.28 | |||
| ‘Melina’ | Unattacked | 7.6 ± 1.3 | 6.4 ± 1.6 | 40 | Very sensitive (FS) |
| Attacked | 5.7 ± 1.2 | 4.9 ± 1.5 | |||
| Diminished appropriation (%) | 25.00 | 23.43 | |||
| ‘Asja’ | Unattacked | 6.2 ± 1.6 | 4.0 ± 1.3 | 9.2 | Sensitive (S) |
| Attacked | 3.7 ± 1.8 | 2.9 ± 1.8 | |||
| Diminished appropriation (%) | 40.32 | 27.5 | |||
| ‘Velvet Fragrance’ | Unattacked | 5.2 ± 1.6 | 3.2 ± 1.3 | 30 | Very sensitive (FS) |
| Attacked | 4.1 ± 1.6 | 2.6 ± 0.9 | |||
| Diminished appropriation (%) | 21.15 | 18.75 | |||
| ‘Cluj 2010′ | Unattacked | 4.5 ± 0.5 | 3.1 ± 0.5 | 40 | Very sensitive (FS) |
| Attacked | 4.2 ± 0.9 | 2.5 ± 0.7 | |||
| Diminished appropriation (%) | 6.67 | 19.34 |
| Frequencies (cm−1) | ||
|---|---|---|
| Healthy Leaves | Diseased Leaves | Peak Assignment |
| 3290 | 3292 | νO–H, N–H |
| 2915 | 2915 | νasym C–H |
| 2848 | 2848 | νsym C–H |
| 1731 | 1726 | νC=O stretching due to lipids |
| 1626 | 1625 | νC=O, Amide I, stretching of protein |
| 1462 | 1462 | δasym CH2 |
| 1369 | 1376 | Deformation N–H, δ CH2 |
| 1315 | 1321 | νsym COO− stretching mainly from pectin |
| 1242 | 1235 | ν C–O from carbohydrates and lignin |
| 1146 | 1152 | νasym O–C–O stretching of polysaccharides |
| 1102 | 1100 | Stretching PO2− symmetric (phosphate II) |
| 1026 | 1029 | νC–O stretching vibration coupled with C–O bending of the C–OH groups of carbohydrates |
| 687 | 685 | CH out-of-plane bending vibrations |
| Frequencies (cm−1) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| ‘Orient Express’ | ‘Paul McCartney’ | ‘Terracotta’ | ‘Glorious’ | ‘Mythos’ | ‘Melina’ | ‘Asja’ | ‘Velvet Fragrance’ | ‘Cluj 2010′ | Peak Assignment |
