Effect of Preharvest Ethephon Application on Selected Biochemical Components and Polyphenol Oxidase Activity in Macadamia Nuts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethephon Spray and Concentration
2.2. Nut Collection and Preparation
2.3. Storage Conditions
2.4. Extraction and Determination of Total Polyphenols Content and Flavonoids
2.4.1. Quantification of Total Phenolic Content
2.4.2. Quantification of Total Flavonoid Content
2.4.3. 2,2,-Diphenyl-1-picrylhydrazyl (DPPH) Assay
2.4.4. Ferric Reducing Ability Power (FRAP) Assay
2.5. Sucrose Analysis
2.6. Protein Extraction
2.6.1. Protein Assay Using the Bradford Method
2.6.2. Quantification of Polyphenol Oxidase
2.7. Data Analysis
3. Results and Discussion
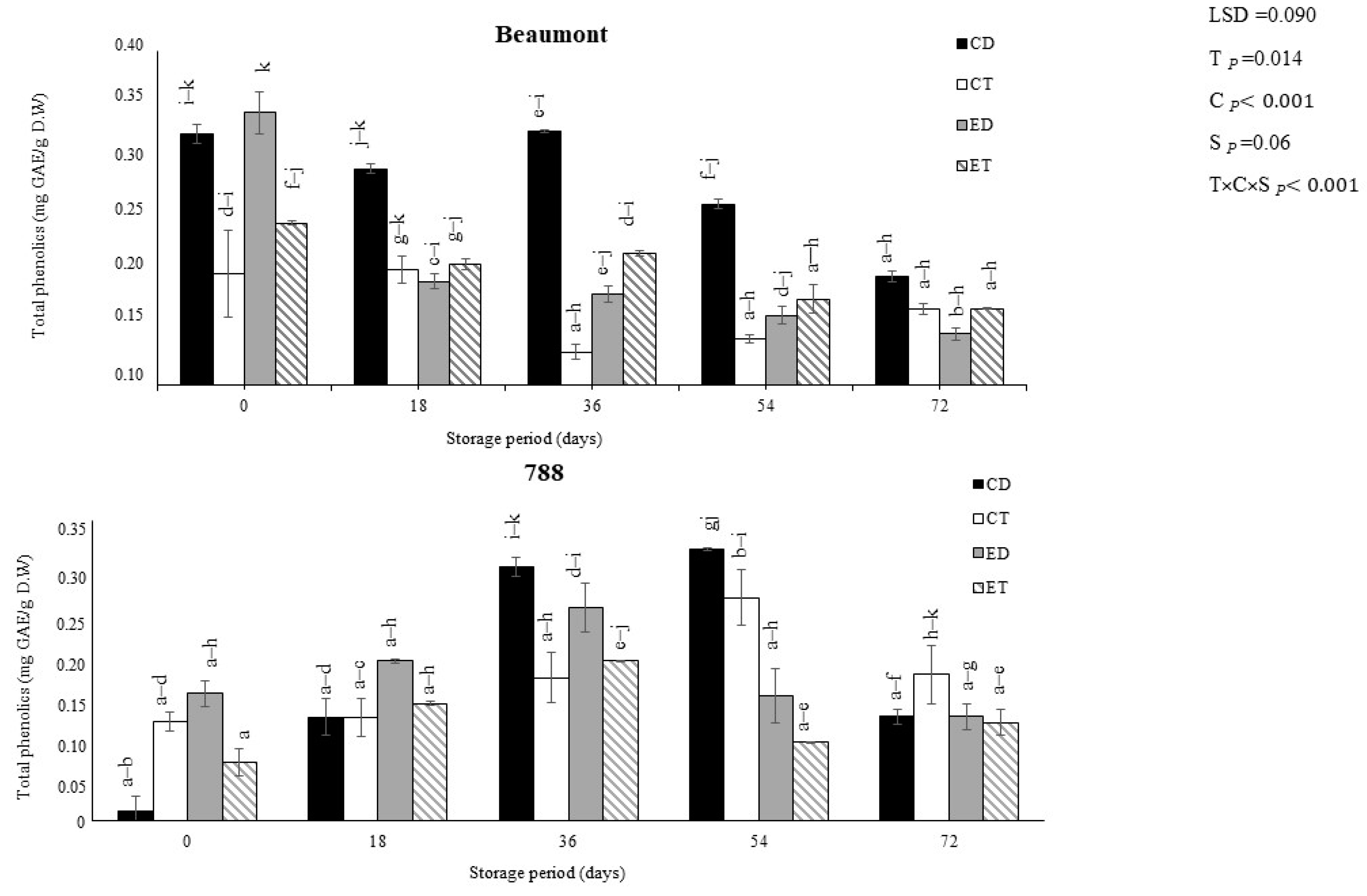
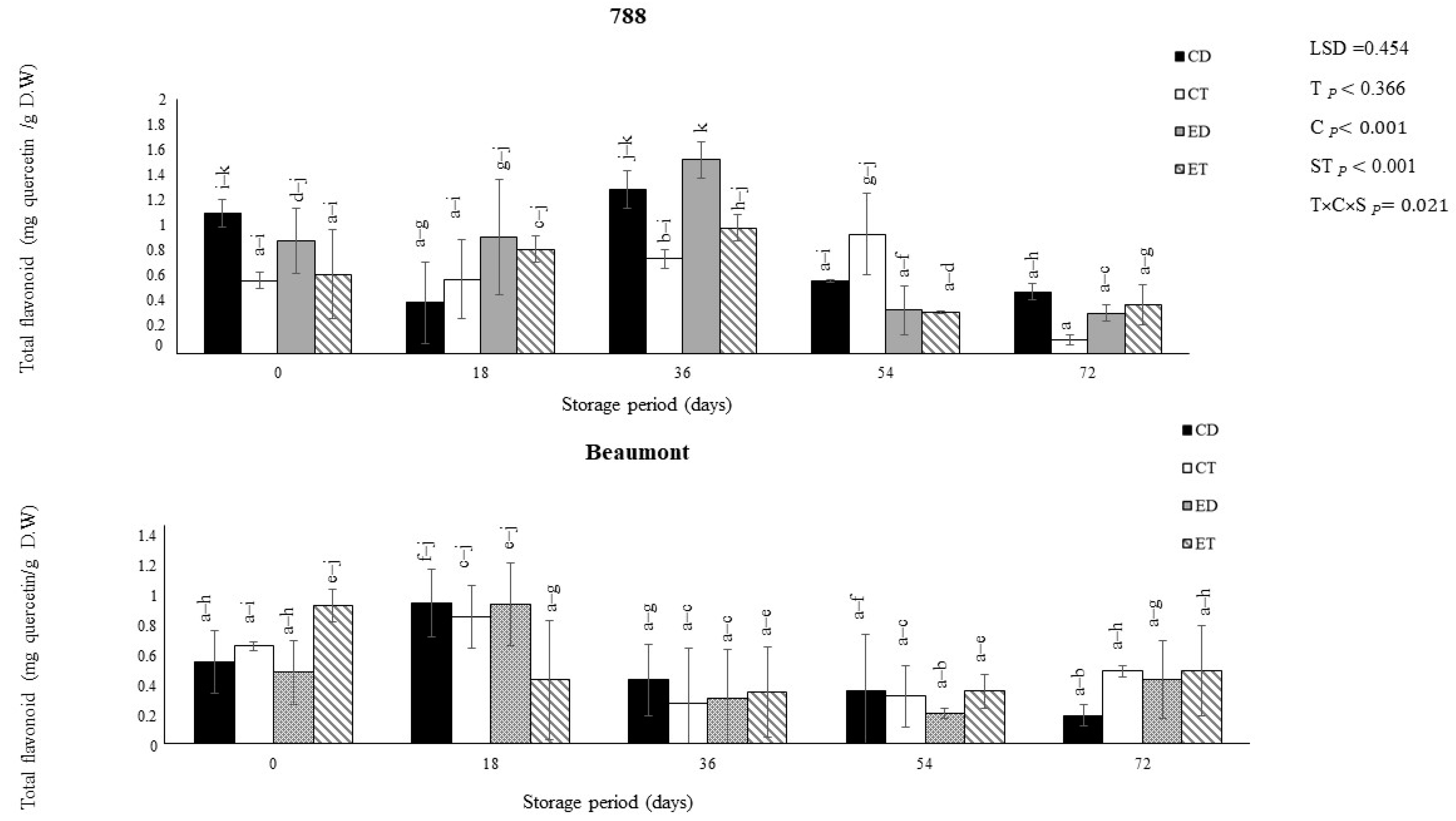
3.1. Total Phenolic Contents (TPC) and Total Flavonoid Contents (TFC)
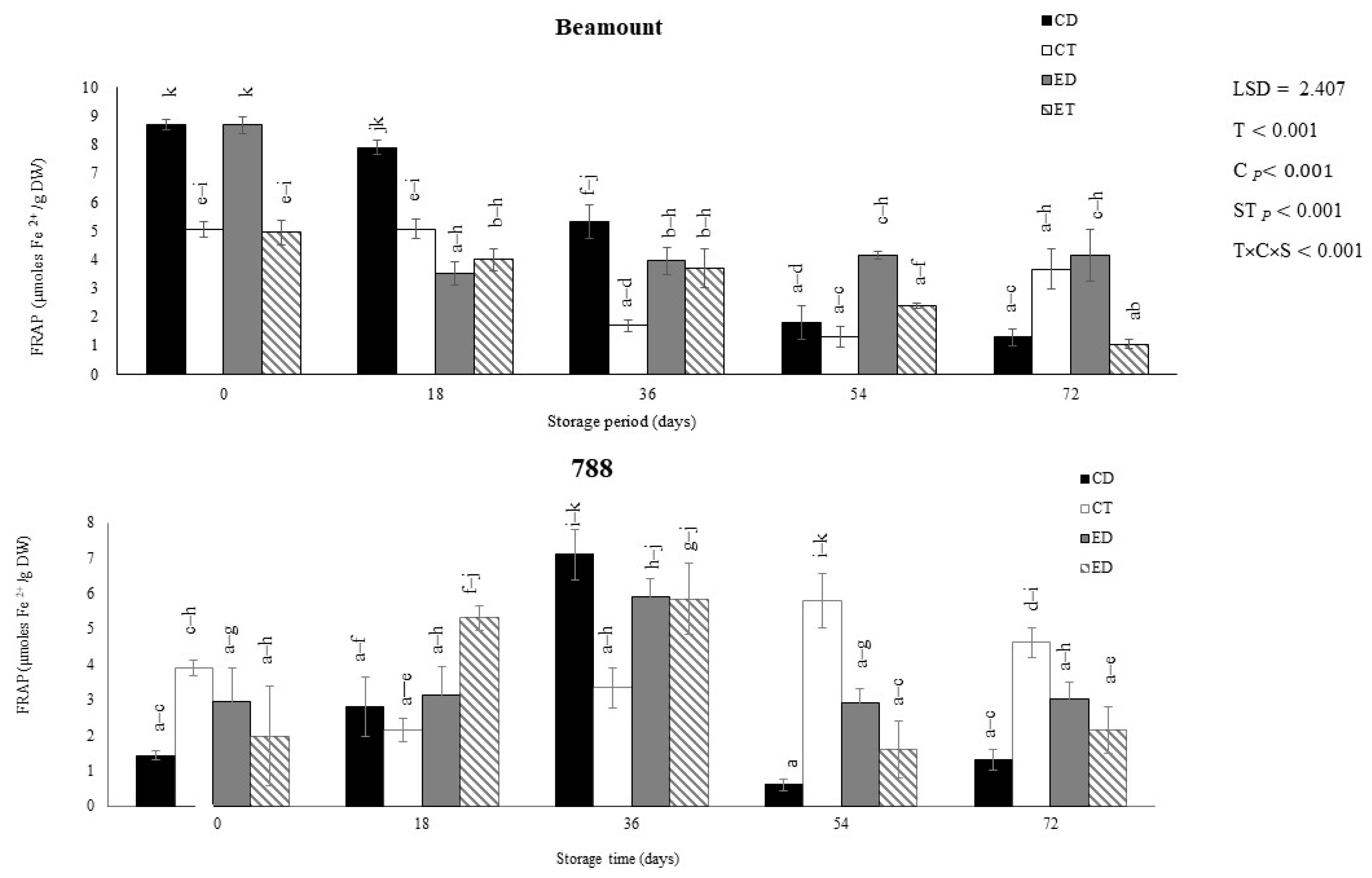
3.2. Antioxidant Activity (DPPH and FRAP)
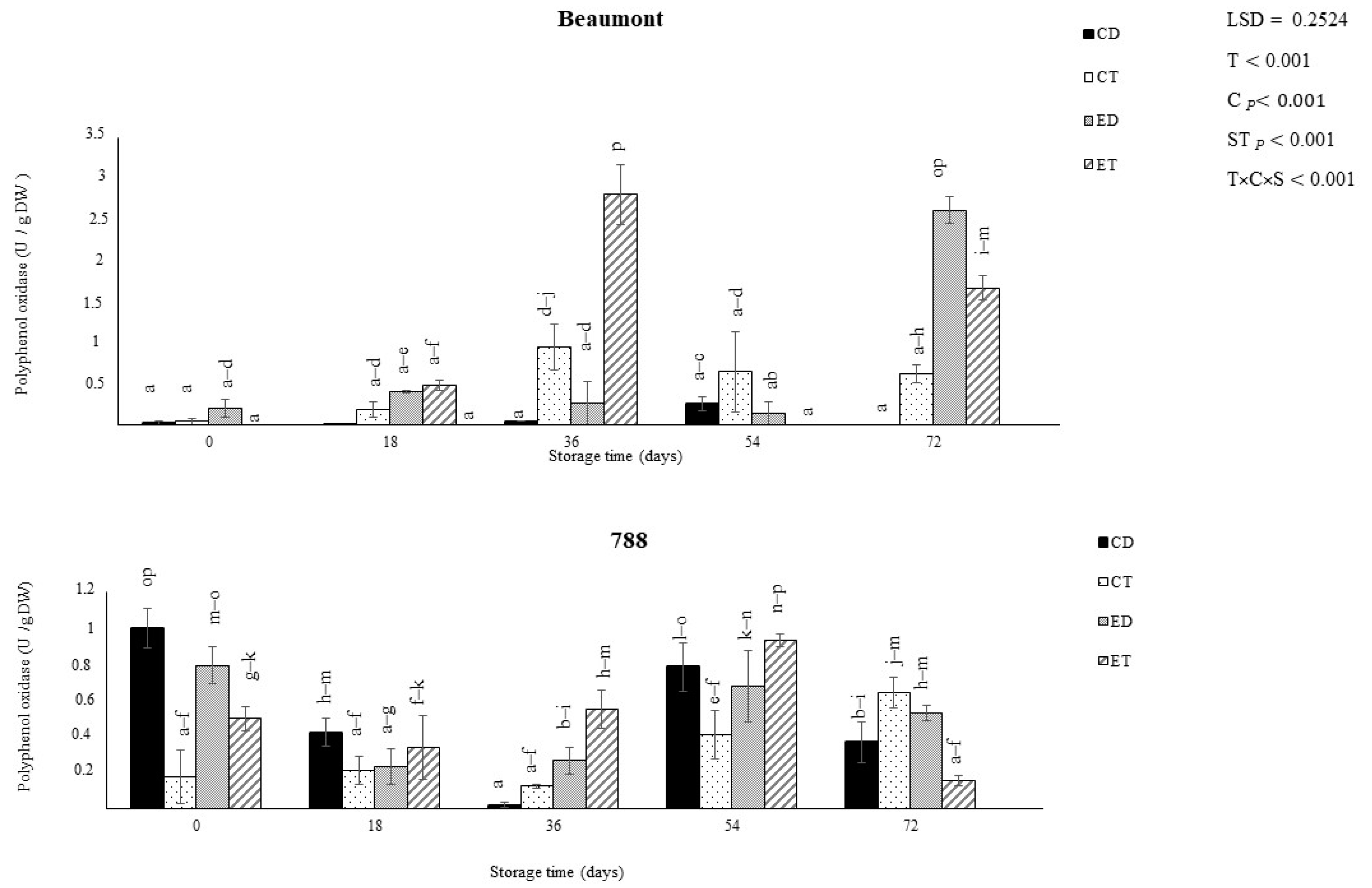
3.3. Polyphenol Oxidase Activity
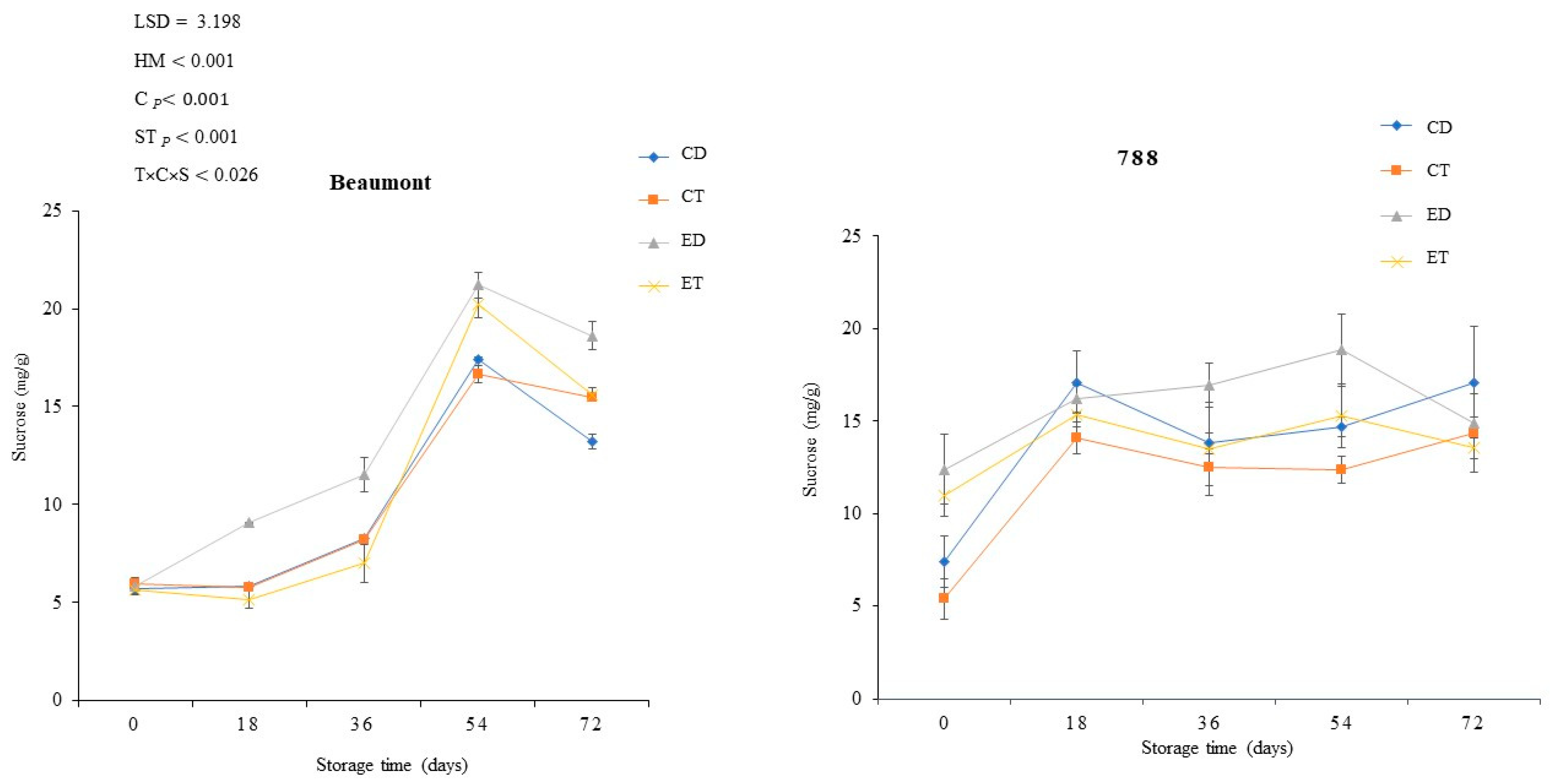
3.4. Sucrose Content
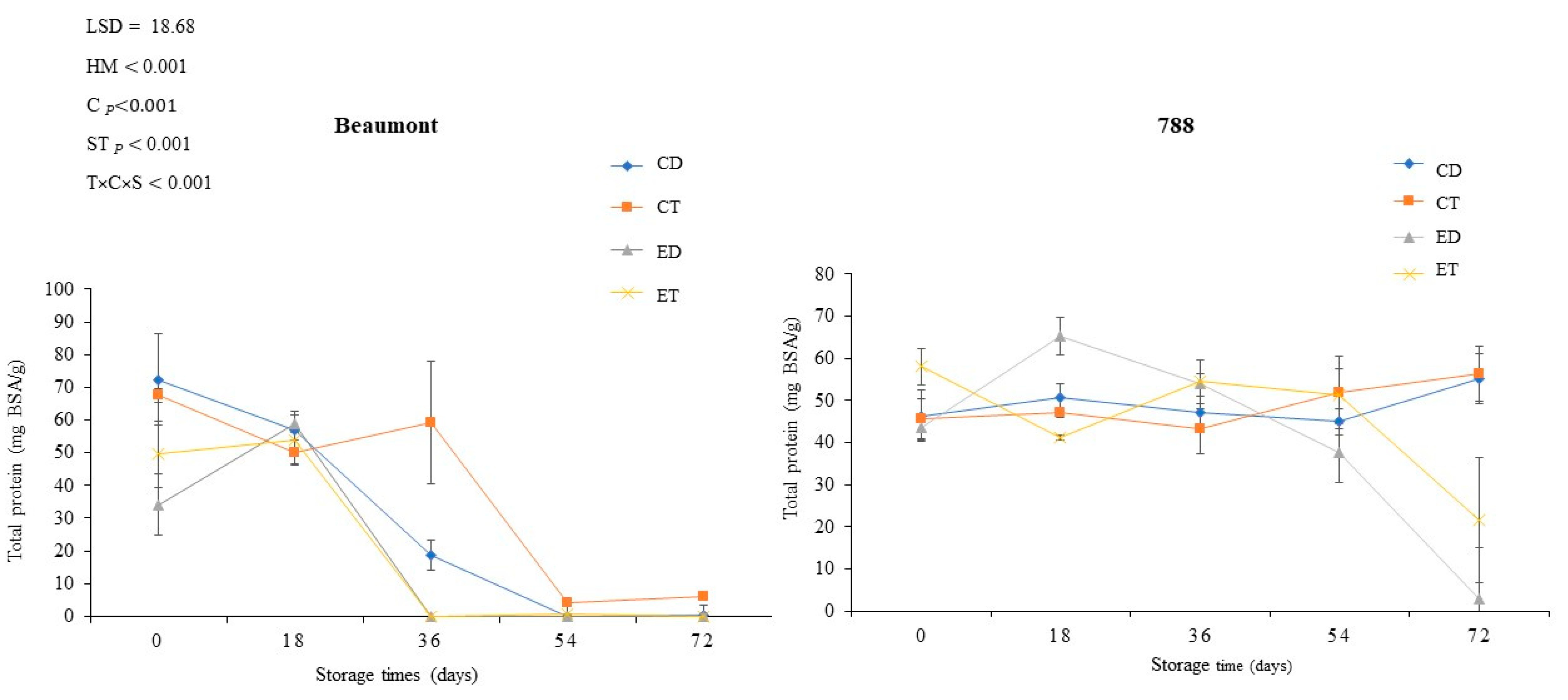
3.5. Total Protein Content
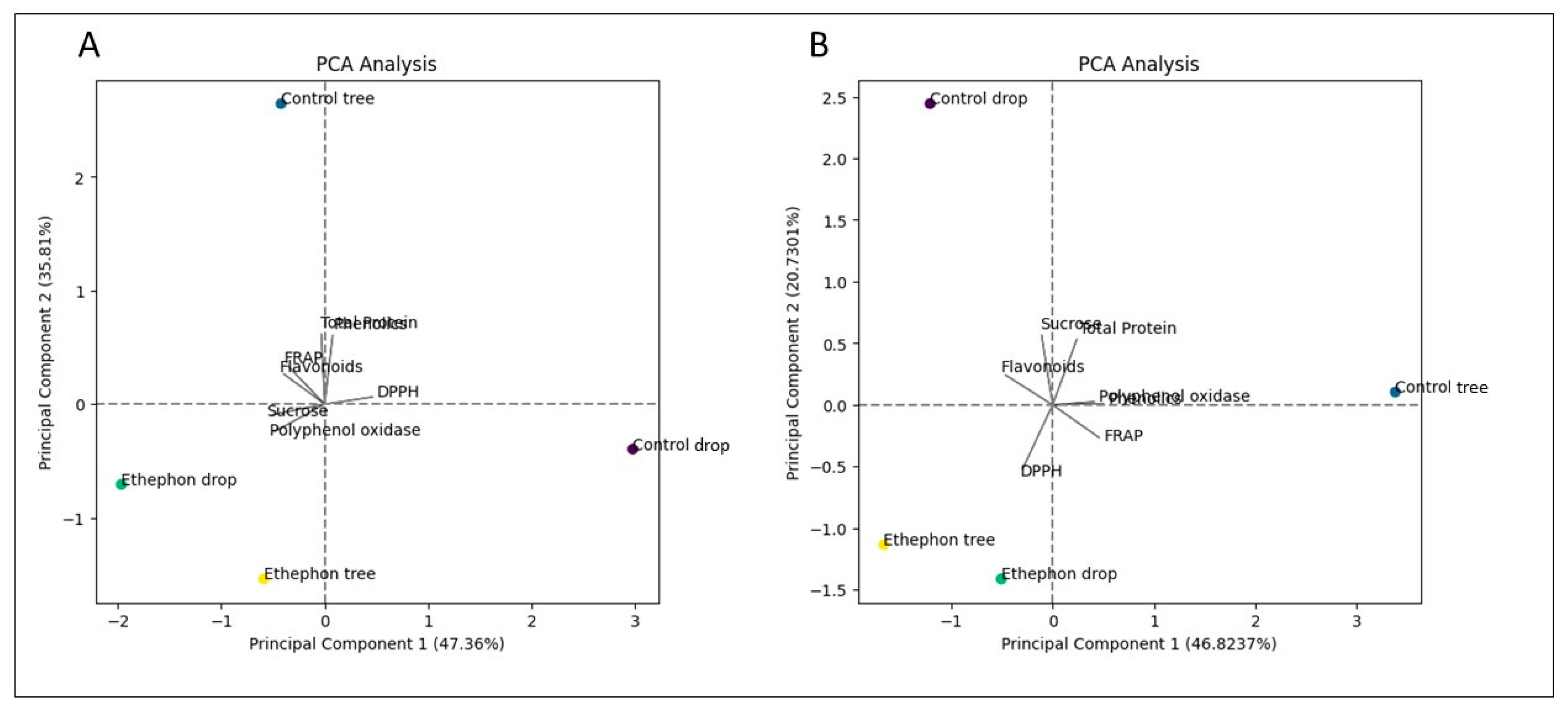
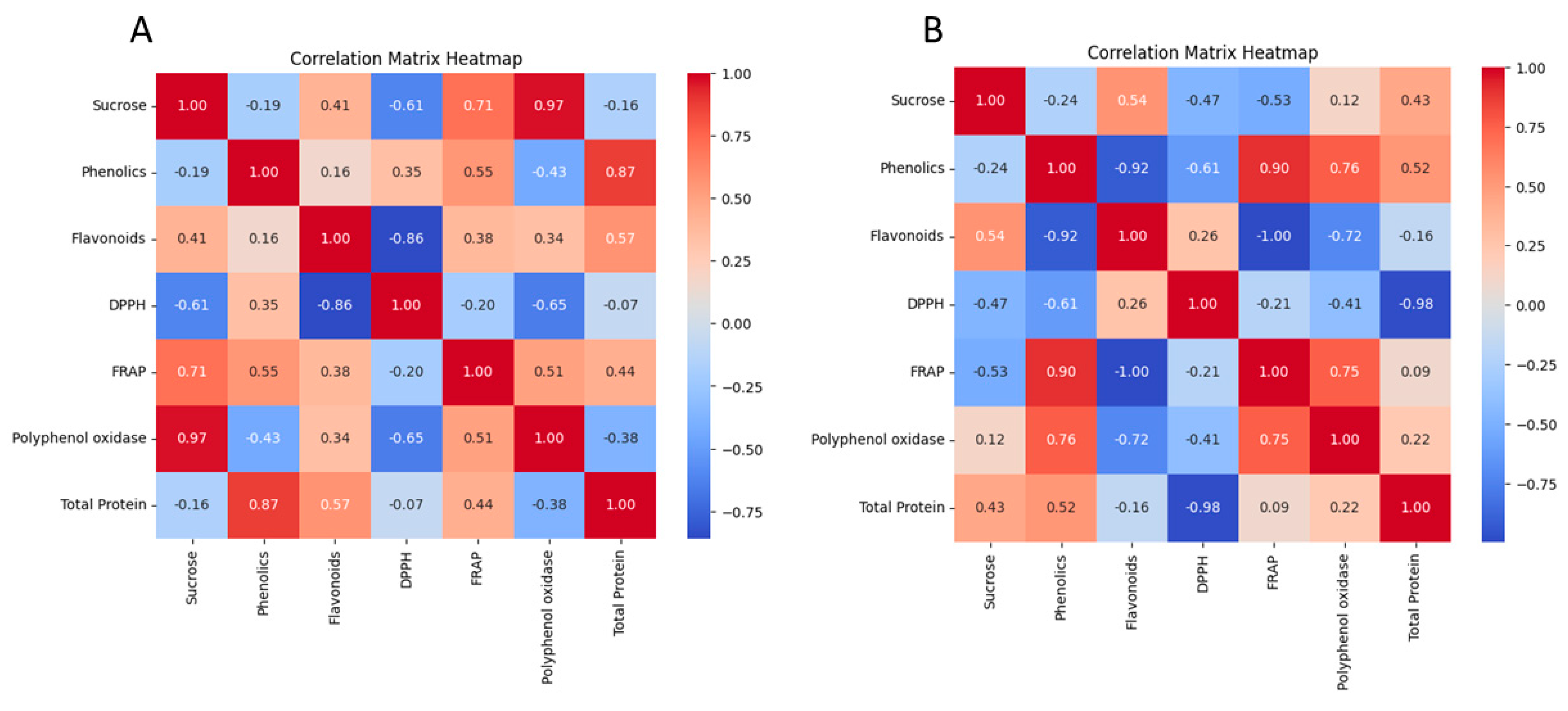
3.6. Correlation Matrix Heatmap and Principal Component Analysis (PCA)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Phatanayindee, S.; Borompichaichartkul, C.; Srzednicki, G.; Craske, J.; Wootton, M. Changes of chemical and physical quality attributes of macadamia nuts during hybrid drying and processing. Dry. Technol. 2012, 30, 1870–1880. [Google Scholar] [CrossRef]
- Wall, M.M. Improving the quality and safety of macadamia nuts. In Improving the Safety and Quality of Nuts; Woodhead Publishing: Sawston, UK, 2013; pp. 274–296. [Google Scholar]
