Growth Responses and Adventitious Root Formation of Cucumber Hybrid Lines in a Waterlogged Condition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
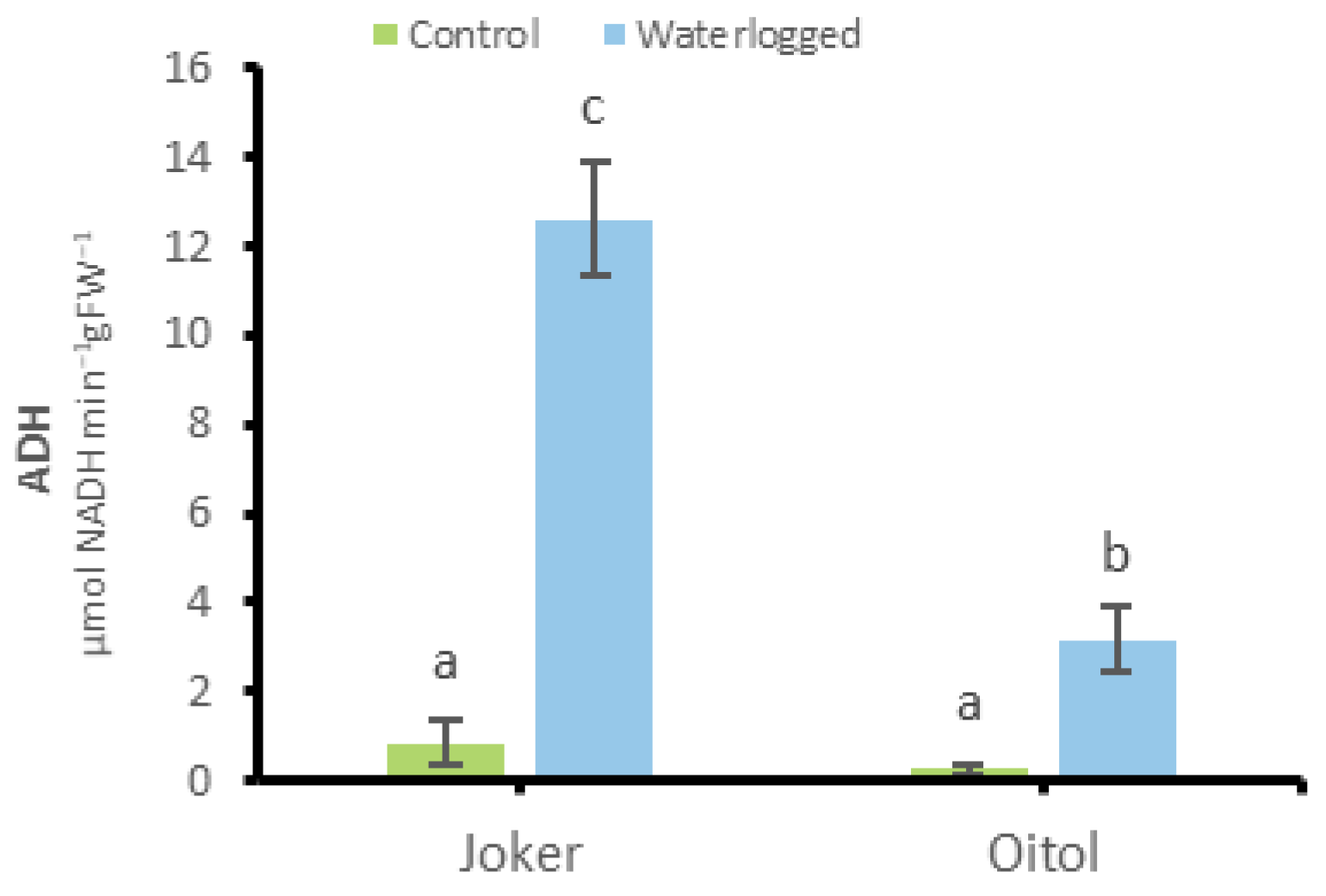
2.2. Alcohol Dehydrogenase (ADH) Activity
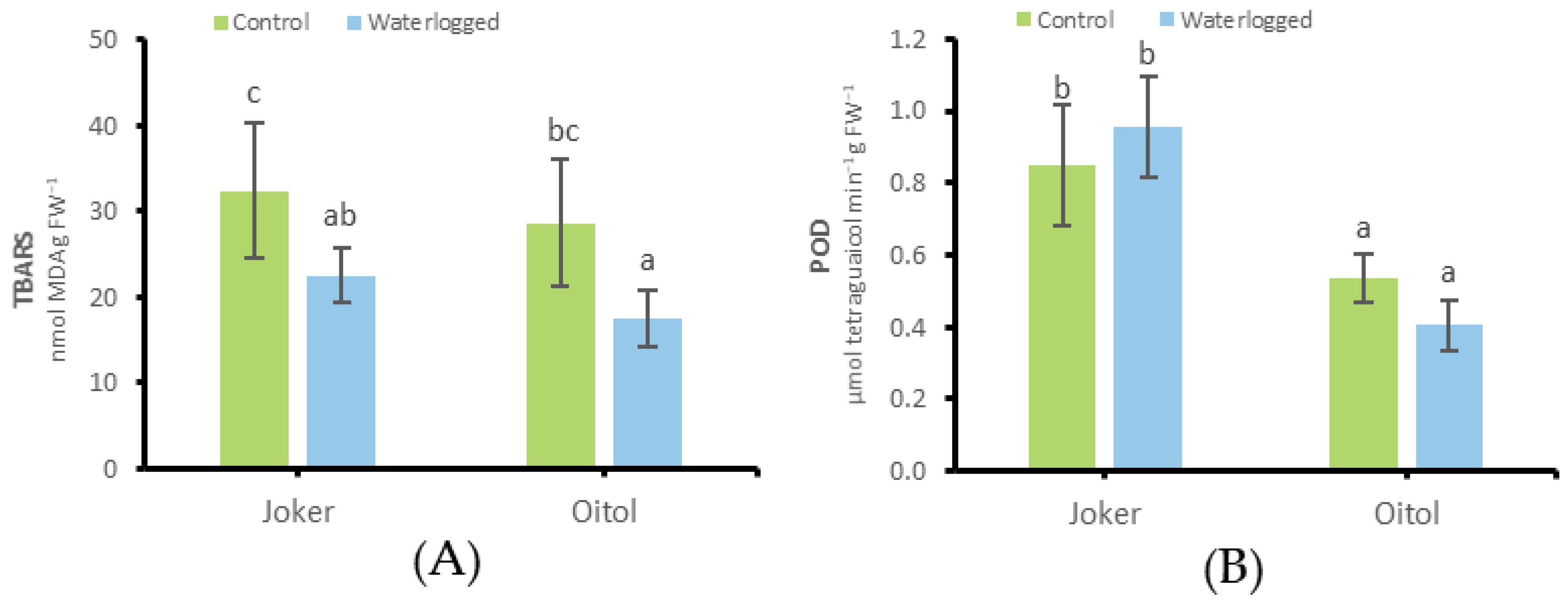
2.3. Malondialdehyde (MDA) Detection and Guaiacol Peroxidase (POD) Assay
2.4. RNA Isolation, cDNA Synthesis, RT-PCR
2.5. Statistical Analysis
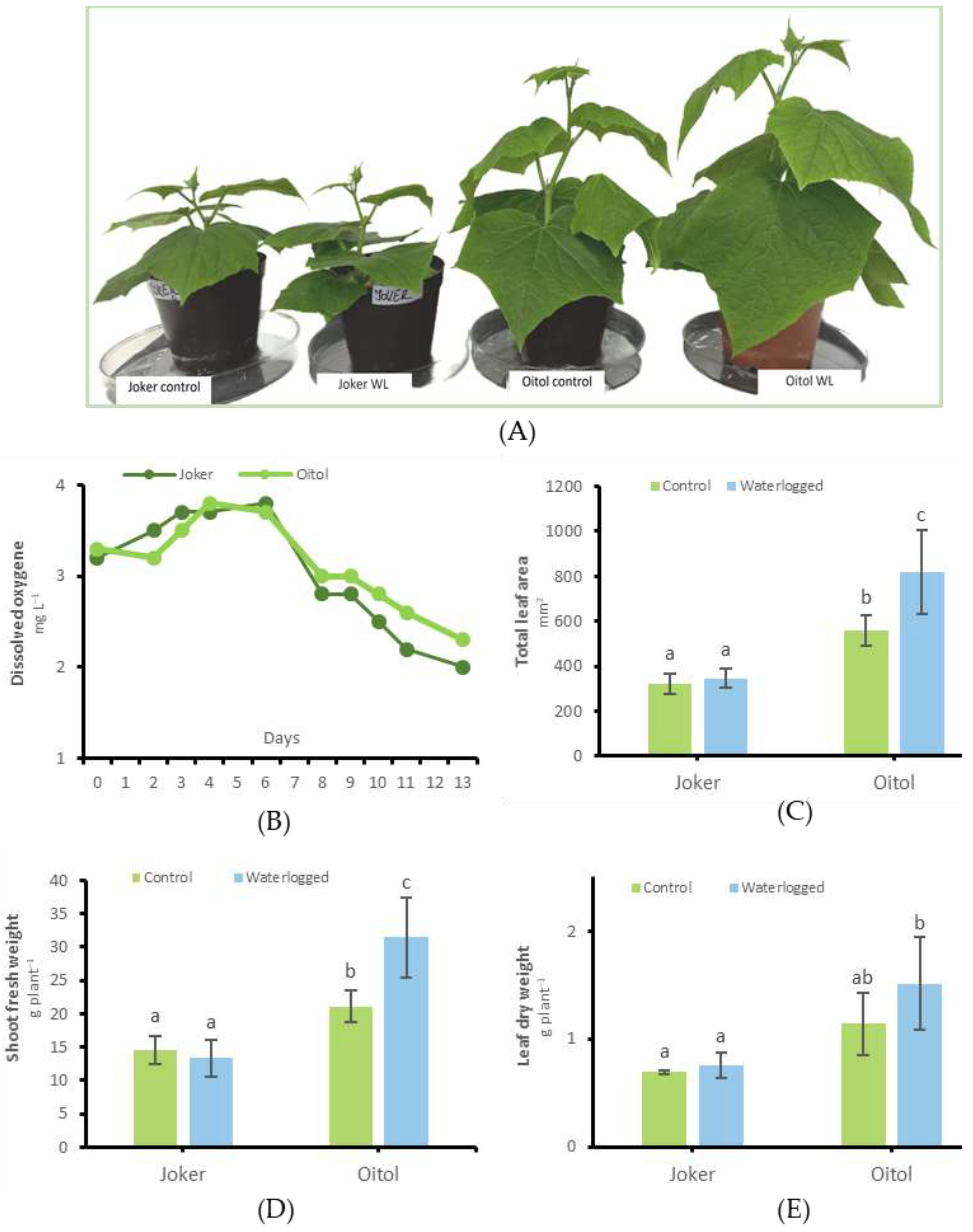
3. Results
3.1. Adventitious Roots (ARs) of Various F1 Hybrids without Stress Treatment
3.2. AR Numbers of ‘Joker’ and ‘Oitol’ Plants under WL Treatments
3.3. Growth Responses and Factors of Redox Balance for ‘Joker’ and ‘Oitol’ Plants under WL Stress
3.4. Alcohol Dehydrogenase Activity in Roots of ‘Joker’ and ‘Oitol’ Plants under WL Treatments
3.5. Expression of Cysteine-Oxidase Genes (PCOs) in Cucumber Roots under WL Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Han, D.; Ma, X.; Zhang, L.; Zhang, S.; Sun, Q.; Li, P.; Shu, J.; Zhao, Y. Serial-Omics and molecular function study provide novel insight into cucumber variety improvement. Plants 2022, 11, 1609. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Ji, J.; Ma, X.; Xu, Q.; Qi, X.; Chen, X. Comparative proteomic analysis provides insight into the key proteins involved in cucumber (Cucumis sativus L.) adventitious root emergence under waterlogging stress. Front. Plant Sci. 2016, 7, 1515. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, A.; Ali, S.; Park, S.; Bae, H. Exploring the potential of multiomics and other integrative approaches for improving waterlogging tolerance in plants. Plants 2023, 12, 1544. [Google Scholar] [CrossRef] [PubMed]
