Abstract
The cape gooseberry (Physalis peruviana L.) is an exotic tropical fruit of great national and international importance due to its nutritional and organoleptic properties. The objective of this study was to evaluate different postharvest treatments—coating, vacuum impregnation, and immersion—on the conservation of several quality characteristics of cape gooseberry fruit. Moreover, the different conditions of the selected treatments were studied. Weight loss was assessed with a gravimetric analysis of the fresh and treated fruit. Firmness was determined by the instrumental texture. A sensory analysis was conducted using a multidimensional profile approach. Of the treatments evaluated, the lowest weight loss was recorded with the use of coating and immersion. However, the immersion process resulted in the product with the highest overall quality according to the sensory analysis and presented the most appropriate texture according to the firmness values. Finally, in the evaluation of the immersion, a significant influence of the CaCl2 immersion time (p < 0.05) on the firmness values of the product was found, resulting in longer times leading to less firm products. Considering 10% as a commercial standard limit for weight loss, the fruit treated under immersion and coating processes can be stored for at least 12 days. The immersion process is highlighted because it improved the sensory characteristics with respect to the control (i.e., without treatment). Therefore, it is a promising alternative for the postharvest treatment of cape gooseberries.
1. Introduction
The cape gooseberry (Physalis peruviana Linnaeus) is a fruit belonging to the Solanaceae family and genus Physalis. This plant is native to the South American Andes and produces juicy, yellow–orange berry-like fruit with a diameter between 1.25 and 2.50 cm and weight between 4 and 10 g, depending on the harvesting conditions []. It is a climacteric fruit characterized by a bittersweet and fresh flavor, pleasant texture, and aroma [,], and it has a high nutritional value due to the notable presence of compounds such as vitamins (ascorbic acid, thiamine, riboflavin, and niacin) and minerals (calcium, phosphorus, and iron) []. Additionally, it has a high content of carotenes and other minority compounds that favor its antioxidant capacity and provide it with a hepatoprotective, antimutagenic, purifying, immune-system strengthening, and cholesterol- and blood-sugar-lowering effect [,]. Thus, the cape gooseberry is now a popular health food product used in various ways, such as in ice cream, yogurt, jams, and more. However, its primary form of commercialization is fresh, which makes it a highly perishable food due to its moisture content, sensitivity to ethylene, high respiratory activity, and the incidence of ripening processes that accelerate the senescence phenomenon and determine the deterioration of its texture, flavor, and aroma conditions, thus limiting its shelf life to approximately between 10 and 12 days [,]. Colombia is the main producer and exporter of cape gooseberry, with about 90% of the world’s production. According to the Colombian Ministry of Agriculture and Rural Development, from 2016 to 2020, the cultivation area increased from 1023.1 to 1372.4 hectares. In the same period, there was an increase in production from 15,111.8 to 19,775.7 tons []. Regarding exportation, according to data provided by the National Foreign Trade Association (ANALDEX), during the period 2016–2020, Colombia exported a net amount of 50,865.7 tons, representing an average growth of around 9.1% per year. Therefore, this fruit has also appreciated within international markets, which makes it a fruit of economic importance for the country.
One challenge that Colombia faces in the export of cape gooseberry is the demand for fresh quality products, since according to data provided by the National Planning Department (DNP), of the total annual production of fruits and vegetables approximately 21% is lost or wasted, which corresponds to between 1813 and 2373 tons of cape gooseberry per year, with the main limiting factors being weight loss during storage and spoilage by microorganisms, such as Cladosporium, Phomopsis, Pestalotia, Botrytis cinerea, and Alternaria spp. []. Among the strategies that have been developed for the conservation of fresh fruits include high pressure, ultrasound, ionizing radiation, pulsed electric fields, cold plasma, and packaging in modified atmospheres [,,]. However, the high cost of processing and the acquisition of specialized equipment limit their use in the Colombian context. Other strategies have used minimal processing methods showing promising results. In the specific case of cape gooseberry, coatings made from beeswax and whey protein [], chitosan and aloe vera [], and chitosan and rosemary essential oil [] have been used. In all of them, a decrease in the weight loss of the fruit during storage was achieved, prolonging its shelf life, since the freshness of fruits and vegetables decreases, according to Pinzón et al. (2015), when samples have weight losses of more than 10% []. Vacuum impregnation has also been applied for the conservation of cape gooseberry, based on the incorporation of various components, such as ethanolic extracts of annatto leaves and seeds [], as well as micronutrients like calcium and vitamins B9, C, D, and E []. Likewise, immersion in technological coadjutants, such as citric and ascorbic acids and calcium chloride have been evaluated for the minimum processing of horticultural and fruit products. Thus, these preservation agents help to prevent browning reactions and provide stability to the matrix against external microbial alterations. In addition, the application of sodium carbonate in fruit products has been proven to inhibit microorganisms such as Botrytis cinerea [] and the addition of calcium salts has been used to improve the textural characteristics of horticultural products []. However, for the evaluation of the quality characteristics of cape gooseberries, their use in combination with other substances in various postharvest treatments has not been studied before. Therefore, in this study the objective was to evaluate different postharvest processing alternatives—coating process (CP), vacuum impregnation process (VIP), and immersion process (IP)—on the conservation of several quality characteristics of cape gooseberry (Physalis peruviana L.), including its sensory analysis. Moreover, different conditions were studied for the selected treatments.
2. Materials and Methods
2.1. Materials
This research used the Colombia ecotype of the cape gooseberry (Physalis peruviana L.) with no mechanical affectations and free of damage caused by pathogens and insects. The fruit was category 1 with caliber D and E (20.1–22.0 and >22.0 mm diameter, respectively) and presented a maturity index between 4 and 6 based on the Colombian Technical Standard NTC 4580 [] and were harvested in the small village of Mesopotamia (5°53′03″ N 75°18′58″ O), located east of Medellín city, Department of Antioquia, Colombia. The cape gooseberries used in the analysis were transported (2 h) with their calyxes in sacks until their arrival at the laboratory, where they were selected and stored in refrigerated conditions at 5 ± 1 °C until processing.
2.2. Postharvest Treatments
The cape gooseberry fruit were treated under three different postharvest treatments, and the methodology used for each was based on previous studies and slightly modified.
