Advances in Postharvest Diseases Management of Fruits and Vegetables: A Review
Abstract
:1. Introduction
2. Postharvest Diseases Management
2.1. Biological Control
2.2. Biosensors
2.3. Nanotechnology
2.4. Plant Growth Regulators (PGRs)
2.5. Edible Coatings
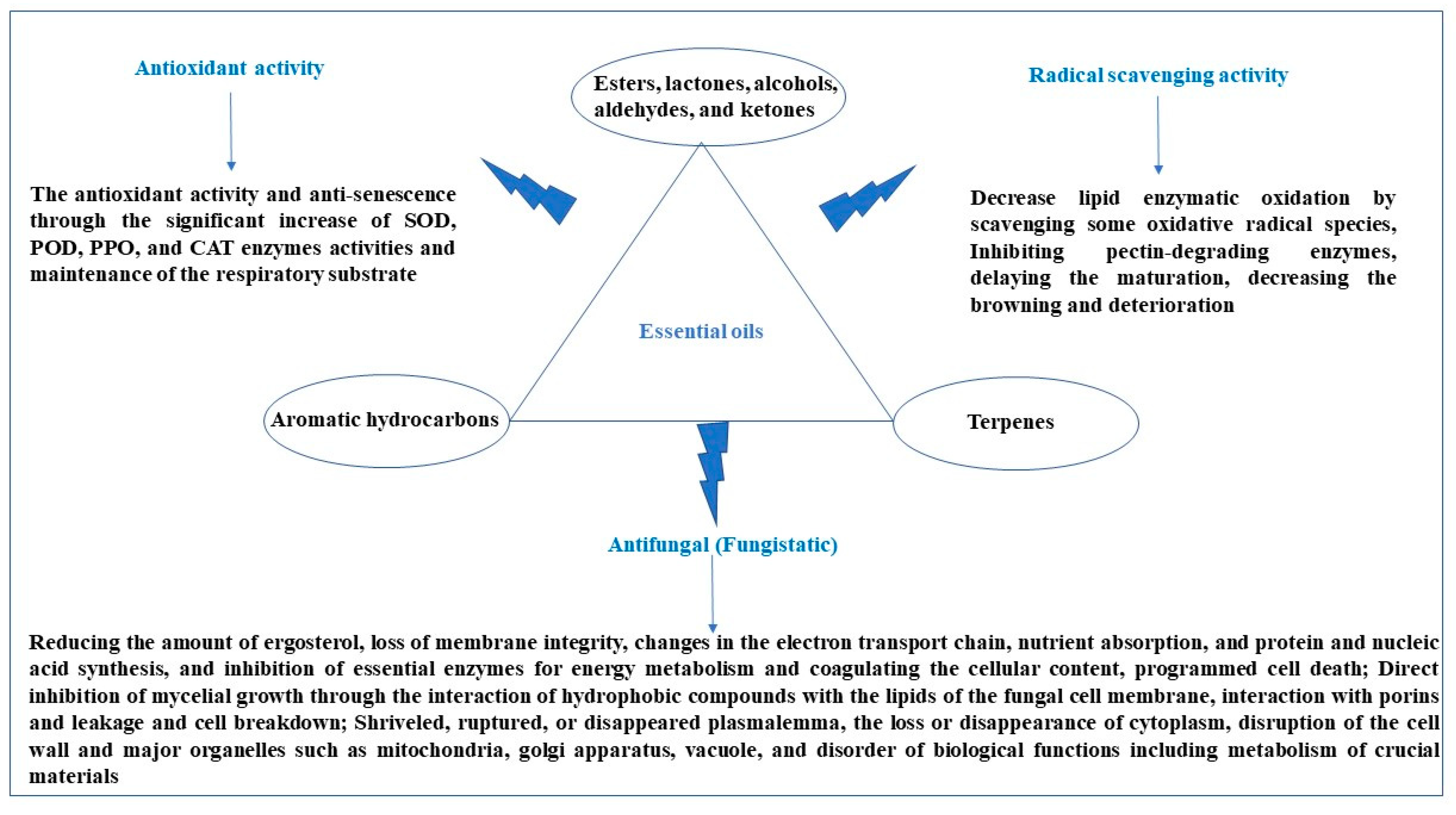
2.6. Essential Oils (EOs)
3. Conclusions and Future Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dale, J.; James, A.; Paul, J.Y.; Khanna, H.; Smith, M.; Peraza-Echeverria, S.; Harding, R. Transgenic Cavendish bananas with resistance to Fusarium wilt tropical race 4. Nat. Commun. 2017, 8, 1496. [Google Scholar] [CrossRef] [PubMed]
- Panth, M.; Hassler, S.C.; Baysal-Gurel, F. Methods for management of soilborne diseases in crop production. Agriculture 2020, 10, 16. [Google Scholar] [CrossRef]
- Xu, J.; Xian, Q.; Wang, K.; Dong, J.; Zhang, C.; Du, S.; Chen, X. Screening and identification of candidate Fusarium wilt-resistance genes from pumpkin. Hortic. Plant J. 2022, 8, 583–592. [Google Scholar] [CrossRef]
- Xu, S.; Bai, T.; Zhang, L.; Fan, H.; Yang, P.; Yin, K.; Zheng, S. Evaluation of different banana varieties on Fusarium wilt TR4 resistance by phenotypic symptom and real-time quantitative PCR. Southwest China J. Agric. Sci. 2017, 30, 1997–2002. [Google Scholar]
- Brogden, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef]
- Rodríguez, A.; San Andrés, V.; Cervera, M.; Redondo, A.; Alquézar, B.; Shimada, T.; Peña, L. Terpene down-regulation in orange reveals the role of fruit aromas in mediating interactions with insect herbivores and pathogens. Plant Physiol. 2011, 156, 793–802. [Google Scholar] [CrossRef]
- Rodríguez, A.; Shimada, T.; Cervera, M.; Alquézar, B.; Gadea, J.; Gómez-Cadenas, A.; Peña, L. Terpene down-regulation triggers defense responses in transgenic orange leading to resistance against fungal pathogens. Plant Physiol. 2014, 164, 321–339. [Google Scholar] [CrossRef]
- Che, J.; Chen, Y.; Wu, Y.; Li, L.; Tao, N. Metabolomics analysis reveals that myrcene stimulates the spore germination of Penicillium digitatum via the upregulation of central carbon and energy metabolism. Postharvest Biol. Technol. 2020, 170, 111329. [Google Scholar] [CrossRef]
- Tao, N.; Chen, Y.; Wu, Y.; Wang, X.; Li, L.; Zhu, A. The terpene limonene induced the green mold of citrus fruit through regulation of reactive oxygen species (ROS) homeostasis in Penicillium digitatum spores. Food Chem. 2019, 277, 414–422. [Google Scholar] [CrossRef]
- Alegbeleye, O.O.; Sant’Ana, A.S. Risks associated with the consumption of irrigation water contaminated produce: On the role of quantitative microbial risk assessment. Curr. Opin. Food Sci. 2021, 41, 88–98. [Google Scholar] [CrossRef]
- Pruvost, O.; Couteau, A.; Luisetti, J. Development of bacterial black spot of mangoes and epiphytic populations of the pathogen (Xanthomonas campestris pv. mangiferaeindicae) under natural conditions in Reunion. Fruits 1990, 45, 125–140. [Google Scholar]
- Dukare, A.S.; Paul, S.; Nambi, V.E.; Gupta, R.K.; Singh, R.; Sharma, K.; Vishwakarma, R.K. Exploitation of microbial antagonists for the control of postharvest diseases of fruits: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1498–1513. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Ren, J.; Li, P.; Feng, S.; Dong, P.; Ren, M. Potential of microbial endophytes to enhance the resistance to postharvest diseases of fruit and vegetables. J. Sci. Food Agric. 2021, 101, 1744–1757. [Google Scholar] [CrossRef] [PubMed]
- Leyva Salas, M.; Mounier, J.; Valence, F.; Coton, M.; Thierry, A.; Coton, E. Antifungal microbial agents for food biopreservation—A review. Microorganisms 2021, 5, 37. [Google Scholar] [CrossRef] [PubMed]
- Oufensou, S.; Ul Hassan, Z.; Balmas, V.; Jaoua, S.; Migheli, Q. Perfume Guns: Potential of Yeast Volatile Organic Compounds in the Biological Control of Mycotoxin-Producing Fungi. Toxins 2023, 15, 45. [Google Scholar] [CrossRef]
- Gallo, A.; Giuberti, G.; Frisvad, J.C.; Bertuzzi, T.; Nielsen, K.F. Review on mycotoxin issues in ruminants: Occurrence in forages, effects of mycotoxin ingestion on health status and animal performance and practical strategies to counteract their negative effects. Toxins 2015, 7, 3057–3111. [Google Scholar] [CrossRef]
- Sangchote, S. Botryodiplodia stem end rot of mango and its control. In III International Mango Symposium; Acta Hortic.: Darvin, NT, Australia, 1989; Volume 291, pp. 296–303. [Google Scholar]
- Lee, H.J.; Ryu, D. Worldwide occurrence of mycotoxins in cereals and cereal-derived food products: Public health perspectives of their co-occurrence. J. Agric. Food Chem. 2017, 65, 7034–7051. [Google Scholar] [CrossRef]
- Wielogórska, E.; MacDonald, S.; Elliott, C.T. A review of the efficacy of mycotoxin detoxifying agents used in feed in light of changing global environment and legislation. World Mycotoxin J. 2016, 9, 419–433. [Google Scholar] [CrossRef]
- Usall, J.; Ippolito, A.; Sisquella, M.; Neri, F. Physical treatments to control postharvest diseases of fresh fruits and vegetables. Postharvest Biol. Technol. 2016, 122, 30–40. [Google Scholar] [CrossRef]
- Papoutsis, K.; Mathioudakis, M.M.; Hasperue, J.H.; Ziogas, V. Non-chemical treatments for preventing the postharvest fungal rotting of citrus caused by Penicillium digitatum (green mold) and Penicillium italicum (blue mold). Trends Food Sci. Technol. 2019, 86, 479–491. [Google Scholar] [CrossRef]
- Bosch, Y.; Britt, E.; Perren, S.; Naef, A.; Frey, J.E.; Buhlmann, A. Dynamics of the apple fruit microbiome after harvest and implications for fruit quality. Microorganisms 2021, 9, 272. [Google Scholar] [CrossRef]
- Perini, M.A.; Sin, I.N.; Jara, A.M.R.; Lobato, M.E.G.; Civello, P.M.; Martínez, G.A. Hot water treatments performed in the base of the broccoli stem reduce postharvest senescence of broccoli (Brassica oleracea L. Var italic) heads stored at 20 C. LWT-Food Sci. Technol. 2017, 77, 314–322. [Google Scholar] [CrossRef]
- Wu, Z.; Yuan, X.; Li, H.; Liu, F.; Wang, Y.; Li, J.; Wang, Y. Heat acclimation reduces postharvest loss of table grapes during cold storage–Analysis of possible mechanisms involved through a proteomic approach. Postharvest Biol. Technol. 2015, 105, 26–33. [Google Scholar] [CrossRef]
- Wassermann, B.; Kusstatscher, P.; Berg, G. Microbiome response to hot water treatment and potential synergy with biological control on stored apples. Front. Microbiol. 2019, 10, 2502. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Vilet, L.; Hernández-Hernández, H.M.; Villanueva-Rodríguez, S.J. Current status of emerging food processing technologies in Latin America: Novel thermal processing. Innov. Food Sci. Emerg. Technol. 2018, 50, 196–206. [Google Scholar] [CrossRef]
- Delorme, M.M.; Guimarães, J.T.; Coutinho, N.M.; Balthazar, C.F.; Rocha, R.S.; Silva, R.; Cruz, A.G. Ultraviolet radiation: An interesting technology to preserve quality and safety of milk and dairy foods. Trends Food Sci. Technol. 2020, 102, 146–154. [Google Scholar] [CrossRef]
- Das, I.; Shah, N.G.; Kumar, G. Properties of walnut influenced by short time microwave treatment for disinfestation of insect infestation. J. Stored Prod. Res. 2014, 59, 152–157. [Google Scholar] [CrossRef]
- Pérez-Lavalle, L.; Carrasco, E.; Valero, A. Strategies for microbial decontamination of fresh blueberries and derived products. Foods 2020, 9, 1558. [Google Scholar] [CrossRef]
- Charles, M.T.; Tano, K.; Asselin, A.; Arul, J. Physiological basis of UV-C induced resistance to Botrytis cinerea in tomato fruit. V. Constitutive defense enzymes and inducible pathogenesis-related proteins. Postharvest Biol. Technol. 2009, 51, 414–424. [Google Scholar] [CrossRef]
- Erkan, M.; Wang, S.Y.; Wang, C.Y. Effect of UV treatment on antioxidant capacity, antioxidant enzyme activity and decay in strawberry fruit. Postharvest Biol. Technol. 2008, 48, 163–171. [Google Scholar] [CrossRef]
- Guerreiro, D.; Madureira, J.; Silva, T.; Melo, R.; Santos, P.M.; Ferreira, A.; Verde, S.C. Post-harvest treatment of cherry tomatoes by gamma radiation: Microbial and physicochemical parameters evaluation. Innov. Food Sci. Emerg. Technol. 2016, 36, 1–9. [Google Scholar] [CrossRef]
- Jeong, R.D.; Chu, E.H.; Lee, G.W.; Cho, C.; Park, H.J. Inhibitory effect of gamma irradiation and its application for control of postharvest green mold decay of Satsuma mandarins. Int. J. Food Microbiol. 2016, 234, 1–8. [Google Scholar] [CrossRef]
- Yoon, Y.S.; Ameer, K.; Song, B.S.; Kim, J.K.; Park, H.Y.; Lee, K.C.; Park, J.H. Effects of X-ray irradiation on the postharvest quality characteristics of ‘Maehyang’ strawberry (Fragaria× ananassa). Food Chem. 2020, 325, 126817. [Google Scholar] [CrossRef] [PubMed]