- Irfan, M.; Hayat, S.; Hayat, Q.; Afroz, S.; Ahmad, A. Physiological and biochemical changes in plants under waterlogging. Protoplasma 2010, 241, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Fukao, T.; Barrera-Figueroa, B.E.; Juntawong, P.; Peña-Castro, J.M. Submergence and waterlogging stress in plants: A review highlighting research opportunities and understudied aspects. Front. Plant Sci. 2019, 10, 340. [Google Scholar] [CrossRef] [PubMed]
- Jethva, J.; Schmidt, R.R.; Sauter, M.; Selinski, J. Try or Die: Dynamics of plant respiration and how to survive low oxygen conditions. Plants 2022, 11, 205. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.E. Aerenchyma formation. New Phytol. 2004, 161, 35–49. [Google Scholar] [CrossRef]
- Mustroph, A.; Lee, S.C.; Oosumi, T.; Zanetti, M.E.; Yang, H.; Ma, K.; Yaghoubi-Masihi, A.; Fukao, T.; Bailey-Serres, J. Cross-Kingdom comparison of transcriptomic adjustments to low-oxygen stress highlights conserved and plant-specific responses. Plant Physiol. 2010, 152, 1484–1500. [Google Scholar] [CrossRef]
- Giuntoli, B.; Perata, P. Group VII ethylene response factors in arabidopsis: Regulation and physiological roles. Plant Physiol. 2018, 176, 1143–1155. [Google Scholar] [CrossRef]
- Taylor-Kearney, L.J.; Madden, S.; Wilson, J.; Myers, W.K.; Gunawardana, D.M.; Pires, E.; Holdship, P.; Tumber, A.; Rickaby, R.E.M.; Flashman, E. Plant cysteine oxidase oxygen-sensing function is conserved in early land plants and algae. ACS Bio. Med. Chem. 2022, 2, 521–528. [Google Scholar] [CrossRef]
- Dalle Carbonare, L.; Jiménez, J.D.L.C.; Lichtenauer, S.; van Veen, H. Plant responses to limited aeration: Advances and future challenges. Plant Direct 2023, 7, e488. [Google Scholar] [CrossRef] [PubMed]
- White, M.; Dalle, L.C.; Lavilla, M.P.; Iacopino, S.; Edwards, M.; Dunne, K.; Pires, E.; Levy, C.; McDonough, M.; Licausi, F.; et al. Structures of Arabidopsis thaliana oxygen-sensing plant cysteine oxidases 4 and 5 enable targeted manipulation of their activity. Proc. Natl. Acad. Sci. USA 2020, 117, 23140–23147. [Google Scholar] [CrossRef] [PubMed]