- Tu, X.H.; Wu, B.F.; Xie, Y.; Xu, S.L.; Wu, Z.Y.; Lv, X.; Chen, H. A comprehensive study of raw and roasted macadamia nuts: Lipid profile, physicochemical, nutritional, and sensory properties. Food Sci. Nutr. 2021, 9, 1688–1697. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fan, L.; Li, J. Flavor and compositional analysis of macadamia nuts during long-term storage. J. Food Process. Preserv. 2022, 46, e16540. [Google Scholar] [CrossRef]
- Zhao, L.; Ai, X.; Pan, F.; Zhou, N.; Zhao, L.; Cai, S.; Tang, X. Novel peptides with xanthine oxidase inhibitory activity identified from macadamia nuts: Integrated in silico and in vitro analysis. Eur. Food Res. Technol. 2022, 248, 2031–2042. [Google Scholar] [CrossRef]
- Lopez, M.J.; Mohiuddin, S.S. Biochemistry, Essential Amino Acids. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Hu, W.; Fitzgerald, M.; Topp, B.; Alam, M.; O’Hare, T.J. A review of biological functions, health benefits, and possible de novo biosynthetic pathway of palmitoleic acid in macadamia nuts. J. Funct. Foods 2019, 62, 103520. [Google Scholar] [CrossRef]
- Stolp, L.J.; Kodali, D.R. Naturally occurring high-oleic oils: Avocado, macadamia, and olive oils. In High Oleic Oils; AOCS Press: Urbana, IL, USA, 2022; pp. 7–52. [Google Scholar]
- Mehmood, A.; Pan, F.; Ai, X.; Tang, X.; Cai, S.; Soliman, M.M.; Albogami, S.; Usman, M.; Murtaza, M.A.; Nie, Y.; et al. Novel angiotensin-converting enzyme (ACE) inhibitory mechanism of peptides from Macadamia integrifolia antimicrobial protein 2 (MiAMP2). J. Food Biochem. 2022, 46, e14168. [Google Scholar] [CrossRef]
- Lara, D.; Vilcacundo, E.; Carrillo, C.; Carpio, C.; Silva, M.; Alvarez, M.; Carrillo, W. Obtention of protein concentrate and polyphenols from macadamia (Macadamia integrifolia) with aqueous extraction method. Asian J. Pharm. Clin. Res. 2017, 10, 138–142. [Google Scholar]