- Misra, N.N.; Yadav, B.; Roopesh, M.S.; Jo, C. Cold plasma for effective fungal and mycotoxin control in foods: Mechanisms, inactivation effects, and applications. Compr. Rev. Food Sci. Food Saf. 2019, 18, 106–120. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.Z.; Mujumdar, A.S.; Pan, Z.; Vidyarthi, S.K.; Xu, J.; Zielinska, M.; Xiao, H.W. Emerging chemical and physical disinfection technologies of fruits and vegetables: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2020, 60, 2481–2508. [Google Scholar] [CrossRef]
- Ouf, S.A.; Basher, A.H.; Mohamed, A.A.H. Inhibitory effect of double atmospheric pressure argon cold plasma on spores and mycotoxin production of Aspergillus niger contaminating date palm fruits. J. Sci. Food Agric. 2015, 95, 3204–3210. [Google Scholar] [CrossRef]
- Cuthbert, R.N.; Dick, J.T.; Callaghan, A.; Dickey, J.W. Biological control agent selection under environmental change using functional responses, abundances and fecundities; the Relative Control Potential (RCP) metric. Biol. Control 2018, 121, 50–57. [Google Scholar] [CrossRef]
- Carmona-Hernandez, S.; Reyes-Pérez, J.J.; Chiquito-Contreras, R.G.; Rincon-Enriquez, G.; Cerdan-Cabrera, C.R.; Hernandez-Montiel, L.G. Biocontrol of postharvest fruit fungal diseases by bacterial antagonists: A review. Agronomy 2019, 9, 121. [Google Scholar] [CrossRef]
- Wisniewski, M.E.; Wilson, C.L. Biological control of postharvest diseases of fruits and vegetables: Recent advances. Hort Sci. 1992, 27, 94–98. [Google Scholar] [CrossRef]
- Hirt, H. Healthy soils for healthy plants for healthy humans: How beneficial microbes in the soil, food and gut are interconnected and how agriculture can contribute to human health. EMBO Rep. 2020, 21, 51069. [Google Scholar] [CrossRef]
- Loo, Y.T.; Howell, K.; Chan, M.; Zhang, P.; Ng, K. Modulation of the human gut microbiota by phenolics and phenolic fiber-rich foods. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1268–1298. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Penton, C.R.; Ruan, Y.; Shen, Z.; Xue, C.; Li, R.; Shen, Q. Inducing the rhizosphere microbiome by biofertilizer application to suppress banana Fusarium wilt disease. Soil Biol. Biochem. 2017, 104, 39–48. [Google Scholar] [CrossRef]
- Lastochkina, O.; Seifikalhor, M.; Aliniaeifard, S.; Baymiev, A.; Pusenkova, L.; Garipova, S.; Maksimov, I. Bacillus spp.: Efficient biotic strategy to control postharvest diseases of fruits and vegetables. Plants 2019, 8, 97. [Google Scholar] [CrossRef] [PubMed]
- Luksa, J.; Vepstaitė-Monstavicė, I.; Apsegaitė, V.; Blazytė-Čereskienė, L.; Staneviciene, R.; Strazdaitė-Žielienė, Ž.; Serviene, E. Fungal microbiota of sea buckthorn berries at two ripening stages and volatile profiling of potential biocontrol yeasts. Microorganisms 2020, 8, 456. [Google Scholar] [CrossRef]
- Romero, J.; Albertos, I.; Díez-Méndez, A.; Poveda, J. Control of postharvest diseases in berries through edible coatings and bacterial probiotics. Sci. Hortic. 2022, 304, 111326. [Google Scholar] [CrossRef]
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.C.C.; Charles, T.; Schloter, M. Microbiome definition re-visited: Old concepts and new challenges. Microbiome 2020, 8, 103. [Google Scholar]
- Kaul, S.; Sharma, T.; Dhar, M.K. “Omics” tools for better understanding the plant–endophyte interactions. Front. Plant Sci. 2016, 7, 955. [Google Scholar] [CrossRef]
- Madbouly, A.K.; Elyousr, K.A.A.; Ismail, I.M. Biocontrol of Monilinia fructigena, causal agent of brown rot of apple fruit, by using endophytic yeasts. Biol. Control 2020, 144, 104239. [Google Scholar] [CrossRef]
- Cruz, A.F.; Barka, G.D.; Blum, L.E.B.; Tanaka, T.; Ono, N.; Kanaya, S.; Reineke, A. Evaluation of microbial communities in peels of Brazilian tropical fruits by amplicon sequence analysis. Braz. J. Microbiol. 2019, 50, 739–748. [Google Scholar] [CrossRef]
- Hall, M.E.; Wilcox, W.F. Identification and frequencies of endophytic microbes within healthy grape berries. Am. J. Enol. Vitic. 2019, 70, 212–219. [Google Scholar] [CrossRef]
- Chen, C.; Cao, Z.; Li, J.; Tao, C.; Feng, Y.; Han, Y. A novel endophytic strain of Lactobacillus plantarum CM-3 with antagonistic activity against Botrytis cinerea on strawberry fruit. Biol. Control 2020, 148, 104306. [Google Scholar] [CrossRef]
- Fresno, D.H.; Munné-Bosch, S. Differential tissue-specific jasmonic acid, salicylic acid, and abscisic acid dynamics in sweet cherry development and their implications in fruit-microbe interactions. Front. Plant Sci. 2021, 12, 640601. [Google Scholar] [CrossRef] [PubMed]
- Lemfack, M.C.; Gohlke, B.O.; Toguem, S.M.T.; Preissner, S.; Piechulla, B.; Preissner, R. mVOC 2.0: A database of microbial volatiles. Nucleic Acids Res. 2018, 46, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Medina-Romero, Y.M.; Roque-Flores, G.; Macías-Rubalcava, M.L. Volatile organic compounds from endophytic fungi as innovative postharvest control of Fusarium oxysporum in cherry tomato fruits. Appl. Microbiol. Biotechnol. 2017, 101, 8209–8222. [Google Scholar] [CrossRef]
- Aiello, D.; Restuccia, C.; Stefani, E.; Vitale, A.; Cirvilleri, G. Postharvest biocontrol ability of Pseudomonas synxantha against Monilinia fructicola and Monilinia fructigena on stone fruit. Postharvest Biol. Technol. 2019, 149, 83–89. [Google Scholar] [CrossRef]
- Mohd Taha, M.D.; Mohd Jaini, M.F.; Saidi, N.B.; Abdul Rahim, R.; Md Shah, U.K.; Mohd Hashim, A. Biological control of Erwinia mallotivora, the causal agent of papaya dieback disease by indigenous seed-borne endophytic lactic acid bacteria consortium. PLoS ONE 2019, 14, 0224431. [Google Scholar] [CrossRef]
- Keswani, C.; Singh, H.B.; García-Estrada, C.; Caradus, J.; He, Y.W.; Mezaache-Aichour, S.; Sansinenea, E. Antimicrobial secondary metabolites from agriculturally important bacteria as next-generation pesticides. Appl. Microbiol. Biotechnol. 2020, 104, 1013–1034. [Google Scholar] [CrossRef]
- Poveda, J. Beneficial effects of microbial volatile organic compounds (MVOCs) in plants. Appl. Soil Ecol. 2021, 168, 104118. [Google Scholar] [CrossRef]
- Ullah, A.; Nisar, M.; Ali, H.; Hazrat, A.; Hayat, K.; Keerio, A.A.; Yang, X. Drought tolerance improvement in plants: An endophytic bacterial approach. Appl. Microbiol. Biotechnol. 2019, 103, 7385–7397. [Google Scholar] [CrossRef]
- Seifi Kalhor, M.; Aliniaeifard, S.; Seif, M.; Javadi, E.; Bernard, F.; Li, T.; Lastochkina, O. Rhizobacterium Bacillus subtilis reduces toxic effects of high electrical conductivity in soilless culture of lettuce. In International Symposium on New Technologies for Environment Control, Energy-Saving and Crop Production in Greenhouse and Plant; Acta Hortic.: Darvin, NT, Australia, 2017; Volume 1227, pp. 471–478. [Google Scholar]
- del Carmen Orozco-Mosqueda, M.; Glick, B.R.; Santoyo, G. ACC deaminase in plant growth-promoting bacteria (PGPB): An efficient mechanism to counter salt stress in crops. Microbiol. Res. 2020, 235, 126439. [Google Scholar]
- Solanki, M.K.; Yandigeri, M.S.; Kumar, S.; Singh, R.K.; Srivastava, A.K. Co-inoculation of different antagonists can enhance the biocontrol activity against Rhizoctonia solani in tomato. Antonie Leeuwenhoek 2019, 112, 1633–1644. [Google Scholar] [CrossRef] [PubMed]
- Thangavelu, R.; Gopi, M. Field suppression of Fusarium wilt disease in banana by the combined application of native endophytic and rhizospheric bacterial isolates possessing multiple functions. Phytopathol. Mediterr. 2015, 54, 241–252. [Google Scholar]
- Beneduzi, A.; Ambrosini, A.; Passaglia, L.M. Plant growth-promoting rhizobacteria (PGPR): Their potential as antagonists and biocontrol agents. Genet. Mol. Biol. 2012, 35, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- Maksimov, I.V.; Veselova, S.V.; Nuzhnaya, T.V.; Sarvarova, E.R.; Khairullin, R.M. Plant growth-promoting bacteria in regulation of plant resistance to stress factors. Russ. J. Plant Physiol. 2015, 62, 715–726. [Google Scholar] [CrossRef]
- Duffy, B.; Schouten, A.; Raaijmakers, J.M. Pathogen self-defense: Mechanisms to counteract microbial antagonism. Annu. Rev. Phytopathol. 2003, 41, 501–538. [Google Scholar] [CrossRef]
- Di Francesco, A.; Martini, C.; Mari, M. Biological control of postharvest diseases by microbial antagonists: How many mechanisms of action? Eur. J. Plant Pathol. 2016, 145, 711–717. [Google Scholar] [CrossRef]
- Fan, H.; Ru, J.; Zhang, Y.; Wang, Q.; Li, Y. Fengycin produced by Bacillus subtilis 9407 plays a major role in the biocontrol of apple ring rot disease. Microbiol. Res. 2017, 199, 89–97. [Google Scholar] [CrossRef]