- -
- Coating process (CP): This methodology was based on Muley and Singhal (2020) []. Firstly, the whey protein (WPC) was hydrated: 30 g of protein in 188.31 mL of distilled water. Then, the pH was adjusted to 7 by adding NaOH, and the solution was heated (55 °C, 10 min) with constant stirring. After, 1.59 g of sodium carbonate was added while maintaining the agitation and temperature, and 30 g of glycerol was also added. After cooling, the solution was stored under refrigeration (5 ± 1 °C) until its application. The fruit was submerged twice for 1 min, and then the samples were drained and placed in an oven for 10 min at 30 °C to ensure the elimination of the surface liquid.
- -
- Vacuum impregnation process (VIP): This methodology was based on Ciro (2012). An impregnation solution containing 1% calcium chloride (CaCl2) and 0.05% sodium carbonate (Na2CO3) was prepared over an isotonic aqueous base (sucrose 20 °Brix). The fruit was placed in a desiccator and the solution ratio was maintained at 1:5 to ensure adequate immersion. A vacuum pump was attached to the desiccator (Welch, Gardner Denver Thomas, Inc. Sheboygan, WI USA), and the system was subjected to a vacuum pressure of 17 KPa for 5 min. Subsequently, the atmospheric pressure was restored, and the fruit remained submerged for an additional amount of time (5 min) without stirring. Finally, the impregnated samples were taken out of the desiccator, drained, and placed in an oven for 10 min at 30 °C to ensure removal of the surface liquid.
- -
- Immersion process (IP): This methodology was based on Pérez-Martínez et al. (2021). The immersion of the fruit was carried out sequentially using technological coadjutants. First, the fruit was immersed in a recipient containing a solution with 500 ppm citric acid and 500 ppm ascorbic acid for 5 min. Then, they were taken out from the recipient and immersed in a solution containing Na2CO3 0.05 M for 5 min. Finally, they were taken out and submerged in a solution containing 1% CaCl2 for 2 min. The immersed samples were drained and placed in the oven for 10 min at 30 °C to ensure the elimination of the surface liquid.
2.3. Characterization of the Fresh and Treated Fruit
The fresh, or untreated, cape gooseberries (control) and those after each treatment (treated) were placed in four (4) polyethylene boxes, each containing between 8 and 12 pieces of fruit. For the initial characterization, one box was selected (box 1) from each treatment. The other boxes were used for the textural analysis (box 2) and the weight loss (boxes 3 and 4).
The color assessment was conducted according to the CIE L*a*b* methodology, taking 5 samples (from box 1) and measuring the color at opposite points of the transverse and longitudinal axes, together with the center of the fruit. The overall average of the samples analyzed was taken. For the determination of the moisture, pH, total soluble solids (TSS), and total acidity of the cape gooseberries, the analyses were carried out after the homogenization of the samples with a fruit processor (Oster®, Model FPSTJE316W, Bogotá, Colombia), with subsequent mixing. Samples were taken from each treatment (box 1), and the tests were performed in triplicate. The moisture content (%) was determined according to the AOAC method []. The pH was measured using a pH meter (Hanna Instruments pHmeter, HI3220, Washington, DC, USA). The total acidity was calculated by titration with NaOH (0.1 N) using phenolphthalein as an indicator until reaching the neutralization of all of the organic acids present and expressed as a percentage of citric acid. Finally, the total soluble solids (TSS) were determined through refractometer readings using Brixco Instruments (ABBE 3030. Medellín, Colombia).
2.4. Determination of Vitamin C, Carotenoids, Total Phenolic Content, and Antioxidant Capacity
For the determination of vitamin C, carotenoids, total phenolic content, and antioxidant capacity, analyses were carried out for the fresh fruit and immediately after applying the treatments (day 0). The analyses were carried out on the homogenized samples (box 1).
- -
- Vitamin C: Vitamin C was determined following the method used by Contreras-Calderón et al. (2011) [] based on titration of the analyzed samples with a 2,6-dichlorophenolindophenol standard solution in an acid environment. The analyses were performed in triplicate, and the results are expressed as mg/100 g.
- -
- Carotenoids: Carotenoids were determined following the methodology described by Rodriguez-Amaya and Kimura (2004) [] using the absorption coefficient for β-carotene in petroleum ether solvent (A = 25 92). Three grams of homogenized fruit was placed in a falcon tube, and 50 mL of cold acetone was added. Then, they were homogenized in a vortex, and filtered in a Buchner funnel with filter paper. The samples were washed with small amounts of acetone until colorless. Subsequently, in a decanting funnel of 500 mL, 40 mL of petroleum ether and the acetone extract (obtained previously) was added. Distilled water (~300 mL) was slowly added to avoid the formation of an emulsion. Three or four washes were carried out to eliminate any residual acetone. Finally, the petroleum ether was collected in a 50 mL volumetric flask by passing the solution through a funnel containing anhydrous sodium sulfate to remove residual water. The separating funnel was washed with petroleum ether, collecting the washings in the volumetric balloon, gauged with petroleum ether, and the absorbance was read at 450 nm CARY 50 BIO, UV–vis (Varian Pty. Ltd., Mulgrave, Australia). The analyses were performed in triplicate, and the results are expressed as μg/100 g.
- -
- Total Phenolic Content (TPC): TPC was determined based on the method of Folin–Ciocalteu, following the steps used by Osorio-Arias et al. (2019) []. Firstly, the fruit was dissolved in a methanol/water (50:50) solution and an acetone-water solution (70:30) to make the extracts. Then 20 mL of extract was mixed with 1580 mL of distilled water and 100 mL of Folin–Ciocalteu reagent; after 2 min 300 mL of sodium carbonate (20 g/100 mL) was added and the final mixture was stored in the dark for 1 h. The absorbance of the solution was measured in a spectrophotometer (CARY 50 BIO, UV–vis) at 725 nm and the absorbance was compared with a calibration curve based on gallic acid. The analyses were performed in triplicate and the results were expressed as mg equivalents of gallic acid per 100 g of fresh sample (mg GAE/100 g).