- Afsah-Hejri, L.; Homayouni, T.; Toudeshki, A.; Ehsani, R.; Ferguson, L.; Castro-García, S. Mechanical harvesting of selected temperate and tropical fruit and nut trees. Hortic. Rev. 2022, 49, 171–242. [Google Scholar]
- Basra, A. (Ed.) Plant Growth Regulators in Agriculture and Horticulture: Their Role and Commercial Uses; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Rademacher, W. Plant growth regulators: Backgrounds and uses in plant production. J. Plant Growth Regul. 2015, 34, 845–872. [Google Scholar] [CrossRef]
- Qiu, Z.L.; Wen, Z.; Yang, K.; Tian, T.; Qiao, G.; Hong, Y.; Wen, X.P. Comparative proteomics profiling illuminates the fruitlet abscission mechanism of sweet cherry as induced by embryo abortion. Int. J. Mol. Sci. 2020, 21, 1200. [Google Scholar] [CrossRef]
- Wang, J.; Liang, S.; Ma, H.; Zhang, P.; Shi, W. Effects of ethephon on fresh in-husk walnut preservation and its possible relationship with phenol metabolism. J. Food Sci. 2016, 81, C1921–C1927. [Google Scholar] [CrossRef]
- Wang, S.; Sun, H.; Zhu, L.; Zhang, K.; Zhang, Y.; Zhang, H.; Liu, L. Effects of Spraying with Ethephon and Early Topping on the Growth, Yield, and Earliness of Cotton under Late-Sowing and High-Density Cultivation Modes. Agronomy 2023, 13, 1244. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Jahan, B.; AlAjmi, M.F.; Rehman, M.T.; Khan, N.A. Ethephon mitigates nickel stress by modulating antioxidant system, glyoxalase system and proline metabolism in Indian mustard. Physiol. Mol. Biol. Plants 2020, 26, 1201–1213. [Google Scholar] [CrossRef] [PubMed]
- Ali, T.; John, A.C.; Subramanian, J.; Peter, K.P. Ethephon-induced abscission of “Redhaven” peach. Am. J. Plant Sci. 2012, 3, 17595. [Google Scholar]