- Triasih, U.; Nugroho, Y.A.; Widyaningsih, S. Antimicrobial Activity of Pseudomonas fluorescens and Bacillus subtilis on Different Dilution Concentrations Against Various Citrus Post-Harvest Pathogens. In 3rd KOBI Congress, International and National Conferences; Atlantis Press: Paris, France, 2021; pp. 535–539. [Google Scholar]
- Bell, S.R.; Montiel, L.G.H.; Estrada, R.R.G.; Martínez, P.G. Main diseases in postharvest blueberries, conventional and eco-friendly control methods: A review. LWT-Food Sci. Technol. 2021, 149, 112046. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, W.; Zeng, J.; Shao, Y. Mechanisms of action of the yeast Debaryomyces nepalensis for control of the pathogen Colletotrichum gloeosporioides in mango fruit. Biol. Control 2018, 123, 111–119. [Google Scholar] [CrossRef]
- Konsue, W.; Dethoup, T.; Limtong, S. Biological control of fruit rot and anthracnose of postharvest mango by antagonistic yeasts from economic crops leaves. Microorganisms 2020, 8, 317. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, R.; Tang, Y.; Cheng, Z.; Qian, M.; Li, W.; Shao, Y. Transcriptome analysis reveals the inducing effect of Bacillus siamensis on disease resistance in postharvest mango fruit. Foods 2022, 11, 107. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, Z.; Zhang, X.; Bai, W.; Zhang, L.; Pei, H.; Zhang, Y. Control effects of Bacillus siamensis G-3 volatile compounds on raspberry postharvest diseases caused by Botrytis cinerea and Rhizopus stolonifer. Biol. Control 2020, 141, 104135. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Wang, L.; Fan, W.; Zhang, X.; Chen, X.; Wang, J. Biocontrol potential of volatile organic compounds from Pseudomonas chlororaphis ZL3 against postharvest gray mold caused by Botrytis cinerea on Chinese cherry. Biol. Control 2021, 159, 104613. [Google Scholar] [CrossRef]
- Gotor-Vila, A.; Teixidó, N.; Di Francesco, A.; Usall, J.; Ugolini, L.; Torres, R.; Mari, M. Antifungal effect of volatile organic compounds produced by Bacillus amyloliquefaciens CPA-8 against fruit pathogen decays of cherry. Food Microbiol. 2017, 64, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, F.; Gu, N.; Yan, X.; Wang, K.; Dhanasekaran, S.; Zhang, H. Postharvest biological control of Rhizopus rot and the mechanisms involved in induced disease resistance of peaches by Pichia membranefaciens. Postharvest Biol. Technol. 2020, 163, 111146. [Google Scholar] [CrossRef]
- Zhong, T.; Wang, Z.; Zhang, M.; Wei, X.; Kan, J.; Zalán, Z.; Du, M. Volatile organic compounds produced by Pseudomonas fluorescens ZX as potential biological fumigants against gray mold on postharvest grapes. Biol. Control 2021, 163, 104754. [Google Scholar] [CrossRef]
- Fang, X.; Duan, Q.; Wang, Z.; Li, F.; Du, J.; Ke, W.; Zhang, Y. Products of Lactobacillus delbrueckii subsp. bulgaricus strain F17 and Leuconostoc lactis strain H52 are bio preservatives for improving postharvest quality of ‘Red Globe’ grapes. Microorganisms 2020, 8, 656. [Google Scholar] [CrossRef]
- Chavez-Diaz, I.F.; Mena-Violante, H.G.; Hernandez-Lauzardo, A.N.; Oyoque-Salcedo, G.; Oregel-Zamudio, E.; Angoa-Perez, M.V. Postharvest control of Rhizopus stolonifer on blackberry (Rubus fruticosus) by blackberry native crop bacteria. Rev. Fac. Cienc. 2019, 51, 306–317. [Google Scholar]
- Kannojia, P.; Choudhary, K.K.; Srivastava, A.K.; Singh, A.K. PGPR bioelicitors: Induced systemic resistance (ISR) and proteomic perspective on biocontrol. In PGPR Amelioration in Sustainable Agriculture; Woodhead Publishing: Cambridge, UK, 2019; pp. 67–84. [Google Scholar]
- Chen, X.; Wang, Y.; Gao, Y.; Gao, T.; Zhang, D. Inhibitory abilities of Bacillus isolates and their culture filtrates against the gray mold caused by Botrytis cinerea on postharvest fruit. Plant Pathol. J. 2019, 35, 425. [Google Scholar] [CrossRef]
- Damasceno, C.L.; Duarte, E.A.A.; dos Santos, L.B.P.R.; de Oliveira, T.A.S.; de Jesus, F.N.; de Oliveira, L.M.; Soares, A.C.F. Postharvest biocontrol of anthracnose in bananas by endophytic and soil rhizosphere bacteria associated with sisal (Agave sisalana) in Brazil. Biol. Control 2019, 137, 104016. [Google Scholar] [CrossRef]
- Wu, L.; Shang, H.; Gu, H.; Zheng, J. Bacterial iturins mediate biocontrol activity of Bacillus sp. against postharvest pear fruit-rotting fungi. J. Phytopathol. 2019, 167, 501–509. [Google Scholar] [CrossRef]
- Pang, L.; Xia, B.; Liu, X.; Yi, Y.; Jiang, L.; Chen, C.; Wang, R. Improvement of antifungal activity of a culture filtrate of endophytic Bacillus amyloliquefaciens isolated from kiwifruit and its effect on postharvest quality of kiwifruit. J. Food Biochem. 2021, 45, 13551. [Google Scholar] [CrossRef] [PubMed]
- Villarreal-Delgado, M.F.; Villa-Rodríguez, E.D.; Cira-Chávez, L.A.; Estrada-Alvarado, M.I.; Parra-Cota, F.I.; Santos-Villalobos, S.D.L. The genus Bacillus as a biological control agent and its implications in the agricultural biosecurity. Rev. Mex. Fitopatol. 2018, 36, 95–130. [Google Scholar]
- Wu, Y.; Lin, H.; Lin, Y.; Shi, J.; Xue, S.; Hung, Y.C.; Wang, H. Effects of biocontrol bacteria Bacillus amyloliquefaciens LY-1 culture broth on quality attributes and storability of harvested litchi fruit. Postharvest Biol. Technol. 2017, 132, 81–87. [Google Scholar] [CrossRef]
- Wang, F.; Xiao, J.; Zhang, Y.; Li, R.; Liu, L.; Deng, J. Biocontrol ability and action mechanism of Bacillus halotolerans against Botrytis cinerea causing grey mold in postharvest strawberry fruit. Postharvest Biol. Technol. 2021, 174, 111456. [Google Scholar] [CrossRef]
- Elsherbiny, E.A.; Taher, M.A.; Abd El-Aziz, M.H.; Mohamed, S.Y. Action mechanisms and biocontrol of Purpureocillium lilacinum against green mold caused by Penicillium digitatum in orange fruit. J. Appl. Microbiol. 2021, 131, 1378–1390. [Google Scholar] [CrossRef]
- Lahlali, R.; Aksissou, W.; Lyousfi, N.; Ezrari, S.; Blenzar, A.; Tahiri, A.; Amiri, S. Biocontrol activity and putative mechanism of Bacillus amyloliquefaciens (SF14 and SP10), Alcaligenes faecalis ACBC1, and Pantoea agglomerans ACBP1 against brown rot disease of fruit. Microb. Pathog. 2020, 139, 103914. [Google Scholar] [CrossRef]
- Christopoulos, M.V.; Tsantili, E. Participation of phenylalanine ammonia-lyase (PAL) in increased phenolic compounds in fresh cold stressed walnut (Juglans regia L.) kernels. Postharvest Biol. Technol. 2015, 104, 17–25. [Google Scholar] [CrossRef]
- Singh, B.; Kaur, N.; Kumar, P.; Hallan, V.; Pati, P.K. Reactive oxygen species generating and scavenging systems play critical role in conferring leaf spot disease resistance in Withania somnifera (L.) Dunal. Ind. Crop. Prod. 2020, 157, 112889. [Google Scholar] [CrossRef]
- Jiang, M.Y.; Wang, Z.R.; Chen, K.W.; Kan, J.Q.; Wang, K.T.; Zalán, Z.S.; Du, M.Y. Inhibition of postharvest gray mold decay and induction of disease resistance by Pseudomonas fluorescens in grapes. Acta Aliment. 2019, 48, 288–296. [Google Scholar] [CrossRef]
- Zhou, Q.; Fu, M.; Xu, M.; Chen, X.; Qiu, J.; Wang, F.; Chen, L. Application of antagonist Bacillus amyloliquefaciens NCPSJ7 against Botrytis cinerea in postharvest Red Globe grapes. Food Sci. Nutr. 2020, 8, 1499–1508. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Ma, D.; He, X.; Wang, F.; Wu, J.; Liu, Y.; Deng, J. Bacillus subtilis KLBC BS6 induces resistance and defense-related response against Botrytis cinerea in blueberry fruit. Physiol. Mol. Plant Pathol. 2021, 114, 101599. [Google Scholar] [CrossRef]
- Sen, S.; Sengupta, P.; Molla, J.; Mukherjee, K.; Acharya, K. Management of postharvest green mold decay in common mandarin and Indian gooseberry with Bacillus licheniformis SR-14. J. Appl. Hortic. 2018, 20, 129–135. [Google Scholar] [CrossRef]
- Deng, J.; Kong, S.; Wang, F.; Liu, Y.; Jiao, J.; Lu, Y.; Li, X. Identification of a new Bacillus sonorensis strain KLBC GS-3 as a biocontrol agent for postharvest green mold in grapefruit. Biol. Control 2020, 151, 104393. [Google Scholar] [CrossRef]
- Madhupani, Y.D.S.; Adikaram, N.K.B. Delayed incidence of stem-end rot and enhanced defences in Aureobasidium pullulans-treated avocado (Persea americana Mill.) fruit. J. Plant Dis. Prot. 2017, 124, 227–234. [Google Scholar] [CrossRef]
- Granada, D.; Lopez-Lujan, L.; Ramirez-Restrepo, S.; Morales, J.; Pelaez-Jaramillo, C.; Andrade, G.; Bedoya-pérez, J.C. Bacterial extracts and bioformulates as a promising control of fruit body rot and root rot in avocado cv. Hass. J. Integr. Agric. 2020, 19, 748–758. [Google Scholar] [CrossRef]
- Kurniawan, O.; Wilson, K.; Mohamed, R.; Avis, T.J. Bacillus and Pseudomonas spp. provide antifungal activity against gray mold and Alternaria rot on blueberry fruit. Biol. Control 2018, 126, 136–141. [Google Scholar] [CrossRef]