- -
- Antioxidant Capacity: Two different methods were used to evaluate the antioxidant capacity of the fresh fruit. The FRAP (ferric-reducing ability of plasma) method is based on the single-electron transfer (SET) mechanism, while the ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)) method acts via both the SET and hydrogen atom transfer (HAT) reactions. The FRAP assay was conducted according to the methodology described by Duarte-Correa et al. (2020) []. For the first method, 900 μL of FRAP reagent (containing TPTZ (2,4,6-Tris(2-pyridyl)-s-triazine), FeCl3, and acetate buffer) was mixed with 90 μL of distilled water and 30 μL of the test sample and incubated at 37 °C/30 min. The absorbance was measured at 595 nm after 30 min (UV–3300 Mapada Instruments, Shanghai, China). Trolox was used for the calibration curve. The results are expressed as micromol of Trolox equivalents (TE) per gram (μmol TE/g). Regarding the second method, the ABTS assay was performed according to Osorio-Arias et al. (2019). Firstly, the radical cation solution (ABTS+) was prepared by mixing 10 mL of an ABTS stock solution (7 mM) with 10 mL of potassium persulfate (2.45 mM). The solution was let to stand overnight and, subsequently, diluted with ethanol until an absorbance reading of 0.7 was achieved (730 nm). Finally, the absorbance of the samples was determined after 30 min of mixing at 30 °C with the radical solution. The reduction in the absorbance was correlated with a Trolox calibration curve. The results are expressed as μM of Trolox equivalent (TE) per gram (μmol TE/g).
2.5. Textural Analysis of Fresh and Treated Fruit
According to the random initial classification, box 2 was selected for the textural analysis. A texture analyzer, TPA TexturePro CT V1.6 Build (Brookfield Engineering Labs. Inc., Stoughton, MA, USA), was used to measure the firmness of the fresh and treated fruit following the methodology described by Olivares-Tenorio et al. (2017) []. The analysis was carried out with the following specifications: Speed of 0.5 mm/s, penetration distance of 5 mm, and a trigger of 4 g. A TA9 stainless-steel needle probe with a 1.0 mm diameter was used. In the penetration test, the firmness was defined as the maximum force required to break the sample. These measurements were made at room temperature (25 ± 2 °C), and the data were recorded in grams of force (gf). At least five samples were used in each treatment.
2.6. Determination of the Weight Loss of Fresh and Treated Fruit
Weight loss was assessed with gravimetric analysis, taking as a reference the day 0 weight. It was calculated according to Balaguera-lópez et al. (2014), using Equation (1). For each treatment and the control, ten separate piece of fruits were placed in two polyethylene containers (with holes), and the weight of each fruit was recorded daily. According to the random initial classification, this corresponded to sample boxes 3 and 4. The samples were stored at 6 ± 2 °C.
where Pi is the fruit weight at the initial time (day 0), and Pf is the fruit weight at the final time (day 12).
2.7. Sensory Analysis for Fresh and Treated Fruit
The sensory analysis of the fresh (control) and treated fruit was conducted in the Food Sensory Analysis Laboratory at the University of Antioquia, Colombia, using a multidimensional profile approach (NTC 3932 (1996) and ISO 11035 (1994) []) with an expert panel. The judges were able to identify a set of relevant descriptors for appearance (A), texture (T), flavor (F), odor (O), and somatosensory sensation (S) to provide the maximum amount of information about the samples’ sensorial attributes to establish a sensory profile. The scale used to rank the descriptors’ intensities was from 0 to 5, where 0 is absence, 1 is very weak, 2 is weak, 3 is moderate, 4 is strong, and 5 is intense. The evaluation was carried out in individual cubicles with an ambient temperature of 24.0 ± 0.5 °C and relative humidity of 64 ± 1%. The overall quality was also evaluated on a scale from 1 to 3, where 1 is low, 2 is medium, and 3 is high quality. The analyses were performed in duplicate following international tenets.
2.8. Statistical Analysis
The results of each analysis are expressed as the means ± standard deviation from at least three estimations. An analysis of variance (ANOVA) with Fisher’s least significant difference (LSD) test was used to determine significant differences among the means at a significance level of p < 0.05 using Statgraphics Centurion XVI software (Statistical Graphics Corporation, Ver. 16.0.07, Rockville, MD, USA). Considering the results obtained with the three postharvest treatments applied to the fruit (CP, VIP, and IP) in terms of physicochemical characterization, weight loss, texture analysis, and sensory analysis, one method was selected to continue with the development of the research. Once a postharvest treatment was selected (IP), a factorial design of experiments was carried out. The independent variables were calcium chloride (1–3%) and immersion time in calcium chloride solution (2–5 min). The dependent variables were moisture content (%), TSS, firmness (gf), and weight loss at 12 days of storage (%).
3. Results and Discussion
3.1. Characterization of Fresh Fruit
Table 1 shows the results of the characterization of the fresh cape gooseberry fruit. The values obtained for moisture content and pH agreed with those reported previously by Balaguera-lópez et al. (2014) for the same ecotype. The total acidity was also between the values reported by Bravo et al. (2015) [] and Pinzón et al. (2015), who found values between 1.49 and 1.90%. Regarding the values for TSS, they are expressed as °Brix, which is an estimate of the sugar content, in addition to organic acids, amino acids, and soluble pectins, an average of 15.20 was found, similar to those reported by Bravo et al. (2015), who also stated that the TSS is strongly affected by cultivar, harvest month, and maturity. For the cape gooseberry color, the CIEL*a*b* system was applied. This system uses brightness (L*), red (a*), and yellow (b*) parameters. The positive values for a* and b* allowed us to establish that the fruit presented tonalities between red and yellow []. These colors are characteristic in fruits with a high content of carotenoids, which are substances responsible for this coloration. On the other hand, the value of L is related to light (white) luminosities.

Table 1.
Physicochemical characterization of cape gooseberry fruit.
