Fruit Quality Parameters, Sugars, Vitamin C, Antioxidant Activity, Organic Acids, and Phenolic Compounds for a New Endemic Apple Variety, “Long Apple”
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Area
2.2. Plant Material
2.3. Reagents and Standards
2.4. Fruit Quality Parameters
2.5. Antioxidant Activity
2.6. Organic Acids, and Phenolic Compounds
2.6.1. Apparatus
2.6.2. Extraction and Analysis of Organic Acids
2.6.3. Extraction and Analysis of Phenolic Compounds in Fruit Samples
2.7. Vitamin C and Sugar Component
2.8. Statistical Analyses
3. Results and Discussion
3.1. Fruit Quality Parameters
3.2. Antioxidant Activity
3.3. Organic Acids, Sugars, and Vitamin C
3.4. Phenolic Compounds
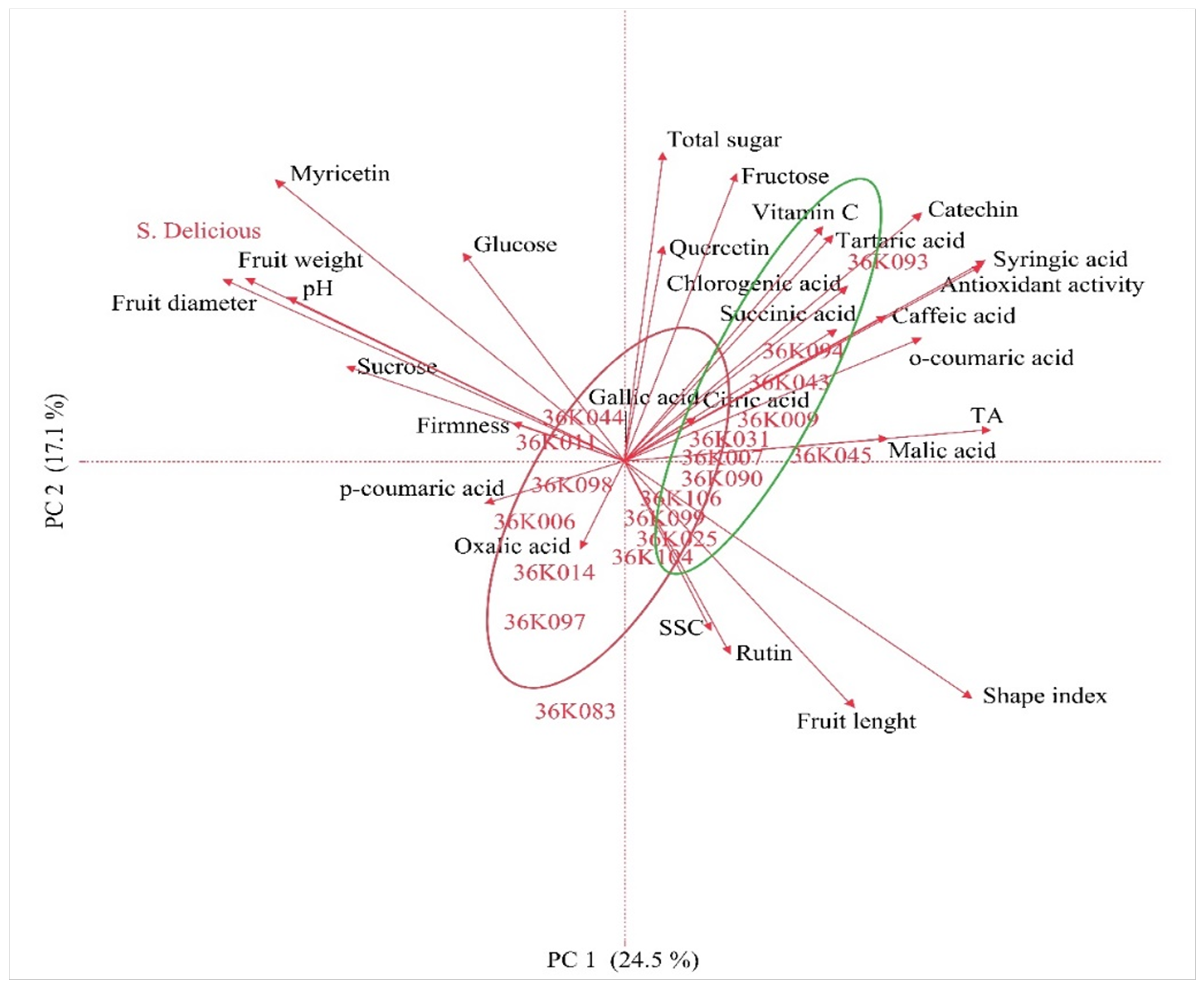
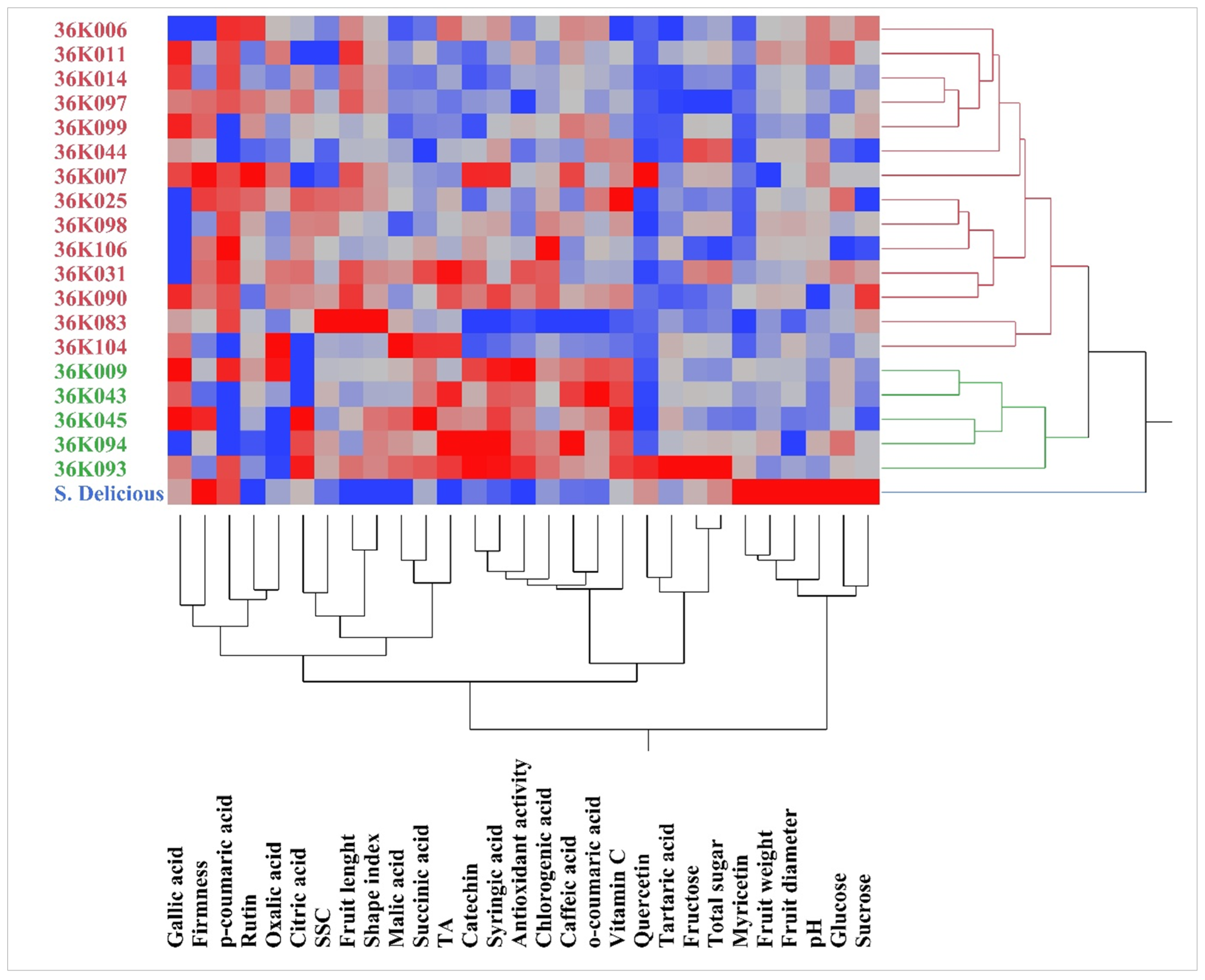
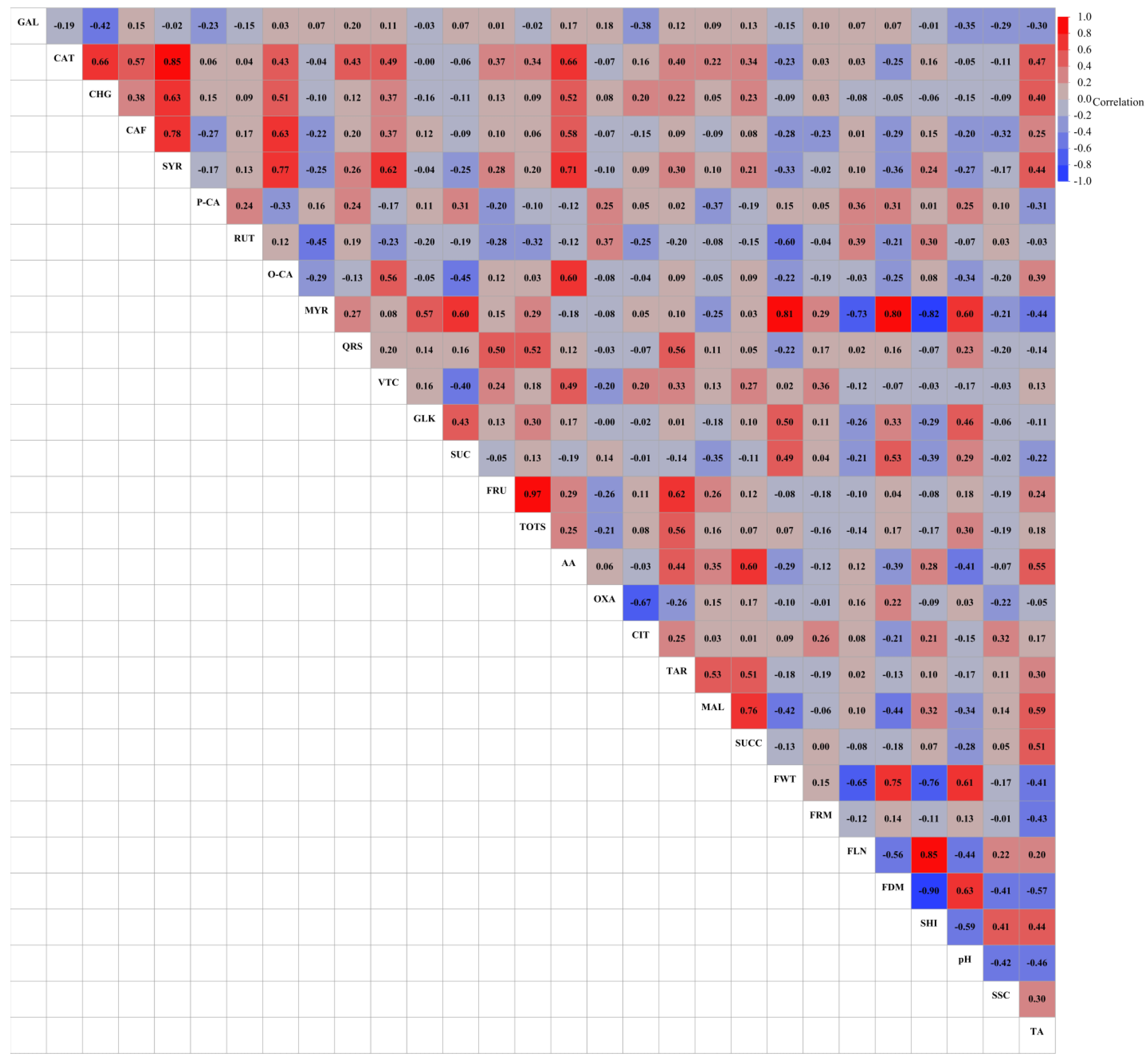
3.5. Principal Component, Heatmap and Correlation Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Janick, J.; Cummins, J.N.; Brown, S.; Hemmat, M. Fruit Breeding. Tree and Tropical Fruit; John Wiley: New York, NY, USA, 1996; Volume 1, pp. 1–77. [Google Scholar]
- Božović, D.; Lazović, B.; Ercisli, S.; Adakalić, M.; Jaćimović, V.; Sezer, I.; Koc, A. Morphological characterization of autochthonous apple genetic resources in Montenegro. Erwerbs-Obstbau 2016, 58, 93–102. [Google Scholar] [CrossRef]
- Ercisli, S. A short review of the fruit germplasm resources of Turkey. Genet. Resour. Crop Evol. 2004, 51, 419–435. [Google Scholar] [CrossRef]