- Qin, X.; Xiao, H.; Xue, C.; Yu, Z.; Yang, R.; Cai, Z.; Si, L. Biocontrol of gray mold in grapes with the yeast Hanseniaspora uvarum alone and in combination with salicylic acid or sodium bicarbonate. Postharvest Biol. Technol. 2015, 100, 160–167. [Google Scholar] [CrossRef]
- Restuccia, C.; Lombardo, M.; Scavo, A.; Mauromicale, G.; Cirvilleri, G. Combined application of antagonistic Wickerhamomyces anomalus BS91 strain and Cynara cardunculus L. leaf extracts for the control of postharvest decay of citrus fruit. Food Microbiol. 2020, 92, 103583. [Google Scholar] [CrossRef]
- Lyousfi, N.; Lahlali, R.; Letrib, C.; Belabess, Z.; Ouaabou, R.; Ennahli, S.; Barka, E.A. Improving the biocontrol potential of bacterial antagonists with salicylic acid against brown rot disease and impact on nectarine fruits quality. Agronomy 2021, 11, 209. [Google Scholar] [CrossRef]
- Genzel, F.; Franken, P.; Witzel, K.; Grosch, R. Systemic induction of salicylic acid-related plant defenses in potato in response to Rhizoctonia solani AG 3 PT. Plant Pathol. 2018, 67, 337–348. [Google Scholar] [CrossRef]
- Negi, N.; Khurana, P.A. salicylic acid inducible mulberry WRKY transcription factor, Mi WRKY53 is involved in plant defense response. Plant Cell Rep. 2021, 40, 2151–2171. [Google Scholar] [CrossRef] [PubMed]
- El Hamss, H.; Kajad, N.; Belabess, Z.; Lahlali, R. Enhancing bio efficacy of Bacillus amyloliquefaciens SF14 with salicylic acid for the control of the postharvest citrus green mold. Plant Stress 2023, 7, 100144. [Google Scholar] [CrossRef]
- Sun, C.; Huang, Y.; Lian, S.; Saleem, M.; Li, B.; Wang, C. Improving the biocontrol efficacy of Meyerozyma guilliermondii Y-1 with melatonin against postharvest gray mold in apple fruit. Postharvest Biol. Technol. 2021, 171, 111351. [Google Scholar] [CrossRef]
- Freimoser, F.M.; Rueda-Mejia, M.P.; Tilocca, B.; Migheli, Q. Biocontrol yeasts: Mechanisms and applications. World J. Microbiol. Biotechnol. 2019, 35, 154. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, Y.; Sui, Y.; Xie, Z.; Liu, Y.; Jiang, M.; Liu, J. Exposure of Candida oleophila to sublethal salt stress induces an antioxidant response and improves biocontrol efficacy. Biol. Control 2018, 127, 109–115. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Liu, J.; Tian, X.; Zhang, D.; Wang, Q. Management of blue mold (Penicillium italicum) on mandarin fruit with a combination of the yeast, Meyerozyma guilliermondii and an alginate oligosaccharide. Biol. Control 2021, 152, 104451. [Google Scholar] [CrossRef]
- Ming, X.; Wang, Y.; Sui, Y. Pretreatment of the antagonistic yeast, Debaryomyces hansenii, with mannitol and sorbitol improves stress tolerance and biocontrol efficacy. Front Microbiol. 2020, 11, 601. [Google Scholar] [CrossRef]
- Sui, Y.; Wang, Z.; Zhang, D.; Wang, Q. Oxidative stress adaptation of the antagonistic yeast, Debaryomyces hansenii, increases fitness in the microenvironment of kiwifruit wound and biocontrol efficacy against postharvest diseases. Biol. Control 2021, 152, 104428. [Google Scholar] [CrossRef]
- Palmieri, D.; Ianiri, G.; Conte, T.; Castoria, R.; Lima, G.; De Curtis, F. Influence of Biocontrol and Integrated Strategies and Treatment Timing on Plum Brown Rot Incidence and Fungicide Residues in Fruits. Agriculture 2022, 12, 1656. [Google Scholar] [CrossRef]
- Zhu, H.; Zhao, L.; Zhang, X.; Foku, J.M.; Li, J.; Hu, W.; Zhang, H. Efficacy of Yarrowia lipolytica in the biocontrol of green mold and blue mold in Citrus reticulata and the mechanisms involved. Biol. Control 2019, 139, 104096. [Google Scholar] [CrossRef]
- Wang, S.; Ruan, C.; Yi, L.; Deng, L.; Yao, S.; Zeng, K. Biocontrol ability and action mechanism of Metschnikowia citriensis against Geotrichum citri-aurantii causing sour rot of postharvest citrus fruit. Food Microbiol. 2020, 87, 103375. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.; Zhang, C.; Jiang, C.; Zhang, R.; Guo, F.; Fan, X. Trehalose increases the oxidative stress tolerance and biocontrol efficacy of Candida oleophila in the microenvironment of pear wounds. Biol. Control 2019, 132, 23–28. [Google Scholar] [CrossRef]
- Bolívar-Anillo, H.J.; Garrido, C.; Collado, I.G. Endophytic microorganisms for biocontrol of the phytopathogenic fungus Botrytis cinerea. Phytochem. Rev. 2020, 19, 721–740. [Google Scholar] [CrossRef]
- Volschenk, Q.; du Plessis, E.M.; Duvenage, F.J.; Korsten, L. Effect of postharvest practices on the culturable filamentous fungi and yeast microbiota associated with the pear carpoplane. Postharvest Biol. Technol. 2016, 118, 87–95. [Google Scholar] [CrossRef]
- Di Francesco, A.; Mari, M.; Ugolini, L.; Baraldi, E. Effect of Aureobasidium pullulans strains against Botrytis cinerea on kiwifruit during storage and on fruit nutritional composition. Food Microbiol. 2018, 72, 67–72. [Google Scholar] [CrossRef]
- Qiao, Z.; Fu, Y.; Lei, C.; Li, Y. Advances in antimicrobial peptides-based biosensing methods for detection of foodborne pathogens: A review. Food Control 2020, 112, 107116. [Google Scholar] [CrossRef]
- Islam, M.A.; Karim, A.; Ethiraj, B.; Raihan, T.; Kadier, A. Antimicrobial peptides: Promising alternatives over conventional capture ligands for biosensor-based detection of pathogenic bacteria. Biotechnol. Adv. 2022, 55, 107901. [Google Scholar] [CrossRef]
- Zhang, Y.; Lai, B.S.; Juhas, M. Recent advances in aptamer discovery and applications. Molecules 2019, 24, 941. [Google Scholar] [CrossRef]
- Fong, D.; Luo, S.X.L.; Andre, R.S.; Swager, T.M. Trace ethylene sensing via wacker oxidation. ACS Cent. Sci. 2020, 6, 507–512. [Google Scholar] [CrossRef]
- Chalupowicz, D.; Veltman, B.; Droby, S.; Eltzov, E. Evaluating the use of biosensors for monitoring of Penicillium digitatum infection in citrus fruit. Sens. Actuators B Chem. 2020, 311, 127896. [Google Scholar] [CrossRef]
- Lee, J.I.; Jang, S.C.; Chung, J.; Choi, W.K.; Hong, C.; Ahn, G.R.; Chung, W.J. Colorimetric allergenic fungal spore detection using peptide-modified gold nanoparticles. Sens. Actuators B Chem. 2021, 327, 128894. [Google Scholar] [CrossRef]
- Sistani, P.; Sofimaryo, L.; Masoudi, Z.R.; Sayad, A.; Rahimzadeh, R.; Salehi, B. A penicillin biosensor by using silver nanoparticles. Int. J. Electrochem. Sci. 2014, 9, 6201–6212. [Google Scholar] [CrossRef]
- Machado, S.; Pacheco, J.G.; Nouws, H.P.A.; Albergaria, J.T.; Delerue-Matos, C. Characterization of green zero-valent iron nanoparticles produced with tree leaf extracts. Sci. Total Environ. 2015, 533, 76–81. [Google Scholar] [CrossRef]
- Wang, P.; Lombi, E.; Zhao, F.J.; Kopittke, P.M. Nanotechnology: A new opportunity in plant sciences. Trends Plant Sci. 2016, 21, 699–712. [Google Scholar] [CrossRef]
- Igiebor, F.A.; Ikhajiagbe, B.; Asia, M. Green Nanotechnology: A modern tool for Sustainable Agriculture in Nigeria–A Review. Int. J. Hortic. Sci. Technol. 2023, 10, 269–286. [Google Scholar]
- Yadav, S.; Sawarni, N.; Dahiya, T.; Rana, J.S.; Sharma, M.; Batra, B. Nanoagriculture: Advantages and Drawbacks. In Agricultural and Environmental Nanotechnology: Novel Technologies and Their Ecological Impact; Springer Nature Singapore: Singapore, 2023; pp. 3–42. [Google Scholar]
- Boxi, S.S.; Mukherjee, K.; Paria, S. Ag doped hollow TiO2 nanoparticles as an effective green fungicide against Fusarium solani and Venturia inaequalis phytopathogens. Nanotechnology 2016, 27, 085103. [Google Scholar] [CrossRef] [PubMed]
- Elmer, W.; De La Torre-Roche, R.; Pagano, L.; Majumdar, S.; Zuverza-Mena, N.; Dimkpa, C.; White, J.C. Effect of metalloid and metal oxide nanoparticles on Fusarium wilt of watermelon. Plant Dis. 2018, 102, 1394–1401. [Google Scholar] [CrossRef]
- Sharma, R.; Garg, R.; Kumari, A. A review on biogenic synthesis, applications and toxicity aspects of zinc oxide nanoparticles. Exp. Clin. Sci. J. 2020, 19, 1325. [Google Scholar]
- Wang, Z.; Wei, F.; Liu, S.Y.; Xu, Q.; Huang, J.Y.; Dong, X.Y.; Chen, H. Electrocatalytic oxidation of phytohormone salicylic acid at copper nanoparticles-modified gold electrode and its detection in oilseed rape infected with fungal pathogen Sclerotinia sclerotiorum. Talanta 2020, 80, 1277–1281. [Google Scholar] [CrossRef]
- Subbenaik, S.C. Physical and chemical nature of nanoparticles. In Plant Nanotechnology: Principles and Practices; Springer: Cham, Switzerland, 2016; pp. 15–27. [Google Scholar]
- Abu-Salah, K.M.; Zourob, M.M.; Mouffouk, F.; Alrokayan, S.A.; Alaamery, M.A.; Ansari, A.A. DNA-based nanobiosensors as an emerging platform for detection of disease. Sensors 2015, 15, 14539–14568. [Google Scholar] [CrossRef]
- Agarwal, M.; Nagar, D.P.; Srivastava, N.; Agarwal, M.K. Chitosan Nanoparticles based Drug Delivery: An Update. Int. J. Adv. Multidiscip. Res. 2015, 2, 1–13. [Google Scholar]
