Abstract
It has been well documented that far-red radiation (FR; 700–799 nm) elicits a shade-avoidance/shade-tolerance response across a wide range of plant species. Most sole-source lighting is relatively low in FR compared to sunlight (i.e., 2% vs. 20% of photons, respectively, integrated between 400 and 799 nm). The objective of this experiment was to determine if the photomorphogenic response to FR is a useful strategy during the seedling stage to promote leaf expansion in the hopes that subsequently transplanted seedlings would increase radiation capture resulting in higher harvestable biomass. Lettuce (cv. ‘Rex’, ‘Red Oak’, and ‘Green Grand Rapids’) seedlings were exposed to 5, 10, 20, or 30 µmol·m−2·s−1 of supplemental FR for a duration of 10 d in a growth chamber for 20 h daily. During this stage, all seedlings received background light levels of 195 µmol·m−2·s−1 PAR light from white LEDs for 20 h daily. Seedlings were transplanted into a nutrient film technique (NFT) hydroponic system in a separate growth chamber with LED fixtures that supplied white light at 295 µmol·m−2·s−1 for 16 h daily (DLI = 17 mol·m−2·d−1) until they were harvested at 35 d from seeding. At transplant, fresh weight, leaf area, and plant height were significantly greater for all cultivars exposed to 30 µmol·m−2·s−1 of supplemental FR radiation compared to the 5 µmol·m−2·s−1 control. Fresh weight increased by an average of 35% under 30 µmol·m−2·s−1 FR. Mature plant dry biomass increased by 14% when seedlings were exposed to 30 µmol·m−2·s−1 of supplemental FR radiation. Increasing far-red radiation consistently increased plant growth at the seedling stage, but these increases were generally overcome by maturation.
1. Introduction
Controlled environment agriculture (CEA) is a novel approach to agriculture in which crops are grown in a protective structure []. CEA’s capacity to produce plant crops on a year-round basis is a key component to its viability. Light, the catalyst of photosynthetic reactions, can be a limiting factor, most notably during winter months in middle to high-latitude regions worldwide []. Producers in these regions have overcome low periods of daily light integral (DLI) using supplemental radiation via electric lamps []. Similarly, sole source lighting production systems (i.e., vertical farms, warehouse farms, or plant factories) rely entirely on light photons produced through electricity. Technological advancements in the illumination space have given these growers significant control and options over this variable. However, there are vast energy implications involved in using horticultural lighting [,]. High-intensity discharge (HID) fixtures, such as high-pressure sodium (HPS) and metal halide (MH), are the most widely used technology in supplemented greenhouses and non-stacked (single-layer) sole source operations. A Department of Energy (DOE) report stated that 98% of greenhouses and 86% of non-stacked sole source operations employed HID fixtures []. These lighting options offer broad-spectrum illumination but do so with poor efficacy (µmol J−1) and control [,,,]. Light-emitting diodes (LED), the most prevalent form of solid-state lighting, have exceeded all other horticultural lighting technologies in terms of efficacy, lifetime, performance, versatility, and color quality [,,]. Among the best-performing HID fixtures in terms of efficacy are double-ended HPS (DE HPS) at 1.72 µmol J−1 [] compared to that of industry-leading LED fixtures at 3.69 µmol J−1 []. According to Lee et al. [], if all sampled supplemental greenhouses and single-layer sole source operations adopted LED lighting, they would save, respectively, 31% and 35% of their lighting electricity costs annually.
Continued advancement of LED technology has also delivered unparalleled spectral control allowing growers to spectrally tune their lighting fixtures to increase yields. Manufacturers produce LEDs in far-red (FR; λ = 700–799 nm), red (R; λ = 600–699 nm), green (G; λ = 500–599 nm), and blue (B; λ = 400–499 nm) narrow-band spectra []. In addition to these narrow-spectrum LEDs, broad-spectrum phosphor-converted white LEDs are available. White LEDs are short-wavelength LEDs (typically blue) with a luminescent phosphor coating that absorbs blue photons and luminesces them at a broader spectrum of lower energy (i.e., higher wavelengths) []. White LEDs are considered the workhorse of SSL (solid-state lighting) due to their application in general lighting used for human vision. Because of their popularity, they are manufactured in larger quantities, which reduces the cost per diode at the production level.