- Ferrara, G.; Mazzeo, A.; Matarrese, A.; Pacucci, C.; Trani, A.; Fidelibus, M.W.; Gambacorta, G. Ethephon as a potential abscission agent for table grapes: Effects on pre-harvest abscission, fruit quality, and residue. Front. Plant Sci. 2016, 7, 620. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, Y.; Ying, P.; Ma, W.; Li, J. Genome-wide digital transcript analysis of putative fruitlet abscission related genes regulated by ethephon in litchi. Front. Plant Sci. 2015, 6, 502. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.R. Beneficial effects of ethephon application on sugarcane under sub-tropical climate of China. Sugar Tech. 2004, 6, 235–240. [Google Scholar] [CrossRef]
- Chen, Z.; Qin, C.; Wang, M.; Liao, F.; Liao, Q.; Liu, X.; Huang, D. Ethylene-mediated improvement in sucrose accumulation in ripening sugarcane involves increased sink strength. BMC Plant Biol. 2019, 19, 285. [Google Scholar] [CrossRef]
- Mertoğlu, K.; Evrenosoğlu, Y.; Polat, M. Combined effects of ethephon and mepiquat chloride on late blooming, fruit set, and phytochemical characteristics of Black Diamond plum. Turk. J. Agric. For. 2019, 43, 544–553. [Google Scholar] [CrossRef]
- Stephenson, R.A.; Gallagher, E.C. Effects of ethephon on macadamia racemes. J. Hortic. Sci. 1987, 62, 539–544. [Google Scholar] [CrossRef]
- Richardson, A.C.; Dawson, T.E. Enhancing abscission of mature macadamia nuts with ethephon. N. Zealand J. Crop Hortic. Sci. 1993, 21, 325–329. [Google Scholar] [CrossRef]
- Trueman, S.J. Yield responses to ethephon for unshaken and mechanically shaken macadamia. Aust. J. Exp. Agric. 2003, 43, 1143–1150. [Google Scholar] [CrossRef]
- Kong, G.; Ma, J.; Liu, J.; Ni, S.; He, X.; Li, Y.; Zhang, H.; Tao, L.; Chen, L.; Tao, L.; et al. Efficient harvest ways of ‘O. C’ Macadamia cultivar using hormones. Southwest China J. Agric. Sci. 2018, 31, 399–403. [Google Scholar]
- Nunn, J.; De Faveri, J.; O’Connor, K.; Alam, M.; Hardner, C.; Akinsanmi, O.; Topp, B. Genome-wide association study for abscission failure of fruit pericarps (stick-tights) in wild macadamia germplasm. Agronomy 2022, 12, 1913. [Google Scholar] [CrossRef]