- Chaudhary, S.; Kumar, S.; Kumar, V.; Sharma, R. Chitosan nano emulsions as advanced edible coatings for fruits and vegetables: Composition, fabrication and developments in last decade. Int. J. Biol. Macromol. 2020, 152, 154–170. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Mukherjee, A.; Dutta, J. Chitosan based nanocomposite films and coatings: Emerging antimicrobial food packaging alternatives. Trends Food Sci. 2020, 97, 196–209. [Google Scholar] [CrossRef]
- Sahab, A.F.; Waly, A.I.; Sabbour, M.M.; Nawar, L.S. Synthesis, antifungal and insecticidal potential of Chitosan (CS)-g-poly (acrylic acid) (PAA) nanoparticles against some seed borne fungi and insects of soybean. Int. J. ChemTech. Res. 2015, 8, 589–598. [Google Scholar]
- Li, Y.; Rokayya, S.; Jia, F.; Nie, X.; Xu, J.; Elhakem, A.; Helal, M. Shelf-life, quality, safety evaluations of blueberry fruits coated with chitosan nano-material films. Sci. Rep. 2021, 11, 55. [Google Scholar] [CrossRef]
- Chowdappa, P.; Gowda, S.; Chethana, C.S.; Madhura, S. Antifungal activity of chitosan-silver nanoparticle composite against Colletotrichum gloeosporioides associated with mango anthracnose. Afr. J. Microbiol. Res. 2014, 8, 1803–1812. [Google Scholar]
- Salem, M.F.; Abd-Elraoof, W.A.; Tayel, A.A.; Alzuaibr, F.M.; Abonama, O.M. Antifungal application of biosynthesized selenium nanoparticles with pomegranate peels and nanochitosan as edible coatings for citrus green mold protection. J. Nanobiotechnol. 2022, 20, 182. [Google Scholar] [CrossRef] [PubMed]
- Correa-Pacheco, Z.N.; Bautista-Baños, S.; Valle-Marquina, M.Á.; Hernández-López, M. The effect of nanostructured chitosan and chitosan-thyme essential oil coatings on Colletotrichum gloeosporioides growth in vitro and on cv Hass avocado and fruit quality. J. Phytopathol. 2017, 165, 297–305. [Google Scholar] [CrossRef]
- Meindrawan, B.; Suyatma, N.E.; Wardana, A.A.; Pamela, V.Y. Nanocomposite coating based on carrageenan and ZnO nanoparticles to maintain the storage quality of mango. Food Packag. Shelf Life 2018, 18, 140–146. [Google Scholar] [CrossRef]
- Saqib, S.; Zaman, W.; Ayaz, A.; Habib, S.; Bahadur, S.; Hussain, S.; Ullah, F. Postharvest disease inhibition in fruit by synthesis and characterization of chitosan iron oxide nanoparticles. Biocatal. Agric. Biotechnol. 2020, 28, 101729. [Google Scholar] [CrossRef]
- Moradinezhad, F.; Khayyat, M.; Ranjbari, F.; Maraki, Z. Physiological and quality responses of Shishe-Kab pomegranates to short-term high CO2 treatment and modified atmosphere packaging. Int. J. Fruit Sci. 2018, 18, 287–299. [Google Scholar] [CrossRef]
- Chávez-Magdaleno, M.E.; González-Estrada, R.R.; Ramos-Guerrero, A.; Plascencia-Jatomea, M.; Gutiérrez-Martínez, P. Effect of pepper tree (Schinus molle) essential oil-loaded chitosan bio-nanocomposites on postharvest control of Colletotrichum gloeosporioides and quality evaluations in avocado (Persea americana) cv. Hass. Food Sci. Biotechnol. 2018, 27, 1871–1875. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Cheng, S.F.; Bhattacharya, B.; Chakkaravarthi, S. Efficacy of free and encapsulated natural antioxidants in oxidative stability of edible oil: Special emphasis on nano emulsion-based encapsulation. Trends Food Sci. 2019, 91, 305–318. [Google Scholar] [CrossRef]
- Naseema, A.; Kovooru, L.; Behera, A.K.; Kumar, K.P.; Srivastava, P. A critical review of synthesis procedures, applications and future potential of nano emulsions. Adv. Colloid Interface Sci. 2021, 287, 102318. [Google Scholar]
- Pongsumpun, P.; Iwamoto, S.; Siripatrawan, U. Response surface methodology for optimization of cinnamon essential oil nano emulsion with improved stability and antifungal activity. Ultrason. Sonochem. 2020, 60, 104604. [Google Scholar] [CrossRef]
- Yang, R.; Miao, J.; Shen, Y.; Cai, N.; Wan, C.; Zou, L.; Chen, J. Antifungal effect of cinnamaldehyde, eugenol and carvacrol nanoemulsion against Penicillium digitatum and application in postharvest preservation of citrus fruit. LWT-Food Sci. Technol. 2021, 141, 110924. [Google Scholar] [CrossRef]
- Ansarifar, E.; Moradinezhad, F. Preservation of strawberry fruit quality via the use of active packaging with encapsulated thyme essential oil in zein nanofiber film. Int. J. Food Sci. Technol. 2021, 56, 4239–4247. [Google Scholar] [CrossRef]
- Wan, C.; Kahramanoğlu, İ.; Okatan, V. Application of plant natural products for the management of postharvest diseases in fruits. Folia Hortic. 2021, 33, 203–215. [Google Scholar] [CrossRef]
- Hu, W.; Kong, H.; Guo, Y.; Zhang, Y.; Ding, Z.; Tie, W.; Guo, A. Comparative physiological and transcriptomic analyses reveal the actions of melatonin in the delay of postharvest physiological deterioration of cassava. Front. Plant Sci. 2016, 7, 736. [Google Scholar] [CrossRef]
- Sun, C.; Liu, L.; Wang, L.; Li, B.; Jin, C.; Lin, X. Melatonin: A master regulator of plant development and stress responses. J. Integ. Plant Biol. 2021, 63, 126–145. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, K.; Chaudhary, R.; Sarwar, A.; Ahmad, B.; Gul, A.; Hano, C.; Anjum, S. Melatonin as master regulator in plant growth, development and stress alleviator for sustainable agricultural production: Current status and future perspectives. Sustainability 2020, 13, 294. [Google Scholar] [CrossRef]
- Li, T.; Wu, Q.; Zhu, H.; Zhou, Y.; Jiang, Y.; Gao, H.; Yun, Z. Comparative transcriptomic and metabolic analysis reveals the effect of melatonin on delaying anthracnose incidence upon postharvest banana fruit peel. BMC Plant Biol. 2019, 19, 289. [Google Scholar] [CrossRef] [PubMed]
- Aghdam, M.S.; Fard, J.R. Melatonin treatment attenuates postharvest decay and maintains nutritional quality of strawberry fruits (Fragaria× anannasa cv. Selva) by enhancing GABA shunt activity. Food Chem. 2017, 221, 1650–1657. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Li, J.; Rao, J. Effect of melatonin on ripening and senescence of postharvest kiwifruits. Food Sci. 2018, 39, 226–232. [Google Scholar]
- Bal, E. Physicochemical changes in ‘Santa Rosa’ plum fruit treated with melatonin during cold storage. J. Food Meas. Charact. 2019, 13, 1713–1720. [Google Scholar] [CrossRef]
- Gao, S.; Ma, W.; Lyu, X.; Cao, X.; Yao, Y. Melatonin may increase disease resistance and flavonoid biosynthesis through effects on DNA methylation and gene expression in grape berries. BMC Plant Biol. 2020, 20, 231. [Google Scholar] [CrossRef]
- Liu, J.; Yang, J.; Zhang, H.; Cong, L.; Zhai, R.; Yang, C.; Xu, L. Melatonin inhibits ethylene synthesis via nitric oxide regulation to delay postharvest senescence in pears. J. Agric. Food Chem. 2019, 67, 2279–2288. [Google Scholar] [CrossRef]
- Zhang, W.; Cao, J.; Fan, X.; Jiang, W. Applications of nitric oxide and melatonin in improving postharvest fruit quality and the separate and crosstalk biochemical mechanisms. Trends Food Sci. 2020, 99, 531–541. [Google Scholar] [CrossRef]
- Lin, Y.; Fan, L.; Xia, X.; Wang, Z.; Yin, Y.; Cheng, Y.; Li, Z. Melatonin decreases resistance to postharvest green mold on citrus fruit by scavenging defense-related reactive oxygen species. Postharvest Biol. Technol. 2019, 153, 21–30. [Google Scholar] [CrossRef]
- Palma, J.M.; Freschi, L.; Rodríguez-Ruiz, M.; González-Gordo, S.; Corpas, F.J. Nitric oxide in the physiology and quality of fleshy fruits. J. Exp. Bot. 2019, 70, 4405–4417. [Google Scholar] [CrossRef]
- Tijero, V.; Munoz, P.; Munné-Bosch, S. Melatonin as an inhibitor of sweet cherries ripening in orchard trees. Plant Physiol. Biochem. 2019, 140, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Arabia, A.; Munne-Bosch, S.; Muñoz, P. Melatonin triggers tissue-specific changes in anthocyanin and hormonal contents during postharvest decay of Angeleno plums. Plant Sci. 2022, 320, 111287. [Google Scholar] [CrossRef]
- Pauwels, L.; Morreel, K.; De Witte, E.; Lammertyn, F.; Van Montagu, M.; Boerjan, W.; Goossens, A. Mapping methyl jasmonate-mediated transcriptional reprogramming of metabolism and cell cycle progression in cultured Arabidopsis cells. Proc. Natl. Acad. Sci. USA 2008, 105, 1380–1385. [Google Scholar] [CrossRef] [PubMed]
- Qi, T.; Song, S.; Ren, Q.; Wu, D.; Huang, H.; Chen, Y.; Xie, D. The Jasmonate-ZIM-domain proteins interact with the WD-Repeat/bHLH/MYB complexes to regulate Jasmonate-mediated anthocyanin accumulation and trichome initiation in Arabidopsis thaliana. Plant Cell. 2011, 23, 1795–1814. [Google Scholar] [CrossRef] [PubMed]