Table 2 shows the total phenolic content (TPC), carotenoids, vitamin C, and antioxidant capacity (FRAP and ABTS) of the fresh cape gooseberry. The TPC of the fresh fruit was similar to that reported by Puente et al. (2011) using a modified Folin–Ciocalteu method. They found values between 33.72 and 44.58 mg GAE/100 g. It has been established that the main phenolic compounds present in cape gooseberries are quercetin, myricetin, and kaempferol []. Regarding the carotenoids content, they are responsible for the orange color in the fruit []. The value was found to be in the range reported by Graça Dias et al. (2017) [] for a similar maturity stage: 388.8–1460 μg/100 g. Twenty-two carotenoid compounds were identified, with the main one being all-trans-β-carotene (76.8%), followed by 9-cis-β-carotene (3.6%) and all-trans-α-cryptoxanthin (3.4%) []. Concerning the vitamin C content, the obtained value was similar to that reported by Valente et al. (2011), who found a 33.1 ± 0.4 mg/100 g edible portion. This vitamin is found in particularly high amounts in cape gooseberry in contrast to other fruits, but there is great variation [], reaching up to 68 mg/100 g []. Regarding the antioxidant capacity, the ABTS value was higher than that reported by Céron, Higuita, and Cardona (2011) [], who reported an antioxidant capacity of 0.426 µmol ET/g. The FRAP value was also higher than those reported by Céron et al. (2011) and La Vega et al. (2019) [], who found a range between 0.055 and 2.661 μmol TE/100 g. These variations may be due to the edaphoclimatic differences of each variety, and these, in turn, induce variable contents of natural phytochemicals, such as phenolic compounds, anthocyanins, carotenoids, and vitamins, that are related to antioxidant capacities [,] These differences may be also associated with the fact that the antioxidant capacity shows increasing values with increasing ripening of the cape gooseberry fruit, and there is no information regarding the state of maturity of the fruit used for the cited studies.

Table 2.
Antioxidant capacity, total phenolic content (TPC), carotenoids, and vitamin C content of cape gooseberry fruit.
3.2. Postharvest Treatments
3.2.1. Physicochemical and Texture Characteristics
Table 3 shows the moisture content, TSS, pH, total acidity, vitamin C, TPC, and firmness of the cape gooseberry fruit after the different postharvest treatments and the control (fresh fruit). The results show that there were no significant differences (p > 0.05) in the moisture content among the treatments. Regarding the TSS, the highest TSS value (p < 0.05) was that of the fruit subjected to the coating process; this may be because of the addition of sodium carbonate (soluble salt), which increases the total soluble solids content of the fruit, and the plasticizing effect of glycerol. For the other treatments, it was possible to maintain a lower concentration of the soluble solids because of the contact with the water solutions, which indicates that they had an impact on the slower ripening of the fruit []. As for the total acidity, significant differences were observed among the treatments, and the immersion process presented the highest acidity (p < 0.05), which was because of the addition of the citric and ascorbic acids in the immersion solutions. This agrees with the results for the pH, in which the immersion process had the lowest value. Regarding the vitamin C content, the coating and immersion treatments led to higher vitamin C values than for the control fruit. In the case of the coating treatment, it had a protective barrier against permeability to O2 and CO2, thus decreasing vitamin autooxidation []. In the case of the immersion treatment, it is worth mentioning that ascorbic acid was added to the immersion solution, thus resulting in a significantly higher content (p < 0.05) compared to the control. For the total phenolic content (TPC), there significant differences among treatments (p < 0.05). On the one hand, it could be related to the measurement technique, since the Folin–Ciocalteu method measures the reduction of the reagent with the formation of a blue complex [], but it has low specificity, as it is able to react with any oxidizable hydroxyl, such as those present in the structure of vitamin C, and, as we stated before, the vitamin C values were higher for the treated fruit. However, some studies indicate that those higher values can be also attributed to the greater extraction of compounds from within the matrix during the treatments []. Concerning the fruit firmness, it was directly affected by the different postharvest treatments. Although calcium has been used in fruit pretreatments, and it is generally associated with increased firmness [], in this case, the effect of the process itself was superior. In the case of vacuum impregnation, it presented the lowest firmness values; this may be related to the pressure received by the fruit during the vacuum pressure stage, which could have affected the surface of the cape gooseberry, breaking its original structure, thus resulting in a general softening of the fruit. In fact, this tissue damage was visually perceived in some fruit. According to Fito et al. (1994), the VIP occurs through a hydrodynamic mechanism (HDM), along with deformation–relaxation phenomena (DRP), that the matrix undergoes because of a pressure gradient. Therefore, the final response to the vacuum impregnation is defined by the coupling of both phenomena, HDM and DRP, which will affect both their kinetics and the final equilibrium of the system. The HDM and DRP obviously concern the food’s microstructure and mechanical properties, as well as the processing conditions []. Several papers have reported the incorporation of active components via vacuum impregnation, presenting successful applications in some fruits and vegetables. Since the cape gooseberry is a highly complex fruit due to the presence of a waxy layer on the surface and a low natural porosity that increases because of microfissures on the surface due to pressure, this indicates that the vacuum process is more complex. Thus, in our case, the specific pressure gradients and food microstructure led to a lower postharvest quality. Sharma and Dash (2019) [] describe the vacuum, which expels intracellular air, forming a high degree of intercellular partitions and voids causing significant changes to the structure, leading to decomposition of the fruit tissue. Concerning the immersion treatment, CaCl2 was added to the immersion solution, so it could be the reason for the improvement in the firmness, as has been reported before for other food matrices []. In this context, it has been reported that Ca2+ establishes bonds with pectins, forming calcium pectates that increase the firmness of the cell wall []. It has been stated that with calcium pretreatment, two calcium processes have been identified: (1) bound to carboxyl groups and (2) free or remaining unbound in the plant tissue []. In addition, according to Pérez-Martínez et al. (2021), the application of antioxidant agents such as ascorbic acid minimizes and prevents enzymatic reactions, texture changes, and the development of unpleasant flavors and aromas, because they have a high affinity for fruit carbohydrates, thus providing acid and reducing properties that sequester oxygen and act as chelating agents that retard the deterioration processes caused by enzymes and are also accelerated by lesions in plant tissue, which directly influences the firmness of the fruit. Therefore, the addition of this acid would also be contributing indirectly to improving the firmness of the fruit treated with immersion.

Table 3.
Quality characteristics of cape gooseberry fruit subjected to different postharvest treatments.