Until recently, most LED fixtures intended for horticultural use incorporated primarily R and B light [,]. The rationale was their close correlation with the chlorophyll absorption spectrum, which shows peak absorption in the blue and red regions within photosynthetically active radiation (PAR; λ = 400–700 nm). At the time, these spectra were also widely available and fairly energy efficient. This spectral combination produces a magenta hue that is commonly associated with horticultural LED fixtures. However, R and B LEDs may omit spectra required for optimum plant morphology, yield, or quality. FR is among these neglected spectra. The traditional paradigm of weighting photons [yield photon flux (YPF)] used for photosynthetic processes was based on the quantum yield of photosynthesis (CO2 assimilation) measurements [,] under narrow wave bands. A sharp reduction in the quantum yield of photosynthesis below 400 nm and above 685 nm across numerous plant species provided researchers with evidence to discount FR’s contribution to photosynthesis, therefore excluding it from the definition of PAR [,,]. However, these measurements did not account for synergistic relationships amongst multiple wavebands. Emerson et al. [] observed a photosynthetic enhancement of long-wave radiation when supplemented with shorter wavelength photons. Similarly, recent research has revealed that FR photons (up to 750 nm) have equivalent effects on photosynthesis to traditional PAR photons when added to shorter wavebands [] and are in fact necessary for efficient photochemistry [].
Calculations of photosynthetic efficiency under electric-sourced lighting do not adequately model growth responses due to the photomorphogenic and thermal effects of radiation adjacent to PAR [,,]. Plants use environmental cues, for example, light perception, to modulate their developmental programs (i.e., reproductive status) and physiological apparatuses (i.e., pigment synthesis and movement) [,]. These adjustments are made as plants perceive specific wavelength radiation through various photoreceptors. FR radiation is absorbed by the photoreceptor phytochrome. Five phytochromes (phyA–E) can be classified into two groups: type I (phyA) is light-labile, and type II (phyB–E) is light-stable. Among these, phyA and phyB are the best characterized [], however, each of these photoreceptor proteins plays critical roles in seed dormancy and germination, seedling de-etiolation, plant height, neighbor perception (shade avoidance), flower induction, inhibition or promotion of cell growth, chloroplast development, and gene expression responses [,]. Phytochrome has a unique ability to change its conformation between an active form, phytochrome far-red (Pfr), which has a maximal absorption at 730 nm, and an inactive form, phytochrome red (Pr) which has a maximal absorption at 660 nm []. These interconvertible forms are photoreversible and stabilize under consistent spectra into a characterizing ratio of Pfr to total phytochrome (Ptotal), called phytochrome photo-equilibrium (PPE) or phytochrome photostationary state (PSS). In high R:FR environments, inactive Pr is converted to active Pfr at a higher rate than the opposite conversion, increasing PPE.
PhyB is responsible for the photomorphogenic response known as shade avoidance syndrome (SAS) or neighbor detection [,]. It is a soluble chromoprotein localized in the cytoplasm in the dark and translocates to the nucleus in a light-dependent manner where it regulates gene expression [,,]. In full sun, active phyB (PfrB) accumulates in the nucleus where it physically binds to phytochrome interacting proteins (PIF) []. PIF family members manage phytochrome responses including shade avoidance []. Their interaction with PfrB targets PIF proteins to degradation via the proteasome, resulting in the deactivation of genes induced by shade []. Conversely, a shaded environment rich in FR photons converts more phyB into the inactive form (PrB). PrB does not interact with PIF proteins, allowing them to bind to a G-box DNA motif, promoting the expression of genes that govern shade avoidance [].
Solar PPE in full sun and shade is roughly 0.70 and 0.20, respectively. The phenotype associated with plants grown in low PPE (shade; FR-enriched light) is generally not desirable in agricultural commodities. Ecologists categorize plants into shade-avoiding and shade-tolerant species [] based on their responses to shaded environments. A low PPE increases stem, internode, and petiole length in Arabidopsis thaliana and other dicots [,,,]. These species are generally intolerant of shade. Shade-tolerant species, like lettuce, favor leaf expansion over stem elongation []. Additionally, leaf length is increased while leaf mass per leaf area (LMA) is reduced [,]. Similarly, though harder to subjectively notice, plants grown under excessively high PPE (very low FR) are also undesirable (i.e., too compact, low yield). As mentioned previously, many LED manufacturers fail to use FR LEDs, resulting in PPE values found in crop canopies being higher than that of full sun. Even broad-spectrum white LEDs often produce minimal FR radiation (~2%). Tomato plants (Solanum lycopersicum L.) grown under PPE above solar have been shown to have decreased leaf area, dry mass, and fruit production [].