- Eysholdt-Derzsó, E.; Renziehausen, T.; Frings, S.; Frohn, S.; von Bongartz, K.; Igisch, C.P.; Mann, J.; Häger, L.; Macholl, J.; Leisse, D.; et al. Endoplasmic reticulum-bound ANAC013 factor is cleaved by RHOMBOID-LIKE 2 during the initial response to hypoxia in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2023, 120, e2221308120. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.Y.; Guo, S.R.; Li, J.; Duan, J.J. Effect of root applied 24-epibrassinolide on carbohydrate status and fermentative enzyme activities in cucumber (Cucumis sativus L.) seedlings under hypoxia. Plant Growth Regul. 2009, 57, 259–269. [Google Scholar] [CrossRef]
- Hodges, D.; DeLong, J.; Forney, C.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Jócsák, I.; Malgwi, I.; Rabnecz, G.; Szegő, A.; Varga-Visi, É.; Végvári, G.; Pónya, Z. Effect of cadmium stress on certain physiological parameters, antioxidative enzyme activities and biophoton emission of leaves in barley (Hordeum vulgare L.) seedlings. PLoS ONE 2020, 15, e0240470. [Google Scholar] [CrossRef] [PubMed]
- Jaakola, L.; Pirttilä, A.M.; Halonen, M.; Hohtola, A. Isolation of high quality RNA from bilberry (Vaccinium myrtillus L.) fruit. Mol. Biotechnol. 2001, 19, 201–203. [Google Scholar] [CrossRef] [PubMed]
- Oszlányi, R.; Mirmazloum, I.; Pónya, Z.; Szegő, A.; Jamal, S.; Bat-Erdene, O.; Papp, I. Oxidative stress level and dehydrin gene expression pattern differentiate two contrasting cucumber F1 hybrids under high fertigation treatment. Int. J. Biol. Macromol. 2020, 161, 864–874. [Google Scholar] [CrossRef]