- Kaya, T.; Balta, F.; Şensoy, S. Fruit quality parameters and molecular analysis of apple germplasm resources from Van Lake Basin, Turkey. Turk. J. Agric. For. 2015, 39, 864–875. [Google Scholar] [CrossRef]
- FAOSTAT—Food and Agricultural Organization of the United Nations. Crops and Livestock Products in 2021. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 1 September 2023).
- Ozbek, S. Temperate Fruits; Çukurova Üniversitesi Ziraat Fakültesi Yayınları: Adana, Turkey, 1978; pp. 272–304. [Google Scholar]
- Macit, İ.; Aydın, E.; Tas, A.; Gundogdu, M. Fruit quality properties of the local apple varieties of Anatolia. Sustainability 2021, 13, 6127. [Google Scholar] [CrossRef]
- Balta, M.F.; Karakaya, O.; Kurt, H.; Yılmaz, M.; Uzun, S.; Balta, F. Phytochemical variation of native apple germplasm resources from the Eastern Black Sea Region, Turkey. Erwerbs-Obstbau 2022, 64, 685–695. [Google Scholar] [CrossRef]
- Wu, J.; Gao, H.; Zhao, L.; Liao, X.; Chen, F.; Wang, Z.; Hu, X. Chemical compositional characterization of some apple cultivars. Food Chem. 2007, 103, 88–93. [Google Scholar] [CrossRef]
- Can, Z.; Dincer, B.; Sahin, H.; Baltas, N.; Yildiz, O.; Kolayli, S. Polyphenol oxidase activity and antioxidant properties of Yomra apple (Malus communis L.) from Turkey. J. Enzym. Inhib. Med. Chem. 2014, 29, 829–835. [Google Scholar] [CrossRef]
- Krawitzky, M.; Arias, E.; Peiro, J.M.; Negueruela, A.I.; Val, J.; Oria, R. Determination of color, antioxidant activity, and phenolic profile of different fruit tissue of Spanish ‘Verde Doncella’ apple cultivar. Int. J. Food Prop. 2014, 17, 2298–2311. [Google Scholar] [CrossRef]