| 3353 3362 | 3359 3344 | 3329 3317 | 3316 3334 | 3312 3307 | 3329 3318 | 3327 3334 | 3326 3325 | 3290 3291 | Stretching O–H, N–H, C–H, N–H asymmetric (asym) [28] |
| 2915 2915 | 2915 2915 | 2916 2915 | 2915 2915 | 2916 2915 | 2915 2915 | 2915 2915 | 2915 2916 | 2915 2915 | νasym C–H |
| 2848 2848 | 2848 2848 | 2848 2848 | 2848 2848 | 2848 2848 | 2848 2848 | 2848 2848 | 2848 2849 | 2848 2848 | νsym C–H |
| 1732 1732 | 1732 1731 | 1731 1730 | 1731 1731 | 1726 1730 | 1731 1730 | 1733 1731 | 1732 1730 | 1731 1731 | νC=O stretching due to lipids |
| 1629 1626 | 1613 1633 | 1626 1615 | 1626 1622 | 1630 1634 | 1620 1625 | 1625 1627 | 1625 1600 | 1626 1614 | νC=O, Amide I, stretching of protein |
| 1462 1462 | 1462 1462 | 1462 1462 | 1462 1462 | 1462 1461 | 1462 1461 | 1462 1462 | 1462 1461 | 1464 1461 | δasym CH2 |
| 1375 | 1375 1375 | 1370 | 1373 | 1370 1366 | 1373 1372 | 1375 1369 | 1374 1371 | 1369 | Stretching C–N [28] Deformation N–H, δ CH2 [29] |
| 1315 1314 | 1315 1317 | 1315 1316 | 1315 1314 | 1323 1310 | 1315 1310 | 1315 1316 | 1315 1313 | 1315 1318 | Amide III band components of proteins [30] |
| 1240 1234 | 1240 1236 | 1242 1240 | 1241 1233 | 1236 1233 | 1240 1234 | 1241 1234 | 1241 1233 | 1242 1238 | νC–O from carbohydrates and lignin |
| 1167 1145 | 1165 1148 | 1159 1159 | 1166 1146 | 1160 1159 | 1147 1144 | 1147 1144 | 1147 1149 | 1146 1164 | νasymO–C–O stretching of polysaccharides |
| 1101 | 1101 | 1100 | 1102 | 1100 | 1099 | 1101 | 1099 | 1100 | Stretching PO2− symmetric (phosphate II) |
| 1028 1029 | 1021 1026 | 1030 1036 | 1021 1031 | 1030 1031 | 1025 1032 | 1019 1026 | 1029 1029 | 1026 1031 | νC–O stretching vibration coupled with C–O bending of the C–OH groups of carbohydrates |