- Buthelezi, N.M.D.; Magwaza, L.S.; Tesfay, S.Z. Postharvest pre-storage processing improves antioxidants, nutritional, and sensory quality of macadamia nuts. Sci. Hortic. 2019, 251, 197–208. [Google Scholar] [CrossRef]
- Ghazzawi, H.A.; Al-Ismail, K. A comprehensive study on the effect of roasting and frying on fatty acids profiles and antioxidant capacity of almonds, pine, cashew, and pistachio. J. Food Qual. 2017, 2017, 9038257. [Google Scholar] [CrossRef]
- Hojjati, M.; Noguera-Artiaga, L.; Wojdyło, A.; Carbonell-Barrachina, Á.A. Effects of microwave roasting on physicochemical properties of pistachios (Pistaciavera L.). Food Sci. Biotechnol. 2015, 24, 1995–2001. [Google Scholar] [CrossRef]
- Eghdami, A.; Sadeghi, F. Determination of total phenolic and flavonoids contents in methanolic and aqueous extract of Achillea millefolium. Org. Chem. J. 2010, 2, 81–84. [Google Scholar]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Nyau, V.; Prakash, S.; Rodrigues, J.; Farrant, J. Antioxidant activities of Bambara groundnuts as assessed by FRAP and DPPH assays. Am. J. Food Nutr. 2015, 3, 7–11. [Google Scholar]
- Liu, X.; Robinson, P.W.; Madore, M.A.; Witney, G.W.; Arpaia, M.L. Hass’ avocado carbohydrate fluctuations. II. Fruit growth and ripening. J. Am. Soc. Hortic. Sci. 1999, 124, 676–681. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Van Lelyveld, L.J.; Gerrish, C.; Dixont, R.A. Enzyme activities and polyphenols related to mesocarp discolouration of avocado fruit. Phytochemistry 1984, 23, 1531–1534. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L. Phenol oxidase, peroxidase and organic matter dynamics of soil. Soil Biol. Biochem. 2010, 42, 391–404. [Google Scholar] [CrossRef]
- Martínková, L.; Kotik, M.; Marková, E.; Homolka, L. Biodegradation of phenolic compounds by Basidiomycota and its phenol oxidases: A review. Chemosphere 2016, 149, 373–382. [Google Scholar] [CrossRef]