- Geçer, M.K.; Ozkan, G.; Sagbas, H.I.; Ilhan, G.; Gundogdu, M.; Ercisli, S. Some important horticultural properties of summer apple genotypes from Coruh Valley in Turkey. Int. J. Fruit Sci. 2020, 20, S1406–S1416. [Google Scholar] [CrossRef]
- Maldonado, F.; Yuri, J.A.; Neira, A.; Razmilic, I. Total phenolics, quercetin glycosides and antioxidant activity in organic and conventional orchards in three apple cultivars during fruit growth. Span. J. Agric. Res. 2022, 20, e0805. [Google Scholar] [CrossRef]
- Zucoloto, M.; Ku, K.M.; Kushad, M.M.; Sawwan, J. Bioactive compounds and quality characteristics of five apples cultivars. Ciência Rural 2015, 45, 1972–1979. [Google Scholar] [CrossRef]
- Noiton, D.A.; Alspach, P.A. Founding clones, inbreeding, coancestry, and status number of modern apple cultivars. J. Am. Soc. Hortic. Sci. 1996, 121, 773–782. [Google Scholar] [CrossRef]
- Sofla, H.S.; Zamani, Z.; Talaei, A.R.; Fatahi, M.R.; Nazari, S.A.; Farokhzad, A.R.; Gharghani, A.; Asgarzadeh, M. Introduction of new promising apple genotypes: A study of quality attributes of apple in crosses between Iranian early ripening and exotic late ripening apple cultivars. Int. J. Fruit Sci. 2016, 16, 210–224. [Google Scholar] [CrossRef]
- Contessa, C.; Botta, R. Comparison of physicochemical traits of red-fleshed, commercial and ancient apple cultivars. Hortic. Sci. 2016, 43, 159–166. [Google Scholar] [CrossRef]
- TURKPATENT-Kağızman Uzun Elması. Coğrafi Işaretler in 2017. Available online: https://ci.turkpatent.gov.tr/cografi-isaretler/detay/38075 (accessed on 1 September 2023).