3.2.2. Weight Loss
Figure 1 shows the weight loss for the CP, VIP, and IP over 12 days of storage. It can be seen that at the end of the study (day 12), the weight loss percentage for the vacuum impregnation process (VIP) was significantly higher (11.6%) when compared to the control (7.9%), and the other treatments (CP 6.1% and IP 7.4%). For its part, the immersion process (IP) led to similar values as the control (C) for all days of the study. Finally, with the coating process (CP), the fruit had the lowest weight loss percentage, going from 0.4% on day 2 to 6.1% on day 12. It has been reported that a coating process on fruit leads to a reduced weight loss. López et al. (2016) reported a significant weight loss reduction, from 23% to 15%, in cape gooseberry samples stored for 15 days at 17 °C, with the use of a whey and beeswax based coating. Muñoz et al. (2017) [] were able to prolong cape gooseberries stored from 9 to 11 days while maintaining a weight loss of less than 10% with the use of a chitosan and aloe vera based coating. According to what was reported by Pinzón et al. (2015), when a samples’ losses exceed 10% of the samples’ weight, the freshness of the fruits and vegetables disappear. The large weight losses of the fruit subjected to the vacuum impregnation process can be explained by the product deformations promoted by the pressure changes due to the viscoelastic properties of the fruit solid matrix. The VIP has been described as a fast mass transfer phenomenon, which occurs when porous structures are immersed in a liquid phase and pressure gradients are generated. This causes the external solution to move towards inside the food structure []. Thus, the cape gooseberries subjected to VIP had a greater weight than the untreated fruit, which was related to external liquid gain. However, in some cases, deformations can lead to the size and shape of the pores being changed [], even resulting in tissue injury that may subsequently promote fluid leakage. Regarding the immersion process, at the end of the study, a weight loss of less than 10% was obtained, keeping the freshness similar to the control. Pauro-Flóres (2016) [] achieved a 10% weight loss after 18 days of refrigerated storage (3 °C) and 13 days at room temperature (13 °C) when using immersion in solution with 3% calcium chloride and also found higher values when using coated fruit: 15 and 21 for room temperature and refrigeration, respectively.
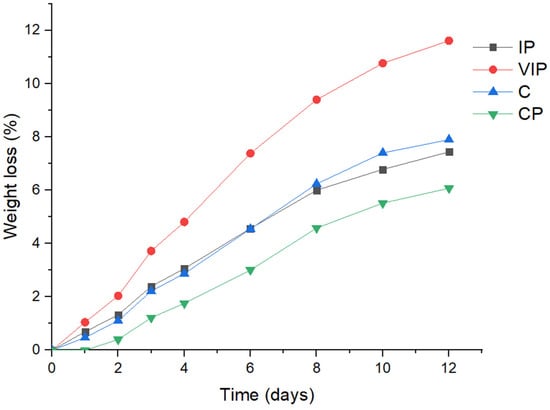
Figure 1.
Weight loss (%) for the control fruit (C) and fruit subjected to three different treatments: coating process (CP); vacuum impregnation process (VIP); immersion process (IP).
3.2.3. Sensory Analysis
Figure 2 shows the descriptors of fresh and treated cape gooseberry found for appearance (A)—brightness (ABG), homogeneous color (AHG), and uniform surface (AUS); for texture (T)—hard (THD), chewable (TCW), fibrous (TFB), dry (TDR), waxy (TWX), and rubbery (TRB); for flavor (F)—sweet (FSW), acid (FAC), floral (FFL), fruity (FFR), herbal (FHB), chemical (FCH), bitter (FBT), saline (FSL), and waxy (FWX); for odor (O)—sweet (OSW), acid (OAC), floral (OFL), fruity (OFR), herbal (OHB), chemical (OCH), dairy (ODR), and perfume (OPF); for somatosensory sensation (S)—astringent (SAT) and spicy (SSP). The figure also shows the overall quality (OQ) of the fresh and treated fruit. The panelists highlighted some aspects of each of the treatments, including the control fruit. The sensory characteristics of the control present different types of notes associated with sweet, fruity, and herbal flavors, which are predominant for this type of fruit product as they indicate their final quality []. The fresh cape gooseberry presents a waxy surface attributed to the fruit’s own natural wax and which constitutes an important point in its long-term preservation, since it prevents moisture loss and delays the phenomena associated with senescence. In the fruit subjected to VIP, panelists found the presence of spicy and chemical notes that provided an uncharacteristic residual to the fruit. It could be attributed to the addition of substances such as sodium carbonate and calcium chloride, since with the use of VIP the porous structure of the matrix is filled with the substances present in the impregnation solution due to the pressure differences []. Regarding the IP, it was characterized by the sensory panel as a balanced treatment in relation to flavor and odor and presented texture characteristics similar to the control, being ideal for a fresh fruit product. This balance is because of the presence of ascorbic and citric acids, together, counteracting the presence of sodium carbonate and calcium chloride, thus, helping to maintain the balance and opaquing the chemical notes. For the fruit subjected to CP, their overall quality was the lowest among all treatments, with an overall rating of two, evidencing that this process had a negative impact on sensory attributes. This low quality is associated with the presence of chemical, astringent, and unpleasant notes, which affect appearance parameters such as brightness and sweet taste, as well as flavor and odor descriptors.
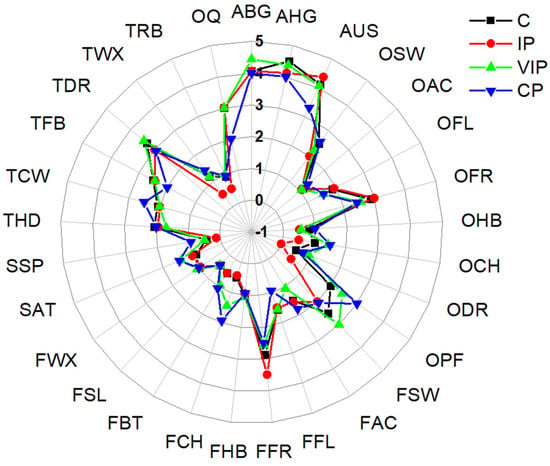
Figure 2.
Sensory analysis of cape gooseberry for the control fruit (C) and fruit subjected to three different treatments: coating process (CP); vacuum impregnation process (VIP); and immersion process (IP). Descriptors for appearance (A)—brightness (ABG), homogeneous color (AHG), and uniform surface (AUS); for texture (T)—hard (THD), chewable (TCW), fibrous (TFB), dry (TDR), waxy (TWX), and rubbery (TRB); for flavor (F)—sweet (FSW), acid (FAC), floral (FFL), fruity (FFR), herbal (FHB), chemical (FCH), bitter (FBT), saline (FSL), and waxy (FWX); for odor (O)—sweet (OSW), acid (OAC), floral (OFL), fruity (OFR), herbal (OHB), chemical (OCH), dairy (ODR), and perfume (OPF); for somatosensory sensation (S)—astringent (SAT) and spicy (SSP).