The effects of supplementary FR on the growth and development of CEA crops grown under LEDs need further exploration, yet trends have emerged in photosynthetic and morphological benefits with supplementary FR on a species-specific level. Lettuce (head and leaf lettuce combined) is the third most consumed vegetable in the United States per capita []. Incorporating FR radiation increases lettuce leaf area in sole-source experiments, which increases incident photon capture throughout the crop canopy leading to higher yields []. However, limits on the fraction of FR incorporation exist. Researchers reported FR-induced bolting in lettuce under spectra that incorporated 25% and 36% FR (percent basis; λ = 400–800 nm) []. Bolting in lettuce is the transitory period in which the plant shifts from vegetative growth to a reproductive stage. Upon bolting, lettuce takes on a bitter taste and marketability decreases sharply []. Thus, FR lighting of lettuce for the whole crop cycle may result in unwanted early bolting. Elucidating target intensities and temporal strategies of supplemental FR radiation used in lettuce growth has the potential to help CEA growers obtain more value out of their LED lights. High yields along with high-quality lettuce harvest improve not only the production cycle of this important and popular crop but also the livelihood of today’s CEA farmers.
The objective of this study was to investigate the effects of increased supplemental FR on the morphology of lettuce seedlings. Additionally, this study aims to track the subsequent impacts of early developmental FR exposure when seedlings are transplanted to the finishing stage in the absence of supplemental FR. It is hypothesized that increasing supplemental FR at the seedling stage will increase plant canopy size resulting in greater photon capture and growth throughout the plant’s life cycle.
2. Materials and Methods
Stone wool hydroponic growing media sheets (200-cell AO plugs; Grodan, Roermond, Neth.) were halved crosswise and placed in 25.4 × 25.4 cm irrigation trays with perforated bases. Seeds of L. sativa (cv. ‘Green Grand Rapids,’ ‘Rex,’ and ‘Salanova® Red Oak’) were placed in dry media sheets at a rate of one seed per cell. Cultivars were individually grouped and randomly assigned a position within the media. Trays were submerged in a nutrient solution containing 150 mg·L−1 N (5 N-12 P-26 K + CalNit; Jacks Nutrients, JR Peters Inc. Allentown, PA, USA) until the media became fully saturated. At that point, they were promptly removed, allowing the excess solution to drain. A translucent humidity dome was placed over the tray upon positioning inside a walk-in growth chamber (M-1; Environmental Growth Chambers, Chagrin Falls, OH, USA). Growth chamber parameters were set to maintain a day-time temperature of 24 °C and a night-time temperature of 19 °C. Illuminance was measured using a spectroradiometer (PS-300; Apogee Instruments, Logan, UT, USA) by mapping the growing area at canopy height in a 15.24 cm grid pattern. All measurements in each grow area were averaged and reported as a single value. Light mapping was performed before and after each experimental replication to confirm fixture output. Photosynthetic radiation was provided by broad-spectrum white LEDs (QB324 V2; Horticulture Lighting Group, Westerville, Ohio, USA) at an intensity of 100 µmol·m−2·s−1 (Table 1; Figure 1a) for 16 h·d−1. Propagules remained in this germination environment for 48 h.

Table 1.
Spectral characteristics of 4000 K white LED with supplemental far-red (FR; 700–799 nm) radiation treatments. The quantum distribution of the germination and finishing stages were equal to the 5 µmol·m−2·s−1 FR treatment.
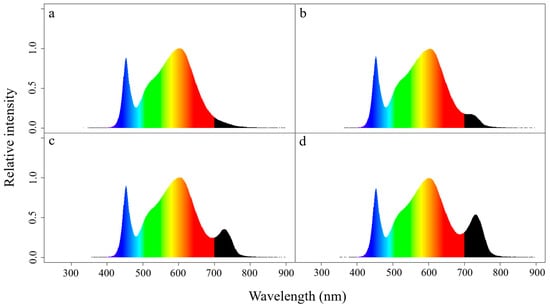
Figure 1.