| 718 716 | 717 716 | 718 718 | 718 717 | 714 713 | 718 716 | 718 718 | 718 718 | 718 718 | out-of-plane bending vibrations |
| Biochemical Parameters | Time | Phytosanitary Status | Cultivar | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ‘Orient Express’ | ‘Paul McCartney’ | ‘Terracotta’ | ‘Glorious’ | ‘Mythos’ | ‘Holstein Perle’ | ‘Melina’ | ‘Asja’ | ‘Velvet Fragrance’ | ‘Cluj 2010′ | |||
| TPC | 28–29 June | Healthy | 980.17 ± 0.20 | 889.20 ± 0.85 | 984.85 ± 3.19 | 975.41 ± 1.28 | 1151.75 ± 0.97 | 1020.77 ± 3.49 | 1237.65 ± 2.64 | 1147.90 ± 0.43 | 1020.98 ± 0.02 | 1090.65 ± 0.67 |
| Affected | 985.13 ± 1.28 | 929.35 ± 1.02 | 1017.98 ± 3.70 | 953.73 ± 1.89 | 1364.24 ± 4.59 | 1091.76 ± 2.73 | 1269.07 ± 5.45 | 1152.61 ± 2.30 | 1105.25 ± 7.57 | 1249.14 ± 5.11 | ||
| 12–13 July | Healthy | 959.32 ± 0.63 | 867.76 ± 0.59 | 950.73 ± 0.74 | 929.04 ± 1.38 | 998.13 ± 0.69 | 959.32 ± 0.63 | 1074.53 ± 1.66 | 1040.09 ± 0.70 | 932.78 ± 0.29 | 999.96 ± 1.53 | |
| Affected | 962.01 ± 0.54 | 887.83 ± 1.77 | 976.60 ± 0.84 | 946.53 ± 0.40 | 1141.84 ± 0.36 | 984.85 ± 3.19 | 1099.61 ± 2.58 | 1052.01 ± 3.09 | 965.71 ± 1.25 | 1076.78 ± 6.69 | ||
| 4–5 September | Healthy | 709.49 ± 1.31 | 784.79 ± 0.97 | 873.12 ± 1.10 | 829.25 ± 1.07 | 833.28 ± 0.39 | 821.04 ± 0.50 | 813.59 ± 0.73 | 908.82 ± 1.60 | 844.15 ± 0.32 | 889.59 ± 1.19 | |
| Affected | 744.83 ± 1.12 | 813.79 ± 0.62 | 886.49 ± 1.12 | 852.34 ± 0.91 | 936.28 ± 2.04 | 847.13 ± 0.61 | 823.44 ± 0.62 | 926.41 ± 1.15 | 864.89 ± 0.83 | 925.98 ± 1.74 | ||
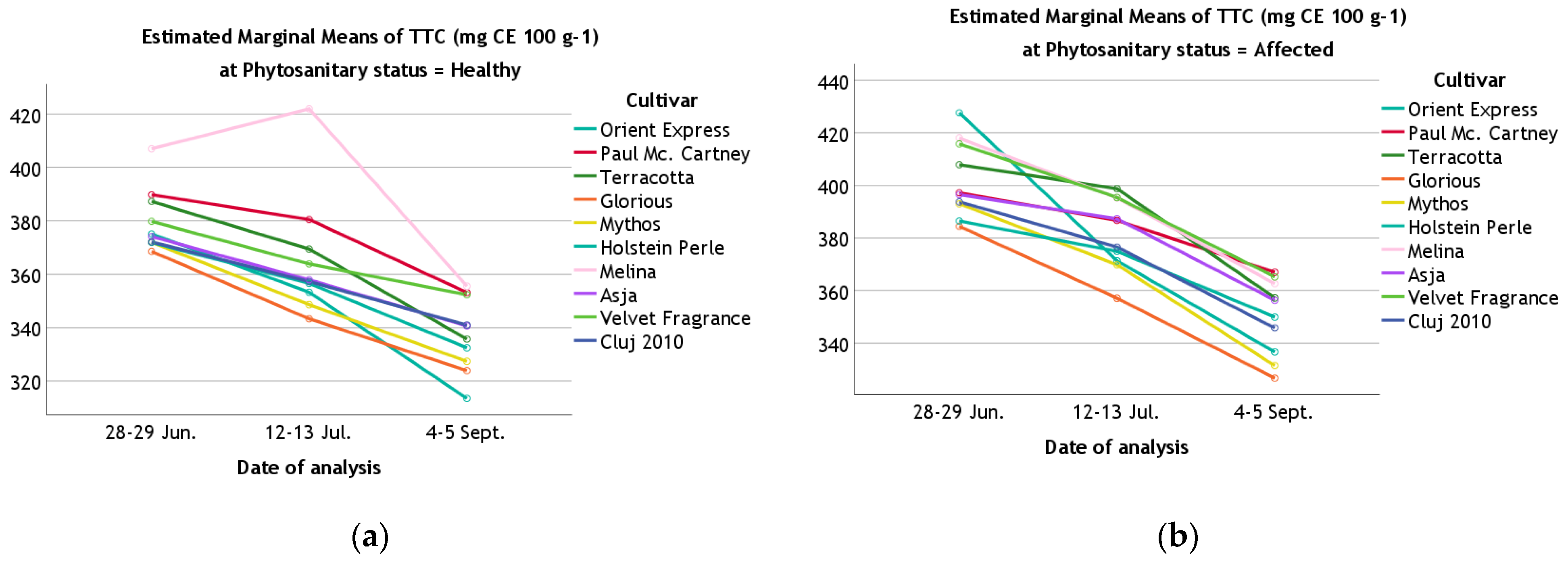
| TTC | 28–29 June | Healthy | 375.14 ± 0.11 | 389.83 ± 0.10 | 387.28 ± 0.08 | 368.57 ± 0.64 | 371.98 ± 0.54 | 371.87 ± 0.54 | 406.99 ± 0.06 | 374.14 ± 0.08 | 379.77 ± 0.48 | 372.09 ± 0.15 |