3.2.4. Postharvest Treatment Selection
Considering the results obtained with the three postharvest treatments applied to the fruit (CP, VIP, and IP) in terms of physicochemical characterization, weight loss, texture analysis, and sensory analysis, the immersion treatment (IP) was selected to continue with the development of the research.
3.3. Immersion Process (IP) under Different Conditions
The evaluation of the IP was carried out using a factorial experimental design (Table 4). Twelve runs were carried out for the treated samples. The value obtained for the moisture of the treated fruit ranged between 81.78% and 84.45%. The value obtained for TSS was in the range of 12.60 and 14.50. The firmness of the fruit was between 22.40 and 34.00 gf. Regarding the weight loss after 12 days of storage, the values were between 6.51 and 10.91%. The results showed that there were no significant differences (p > 0.05) among any of the response variables including firmness by the effect of the calcium concentration (1–3%). Although a higher firmness might be expected with a higher calcium concentration used, and this behavior could be attributed to the calcium, which can precipitate at the entrance of the pores blocking the entrance of more compounds, as has been stated before []. However, comparing the values of firmness for the treated fruit and the control, it is noticeable that the treated fruit showed higher values. Since the use of different concentrations of calcium chloride led to indistinct firmness values, low concentrations can be used to obtain good results at a lower economic cost. On the other hand, there were significant differences (p < 0.05) among the firmness values because of the immersion time in CaCl2. The longer the immersion time, the lower the firmness of the product. This may be because of the relaxation of the fruit tissue which favored calcium loss, especially those free or not strongly bound to pectins within the tissue []. In addition, after reaching equilibrium, an osmotic effect may have occured. Barrera et al. (2009) [] reported a reduction in calcium content after osmosis due to the concentration gradient, and Martínez-Sánchez et al. (2022) [] observed a leaching effect in banana tissue at higher concentrations and temperatures of the osmotic solution. Finally, a correlation analysis by Person showed a significant (p < 0.05) negative correlation (−0.6397) between the moisture content and total soluble solids. This is compositionally related, since the variable TSS is inversely proportional to the moisture content; thus, the higher the humidity, the lower the solids and vice versa.

Table 4.
Results of the experimental design and immersion process.
4. Conclusions
Of the three treatments evaluated (CP, VIP, and IP), the lowest weight loss was recorded with the use of coating (CP) and immersion (IP). However, the immersion process resulted in the product with the highest overall quality according to the sensory analysis, being comparable with the fresh fruit. Regarding the firmness, one of the most important quality attributes for the commercialization and export of fruits, the IP also presented the firmest products. Texture is related to several parameters such as water loss. In this sense, considering 10% as a commercial standard limit for weight loss, fruit treated with immersion and coating processes can be stored for at least 12 days. However, the CP had the lowest overall sensory quality. Therefore, the immersion process is highlighted, since this treatment helps to maintain fruit quality, so it is presented as a promising alternative for the postharvest treatment of cape gooseberries but that it is necessary to continue exploring. For future studies, it will be interesting to incorporate different temperatures for the immersion solutions for IP and to include the use of agitation during the period the fruit is submerged. As a general conclusion, it is important to mention that the quality characteristics of cape gooseberry cannot be determined by a single variable but must be a set of attributes, and it is important to include sensory analysis, as it will determine the consumer’s acceptance of the product.
Author Contributions
S.A.-S.: Conceptualization, formal analysis, investigation, methodology, validation, and writing—original draft; Y.M.-P.: Conceptualization, formal analysis, investigation, methodology, validation, and writing—original draft; D.D.-Ú.: Conceptualization, data curation, formal analysis, investigation, methodology, validation, and writing—original draft; S.C.-M.: Formal analysis, methodology, validation, and writing—original draft; Y.D.-C.: Supervision, project administration, resources, writing—original draft, and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Faculty of Pharmaceutical and Food Sciences (CIFAL), Universidad de Antioquia, Medellín, Colombia (Project CIFAL-336, 2022). The role of the funding source was limited to providing resources to develop the research.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
This work was financially supported by resources from the Universidad de Antioquia (Medellín, Antioquia, Colombia) through the project CIFAL-336, for the Small Projects for undergraduate students call for proposals 2022.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cardenas-Barboza, L.C.; Paredes-Cordoba, A.C.; Serna-Cock, L.; Guancha-Chalapud, M.; Torres-Leon, C. Quality of Physalis peruviana Fruits Coated with Pectin and Pectin Reinforced with Nanocellulose from P. Peruviana Calyces. Heliyon 2021, 7, e07988. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Chaudhari, S.K.; Arshad, S.; Fatima, H.; Amjad, M.S. Saboon Biological Activities and Nutritional Value of Physalis peruviana L. Nat. Bio-Active Compd. Vol. 1 Prod. Appl. 2019, 587–598. [Google Scholar] [CrossRef]
- González-Locarno, M.; Pautt, Y.M.; Albis, A.; López, E.F.; Tovar, C.D.G. Assessment of Chitosan-Rue (Ruta Graveolens l.) Essential Oil-Based Coatings on Refrigerated Cape Gooseberry (Physalis peruviana L.) Quality. Appl. Sci. 2020, 10, 2684. [Google Scholar] [CrossRef]
- Etzbach, L.; Pfeiffer, A.; Weber, F.; Schieber, A. Characterization of Carotenoid Profiles in Goldenberry (Physalis peruviana L.) Fruits at Various Ripening Stages and in Different Plant Tissues by HPLC-DAD-APCI-MSn. Food Chem. 2018, 245, 508–517. [Google Scholar] [CrossRef]
- Ministerio de Agricultura y Desarrollo Rural AGRONET-Rendimiento Nacional Por Cultivo. Uchuva. Available online: https://www.agronet.gov.co/estadistica/Paginas/home.aspx?cod=1 (accessed on 31 January 2022).