- Murathan, Z.T.; Arslan, M.; Erbil, N. Effect of growing region on antioxidant and antibacterial activity and mutagenic effect of Uzun apple genotype. Gümüşhane Univ. J. Sci. Technol. 2022, 12, 275–282. [Google Scholar]
- Erinç, S.; Tunçdilek, N. The agricultural regions of Turkey. Geogr. Rev. 1952, 42, 179–203. [Google Scholar] [CrossRef]
- Atış, E.; Çelikoğlu, Ş. Apricot producing in Kağızman district and its contribution to the economy of territory. Marmara Geogr. Rev. 2017, 36, 191–205. [Google Scholar]
- Ben-Arie, R.; Kislev, N. Ultrastructural changes in the cell walls of ripening apple and pear fruit. Plant Physiol. 1979, 64, 197–202. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 14th ed.; Association of Official Analytical Chemists (AOAC): Arlington, VA, USA, 1984. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Rad. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Ozgen, M.; Reese, R.N.; Tulio, A.Z.; Scheerens, J.C.; Miller, A.R. Modified 2,2-azino-bis-3-ethyl benzo thiazoline-6-sulfonic acid (ABTS) method to measure antioxidant capacity of selected small fruits and a comparison to ferric reducing antioxidant power (FRAP) and 2,2-diphenyl-1-picrylhdrazyl (DPPH) methods. J. Agric. Food Chem. 2006, 54, 1151–1157. [Google Scholar] [CrossRef]
- Bevilacqua, A.E.; Califano, A.N. Determination of organic acids in dairy products by high performance liquid chromatography. J. Food Sci. 1989, 54, 1076–1079. [Google Scholar] [CrossRef]
- Aaby, K.; Ekeberg, D.; Skrede, D. Characterization of phenolic compounds in strawberry (Fragaria × ananassa) fruits by different HPLC detectors and contribution of individual compounds to total antioxidant capacity. J. Agric. Food Chem. 2007, 55, 4395–4406. [Google Scholar] [CrossRef] [PubMed]
- Mertoğlu, K.; Akkurt, E.; Evrenosoğlu, Y.; Çolak, A.M.; Esatbeyoglu, T. Horticultural characteristics of summer apple cultivars from Turkey. Plants 2022, 11, 771. [Google Scholar] [CrossRef] [PubMed]
- Karatas, N.; Ercisli, S.; Bozhuyuk, M.R.; Cakir, O.; Necas, T.; Ondrasek, I. Seed-Propagated Summer Apples: Great Morphological and Biochemical Diversity. Sustainability 2021, 13, 8359. [Google Scholar] [CrossRef]
- Schempp, H.; Christof, S.; Mayr, U.; Treutter, D. Phenolic compounds in juices of apple cultivars and their relation to antioxidant activity. J. Appl. Bot. Food Qual. 2016, 89, 11–20. [Google Scholar]
- Güleryüz, M.; Ercişli, S. Kağızman ilçesinde yetiştirilen mahalli elma çeşitleri üzerinde biyolojik ve pomolojik araştırmalar. Atatürk Üniv. Ziraat Fak. Derg. 1995, 26, 183–193. [Google Scholar]
- Dennis, F.G. Fruit Development. In Tree Fruit Physiology: Growth and Development Books; Good Fruit Grower: Washington, DC, USA, 1996; pp. 107–116. [Google Scholar]
- Crosby, J.A.; Janick, J.; Pecknold, P.C. “Enterprise” Apple. HortScience 1994, 29, 825–826. [Google Scholar] [CrossRef]
- Chang, Y.; Sun, R.; Sun, H.; Zhao, Y.; Han, Y.; Chen, D.; Wang, Y.; Zhang, X.; Han, Z. Mapping of quantitative trait loci corroborates independent genetic control of apple size and shape. Sci. Hortic. 2014, 174, 126–132. [Google Scholar] [CrossRef]
- Gliszczynska-Swiglo, A.; Tyrakowska, B. Quality of commercial apple juices evaluated on the basis of the polyphenol content and the TEAC antioxidant activity. J. Food Sci. 2003, 68, 1844–1849. [Google Scholar] [CrossRef]
- Vieira, F.G.K.; Borges, G.D.S.C.; Copetti, C.; Di Pietro, P.F.; da Costa Nunes, E.; Fett, R. Phenolic compounds and antioxidant activity of the apple flesh and peel of eleven cultivars grown in Brazil. Sci. Hortic. 2011, 128, 261–266. [Google Scholar] [CrossRef]