| Affected | 427.60 ± 0.08 | 397.19 ± 0.07 | 407.88 ± 0.06 | 384.42 ± 0.06 | 393.02 ± 0.16 | 386.50 ± 0.02 | 418.02 ± 0.12 | 396.49 ± 0.04 | 415.86 ± 0.14 | 393.79 ± 0.52 | ||
| 12–13 July | Healthy | 353.24 ± 0.06 | 380.49 ± 0.15 | 369.42 ± 0.57 | 343.32 ± 0.08 | 348.61 ± 0.94 | 356.58 ± 0.53 | 421.92 ± 57.71 | 357.92 ± 0.85 | 363.87 ± 0.38 | 357.25 ± 0.32 | |
| Affected | 371.35 ± 0.14 | 386.71 ± 1.09 | 398.78 ± 1.14 | 357.11 ± 0.22 | 369.82 ± 0.08 | 374.91 ± 0.58 | 395.25 ± 0.64 | 387.29 ± 0.41 | 395.42 ± 0.22 | 376.55 ± 1.23 | ||
| 4–5 September | Healthy | 313.50 ± 0.19 | 353.14 ± 0.06 | 335.72 ± 0.59 | 323.93 ± 0.16 | 327.38 ± 0.01 | 332.46 ± 0.60 | 355.53 ± 0.70 | 340.66 ± 0.60 | 352.33 ± 0.48 | 340.97 ± 0.33 | |
| Affected | 336.65 ± 0.22 | 366.99 ± 0.04 | 357.25 ± 0.11 | 326.73 ± 0.07 | 331.48 ± 0.07 | 349.94 ± 0.61 | 362.65 ± 0.56 | 356.34 ± 1.16 | 365.24 ± 0.58 | 345.79 ± 0.50 | ||
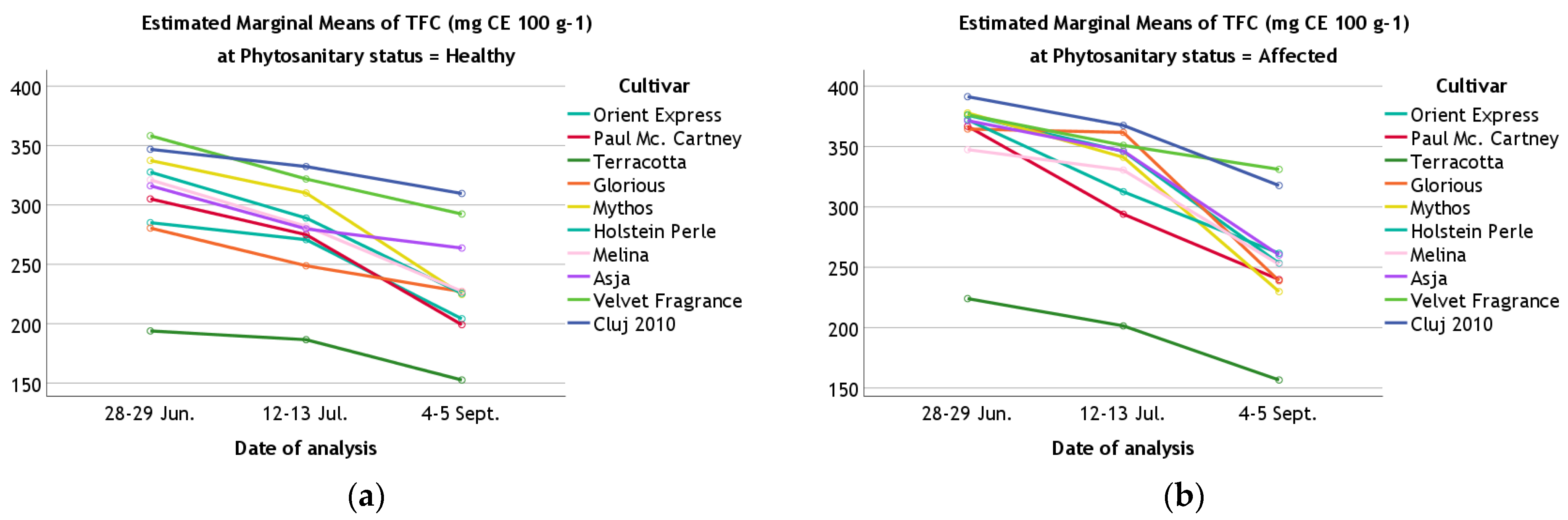
| TFC | 28–29 June | Healthy | 285.05 ± 1.78 | 305.04 ± 4.37 | 193.87 ± 3.55 | 280.47 ± 3.94 | 337.41 ± 4.07 | 327.63 ± 2.91 | 321.06 ± 1.65 | 316.09 ± 3.66 | 358.25 ± 1.45 | 346.83 ± 1.41 |
| Affected | 375.94 ± 4.19 | 366.79 ± 4.21 | 224.04 ± 3.09 | 364.49 ± 2.81 | 377.83 ± 1.92 | 372.19 ± 4.01 | 347.45 ± 3.90 | 371.46 ± 2.99 | 376.35 ± 4.97 | 391.29 ± 3.82 | ||
| 12–13 July | Healthy | 270.76 ± 3.46 | 274.95 ± 2.15 | 186.53 ± 2.89 | 248.77 ± 2.53 | 310.03 ± 2.01 | 288.95 ± 1.42 | 282.04 ± 1.49 | 279.84 ± 3.24 | 321.83 ± 1.63 | 332.31 ± 3.47 | |
| Affected | 345.99 ± 2.17 | 293.98 ± 1.49 | 201.46 ± 1.79 | 361.72 ± 3.25 | 341.12 ± 1.96 | 312.57 ± 1.45 | 330.49 ± 1.56 | 346.25 ± 4.25 | 350.96 ± 2.80 | 367.48 ± 2.17 | ||