- Heredia, A.M.; Quiroga, R.J.; Kirschbaum, D.S. Primer Reporte de Géneros Fúngicos Causando Decaimiento Poscosecha En Goldenberry (Physalis peruviana L.) En Argentina. Libro De Resúmenes. 4 Congr. Argent. De Fitopatol. 2017, 219, 2. [Google Scholar]
- Bisht, B.; Bhatnagar, P.; Gururani, P.; Kumar, V.; Tomar, M.S.; Sinhmar, R.; Rathi, N.; Kumar, S. Food Irradiation: Effect of Ionizing and Non-Ionizing Radiations on Preservation of Fruits and Vegetables—A Review. Trends Food Sci. Technol. 2021, 114, 372–385. [Google Scholar] [CrossRef]
- Denoya, G.I.; Colletti, A.C.; Vaudagna, S.R.; Polenta, G.A. Application of Non-Thermal Technologies as a Stress Factor to Increase the Content of Health-Promoting Compounds of Minimally Processed Fruits and Vegetables. Curr. Opin. Food Sci. 2021, 42, 224–236. [Google Scholar] [CrossRef]
- Pérez-Martínez, B.S.; Ramos-Dubón, E.J.; Ramos-Cortez, S.; Munguía, H.E. Evaluación de Dos Combinaciones de Conservantes y Su Efecto Sobre Un Producto Hortícola de IV Gama. Rev. Agrociencia-Rev. Conten. Científico La Fac. Cienc. Agronómicas La Univ. El Salvador 2021, 4, 38–49. [Google Scholar]
- Balaguera-lópez, H.E.; Martínez, C.A.; Herrera-Arévalo, A. The Role of the Calyx in the Postharvest Behavior of Cape Gooseberry Fruits Ecotype Colombia. Rev. Colomb. Cienc. Hortícolas 2014, 8, 181–191. [Google Scholar]
- Pinzón, E.H.; Reyes, A.J.; Álvarez-Herrera, J.G.; Leguizamo, M.F.; Joya, J.G. Comportamiento Del Fruto de Uchuva Physalis peruviana L., Bajo Diferentes Temperaturas de Almacenamiento. Rev. Cienc. Agrícolas 2015, 32, 26–35. [Google Scholar] [CrossRef]
- Ciro, G. Conservación de Uchuva Basada En La Impregnación a Vacío de Extractos de Plantas Con Actividad Antimicrobiana y Antioxidante. Ph.D. Thesis, Universidad de Antioquia, 2012. [Google Scholar]
- Puente, L.A.; Pinto-Muñoz, C.A.; Castro, E.S.; Cortés, M. Physalis peruviana Linnaeus, the Multiple Properties of a Highly Functional Fruit: A Review. Food Res. Int. 2011, 44, 1733–1740. [Google Scholar] [CrossRef]
- Turkkan, M.; Ozcan, M.; Erper, İ. Antifungal Effect of Carbonate and Bicarbonate Salts against Botrytis Cinerea, the Casual Agent of Grey Mould of Kiwifruit. Akad. Ziraat Derg. 2017, 6, 107–114. [Google Scholar] [CrossRef]
- Duarte-Correa, Y.; Díaz-Osorio, A.; Osorio-Arias, J.; Sobral, P.J.A.; Vega-Castro, O. Development of Fortified Low-Fat Potato Chips through Vacuum Impregnation and Microwave Vacuum Drying. Innov. Food Sci. Emerg. Technol. 2020, 64, 102437. [Google Scholar] [CrossRef]
- Instituto Colombiano de Normas Técnicas y Certificación (ICONTEC) Norma Técnica Colombiana (NTC) 4580, Frutas Frescas. Uchuva. Especificaciones; ICONTEC: Bogotá (D.C.), Colombia, 1999; Available online: https://kontii.files.wordpress.com/2012/10/ntc-4580.pdf (accessed on 31 January 2022).
- Muley, A.B.; Singhal, R.S. Extension of Postharvest Shelf Life of Strawberries (Fragaria Ananassa) Using a Coating of Chitosan-Whey Protein Isolate Conjugate. Food Chem. 2020, 329, 127213. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 17th ed.; Latimer, D.G.W., Ed.; Association of Offical Analytical Chemists: Gaithersburg, MD, USA, 2000; ISBN 935584870. [Google Scholar]
- Contreras-Calderón, J.; Calderón-Jaimes, L.; Guerra-Hernández, E.; García-Villanova, B. Antioxidant Capacity, Phenolic Content and Vitamin C in Pulp, Peel and Seed from 24 Exotic Fruits from Colombia. Food Res. Int. 2011, 44, 2047–2053. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.; Kimura, M. Handbook for Carotenoid Analysis. Harvest. Tech. Monogr. 2004, 8–19. [Google Scholar]
- Osorio-Arias, J.; Delgado-Arias, S.; Cano, L.; Zapata, S.; Quintero, M.; Nuñez, H.; Ramírez, C.; Simpson, R.; Vega-Castro, O. Sustainable Management and Valorization of Spent Coffee Grounds Through the Optimization of Thin Layer Hot Air-Drying Process. Waste Biomass Valorization 2019, 11, 5015–5026. [Google Scholar] [CrossRef]
- Olivares-Tenorio, M.L.; Dekker, M.; van Boekel, M.A.J.S.; Verkerk, R. Evaluating the Effect of Storage Conditions on the Shelf Life of Cape Gooseberry (Physalis peruviana L.). Lwt 2017, 80, 523–530. [Google Scholar] [CrossRef]
- Instituto Colombiano de Normas Técnicas y Certificación (ICONTEC) Norma Tecnica Colombiana (NTC) 3932. Análisis Sensorial. In Identificación y Selección de Descriptores Para Establecer Un Perfil Sensorial Por Una Aproximación Multidimensional; Instituto Colombiano de Normas Técnicas y Certificación: Bogotá (D.C.), Colombia, 1996. [Google Scholar]
- Bravo, K.; Sepulveda-Ortega, S.; Lara-Guzman, O.; Navas-Arboleda, A.A.; Osorio, E. Influence of Cultivar and Ripening Time on Bioactive Compounds and Antioxidant Properties in Cape Gooseberry (Physalis peruviana L.). J. Sci. Food Agric. 2015, 95, 1562–1569. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, M.F. Bioactive Phytochemicals, Nutritional Value, and Functional Properties of Cape Gooseberry (Physalis peruviana): An Overview. Food Res. Int. 2011, 44, 1830–1836. [Google Scholar] [CrossRef]