- Dong, X.; He, Y.; Yuan, C.; Cheng, X.; Li, G.; Shan, Y.; Zhu, X. Controlled atmosphere improves the quality, antioxidant activity, and phenolic content of yellow peach during the shelf life. Antioxidants 2022, 11, 2278. [Google Scholar] [CrossRef]
- Khalid, N.; Sammi, S.; Miskeen, S.; Khan, I.; Liaquat, M.; Anwar, K.; Jahangir, M. Impact of salicylic acid and calcium chloride on quality attributes of peach stored at refrigeration temperature. Food Sci. Biotechnol. 2023, 32, 1281–1296. [Google Scholar] [CrossRef]
- Nath, P.; Pandey, N.; Samota, M.; Sharma, K.; Kale, S.; Kannaujia, P.; Sethi, S.; Chauhan, O.P. Browning reactions in foods. In Advances in Food Chemistry: Food Components, Processing and Preservation; Springer Nature: Singapore, 2022; pp. 117–159. [Google Scholar]
- Shen, W.; Li, W.; Shao, Y.; Zeng, J. Proanthocyanidin delays litchi peel browning by inhibiting ethylene biosynthesis, respiratory metabolism, and phenol oxidase activities. Sci. Hortic. 2023, 309, 111677. [Google Scholar] [CrossRef]
- Gong, B.; Huang, S.; Ye, N.; Yuan, X.; Ma, H. Pre-harvest ethylene control affects vase life of cut rose ‘Carola’ by regulating energy metabolism and antioxidant enzyme activity. Hortic. Environ. Biotechnol. 2018, 59, 835–845. [Google Scholar] [CrossRef]
- Wang, W.; Wang, H.L.; Xiao, X.Z.; Xu, X.Q. Wild almond (Amygdalus pedunculata Pall.) as potential nutritional resource for the future: Studies on its chemical composition and nutritional value. J. Food Meas. Charact. 2019, 13, 250–258. [Google Scholar] [CrossRef]
- Kalli, V.; Kollia, E.; Roidaki, A.; Proestos, C.; Markaki, P. Cistus incanus L. extract inhibits aflatoxin B1 production by Aspergillus parasiticus in macadamia nuts. Ind. Crops Prod. 2018, 111, 63–68. [Google Scholar] [CrossRef]