- Di, X.; Takken, F.L.; Tintor, N. How phytohormones shape interactions between plants and the soil-borne fungus Fusarium oxysporum. Front. Plant Sci. 2016, 7, 170. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Lu, X.; Hu, Y.; Li, W.; Hong, K.; Mo, Y.; Xie, J. Methyl jasmonate induced defense responses increase resistance to Fusarium oxysporum f. sp. cubense race 4 in banana. Sci. Hortic. 2013, 164, 484–491. [Google Scholar] [CrossRef]
- Cao, J.; Yan, J.; Zhao, Y.; Jiang, W. Effects of postharvest salicylic acid dipping on Alternaria rot and disease resistance of jujube fruit during storage. J. Sci. Food Agric. 2013, 93, 3252–3258. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, S.; Xue, J.; Mu, B.; Song, H.; Liu, Y. Exogenous melatonin treatment induces disease resistance against Botrytis cinerea on post-harvest grapes by activating defence responses. Foods 2022, 11, 2231. [Google Scholar] [CrossRef]
- Li, S.; Huan, C.; Liu, Y.; Zheng, X.; Bi, Y. Melatonin induces improved protection against Botrytis cinerea in cherry tomato fruit by activating salicylic acid signaling pathway. Sci. Hortic. 2022, 304, 111299. [Google Scholar] [CrossRef]
- Promyou, S.; Raruang, Y.; Chen, Z.Y. Melatonin Treatment of Strawberry Fruit during Storage Extends Its Post-Harvest Quality and Reduces Infection Caused by Botrytis cinerea. Foods 2023, 12, 1445. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Lu, Z.; Yang, Y.; Wang, D.; Yang, T.; Cao, M.; Cao, W. Melatonin treatment reduces chilling injury in peach fruit through its regulation of membrane fatty acid contents and phenolic metabolism. Food Chem. 2018, 245, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Back, K. Melatonin is required for H2O2-and NO-mediated defense signaling through MAPKKK3 and OXI 1 in Arabidopsis thaliana. J. Pineal Res. 2017, 62, e12379. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Yang, J.; Li, X.; Zhang, Y. Salicylic acid: Biosynthesis and signaling. Annu Rev. Plant Biol. 2021, 72, 761–791. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, F.; Wang, J.; Yang, Q.; Wang, P.; Zhao, H.; Xu, X. Salicylic acid inhibits the postharvest decay of goji berry (Lycium barbarum L.) by modulating the antioxidant system and phenylpropanoid metabolites. Postharvest Biol. Technol. 2021, 178, 111558. [Google Scholar] [CrossRef]
- Zeier, J. Metabolic regulation of systemic acquired resistance. Curr. Opin. Plant Biol. 2021, 62, 102050. [Google Scholar] [CrossRef]
- Vlot, A.C.; Dempsey, D.M.A.; Klessig, D.F. Salicylic acid, a multifaceted hormone to combat disease. Annu. Rev. Phytopathol. 2009, 47, 177–206. [Google Scholar] [CrossRef]
- da Rocha Neto, A.C.; Luiz, C.; Maraschin, M.; Di Piero, R.M. Efficacy of salicylic acid to reduce Penicillium expansum inoculum and preserve apple fruits. Int. J. Food Microbiol. 2016, 221, 54–60. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.; Bian, W.; Yang, X.; Ye, L.; He, S.; Song, X. Control Efficacy of Salicylic Acid Microcapsules against Postharvest Blue Mold in Apple Fruit. Molecules 2022, 27, 8108. [Google Scholar] [CrossRef]
- Glowacz, M.; Roets, N.; Sivakumar, D. Control of anthracnose disease via increased activity of defense related enzymes in ‘Hass’ avocado fruit treated with methyl jasmonate and methyl salicylate. Food Chem. 2017, 234, 163–167. [Google Scholar] [CrossRef]
- Boshadi, T.; Moradinezhad, F.; Jahani, M. Effect of pre-and postharvest application of salicylic acid on quality attributes and decay of pomegranate fruit (cv. Shishe-Kab). J. Appl. Hortic. 2018, 20, 154–160. [Google Scholar] [CrossRef]
- Zeraatgar, H.; Davarynejad, G.H.; Moradinezhad, F.; Abedi, B. Preharvest application effect of salicylic acid and calcium nitrate on physicochemical characteristics of fresh jujube fruit (Ziziphus jujuba. Mill) during storage. Erwerbs-Obstbau 2019, 61, 119–127. [Google Scholar] [CrossRef]
- Kumar, S.; Kaur, G. Effect of pre and postharvest applications of salicylic acid on quality attributes and storage behaviour of strawberry cv. Chandler. J. Pharmacogn. Phytochem. 2019, 8, 516–522. [Google Scholar]
- Moradinezhad, F. Quality improvement and shelf life extension of minimally fresh-cut mango fruit using chemical preservatives. J. Hortic. Postharvest Res. 2021, 4, 13–24. [Google Scholar]
- Wang, Z.; Jia, C.; Li, J.; Huang, S.; Xu, B.; Jin, Z. Activation of salicylic acid metabolism and signal transduction can enhance resistance to Fusarium wilt in banana (Musa acuminata L. AAA group, cv. Cavendish). Funct. Integr. Genom. 2015, 15, 47–62. [Google Scholar] [CrossRef]
- Moosa, A.; Sahi, S.T.; Khan, S.A.; Malik, A.U. Salicylic acid and jasmonic acid can suppress green and blue molds of citrus fruit and induce the activity of polyphenol oxidase and peroxidase. Folia Hortic. 2019, 31, 195–204. [Google Scholar] [CrossRef]
- Pan, L.; Zhao, X.; Chen, M.; Fu, Y.; Xiang, M.; Chen, J. Effect of exogenous methyl jasmonate treatment on disease resistance of postharvest kiwifruit. Food Chem. 2020, 305, 125483. [Google Scholar] [CrossRef]
- Ji, N.; Wang, J.; Li, Y.; Li, M.; Jin, P.; Zheng, Y. Involvement of PpWRKY70 in the methyl jasmonate primed disease resistance against Rhizopus stolonifer of peaches via activating phenylpropanoid pathway. Postharvest Biol. Technol. 2021, 174, 111466. [Google Scholar] [CrossRef]
- Andriani, V.; Handayani, N.A. Recent technology of edible coating production: A review. Mater. Today Proc. 2023, 87, 200–206. [Google Scholar] [CrossRef]
- Teixeira-Costa, B.E.; Andrade, C.T. Natural polymers used in edible food packaging—History, function and application trends as a sustainable alternative to synthetic plastic. Polysaccharides 2021, 3, 32–58. [Google Scholar] [CrossRef]
- Llanes, L.; Dubessay, P.; Pierre, G.; Delattre, C.; Michaud, P. Biosourced polysaccharide-based superabsorbents. Polysaccharides 2020, 1, 51–79. [Google Scholar] [CrossRef]
- Fernández-Pan, I.; Carrión-Granda, X.; Maté, J.I. Antimicrobial efficiency of edible coatings on the preservation of chicken breast fillets. Food Control 2014, 36, 69–75. [Google Scholar] [CrossRef]
- Kumar, N. Polysaccharide-based component and their relevance in edible film/coating: A review. Nutr. Food Sci. 2019, 49, 793–823. [Google Scholar] [CrossRef]
- Marcuzzo, E.; Sensidoni, A.; Debeaufort, F.; Voilley, A. Encapsulation of aroma compounds in biopolymeric emulsion based edible films to control flavour release. Carbohydr. Polym. 2010, 80, 984–988. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, J.; Liu, Z. Starch-based edible films. In Environmentally Compatible Food Packaging; Woodhead Publishing: Sawston, UK, 2008; pp. 108–136. [Google Scholar]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Liu, J.; Liu, S.; Chen, Y.; Zhang, L.; Kan, J.; Jin, C. Physical, mechanical and antioxidant properties of chitosan films grafted with different hydroxybenzoic acids. Food Hydrocoll. 2017, 71, 176–186. [Google Scholar] [CrossRef]
- Dhall, R.K. Advances in edible coatings for fresh fruits and vegetables: A review. Crit. Rev. Food Sci. Nutr. 2013, 53, 435–450. [Google Scholar] [CrossRef]
- Firdous, N.; Moradinezhad, F.; Farooq, F.; Dorostkar, M. Advances in formulation, functionality, and application of edible coatings on fresh produce and fresh-cut products: A review. Food Chem. 2022, 407, 135186. [Google Scholar] [CrossRef]
- Tadros, T. Surfactants. In Encyclopedia of Colloid and Interface Science; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1249–1250. [Google Scholar]
- Romanazzi, G.; Feliziani, E.; Sivakumar, D. Chitosan, a biopolymer with triple action on postharvest decay of fruit and vegetables: Eliciting, antimicrobial and film-forming properties. Front. Microbiol. 2018, 9, 2745. [Google Scholar] [CrossRef]
- Obianom, C.; Romanazzi, G.; Sivakumar, D. Effects of chitosan treatment on avocado postharvest diseases and expression of phenylalanine ammonia-lyase, chitinase and lipoxygenase genes. Postharvest Biol. Technol. 2019, 147, 214–221. [Google Scholar] [CrossRef]
- Lopez-Moya, F.; Suarez-Fernandez, M.; Lopez-Llorca, L.V. Molecular mechanisms of chitosan interactions with fungi and plants. Int. J. Mol. Sci. 2019, 20, 332. [Google Scholar] [CrossRef]
- Perinelli, D.R.; Fagioli, L.; Campana, R.; Lam, J.K.; Baffone, W.; Palmieri, G.F.; Bonacucina, G. Chitosan-based nanosystems and their exploited antimicrobial activity. Eur. J. Pharm. Sci. 2018, 117, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, I.A.; Martin-Diana, A.B.; Rico, D. Fish-gelatin and Carob Seed Peel By-product for Developing Novel Edible Films. In Food Packaging; CRC Press: Boca Raton, FL, USA, 2019; pp. 125–150. [Google Scholar]
- Zheng, F.; Zheng, W.; Li, L.; Pan, S.; Liu, M.; Zhang, W.; Zhu, C. Chitosan controls postharvest decay and elicits defense response in kiwifruit. Food Bioprocess Technol. 2017, 10, 1937–1945. [Google Scholar] [CrossRef]