- Kęska, K.; Szcześniak, M.W.; Makałowska, I.; Czernicka, M. Long-term waterlogging as factor contributing to hypoxia stress tolerance enhancement in cucumber: Comparative transcriptome analysis of waterlogging sensitive and tolerant accessions. Genes 2021, 12, 189. [Google Scholar] [CrossRef]
- Hesari, N.; Szegő, A.; Mirmazloum, I.; Pónya, Z.; Kiss-Bába, E.; Kolozs, H.; Gyöngyik, M.; Vasas, D.; Papp, I. High-nitrate-supply-induced transcriptional upregulation of ascorbic acid biosynthetic and recycling pathways in cucumber. Plants 2023, 12, 1292. [Google Scholar] [CrossRef]
- Men, S.; Chen, H.; Chen, S.; Zheng, S.; Shen, X.; Wang, C.; Yang, Z.; Liu, D. Effects of supplemental nitrogen application on physiological characteristics, dry matter and nitrogen accumulation of winter rapeseed (Brassica napus L.) under waterlogging stress. Sci. Rep. 2020, 10, 10201. [Google Scholar] [CrossRef] [PubMed]
- Xuewen, X.; Huihui, W.; Xiaohua, Q.; Qiang, X.; Xuehao, C. Waterlogging-induced increase in fermentation and related gene expression in the root of cucumber (Cucumis sativus L.). Sci. Hortic. 2014, 179, 388–395. [Google Scholar] [CrossRef]
- Cho, H.Y.; Loreti, E.; Shih, M.C.; Perata, P. Energy and sugar signaling during hypoxia. New Phytol. 2021, 229, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Weits, D.A.; Giuntoli, B.; Kosmacz, M.; Parlanti, S.; Hubberten, H.M.; Riegler, H.; Hoefgen, R.; Perata, P.; van Dongen, J.T.; Licaus, F. Plant cysteine oxidases control the oxygen-dependent branch of the N-end-rule pathway. Nat. Commun. 2014, 5, 3425. [Google Scholar] [CrossRef] [PubMed]
- Manik, S.M.; Pengilley, G.; Dean, G.; Field, B.; Shabala, S.; Zhou, M. Soil and crop management practices to minimize the impact of waterlogging on crop productivity. Front. Plant Sci. 2019, 10, 140. [Google Scholar] [CrossRef] [PubMed]