- Scalzo, J.; Politi, A.; Pellegrini, N.; Mezzetti, B.; Battino, M. Plant genotype affects total antioxidant capacity and phenolic contents in fruit. Nutrition 2005, 21, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Bahukhandi, A.; Dhyani, P.; Bhatt, I.D.; Rawal, R.S. Variation in polyphenolics and antioxidant activity of traditional apple cultivars from West Himalaya, Uttarakhand. Hortic. Plant J. 2018, 4, 151–157. [Google Scholar] [CrossRef]
- Gökmen, V.; Artık, N.; Acar, J.; Kahraman, N.; Poyrazoğlu, E. Effects of various clarification treatments on patulin, phenolic compound and organic acid compositions of apple juice. Eur. Food Res. Technol. 2001, 213, 194–199. [Google Scholar] [CrossRef]
- Coklar, H.; Akbulut, M.; Alhassan, I.; Kirpitci, Ş.; Korkmaz, E. Organic acids, sugars, phenolic compounds and antioxidant activity of Malus floribunda coccinella fruit, peel and flesh. Acta Sci. Pol. Hortorum Cultus 2018, 17, 47–59. [Google Scholar] [CrossRef]
- Gundogdu, M.; Canan, I.; Okatan, V. Bioactive contents and some horticultural characteristics of local apple genotypes from Turkey. JAPS J. Anim. Plant Sci. 2018, 28, 865–874. [Google Scholar]
- Celik, F.; Gundogdu, M.; Ercisli, S.; Kaki, B.; Berk, S.; Ilhan, G.; Sagbas, H.I. Variation in organic acid, sugar and phenolic compounds in fruits of historical apple cultivars. Not. Bot. Horti Agrobot. Cluj-Napoca 2018, 46, 622–629. [Google Scholar] [CrossRef]
- Kim, I.; Ku, K.H.; Jeong, M.C.; Kwon, S.I.; Lee, J. Metabolite profiling and antioxidant activity of 10 new early-to mid-season apple cultivars and 14 traditional cultivars. Antioxidants 2020, 9, 443. [Google Scholar] [CrossRef]
- Średnicka-Tober, D.; Barański, M.; Kazimierczak, R.; Ponder, A.; Kopczyńska, K.; Hallmann, E. Selected antioxidants in organic vs. conventionally grown apple fruits. Appl. Sci. 2020, 10, 2997. [Google Scholar] [CrossRef]
- Kim, I.; Ku, K.H.; Jeong, M.C.; Kim, S.S.; Mitchell, A.E.; Lee, J. A comparison of the chemical composition and antioxidant activity of several new early-to mid-season apple cultivars for a warmer climate with traditional cultivars. J. Sci. Food Agric. 2019, 99, 4712–4724. [Google Scholar] [CrossRef]
- Alarcón-Flores, M.I.; Romero-González, R.; Martínez Vidal, J.L.; Garrido Frenich, A. Evaluation of the presence of phenolic compounds in different varieties of apple by ultra-high-performance liquid chromatography coupled to tandem mass spectrometry. Food Anal. Methods 2015, 8, 696–709. [Google Scholar] [CrossRef]
- Fotirić Akšić, M.; Dabić Zagorac, D.; Gašić, U.; Tosti, T.; Natić, M.; Meland, M. Analysis of apple fruit (Malus × domestica Borkh.) quality attributes obtained from organic and integrated production systems. Sustainability 2022, 14, 5300. [Google Scholar] [CrossRef]
- Mignard, P.; Beguería, S.; Giménez, R.; Font i Forcada, C.; Reig, G.; Moreno, M.Á. Effect of genetics and climate on apple sugars and organic acids profiles. Agronomy 2022, 12, 827. [Google Scholar] [CrossRef]
- Ma, B.; Chen, J.; Zheng, H.; Fang, T.; Ogutu, C.; Li, S.; Han, Y.; Wu, B. Comparative assessment of sugar and malic acid composition in cultivated and wild apples. Food Chem. 2015, 172, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Özden, M.; Özden, A.N. Comparison of different coloured fruits in terms of total anthocyanins total phenolics and total antioxidant capacity. Electron. J. Food Technol. 2014, 9, 1–12. [Google Scholar]

| Genotype | FWT | FRM | FLN | FDM | SHI | pH | SSC | TA | TEAC | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 36K006 | 121.35 | efg | 6.47 | j | 72.50 | b–f | 58.02 | bcd | 1.25 | efg | 3.67 | b | 12.20 | hi | 0.60 | de | 2.08 | hij |
| 36K007 | 87.21 | n | 8.27 | a | 73.67 | a–d | 57.55 | bcd | 1.28 | b–f | 3.65 | bc | 11.63 | jk | 0.49 | j | 2.35 | fg |