- Hua, C.; Li, Y.; Wang, X.; Kai, K.; Su, M.; Zhang, D.; Liu, Y. The effect of low and high molecular weight chitosan on the control of gray mold (Botrytis cinerea) on kiwifruit and host response. Sci. Hortic. 2019, 246, 700–709. [Google Scholar] [CrossRef]
- Youssef, K.; de Oliveira, A.G.; Tischer, C.A.; Hussain, I.; Roberto, S.R. Synergistic effect of a novel chitosan/silica nanocomposites-based formulation against gray mold of table grapes and its possible mode of action. Int. J. Biol. Macromol. 2019, 141, 247–258. [Google Scholar] [CrossRef]
- Vilaplana, R.; Guerrero, K.; Guevara, J.; Valencia-Chamorro, S. Chitosan coatings to control soft mold on fresh blackberries (Rubus glaucus Benth.) during postharvest period. Sci. Hortic. 2020, 262, 109049. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, P.; Zhang, P.; Su, L.; Jia, H.; Wei, X.; Jia, H. Integrative transcriptomics and metabolomics data exploring the effect of chitosan on postharvest grape resistance to Botrytis cinerea. Postharvest Biol. Technol. 2020, 167, 111248. [Google Scholar] [CrossRef]
- Melo, N.F.C.B.; de Lima, M.A.B.; Stamford, T.L.M.; Galembeck, A.; Flores, M.A.; de Campos Takaki, G.M.; Montenegro Stamford, T.C. In vivo and in vitro antifungal effect of fungal chitosan nanocomposite edible coating against strawberry phytopathogenic fungi. Int. J. Food Sci. Technol. 2020, 55, 3381–3391. [Google Scholar] [CrossRef]
- de Oliveira, C.E.V.; Magnani, M.; de Sales, C.V.; de Souza Pontes, A.L.; Campos-Takaki, G.M.; Stamford, T.C.M.; de Souza, E.L. Effects of chitosan from Cunninghamella elegans on virulence of post-harvest pathogenic fungi in table grapes (Vitis labrusca L.). Int. J. Food Microbiol. 2014, 171, 54–61. [Google Scholar] [CrossRef]
- de Oliveira, C.E.V.; Magnani, M.; de Sales, C.V.; de Souza Pontes, A.L.; Campos-Takaki, G.M.; Stamford, T.C.M.; de Souza, E.L. Effects of post-harvest treatment using chitosan from Mucor circinelloides on fungal pathogenicity and quality of table grapes during storage. Food Microbiol. 2014, 44, 211–219. [Google Scholar] [CrossRef]
- Poveda, J. Use of plant-defense hormones against pathogen-diseases of postharvest fresh produce. Physiol. Mol. Plant Pathol. 2020, 111, 101521. [Google Scholar] [CrossRef]
- Peian, Z.; Haifeng, J.; Peijie, G.; Sadeghnezhad, E.; Qianqian, P.; Tianyu, D.; Jinggui, F. Chitosan induces jasmonic acid production leading to resistance of ripened fruit against Botrytis cinerea infection. Food Chem. 2021, 337, 127772. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Oropeza, D.; Aguirre-Joya, J.; Herrera, R.R.; Sepúlveda-Torre, L.; Aguilar, C.N. A Green Technology for Control of Avocado Necrotic Fungi Using Bioactive Coatings. Int. J. 2018, 4, 25. [Google Scholar]
- Xoca-Orozco, L.Á.; Aguilera-Aguirre, S.; Vega-Arreguín, J.; Acevedo-Hernández, G.; Tovar-Pérez, E.; Stoll, A.; Chacón-López, A. Activation of the phenylpropanoid biosynthesis pathway reveals a novel action mechanism of the elicitor effect of chitosan on avocado fruit epicarp. Food Res. Int. 2019, 121, 586–592. [Google Scholar] [CrossRef]
- Badawy, M.E.; Rabea, E.I. Potential of the biopolymer chitosan with different molecular weights to control postharvest gray mold of tomato fruit. Postharvest Biol. Technol. 2009, 51, 110–117. [Google Scholar] [CrossRef]
- Tahir, H.E.; Xiaobo, Z.; Jiyong, S.; Mahunu, G.K.; Zhai, X.; Mariod, A.A. Quality and postharvest-shelf life of cold-stored strawberry fruit as affected by gum arabic (Acacia senegal) edible coating. J. Food Biochem. 2018, 42, e12527. [Google Scholar] [CrossRef]
- Shi, Z.; Deng, J.; Wang, F.; Liu, Y.; Jiao, J.; Wang, L.; Zhang, J. Individual and combined effects of bamboo vinegar and peach gum on postharvest grey mold caused by Botrytis cinerea in blueberry. Postharvest Biol. Technol. 2019, 155, 86–93. [Google Scholar] [CrossRef]
- Pobiega, K.; Igielska, M.; Włodarczyk, P.; Gniewosz, M. The use of pullulan coatings with propolis extract to extend the shelf life of blueberry (Vaccinium corymbosum) fruit. Int. J. Food Sci. Technol. 2021, 56, 1013–1020. [Google Scholar] [CrossRef]
- Nasirifar, S.Z.; Maghsoudlou, Y.; Oliyaei, N. Effect of active lipid-based coating incorporated with nanoclay and orange peel essential oil on physicochemical properties of Citrus sinensis. Food Sci. Nutr. 2018, 6, 1508–1518. [Google Scholar] [CrossRef]
- Jahanshahi, B.; Jafari, A.; Vazifeshenas, M.R.; Gholamnejad, J. A novel edible coating for apple fruits. J. Hortic. Postharvest Res. 2018, 1, 63–72. [Google Scholar]
- Chithra, M.; Sathees, N.; Venkatesan, S.; Thirupathi, M. Effect of edible herbal coatings to extend the shelf life of banana cv. ‘Ney Poovan’ (not exposed to smoke) stored at room temperature. J. Pharmacogn. Phytochem. 2022, 11, 185–188. [Google Scholar]
- Ganiari, S.; Choulitoudi, E.; Oreopoulou, V. Edible and active films and coatings as carriers of natural antioxidants for lipid food. Trends Food Sci. Technol. 2017, 68, 70–82. [Google Scholar] [CrossRef]
- Gunny, A.A.N.; Leem, S.J.; Makhtar, M.M.Z.; Zainuddin, N.I.; Mohd Roslim, M.H.; Raja Hashim, R.H.; Rafatullah, M. The Use of Essential Oil Embedded in Polylactic Acid/Chitosan-Based Film for Mango Post-Harvest Application against Pathogenic Fungi. Polymers 2023, 15, 2722. [Google Scholar] [CrossRef] [PubMed]
- Demitri, C.; Tarantino, A.S.; Moscatello, A.; De Benedictis, V.M.; Madaghiele, M.; Sannino, A.; Maffezzoli, A. Graphene reinforced Chitosan-Cinnamaldehyde derivatives films: Antifungal activity and mechanical properties. In Proceedings of the 1st Workshop on Nanotechnology in Instrumentation and Measurement (NANOFIM), Lecce, Italy, 24–25 July 2015; IEEE: Piscataway, NJ, USA, 2015; pp. 25–29. [Google Scholar]
- Mo, X.; Peng, X.; Liang, X.; Fang, S.; Xie, H.; Chen, J.; Meng, Y. Development of antifungal gelatin-based nanocomposite films functionalized with natamycin-loaded zein/casein nanoparticles. Food Hydrocoll. 2021, 113, 106506. [Google Scholar] [CrossRef]
- Araújo, M.G.d.F.; Hilário, F.; Vilegas, W.; Dos Santos, L.C.; Brunetti, I.L.; Sotomayor, C.E.; Bauab, T.M. Correlation among antioxidant, antimicrobial, hemolytic, and antiproliferative properties of Leiothrix spiralis leaves extract. Int. J. Mol. Sci. 2012, 13, 9260–9277. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Gao, C.; Feng, X.; Yang, Y.; Shen, X.; Tang, X. Structure, physical and antioxidant properties of chitosan-gum arabic edible films incorporated with cinnamon essential oil. Int. J. Biol. Macromol. 2019, 134, 230–236. [Google Scholar] [CrossRef]
- Alvarez, M.V.; Palou, L.; Taberner, V.; Fernández-Catalán, A.; Argente-Sanchis, M.; Pitta, E.; Pérez-Gago, M.B. Natural Pectin-Based Edible Composite Coatings with Antifungal Properties to Control Green Mold and Reduce Losses of ‘Valencia’ Oranges. Foods 2022, 11, 1083. [Google Scholar] [CrossRef]
- Yousuf, B.; Wu, S.; Siddiqui, M.W. Incorporating essential oils or compounds derived thereof into edible coatings: Effect on quality and shelf life of fresh/fresh-cut produce. Trends Food Sci. Technol. 2021, 108, 245–257. [Google Scholar] [CrossRef]
- Salgado-Cruz, M.D.L.P.; Salgado-Cruz, J.; García-Hernández, A.B.; Calderón-Domínguez, G.; Gómez-Viquez, H.; Oliver-Espinoza, R.; Yáñez-Fernández, J. Chitosan as a coating for biocontrol in postharvest products: A bibliometric review. Membranes 2021, 11, 421. [Google Scholar] [CrossRef] [PubMed]
- Barragán-Menéndez, C.; Gálvez-López, D.; Rosas-Quijano, R.; Salvador-Figueroa, M.; Ovando-Medina, I.; Vázquez-Ovando, A. Films of chitosan and Aloe vera for maintaining the viability and antifungal activity of Lactobacillus paracasei TEP6. Coatings 2020, 10, 259. [Google Scholar] [CrossRef]
- Oregel-Zamudio, E.; Angoa-Pérez, M.V.; Oyoque-Salcedo, G.; Aguilar-González, C.N.; Mena-Violante, H.G. Effect of candelilla wax edible coatings combined with biocontrol bacteria on strawberry quality during the shelf-life. Sci. Hortic. 2017, 214, 273–279. [Google Scholar] [CrossRef]
- Lappa, I.K.; Mparampouti, S.; Lanza, B.; Panagou, E.Z. Control of Aspergillus carbonarius in grape berries by Lactobacillus plantarum: A phenotypic and gene transcription study. Int. J. Food Microbiol. 2018, 275, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Marín, A.; Plotto, A.; Atarés, L.; Chiralt, A. Lactic acid bacteria incorporated into edible coatings to control fungal growth and maintain postharvest quality of grapes. Hort Sci. 2019, 54, 337–343. [Google Scholar] [CrossRef]