Spectral distribution of individual lighting treatments; 4000 K white LED containing 195 µmol·m−2·s−1 PAR with (a) 5 µmol·m−2·s−1 (control), (b) 10 µmol·m−2·s−1, (c) 20 µmol·m−2·s−1, and (d) 30 µmol·m−2·s−1 of FR radiation.
Post-germination period, humidity domes were removed from seedling trays and seedlings were exposed to a novel lighting regime that consisted of broad-spectrum white LEDs (QB324 V2) provided at 200 µmol·m−2·s−1 (λ = 400–799 nm) supplemented by narrow spectrum FR LEDs (λ = 736 nm) (XLamp XP-E; Cree, Research Triangle Park, NC, USA) for 20 h (Figure 1a). Seedling trays were randomly assigned to FR radiation treatments: 5 µmol·m−2·s−1 (control; ambient FR from the white LEDs, no additional FR), 10 µmol·m−2·s−1, 20 µmol·m−2·s−1, or 30 µmol·m−2·s−1 (Figure 1a–d). Seedling trays were placed within individual 0.61 m × 0.91 m partitions where they remained for 10 d (Figure 2a–b). Randomly selected seedlings from each treatment and each cultivar were transplanted into a hydroponic nutrient film technique (NFT) system to continue growth to reach maturation (Figure 2c). The NFT system was located in an adjacent and identical growth chamber (M-1) with identical environmental set points. Supplemental FR was removed at this time and transplants grew the rest of their life cycle under broad spectrum white LEDs (QB324 V2) with an intensity of 295 µmol·m−2·s−1 for 16 h·d−1. Plants were randomly assigned a location in the NFT system on an individual basis.

Figure 2.
Experimental grow configuration of lettuce seedling growth chamber with (a) only far-red LEDs and (b) with base white LEDs. (c) Nutrient film technique chamber with white LEDs.
Destructive harvest of lettuce plants for growth assessment occurred at two separate time points: at the time of transplant and maturation (12 and 35 d from seeding, respectively). Seedling parameters collected at the transplant stage consisted of shoot fresh weight, shoot dry weight, length of most mature leaf, hypocotyl length, plant height, leaf area, and leaf number per seedling. For ‘Red Oak’ only, red pigmentation was evaluated using a colorimeter (PCE-CSM 4; PCE Instruments, Jupiter, FL, USA). Leaf colors were defined using L*a*b* color space (CIELAB) coordinates, which standardize color metrics based on three scales: (1) light to dark, (2) red to green, and (3) yellow to blue. Seedlings were lightly pulled from the media and severed at the base of the hypocotyl and immediately weighed on an analytical balance (MS104S; Mettler Toledo, Vernon Hills, IL, USA) for fresh weight. The most mature leaf was selected as the largest leaf above the cotyledons. This leaf was measured with a ruler along with the hypocotyl and total plant height. Leaf area was quantified using a leaf area meter (LI-3100C; LI-COR Biosciences, Lincoln, NE, USA). Seedlings were placed in a paper bag and into a mechanical convection oven (Freas 645; Thermo Electron Corp., Marietta, OH, USA) maintained at 70 °C for a minimum of 3 d. After this period, shoot dry weight was recorded (MS104S). Specific leaf area was calculated as leaf area divided by shoot dry weight. Moisture content (percentage of water mass within plant tissue) was calculated as the difference between fresh and dry weights divided by fresh weight [(Fresh weight–dry weight)/fresh weight × 100].
Mature lettuce heads were harvested by cutting the stem at the top level of the stone wool growing media. Parameters of mature lettuce heads included shoot fresh weight, shoot dry weight, plant height, and leaf area and were conducted as previously described. Additionally, plant diameter, number of leaves exhibiting tip burn, and stem length were recorded. Plant diameter was calculated by measuring a mature lettuce head with a ruler at its widest point, a second measurement perpendicular to the widest point, and averaging these two measurements. Tip burn was evaluated by manually counting the number of leaves per head of lettuce that experienced some degree of tip burn (10 being the maximum score). Stem length was obtained post-leaf separation with a ruler, measuring from the severed stem to the apical meristem.