| 4–5 September | Healthy | 204.13 ± 0.42 | 199.13 ± 1.60 | 152.55 ± 1.87 | 226.94 ± 1.62 | 224.65 ± 2.69 | 225.55 ± 1.67 | 227.34 ± 1.95 | 263.69 ± 2.35 | 292.33 ± 1.32 | 309.63 ± 1.82 | |
| Affected | 253.29 ± 1.28 | 239.77 ± 2.90 | 156.60 ± 1.82 | 238.79 ± 1.42 | 229.79 ± 1.64 | 261.57 ± 2.29 | 252.38 ± 2.43 | 260.19 ± 2.04 | 331.06 ± 4.24 | 317.72 ± 4.93 | ||
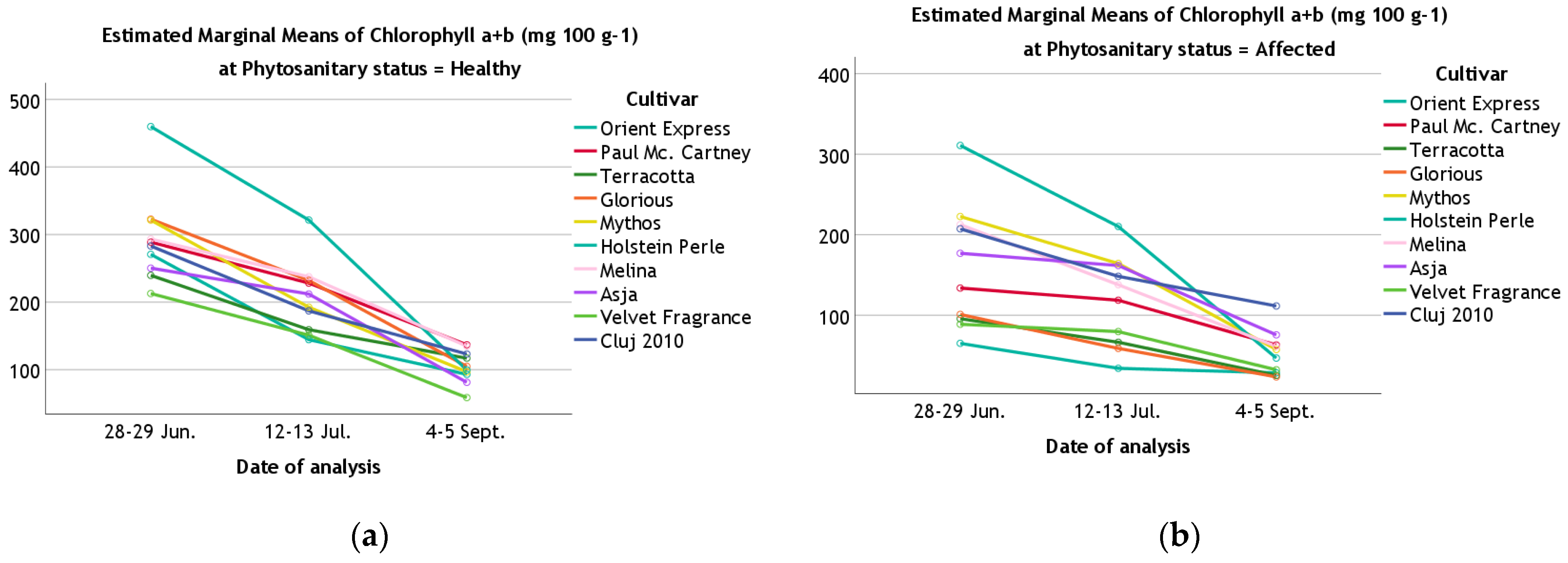
| Chlorophyll a + b | 28–29 June | Healthy | 270.69 ± 1.60 | 288.73 ± 1.24 | 239.62 ± 1.11 | 322.73 ± 1.17 | 321.40 ± 1.04 | 459.72 ± 5.80 | 293.27 ± 0.57 | 250.27 ± 0.60 | 212.73 ± 0.75 | 283.08 ± 0.89 |
| Affected | 65.06 ± 0.91 | 133.72 ± 0.19 | 95.64 ± 0.10 | 101.00 ± 0.47 | 222.61 ± 4.56 | 310.86 ± 5.34 | 212.51 ± 2.44 | 176.92 ± 0.09 | 88.78 ± 2.62 | 207.31 ± 0.48 | ||
| 12–13 July | Healthy | 144.47 ± 5.45 | 228.17 ± 0.70 | 150.24 ± 0.82 | 232.07 ± 0.41 | 192.49 ± 0.75 | 321.45 ± 0.99 | 237.49 ± 5.39 | 211.99 ± 5.24 | 151.02 ± 0.22 | 187.09 ± 5.28 | |
| Affected | 34.05 ± 0.02 | 118.63 ± 0.22 | 66.46 ± 1.00 | 58.75 ± 0.27 | 164.26 ± 5.67 | 210.20 ± 0.63 | 137.84 ± 0.43 | 161.71 ± 0.83 | 79.66 ± 1.70 | 148.30 ± 0.08 | ||
| 4–5 September | Healthy | 93.09 ± 0.67 | 136.84 ± 4.21 | 117.02 ± 4.71 | 104.80 ± 5.87 | 96.93 ± 0.57 | 99.60 ± 0.43 | 134.82 ± 0.32 | 81.30 ± 3.95 | 58.69 ± 0.20 | 123.26 ± 3.00 | |
| Affected | 28.44 ± 0.35 | 63.07 ± 2.58 | 25.31 ± 0.01 | 23.40 ± 0.11 | 57.37 ± 0.33 | 46.82 ± 0.16 | 61.35 ± 0.26 | 75.61 ± 0.70 | 32.21 ± 0.24 | 111.47 ± 4.51 | ||
| Alkaloids | 28–29 June | Healthy | 78.50 ± 0.05 | 86.41 ± 0.09 | 87.90 ± 0.11 | 79.22 ± 0.05 | 88.24 ± 0.05 | 82.90 ± 0.56 | 88.07 ± 0.02 | 75.88 ± 0.06 | 80.12 ± 0.31 | 82.02 ± 0.01 |
| Affected | 89.16 ± 0.09 | 91.45 ± 0.02 | 89.58 ± 0.04 | 80.08 ± 0.03 | 94.62 ± 0.09 | 85.97 ± 0.13 | 90.57 ± 0.07 | 78.85 ± 0.05 | 85.53 ± 0.06 | 89.42 ± 0.08 | ||