- Gao, G.; Duan, X.; Jiang, H.; Yang, F.; Qi, H. CmMYB113 regulates ethylene-dependent sucrose accumulation in postharvest climacteric melon fruit. Postharvest Biol. Technol. 2021, 181, 111682. [Google Scholar] [CrossRef]
- Sun, Y.; Shi, Z.; Jiang, Y.; Zhang, X.; Li, X.; Li, F. Effects of preharvest regulation of ethylene on carbohydrate metabolism of apple (Malus domestica Borkh cv. Starkrimson) fruit at harvest and during storage. Sci. Hortic. 2021, 276, 109748. [Google Scholar] [CrossRef]
- Day, L.; Cakebread, J.A.; Loveday, S.M. Food proteins from animals and plants: Differences in the nutritional and functional properties. Trends Food Sci. Technol. 2022, 119, 428–442. [Google Scholar] [CrossRef]
- Lu, P.; Wu, H.; Gu, J.; Nawaz, M.A.; Ma, X.; Suleria, H.A. Impact of processing on bioaccessibility of phytochemicals in nuts. Food Rev. Int. 2022, 39, 5968–5985. [Google Scholar] [CrossRef]
- Chen, Y.F.; Shakeel, S.N.; Bowers, J.; Zhao, X.C.; Etheridge, N.; Schaller, G.E. Ligand-induced degradation of the ethylene receptor ETR2 through a proteasome-dependent pathway in Arabidopsis. J. Biol. Chem. 2007, 282, 24752–24758. [Google Scholar] [CrossRef]
- Girgžde, E.; Samsone, I.; Gailis, A. Peroxidase, polyphenol oxidase activity, and total phenolic concentration in birch (Betula pendula) in vitro shoots during rejuvenation. Environ. Exp. Biol. 2019, 17, 15–19. [Google Scholar]
- Abdel-Sattar, M.; Al-Obeed, R.S.; Lisek, A.; Eshra, D.H. Enhancing Anna Apples’ Productivity, Physico-Chemical Properties, and Marketability Using Sprays of Naphthalene Acetic Acid and Inhibitors of Ethylene for Alleviating Abiotic Stresses. Horticulturae 2023, 9, 755. [Google Scholar] [CrossRef]
- Croguennec, T. Enzymatic browning. In Handbook of Food Science and Technology 1: Food Alteration and Food Quality; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 159–181. [Google Scholar]