This experiment was arranged in a randomized complete block design using two factors: intensity of supplemental FR radiation (4 levels) and cultivar of lettuce (3 levels). Each experiment consisted of three replicate blocks for each of the 12 treatment combinations. Each of the four FR lighting factors was randomly assigned to a location within each block at the start of each experiment. Each of the three cultivars of lettuce was randomly assigned a location within each FR radiation factor. All seedlings were randomly assigned a location when transplanted to the NFT system. This experiment was repeated three times. Within each cultivar, three seedlings were randomly selected at transplant per block per treatment (27 replicates) for data collection and two were selected to grow to maturity and subsequent data collection (18 replicates). L*a*b* coordinates of ‘Red Oak’ seedlings were averaged using three randomly selected plants from each block of each experimental replication. L*a*b* coordinates of mature ‘Red Oak’ heads averaged one reading from each of three randomly selected leaves per head of lettuce harvested.
Data were analyzed as a full factorial arrangement using JMP software (version 14.0.0; SAS Institute, Cary, NC, USA). Pairwise comparisons and mean separation by Tukey’s honestly significant difference post-hoc test at p ≤ 0.05 were performed. Data columns that did not meet normalized residual distribution requirements (seedling fresh weight, seedling dry weight, seedling leaf area, and seedling hypocotyl length) were log (ln) transformed.
3. Results
3.1. Seedling Mass
Lettuce seedlings harvested after 12 d had increased fresh weight with increasing FR radiation (p = 0.0261). Seedlings had 35% higher fresh weights when exposed to 30 µmol·m−2·s−1 of supplemental FR radiation when compared to those exposed to 5 µmol·m−2·s−1 of FR across all cultivars (Table 2). Incremental increases of supplemental FR photons, thereby decreasing the R:FR ratio, led to commensurate increases in fresh weight for all cultivars. Seedlings exposed to moderate levels of supplemental FR (10 and 20 µmol·m−2·s−1) had a numerical but not statistically significant increase in fresh weight. The interaction of cultivar with treatment was not significant; all cultivars behaved similarly to increasing amounts of supplemental FR radiation (Figure 3). Dry biomass was not affected by the amount of supplemental FR radiation across the selected cultivars at the seedlings stage (p = 0.1099). The moisture content of seedlings was similar under all FR radiation treatments (p = 0.0626).

Table 2.
Seedling plant mass characteristics. Fresh weight (FW), dry weight (DW), and moisture content of ‘Rex’, ‘Red Oak’, and ‘Grand Rapids’ lettuce seedlings grown under white light containing 195 µmol·m−2·s−1 of PAR with 5 µmol·m−2·s−1 (control), 10 µmol·m−2·s−1, 20 µmol·m−2·s−1, or 30 µmol·m−2·s−1 of far-red (FR) radiation treatment (TRT).
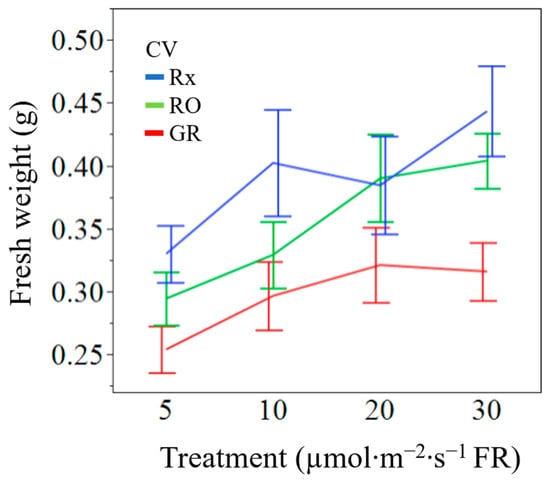
Figure 3.
Cultivar (CV) separated fresh weight means (g) of ‘Rex’ (Rx), ‘Red Oak’ (RO), and ‘Grand Rapids’ (GR) lettuce seedlings grown under white light containing 195 µmol·m−2·s−1 of PAR with 5 µmol·m−2·s−1 (control), 10 µmol·m−2·s−1, 20 µmol·m−2·s−1, or 30 µmol·m−2·s−1 of far-red (FR) radiation. Error bars are constructed using one standard error from the mean.