| 12–13 July | Healthy | 88.99 ± 0.05 | 91.01 ± 0.10 | 89.17 ± 0.16 | 88.02 ± 0.09 | 92.42 ± 0.08 | 89.16 ± 0.07 | 92.83 ± 0.04 | 87.83 ± 0.09 | 85.81 ± 0.02 | 86.66 ± 0.01 | |
| Affected | 92.97 ± 0.10 | 95.92 ± 0.11 | 91.17 ± 0.16 | 92.16 ± 0.04 | 92.88 ± 0.10 | 91.69 ± 0.07 | 93.55 ± 0.09 | 90.71 ± 0.09 | 91.58 ± 0.03 | 92.55 ± 0.03 | ||
| 4–5 September | Healthy | 93.31 ± 0.13 | 93.86 ± 0.15 | 97.13 ± 0.18 | 94.07 ± 0.03 | 96.21 ± 0.06 | 90.10 ± 0.09 | 94.09 ± 0.09 | 89.42 ± 0.05 | 90.79 ± 0.05 | 89.76 ± 0.11 | |
| Affected | 98.25 ± 0.13 | 96.53 ± 0.16 | 97.70 ± 0.13 | 97.82 ± 0.08 | 99.05 ± 05 | 92.17 ± 0.11 | 94.91 ± 0.09 | 97.66 ± 0.04 | 94.52 ± 0.15 | 95.28 ± 0.06 | ||
| Investigated Factor | TPC (mg GAE 100 g−1) | TFC (mg CE 100 g−1) | TFC (mg CE 100 g−1) | Chlorophyll a + b (mg 100 g−1) | Alkaloids (mg AE 100 g−1) |
|---|---|---|---|---|---|
| Cultivar | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Harvest time | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Phytosanitary status | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Cultivar x Harvest time | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Cultivar x Phytosanitary status | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Harvest time x Phytosanitary status | 0.000 | 0.002 | 0.000 | 0.000 | 0.000 |
| Cultivar x Time x Phytosanitary status | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| AD (%) | TPC (mg GAE 100 g−1) | TTC (mg GAE 100 g−1) | TFC (mg CE 100 g−1) | Chlorophyll a + b (mg 100 g−1) | Alkaloids (mg AE 100 g−1) | ||
|---|---|---|---|---|---|---|---|
| AD | Pearson correlation | 1 | −0.107 | −0.189 * | 0.073 | −0.517 *** | 0.510 *** |