| 36K009 | 118.42 | fgh | 7.51 | ef | 70.64 | e–h | 57.15 | cd | 1.24 | fgh | 3.52 | def | 13.00 | ef | 0.54 | gh | 3.30 | a |
| 36K011 | 139.11 | b | 7.34 | fg | 74.48 | ab | 59.34 | b | 1.26 | def | 3.67 | b | 11.30 | k | 0.47 | kl | 2.55 | def |
| 36K014 | 117.44 | ghi | 7.09 | ghi | 73.15 | a–d | 57.51 | bcd | 1.27 | c–f | 3.52 | def | 12.20 | hi | 0.52 | i | 2.27 | ghi |
| 36K025 | 122.23 | ef | 8.08 | abc | 73.03 | a–e | 57.04 | cd | 1.28 | b–e | 3.58 | b–e | 14.90 | b | 0.56 | f | 2.09 | hij |
| 36K031 | 114.90 | hij | 7.90 | c | 73.79 | abc | 56.93 | cd | 1.30 | bcd | 3.61 | b–e | 12.90 | fg | 0.66 | a | 2.94 | bc |
| 36K043 | 113.70 | ijk | 6.85 | i | 67.53 | I | 57.38 | bcd | 1.18 | i | 3.50 | ef | 13.40 | de | 0.65 | ab | 2.87 | bc |
| 36K044 | 122.64 | ef | 7.62 | de | 70.20 | fgh | 57.94 | bcd | 1.21 | ghi | 3.62 | bcd | 11.80 | ij | 0.54 | ghi | 1.98 | jk |
| 36K045 | 112.66 | jkl | 8.18 | ab | 71.38 | c–g | 54.28 | ef | 1.32 | b | 3.50 | ef | 12.50 | gh | 0.59 | e | 2.75 | cd |
| 36K083 | 109.42 | lm | 7.59 | ef | 75.38 | a | 53.22 | fg | 1.42 | a | 3.53 | c–f | 16.70 | a | 0.54 | ghi | 1.80 | kl |
| 36K090 | 127.30 | cd | 7.85 | cd | 74.34 | ab | 58.45 | bc | 1.27 | c–f | 3.43 | f | 13.80 | d | 0.62 | cd | 2.71 | cde |
| 36K093 | 106.08 | m | 7.05 | hi | 72.96 | a–e | 56.22 | de | 1.30 | bc | 3.52 | def | 13.60 | d | 0.63 | bc | 3.11 | ab |
| 36K094 | 129.82 | c | 7.61 | de | 67.37 | i | 51.79 | g | 1.30 | bc | 3.60 | b–e | 13.80 | d | 0.66 | a | 2.85 | c |
| 36K097 | 116.11 | hij | 7.91 | c | 73.50 | a–d | 57.60 | bcd | 1.28 | b–f | 3.54 | c–f | 12.90 | fg | 0.46 | l | 1.72 | l |
| 36K098 | 127.43 | cd | 7.17 | gh | 71.22 | d–g | 59.31 | b | 1.20 | hi | 3.59 | b–e | 14.40 | c | 0.55 | fg | 2.06 | ij |
| 36K099 | 109.61 | klm | 7.94 | bc | 69.47 | ghi | 56.15 | de | 1.24 | fgh | 3.49 | ef | 13.10 | ef | 0.48 | jk | 2.46 | efg |
| 36K104 | 110.50 | kl | 7.03 | hi | 68.72 | hi | 58.34 | bc | 1.18 | i | 3.54 | c–f | 12.80 | fg | 0.64 | bc | 2.09 | hij |
| 36K106 | 124.31 | de | 7.86 | cd | 68.66 | hi | 57.94 | bcd | 1.19 | i | 3.58 | b–e | 13.10 | ef | 0.53 | hi | 2.32 | fgh |
| Stark.D * | 187.70 | a | 8.28 | a | 60.03 | j | 70.15 | a | 0.86 | j | 3.81 | a | 12.00 | ij | 0.39 | m | 1.71 | l |
| P.St.Dev. × | 2.569 | 0.157 | 1.487 | 1.213 | 0.026 | 0.075 | 0.273 | 0.012 | 0.156 | |||||||||
| F-Value | 164.93 | 31.30 | 16.98 | 25.15 | 50.78 | 3.85 | 62.75 | 128.77 | 27.20 | |||||||||
| *** | *** | *** | *** | *** | *** | *** | *** | *** | ||||||||||
| Genotype | OXA | CIT | TAR | MAL | SUC | VIT-C | GLK | SCR | FRU | TOT-S | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 36K006 | 0.51 | h | 0.98 | i | 0.31 | j | 4.34 | kl | 1.19 | j | 66.67 | n | 24.00 | b–e | 16.80 | c | 57.53 | Gh | 96.17 | h |
| 36K007 | 0.82 | c | ND | 0.57 | gh | 5.92 | ef | 1.46 | hi | 117.33 | f | 22.67 | b–f | 12.80 | f | 80.51 | D | 115.08 | e | |
| 36K009 | 1.16 | b | ND | 0.84 | d | 6.38 | de | 2.17 | d | 124.67 | cde | 23.67 | b–e | 10.90 | g | 55.72 | H | 86.90 | i | |
| 36K011 | 0.76 | de | ND | 0.68 | ef | 5.28 | ghi | 1.84 | ef | 109.00 | gh | 28.00 | ab | 12.77 | f | 70.62 | F | 111.89 | ef | |
| 36K014 | 0.25 | l | 1.33 | g | 0.35 | ij | 4.90 | ij | 1.20 | j | 98.33 | jk | 22.33 | b–g | 11.20 | g | 53.33 | i | 87.07 | i |
| 36K025 | 0.57 | g | 1.66 | c | 0.60 | fg | 6.10 | def | 1.48 | gh | 135.67 | a | 27.33 | abc | 7.57 | h | 48.55 | j | 85.06 | ij |
| 36K031 | 0.78 | cd | 1.55 | d | 0.49 | h | 7.23 | c | 2.51 | bc | 101.00 | ijk | 26.00 | bcd | 14.67 | d | 103.48 | c | 143.69 | c |
| 36K043 | 0.35 | ij | ND | 0.73 | e | 5.77 | fg | 2.25 | d | 127.33 | bc | 23.67 | b–e | 10.37 | g | 74.11 | e | 105.05 | g | |