- Graça Dias, M.; Olmedilla-Alonso, B.; Hornero-Méndez, D.; Mercadante, A.Z.; Osorio, C.; Vargas, L.; Meléndez-Martínez, A. Tabla de Contenido de Alimentos Iberoamericanos. En Carotenoides en agroalimentación y salud (Cap. 18); Programa Iberoamericano de Ciencia y Tecnología para el Desarrollo: Madrid, Spain, 2017; pp. 354–429. [Google Scholar]
- Valente, A.; Albuquerque, T.G.; Sanches-Silva, A.; Costa, H.S. Ascorbic Acid Content in Exotic Fruits: A Contribution to Produce Quality Data for Food Composition Databases. Food Res. Int. 2011, 44, 2237–2242. [Google Scholar] [CrossRef]
- Céron, I.; Higuita, J.; Cardona, C. Capacidad Antioxidante y Contenido Fenólico Total de Tres Frutas Cultivadas En La Región Andina. Vector 2011, 18, 2–10. [Google Scholar]
- La Vega, J.C.D.; Cañarejo, M.A.; Cabascango, O.N.; Lara, M.V. Deshidratado de Physalis peruviana L. En Dos Estados de Madurez y Su Efecto Sobre El Contenido de Polifenoles Totales, Capacidad Antioxidante, Carotenos, Color y Ácido Ascórbico. Inf. Tecnológica 2019, 30, 91–100. [Google Scholar] [CrossRef]
- Duarte-Correa, Y.; Vargas-Carmona, M.I.; Vásquez-Restrepo, A.; Ruiz Rosas, I.D.; Pérez Martínez, N. Native Potato (Solanum Phureja) Powder by Refractance Window Drying: A Promising Way for Potato Processing. J. Food Process Eng. 2021, 44, e13819. [Google Scholar] [CrossRef]
- López, D.; Cuatin, L.; Andrade, J.; Osorio, O. Evaluation of an Edible Coating Based Whey Protein and Beeswax on the Physical and Chemical Quality of Gooseberry (Physalis peruviana L.). Acta Agron. 2016, 65, 326–333. [Google Scholar] [CrossRef]
- Sogvar, O.B.; Koushesh Saba, M.; Emamifar, A. Aloe Vera and Ascorbic Acid Coatings Maintain Postharvest Quality and Reduce Microbial Load of Strawberry Fruit. Postharvest Biol. Technol. 2016, 114, 29–35. [Google Scholar] [CrossRef]
- Barba, F.J.; Esteve, M.J.; Frigola, A. Physicochemical and Nutritional Characteristics of Blueberry Juice after High Pressure Processing. Food Res. Int. 2013, 50, 545–549. [Google Scholar] [CrossRef]
- Fito, P.; Andrb, A.; Chiralt, A.; Pardo, P. Coupling of Hydrodynamic Mechanism and Phenomena During Vacuum Treatments in Solid Porous Food-Liquid Systems. J. Food Eng. 1996, 21, 229–240. [Google Scholar] [CrossRef]
- Sharma, M.; Dash, K.K. Effect of Ultrasonic Vacuum Pretreatment on Mass Transfer Kinetics during Osmotic Dehydration of Black Jamun Fruit. Ultrason. Sonochem. 2019, 58, 104693. [Google Scholar] [CrossRef]
- Radziejewska-kubzdela, E.; Kido, M. Applicability of Vacuum Impregnation to Modify Physico-Chemical, Sensory and Nutritive Characteristics of Plant Origin Products—A Review. Int. J. Mol. Sci. 2014, 15, 16577–16610. [Google Scholar] [CrossRef]
- Rodríguez-ramírez, J.; Barragán-iglesias, J.; Ramírez-palma, A.J.; Méndez-lagunas, L.L. Effect of Calcium and Osmotic Pretreatments on Mass Transfer and Texture Parameters during Processing of Chilacayote (Cucurbita Ficifolia Bouché). 2023, 2023, 3873662. J. Food Process. Preserv. 2023, 2023, 3873662. [Google Scholar] [CrossRef]
- Muñoz, A.; Barbosa, A.; Bustos, D.; Ramírez, Y.; Vásquez, Y.; García, J.; Guancha, M. Conservation of Uchuva (Physalis Peruviana) by Applying a Coating Based on Chitosan and Aloe Vera, Using the Spray Method. Inf. Técnico 2017, 81, 86–94. [Google Scholar] [CrossRef]
- Duarte-Correa, Y.; Granda-Restrepo, D.; Cortés, M.; Vega-Castro, O. Potato Snacks Added with Active Components: Effects of the Vacuum Impregnation and Drying Processes. J. Food Sci. Technol. 2020, 57, 1523–1534. [Google Scholar] [CrossRef]
- Pauro-Flóres, V. Aplicación de Dos Métodos (Encerado o Inmersión En Cloruro de Calcio) Para La Conservación Poscosecha Del Aguaymanto (Physalis peruviana) Sin Cáliz; Universidad Nacional del Altiplano: Puno, Peru, 2016. [Google Scholar]
- Del Rodríguez, S.C.; Generoso, S.; Gutiérrez, D.; Questa, A. Application of Sensory Analysis in the Evaluation of Quality Fresh-cut Vegetables. Simiente 2015, 85, 1–27. [Google Scholar]
- Castagnini, J.M. Estudio Del Proceso de Obtención de Zumo de Arándanos y Su Utilización Como Ingrediente Para La Obtención de Un Alimento Funcional Por Impregnación a Vacío; Universidad Politécnica de Valencia: Valencia, Spain, 2015. [Google Scholar]
- Barrera, C.; Betoret, N.; Corell, P.; Fito, P. Effect of Osmotic Dehydration on the Stabilization of Calcium-Fortified Apple Slices (Var. Granny Smith): Influence of Operating Variables on Process Kinetics and Compositional Changes. J. Food Eng. 2009, 92, 416–424. [Google Scholar] [CrossRef]
- Martínez-Sánchez, C.; Solis-Ramos, A.; Rodríguez-Miranda, J.; Juárez-Barrientos, J.; Ramírez-Rivera, E.; Ruíz-López, I.; Gómez-Aldapa, C.; Herman-Lara, E. Evaluation of Ascorbic Acid Impregnation by Ultrasound-Assisted Osmotic Dehydration in Plantain. J. Food Process. Preserv. 2022, 46, e16839. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).