- Bambace, M.F.; Alvarez, M.V.; del Rosario Moreira, M. Novel functional blueberries: Fructo-oligosaccharides and probiotic lactobacilli incorporated into alginate edible coatings. Food Res. Int. 2019, 122, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Temiz, N.N.; Özdemir, K.S. Microbiological and physicochemical quality of strawberries (Fragaria× ananassa) coated with Lactobacillus rhamnosus and inulin enriched gelatin films. Postharvest Biol. Technol. 2021, 173, 111433. [Google Scholar] [CrossRef]
- Hatmi, R.U.; Apriyati, E.; Cahyaningrum, N. Edible coating quality with three types of starch and sorbitol plasticizer. E3S Web Conf. 2020, 142, 02003. [Google Scholar] [CrossRef]
- Chauhan, O.P.; Nanjappa, C.; Ashok, N.; Ravi, N.; Roopa, N.; Raju, P.S. Shellac and Aloe vera gel-based surface coating for shelf life extension of tomatoes. J. Food Sci. Technol. 2015, 52, 1200–1205. [Google Scholar] [CrossRef]
- Ziani, K.; Oses, J.; Coma, V.; Mate, J.I. Effect of the presence of glycerol and Tween 20 on the chemical and physical properties of films based on chitosan with different degree of deacetylation. Food Sci. Technol. 2008, 41, 2159–2165. [Google Scholar] [CrossRef]
- Sharma, P.; Shehin, V.P.; Kaur, N.; Vyas, P. Application of edible coatings on fresh and minimally processed vegetables: A review. Int. J. Veg. Sci. 2019, 25, 295–314. [Google Scholar] [CrossRef]
- Fan, Y.; Yang, J.; Duan, A.; Li, X. Pectin/sodium alginate/xanthan gum edible composite films as the fresh-cut package. Int. J. Biol. Macromol. 2021, 181, 1003–1009. [Google Scholar] [CrossRef]
- Sarkhosh, A.; Schaffer, B.; Vargas, A.I.; Palmateer, A.J.; Lopez, P.; Soleymani, A.; Farzaneh, M. Antifungal activity of five plant-extracted essential oils against anthracnose in papaya fruit. Biol. Agric. Hortic. 2018, 34, 18–26. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; Feo, V.D. Essential oils and antifungal activity. Pharmaceuticals 2017, 10, 86. [Google Scholar] [CrossRef] [PubMed]
- Carson, C.F.; Hammer, K.A. Chemistry and Bioactivity of Essential Oils. In Lipids and Essential Oils as Antimicrobial Agents; Thormar, H., Ed.; John Wiley & Sons, Ltd.: Chichester West Sussex, UK, 2010. [Google Scholar]
- Singh, P.; Pandey, A.K. Prospective of essential oils of the genus Mentha as biopesticides: A review. Front. Plant Sci. 2018, 9, 1295. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.R.; Chonhenchob, V.; Huang, C.; Suwanamornlert, P. Antifungal activity of propyl disulfide from neem (Azadirachta indica) in vapor and agar diffusion assays against anthracnose pathogens (Colletotrichum gloeosporioides and Colletotrichum acutatum) in mango fruit. Microorganisms 2021, 9, 839. [Google Scholar] [CrossRef] [PubMed]
- Yin, G.; Zhang, Y.; Pennerman, K.K.; Wu, G.; Hua, S.S.T.; Yu, J.; Bennett, J.W. Characterization of blue mold Penicillium species isolated from stored fruits using multiple highly conserved loci. J. Fungi 2017, 3, 12. [Google Scholar] [CrossRef]
- Barrera-Necha, L.L.; Bautista-Banos, S.; Flores-Moc, H.E.; Estudillo, A.R. Efficacy of Essential Oils on the Conidial Germination, Growth of Colletotrichum Gloeosporioides (Penz.) Penz. and Sacc and Control of Postharvest Diseases in Papaya (Carica Papaya L.). Plant Pathol. J. 2008, 7, 174–178. [Google Scholar] [CrossRef]
- Pangallo, S.; Li Destri Nicosia, M.G.; Raphael, G.; Levin, E.; Ballistreri, G.; Cacciola, S.O.; Schena, L. Elicitation of resistance responses in grapefruit and lemon fruits treated with a pomegranate peel extract. Plant Pathol. 2017, 66, 633–640. [Google Scholar] [CrossRef]
- Dania, V.O.; Esiobu, M.G. Efficacy of plant-derived essential oils in post-harvest management of anthracnose disease on mango fruits. Makerere Univ. J. Agric. Environ. Sci. 2022, 11, 90–106. [Google Scholar]
- Najmi, Z.; Scalia, A.C.; De Giglio, E.; Cometa, S.; Cochis, A.; Colasanto, A.; Rimondini, L. Screening of Different Essential Oils Based on Their Physicochemical and Microbiological Properties to Preserve Red Fruits and Improve Their Shelf Life. Foods 2023, 12, 332. [Google Scholar] [CrossRef]
- Bill, M.; Sivakumar, D.; Beukes, M.; Korsten, L. Expression of pathogenesis-related (PR) genes in avocados fumigated with thyme oil vapors and control of anthracnose. Food Chem. 2016, 194, 938–943. [Google Scholar] [CrossRef]
- Bill, M.; Korsten, L.; Remize, F.; Glowacz, M.; Sivakumar, D. Effect of thyme oil vapors exposure on phenylalanine ammonia-lyase (PAL) and lipoxygenase (LOX) genes expression, and control of anthracnose in ‘Hass’ and ‘Ryan’ avocado fruit. Sci. Hortic. 2017, 224, 232–237. [Google Scholar] [CrossRef]
- Chen, C.; Cai, N.; Chen, J.; Wan, C. Clove essential oil as an alternative approach to control postharvest blue mold caused by Penicillium italicum in citrus fruit. Biomolecules 2019, 9, 197. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, A.; Ramezanian, A.; Shekarforoush, S.; Niakousari, M.; Eshghi, S. Antifungal activity of thymol against the main fungi causing pomegranate fruit rot by suppressing the activity of cell wall degrading enzymes. LWT-Food Sci. Technol. 2022, 161, 113303. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, S.; Du, S.; Chen, S.; Sun, H. Antifungal activity of thymol and carvacrol against postharvest pathogens Botrytis cinerea. J. Food Sci. Technol. 2019, 56, 2611–2620. [Google Scholar] [CrossRef]
- Olea, A.F.; Bravo, A.; Martínez, R.; Thomas, M.; Sedan, C.; Espinoza, L.; Carrasco, H. Antifungal activity of eugenol derivatives against Botrytis cinerea. Molecules 2019, 24, 1239. [Google Scholar] [CrossRef]
- Tian, J.; Ban, X.; Zeng, H.; He, J.; Chen, Y.; Wang, Y. The mechanism of antifungal action of essential oil from dill (Anethum graveolens L.) on Aspergillus flavus. PLoS ONE 2012, 7, e30147. [Google Scholar] [CrossRef]
- Ahmadi, R.; Kalbasi-Ashtari, A.; Oromiehie, A.; Yarmand, M.S.; Jahandideh, F. Development and characterization of a novel biodegradable edible film obtained from psyllium seed (Plantago ovata Forsk). J. Food Eng. 2012, 109, 745–751. [Google Scholar] [CrossRef]
- Skandamis, P.N.; Nychas, G.J.E. Preservation of fresh meat with active and modified atmosphere packaging conditions. Int. J. Food Microbiol. 2002, 79, 35–45. [Google Scholar] [CrossRef]
- Kang, K.; Fong, W.P.; Tsang, P.W.K. Novel antifungal activity of purpurin against Candida species in vitro. Med. Mycol. J. 2010, 48, 904–911. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, J.; Kong, W.; Zhao, G.; Yang, M. Mechanisms of antifungal and anti-aflatoxigenic properties of essential oil derived from turmeric (Curcuma longa L.) on Aspergillus flavus. Food Chem. 2017, 220, 1–8. [Google Scholar] [CrossRef]
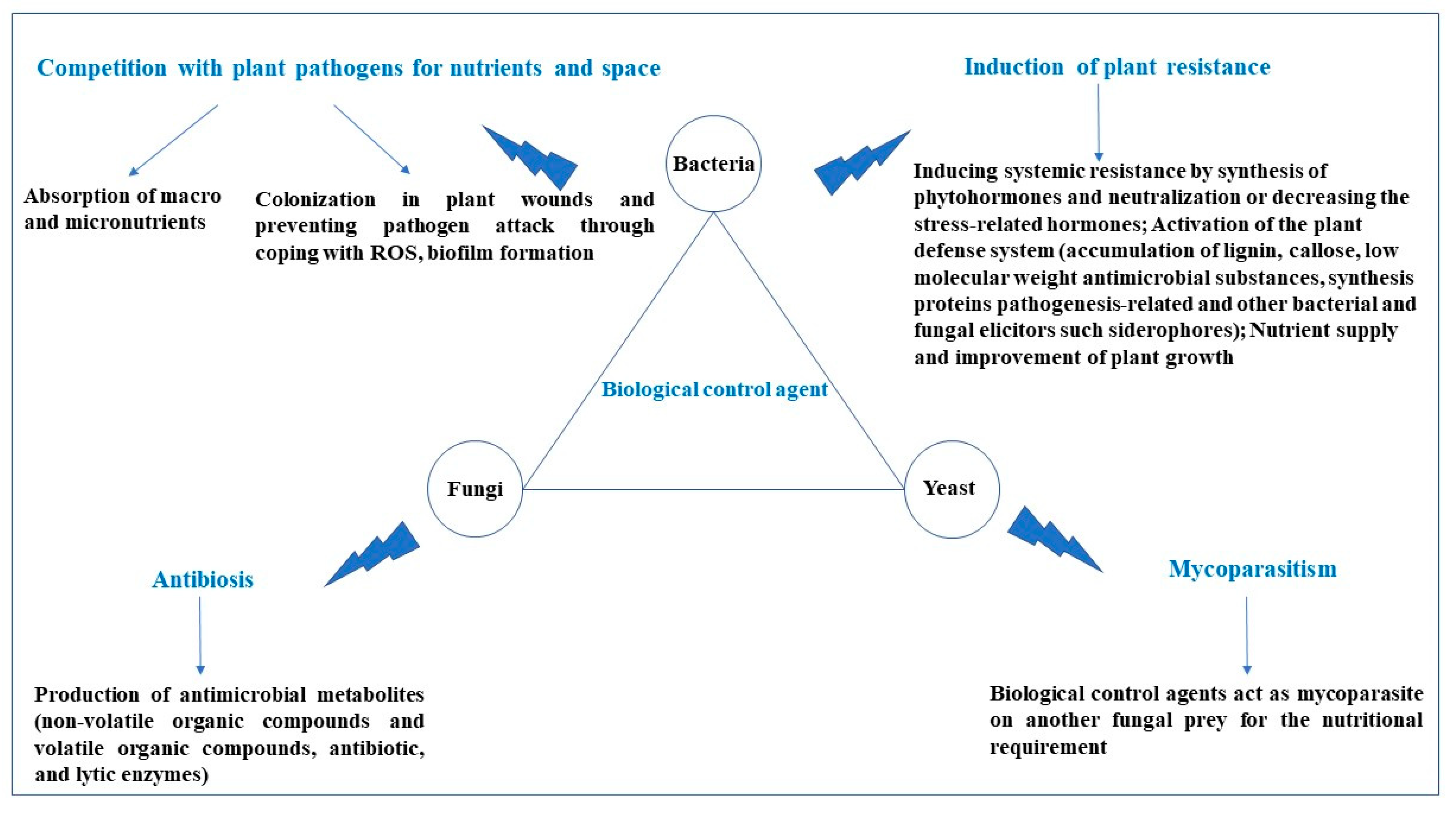

| Biological Control Agents | Mode of Action Biological Control Agents | Main Function of Biological Control Agents | References |
|---|---|---|---|
| Antagonistic bacteria | Rapid colonization in host tissue; Synthesize and release secondary metabolites (non-volatile organic compounds and volatile organic compounds); Produce siderophores and inhibit the growth and colonization of iron-dependent microorganisms; Suppress the activity of pathogens through competition for nutrients, production of lytic enzymes, inhibition of the synthesis of toxins; Activation of the plant defense system; Neutralization or decrease the stress-related hormones and stimulation of plant growth; Nutrient supply and improvement of host plant growth | Inhibition of postharvest pathogens | [39,44,45,46,59,62,63,82,87] |
| Antagonistic fungi | Synthesis of phytohormones and volatile organic compounds; Destruction cell membrane and the morphology of fungal hyphae | Inhibition of postharvest pathogen | [90,91] |
| Antagonistic yeasts | Suppress the activity of pathogens through competition for nutrients, production of lytic enzymes, inhibition of the synthesis of toxins; Increasing the expression of antioxidant genes; Induction of defense system and mycoparasitism | Inhibition of postharvest pathogens | [78,109] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moradinezhad, F.; Ranjbar, A. Advances in Postharvest Diseases Management of Fruits and Vegetables: A Review. Horticulturae 2023, 9, 1099. https://doi.org/10.3390/horticulturae9101099
Moradinezhad F, Ranjbar A. Advances in Postharvest Diseases Management of Fruits and Vegetables: A Review. Horticulturae. 2023; 9(10):1099. https://doi.org/10.3390/horticulturae9101099
Chicago/Turabian StyleMoradinezhad, Farid, and Azam Ranjbar. 2023. "Advances in Postharvest Diseases Management of Fruits and Vegetables: A Review" Horticulturae 9, no. 10: 1099. https://doi.org/10.3390/horticulturae9101099
APA StyleMoradinezhad, F., & Ranjbar, A. (2023). Advances in Postharvest Diseases Management of Fruits and Vegetables: A Review. Horticulturae, 9(10), 1099. https://doi.org/10.3390/horticulturae9101099