- Kuroha, T.; Nagai, K.; Gamuyao, R.; Wang, D.R.; Furuta, T.; Nakamori, M.; Kitaoka, T.; Adachi, K.; Minami, A.; Mori, Y.; et al. Ethylene-gibberellin signaling underlies adaptation of rice to periodic flooding. Science 2018, 361, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Li, Q.; Ma, X.; Qian, C.; Wang, H.; Ren, N.; Shen, C.; Huang, S.; Xu, X.; Xu, Q.; et al. Waterlogging-induced adventitious root formation in cucumber is regulated by ethylene and auxin through reactive oxygen species signalling. Plant Cell Environ. 2019, 42, 1458–1470. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, W.; Brändle, R.; Jackson, M.B. Mechanisms of flood tolerance in plants. Acta Bot. 1994, 43, 307–358. [Google Scholar] [CrossRef]
- Sasidharan, R.; Voesenek, L.A. Ethylene-mediated acclimations to flooding stress. Plant Physiol. 2015, 169, 3–12. [Google Scholar] [CrossRef]
- Yamauchi, T.; Colmer, T.D.; Pedersen, O.; Nakazono, M. Regulation of root traits for internal aeration and tolerance to soil waterlogging-flooding stress. Plant Physiol. 2018, 176, 1118–1130. [Google Scholar] [CrossRef]
- Ma, Y.; Guo, S.; Sun, J.; Shu, S. Effect of hypoxia stress on growth, morpho-anatomical acclimation and activity of involved enzymes of cucumber seedlings. J. Nanjing Agric. Univ. 2016, 39, 213–219. [Google Scholar]

| Gene | Accession No | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | bp cDNA | bp gDNA |
|---|---|---|---|---|---|
| CysOx1 | CsaV3_6G015320 | GCAAAGCTGCATTGTCTCCT | GGAATGATTGTCGTCTCCCA | 171 | 1926 |
| CysOx2 | CsaV3_3G041620 | CCTTCAGGTGTCATTCCAC | GCTGTGAAGTCTGCATCTAC | 206 | 837 |
| CysOx3 | CsaV3_4G012050 | GCTGTCTTCACTTCACCC | CATCCATAACCCTCACCC | 227 | 855 |
| CysOx4 | CsaV3_1G003120 | GGTTGGCTAAGTTAGCCG | GCAGGACCAACATACATTCC | 277 | 384 |
| ACTIN | CsaV3_2G01809 | TCGTGCTTGACTCTGGTGATGG | ACAACCACTGCCGAACGGGAAA | 171 | 171 |