| 36K044 | 0.22 | l | 1.05 | hi | 0.40 | i | 5.60 | fgh | 0.79 | k | 118.67 | def | 16.67 | gh | 7.93 | h | 131.83 | b | 155.13 | b |
| 36K045 | 0.02 | m | 2.13 | a | 1.11 | b | 8.42 | b | 2.88 | a | 134.00 | ab | 22.67 | b–f | 8.37 | h | 58.98 | g | 79.88 | j |
| 36K083 | 0.25 | l | 1.09 | h | 0.63 | fg | 6.59 | d | 1.57 | gh | 76.00 | m | 21.67 | c–g | 14.70 | d | 47.27 | j | 83.67 | ij |
| 36K090 | 0.73 | e | 1.37 | fg | 0.40 | i | 5.29 | ghi | 1.78 | f | 118.33 | ef | 21.33 | d–g | 20.13 | b | 41.16 | k | 81.73 | ij |
| 36K093 | 0.04 | m | 2.04 | a | 3.46 | a | 8.34 | b | 2.42 | c | 128.67 | abc | 23.33 | b–f | 12.80 | f | 165.83 | a | 196.29 | a |
| 36K094 | 0.02 | m | 1.77 | b | 0.72 | e | 7.15 | c | 1.89 | ef | 125.67 | cd | 27.00 | a–d | 12.77 | f | 74.75 | e | 111.59 | ef |
| 36K097 | 0.62 | f | 1.52 | de | 0.33 | ij | 5.19 | hi | 1.43 | hi | 105.00 | hij | 17.67 | fgh | 13.63 | def | 28.09 | m | 58.28 | k |
| 36K098 | 0.30 | k | 1.44 | ef | 0.55 | gh | 4.36 | kl | 1.42 | hi | 115.67 | fg | 21.33 | d–g | 13.27 | ef | 80.34 | d | 114.52 | e |
| 36K099 | 0.31 | jk | 1.15 | h | 0.40 | i | 4.57 | jk | 1.32 | ij | 95.00 | k | 23.00 | b–f | 14.47 | de | 73.90 | e | 106.02 | fg |
| 36K104 | 1.20 | a | ND | 0.99 | c | 10.50 | a | 2.65 | b | 84.33 | l | 19.00 | e–h | 12.57 | f | 73.39 | e | 103.10 | g | |
| 36K106 | 0.34 | ijk | 1.34 | fg | 0.92 | cd | 6.05 | ef | 1.95 | e | 105.67 | hi | 13.67 | h | 8.23 | h | 37.23 | l | 60.46 | k |
| Stark.D * | 0.38 | i | 1.13 | h | 0.61 | fg | 3.93 | l | 1.63 | g | 108.00 | hi | 32.33 | a | 22.47 | a | 79.77 | d | 133.75 | d |
| P.St.Dev. × | 0.028 | 0.063 | 0.051 | 0.304 | 0.091 | 4.291 | 3.505 | 0.777 | 1.241 | 3.688 | ||||||||||
| F-Value | 468.62 | 376.18 | 525.28 | 84.51 | 107.86 | 57.93 | 4.33 | 72.61 | 2033.49 | 236.40 | ||||||||||
| *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | |||||||||||
| Genotype | GAL | CAT | CHG | CAF | SYR | P-CA | RUT | O-CA | MYR | QRS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 36K006 | ND | 24.49 | ef | 81.68 | f | 2.99 | cde | 17.74 | e | 0.81 | bc | 1.40 | ab | 11.80 | de | 1.73 | gh | 1.26 | def | |
| 36K007 | 1.42 | c | 33.32 | ab | 85.35 | f | 3.30 | b | 21.61 | b | 0.76 | de | 1.46 | A | 9.65 | h | 1.76 | e–h | 2.74 | a |
| 36K009 | 1.64 | a | 31.38 | b | 91.62 | de | 3.09 | c | 22.23 | a | 0.82 | b | 1.27 | c–f | 13.64 | b | 1.86 | c | 1.25 | d–h |
| 36K011 | 1.56 | b | 15.84 | ij | 64.88 | i | 2.88 | efg | 13.61 | h | 0.76 | de | 1.11 | jkl | 9.50 | h | 1.78 | efg | 1.26 | d–g |
| 36K014 | 1.46 | c | 14.63 | ijk | 70.91 | gh | 2.74 | gh | 9.60 | i | 0.77 | de | 1.16 | hij | 9.69 | h | 1.74 | fgh | 1.24 | e–h |
| 36K025 | ND | 16.06 | ij | 86.63 | ef | 2.21 | ij | 14.77 | g | 0.74 | e | 1.33 | bc | 12.48 | c | 1.73 | gh | 1.23 | fgh | |
| 36K031 | ND | 30.45 | bc | 101.60 | b | 2.35 | i | 15.34 | fg | 0.81 | b | 1.23 | efg | 9.37 | h | 1.79 | def | 1.22 | fgh | |
| 36K043 | 1.34 | d | 24.55 | ef | 82.63 | f | 3.34 | b | 19.87 | d | ND | 1.22 | e–h | 15.54 | a | 1.80 | de | 1.22 | fgh | |
| 36K044 | 1.09 | g | 22.72 | fg | 70.66 | gh | 2.35 | i | 17.41 | e | ND | 1.08 | klm | 12.35 | c | 1.72 | h | 1.23 | fgh | |
| 36K045 | 1.64 | a | 26.55 | de | 71.01 | gh | 2.88 | efg | 20.89 | c | ND | 1.18 | ghi | 12.19 | cd | 1.76 | e–h | 1.22 | gh | |
| 36K083 | 1.08 | g | 12.62 | k | 35.75 | j | 1.67 | k | 4.94 | l | 0.77 | de | 1.20 | f–i | 4.26 | l | 1.70 | h | 1.28 | de |
| 36K090 | 1.53 | b | 27.90 | cd | 101.97 | b | 2.82 | fgh | 20.75 | c | 0.76 | de | 1.10 | jkl | 11.62 | ef | 1.84 | cd | 1.28 | d |
| 36K093 | 1.21 | f | 35.04 | a | 98.19 | bc | 2.93 | def | 22.45 | a | 0.76 | de | 1.14 | ijk | 11.23 | f | 1.95 | b | 2.52 | b |
| 36K094 | ND | 35.11 | a | 95.10 | cd | 3.58 | a | 22.71 | a | ND | 1.06 | lm | 11.26 | f | 1.86 | c | 1.26 | d–g | ||