| Sig. (2-tailed) | 0.154 | 0.011 | 0.327 | 0.000 | 0.000 | ||
| TPC | Pearson correlation | 1 | 0.636 *** | 0.629 *** | 0.506 *** | −0.403 *** | |
| Sig. (2-tailed) | 0.000 | 0.000 | 0.000 | 0.000 | |||
| TTC | Pearson correlation | 1 | 0.516 *** | 0.279 *** | −0.339 *** | ||
| Sig. (2-tailed) | 0.000 | 0.000 | 0.000 | ||||
| TFC | Pearson correlation | 1 | 0.280 *** | −0.409 *** | |||
| Sig. (2-tailed) | 0.000 | 0.000 | |||||
| Chlorophyll a + b | Pearson correlation | 1 | −0.604 *** 0.000 | ||||
| Sig. (2-tailed) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munteanu, A.L.; Vijan, L.E.; Topală, C.M.; Mitrea, R. Influence of the Phytosanitary Status, Cultivar, and Harvest Time on the Phenolic, Chlorophyll, and Alkaloid Content of Rosa sp. Leaves. Horticulturae 2023, 9, 1169. https://doi.org/10.3390/horticulturae9111169
Munteanu AL, Vijan LE, Topală CM, Mitrea R. Influence of the Phytosanitary Status, Cultivar, and Harvest Time on the Phenolic, Chlorophyll, and Alkaloid Content of Rosa sp. Leaves. Horticulturae. 2023; 9(11):1169. https://doi.org/10.3390/horticulturae9111169
Chicago/Turabian StyleMunteanu, Adelina Larisa, Loredana Elena Vijan, Carmen Mihaela Topală, and Rodi Mitrea. 2023. "Influence of the Phytosanitary Status, Cultivar, and Harvest Time on the Phenolic, Chlorophyll, and Alkaloid Content of Rosa sp. Leaves" Horticulturae 9, no. 11: 1169. https://doi.org/10.3390/horticulturae9111169
APA StyleMunteanu, A. L., Vijan, L. E., Topală, C. M., & Mitrea, R. (2023). Influence of the Phytosanitary Status, Cultivar, and Harvest Time on the Phenolic, Chlorophyll, and Alkaloid Content of Rosa sp. Leaves. Horticulturae, 9(11), 1169. https://doi.org/10.3390/horticulturae9111169