| Cultivar | Number of Adventitious Roots | |
|---|---|---|
| Greenhouse-type hybrids | Oitol | 9.13 ± 7.0 bc |
| Diapason | 8.44 ± 4.0 bc | |
| Grafito | 14.13 ± 8.7 c | |
| Forami | 7.75 ± 3.4 b | |
| Open-field-type hybrids | Harmony | 1.88 ± 1.7 a |
| Dirigent | 0.0 a | |
| Promissa | 0.0 a | |
| Joker | 0.0 a | |
| Cultivar | Treatment | Number of Adventitious Roots | |
|---|---|---|---|
| 1 Week after WL | 2 Weeks after WL | ||
| Oitol | Control | 17.3 ± 4.5 b | 40.6 ± 3.2 c |
| Waterlogged | 31 ± 13.5 c | 60.1 ± 5.8 d | |
| Joker | Control | 0.86 ± 0.9 a | 12.4 ± 2.3 a |
| Waterlogged | 3.8 ± 2.9 a | 19.5 ± 5.2 b | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolozs, H.; Szegő, A.; Kiss-Bába, E.; Hesari, N.; Cardoso, J.T.; Mirmazloum, I.; Papp, I. Growth Responses and Adventitious Root Formation of Cucumber Hybrid Lines in a Waterlogged Condition. Horticulturae 2023, 9, 1102. https://doi.org/10.3390/horticulturae9101102
Kolozs H, Szegő A, Kiss-Bába E, Hesari N, Cardoso JT, Mirmazloum I, Papp I. Growth Responses and Adventitious Root Formation of Cucumber Hybrid Lines in a Waterlogged Condition. Horticulturae. 2023; 9(10):1102. https://doi.org/10.3390/horticulturae9101102
Chicago/Turabian StyleKolozs, Henriett, Anita Szegő, Erzsébet Kiss-Bába, Neda Hesari, Juliana Teles Cardoso, Iman Mirmazloum, and István Papp. 2023. "Growth Responses and Adventitious Root Formation of Cucumber Hybrid Lines in a Waterlogged Condition" Horticulturae 9, no. 10: 1102. https://doi.org/10.3390/horticulturae9101102
APA StyleKolozs, H., Szegő, A., Kiss-Bába, E., Hesari, N., Cardoso, J. T., Mirmazloum, I., & Papp, I. (2023). Growth Responses and Adventitious Root Formation of Cucumber Hybrid Lines in a Waterlogged Condition. Horticulturae, 9(10), 1102. https://doi.org/10.3390/horticulturae9101102