| 36K097 | 1.22 | ef | 19.68 | gh | 70.59 | gh | 2.72 | h | 13.23 | h | 0.74 | e | 1.31 | cd | 8.46 | i | 1.75 | e–h | 1.25 | d–h |
| 36K098 | ND | 24.73 | ef | 93.68 | cd | 2.88 | efg | 17.15 | e | 0.78 | cd | 1.25 | d–g | 9.67 | h | 1.73 | gh | 1.21 | h | |
| 36K099 | 1.56 | b | 14.02 | jk | 82.28 | f | 3.06 | cd | 15.71 | f | ND | 1.28 | cde | 11.88 | de | 1.73 | gh | 1.25 | d–h | |
| 36K104 | 1.29 | de | 14.59 | ijk | 74.03 | g | 2.27 | i | 8.76 | j | ND | 1.22 | e–h | 7.59 | j | 1.74 | fgh | 1.26 | def | |
| 36K106 | ND | 25.68 | def | 117.68 | a | 2.30 | i | 15.79 | f | 0.87 | a | 1.22 | e–h | 10.67 | g | 1.75 | e–h | 1.28 | d | |
| Stark.D * | 1.08 | g | 17.48 | hi | 66.93 | hi | 2.08 | j | 7.55 | k | 0.76 | de | 1.03 | M | 6.46 | k | 3.06 | a | 1.76 | c |
| P.St.Dev. × | 0.041 | 1.873 | 3.415 | 0.091 | 0.363 | 0.021 | 0.040 | 0.277 | 0.036 | 0.025 | ||||||||||
| F-Value | 781.40 | 46.97 | 80.94 | 83.41 | 631.40 | 950.06 | 22.60 | 254.65 | 202.81 | 880.77 | ||||||||||
| *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | |||||||||||
| Variables | PC 1 | PC 2 | PC 3 |
|---|---|---|---|
| Gallic acid | 0.0023 | 0.0166 | −0.1022 |
| Catechin | 0.5953 * | 0.5606 | −0.1797 |
| Chlorogenic acid | 0.4470 * | 0.3921 | −0.3201 |
| Caffeic acid | 0.5228 | 0.3282 | −0.5641 * |
| Syringic acid | 0.7247 * | 0.4520 | −0.4074 |
| p-coumaric acid | −0.2737 | -0.0940 | −0.1025 |
| Rutin | 0.2112 | -0.4324 | −0.4444 * |
| o-coumaric acid | 0.5935 * | 0.2760 | −0.5108 |
| Myricetin | −0.6992 * | 0.6353 | 0.0937 |
| Quercetin | 0.0784 | 0.4831 | 0.0459 |
| Vitamin C | 0.3985 | 0.5272 * | −0.1329 |
| Glucose | −0.3222 | 0.4687 | 0.0012 |
| Sucrose | −0.5540 * | 0.2125 | 0.0193 |
| Fructose | 0.2249 | 0.6463 * | 0.3777 |
| Total sugar | 0.0780 | 0.6959 * | 0.3652 |
| Antioxidant activity | 0.7164 * | 0.4403 | −0.1396 |
| Oxalic acid | −0.0855 | -0.1913 | −0.4254 |
| Citric acid | 0.1372 | 0.0948 | 0.4909 |
| Tartaric acid | 0.4179 | 0.5093 | 0.5013 * |
| Malic acid | 0.5269 * | 0.0500 | 0.5040 |
| Succinic acid | 0.4230 | 0.2964 | 0.2736 |
| Fruit weight | −0.7583 * | 0.4119 | 0.0204 |
| Firmness | −0.2179 | 0.0855 | −0.0383 |
| Fruit length | 0.4601 | −0.5560 * | 0.0451 |
| Fruit diameter | −0.8038 * | 0.4105 | −0.1886 |
| Shape index | 0.6968 * | −0.5345 | 0.1952 |
| pH | −0.6735 * | 0.3690 | −0.0883 |
| SSC | 0.1717 | −0.3772 | 0.5019 * |
| TA | 0.7349 * | 0.0710 | 0.2459 |
| Eigenvalue | 7.1 | 4.9 | 2.8 |
| Percent | 24.5 | 17.1 | 9.7 |
| Cumulative Percent | 24.5 | 41.6 | 51.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balık, S.; Kaya, T.; Aslantaş, R. Fruit Quality Parameters, Sugars, Vitamin C, Antioxidant Activity, Organic Acids, and Phenolic Compounds for a New Endemic Apple Variety, “Long Apple”. Horticulturae 2023, 9, 1171. https://doi.org/10.3390/horticulturae9111171
Balık S, Kaya T, Aslantaş R. Fruit Quality Parameters, Sugars, Vitamin C, Antioxidant Activity, Organic Acids, and Phenolic Compounds for a New Endemic Apple Variety, “Long Apple”. Horticulturae. 2023; 9(11):1171. https://doi.org/10.3390/horticulturae9111171
Chicago/Turabian StyleBalık, Serdar, Tuncay Kaya, and Rafet Aslantaş. 2023. "Fruit Quality Parameters, Sugars, Vitamin C, Antioxidant Activity, Organic Acids, and Phenolic Compounds for a New Endemic Apple Variety, “Long Apple”" Horticulturae 9, no. 11: 1171. https://doi.org/10.3390/horticulturae9111171
APA StyleBalık, S., Kaya, T., & Aslantaş, R. (2023). Fruit Quality Parameters, Sugars, Vitamin C, Antioxidant Activity, Organic Acids, and Phenolic Compounds for a New Endemic Apple Variety, “Long Apple”. Horticulturae, 9(11), 1171. https://doi.org/10.3390/horticulturae9111171