- Ghoora, M.D.; Haldipur, A.C.; Srividya, N. Comparative evaluation of phytochemical content, antioxidant capacities, and overall antioxidant potential of select culinary microgreens. J. Agric. Food Res. 2020, 2, 100046. [Google Scholar] [CrossRef]
- Machado, R.M.; Alves-Pereira, I.; Faty, Y.; Perdigão, S.; Ferreira, R. Influence of nitrogen sources applied by fertigation to an enriched soil with organic compost on growth, mineral nutrition, and phytochemicals content of coriander (Coriandrum sativum L.) in two successive harvests. Plants 2021, 11, 22. [Google Scholar] [CrossRef]
- Gama, T.; Wallace, H.M.; Trueman, S.J.; Jones, K.; Hosseini-Bai, S. Late-dropping macadamia nuts have reduced shelf life. Sci. Hortic. 2020, 268, 109378. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aruwajoye, N.N.; Mditshwa, A.; Magwaza, L.S.; Ngidi, M.S.C.; Tesfay, S.Z. Effect of Preharvest Ethephon Application on Selected Biochemical Components and Polyphenol Oxidase Activity in Macadamia Nuts. Horticulturae 2023, 9, 1101. https://doi.org/10.3390/horticulturae9101101
Aruwajoye NN, Mditshwa A, Magwaza LS, Ngidi MSC, Tesfay SZ. Effect of Preharvest Ethephon Application on Selected Biochemical Components and Polyphenol Oxidase Activity in Macadamia Nuts. Horticulturae. 2023; 9(10):1101. https://doi.org/10.3390/horticulturae9101101
Chicago/Turabian StyleAruwajoye, Noluthando Noxolo, Asanda Mditshwa, Lembe Samukelo Magwaza, Mjabuliseni Simon Cloapas Ngidi, and Samson Zeray Tesfay. 2023. "Effect of Preharvest Ethephon Application on Selected Biochemical Components and Polyphenol Oxidase Activity in Macadamia Nuts" Horticulturae 9, no. 10: 1101. https://doi.org/10.3390/horticulturae9101101
APA StyleAruwajoye, N. N., Mditshwa, A., Magwaza, L. S., Ngidi, M. S. C., & Tesfay, S. Z. (2023). Effect of Preharvest Ethephon Application on Selected Biochemical Components and Polyphenol Oxidase Activity in Macadamia Nuts. Horticulturae, 9(10), 1101. https://doi.org/10.3390/horticulturae9101101