3.2. Seedling Morphology
Increases in supplemental FR radiation increased seedling leaf area (p = 0.0487). Averaged across cultivars, seedlings exposed to 30 µmol·m−2·s−1 of FR radiation had 38% greater leaf area than the control plants (Table 3). Though statistically similar, seedlings treated with 10 or 20 µmol·m−2·s−1 FR had a 17% and 29% greater leaf area than the control, respectively. Specific leaf area was not found to be significantly different amongst treatments at the main effect level (p = 0.0993). However, the interaction of treatment by cultivar resulted in significance among the ‘Red Oak’ cultivar only (Figure 4). Increasing levels of supplemental FR radiation resulted in less dry biomass per unit area in ‘Red Oak’ lettuce seedlings at all treatments. The main effect of FR radiation treatment on leaf length resulted in leaves that were on average 26% and 39% longer when exposed to 20 µmol·m−2·s−1 and 30 µmol·m−2·s−1 of supplemental FR radiation, respectively, when compared to the control 5 µmol·m−2·s−1 of FR (Figure 5). The interaction effect of cultivar by treatment was significant regarding leaf length (p = 0.0199). Cultivars ‘Rex’ and ‘Grand Rapids’ produced larger leaf lengths under all levels of supplemental FR radiation when compared to the control. ‘Red Oak’, however, only produced larger leaf lengths under 20 µmol·m−2·s−1 and 30 µmol·m−2·s−1 of supplemental FR radiation (Figure 6). The main effect of FR treatment and the interaction between cultivars by treatment were significant on seedling hypocotyl length (p = 0.0031 and p = 0.0338, respectively). Cultivars ‘Rex’ and ‘Red Oak’ did not exhibit differences between FR treatments, however, ‘Grand Rapids’ hypocotyls were 24% larger when exposed to 20 µmol·m−2·s−1 and 30 µmol·m−2·s−1 of supplemental FR radiation when compared to 5 µmol·m−2·s−1 and 10 µmol·m−2·s−1 of supplemental FR radiation (Figure 7a). Similarly, the main effect of FR treatment and the interaction of cultivar by treatment were significant on plant height (p = 0.0007 and p = 0.0027, respectively). The number of leaves at 12 d was similar amongst all FR treatments (p = 0.2361).

Table 3.
Seedling plant morphology characteristics for ‘Rex’, ‘Red Oak’, and ‘Grand Rapids’ lettuce seedlings grown under white light containing 195 µmol·m−2·s−1 of PAR with 5 µmol·m−2·s−1 (control), 10 µmol·m−2·s−1, 20 µmol·m−2·s−1, or 30 µmol·m−2·s−1 of far-red (FR) radiation treatment (TRT). Specific leaf area (SLA) was calculated as leaf area divided by leaf dry weight (DW).
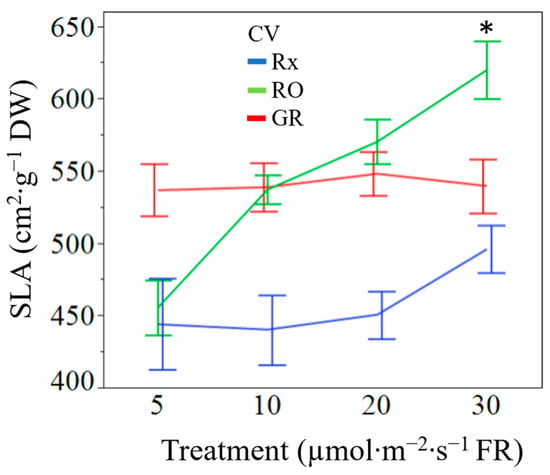
Figure 4.
Cultivar separated specific leaf area (cm2·g−1 leaf dry weight) means of ‘Rex’ (Rx), ‘Red Oak’ (RO), and ‘Grand Rapids’ (GR) lettuce seedlings grown under white light containing 195 µmol·m−2·s−1 of PAR with 5 µmol·m−2·s−1 (control), 10 µmol·m−2·s−1, 20 µmol·m−2·s−1, or 30 µmol·m−2·s−1 of far-red (FR) radiation. Error bars are constructed using one standard error from the mean. Means with asterisk (*) are significantly different from the control (5 µmol·m−2·s−1) within the cultivar using Tukey’s honestly significance difference test at p ≤ 0.05.

Figure 5.
Lettuce seedlings grown under white light containing 195 µmol·m−2·s−1 of PAR with 5 µmol·m−2·s−1 (control), 10 µmol·m−2·s−1, 20 µmol·m−2·s−1, or 30 µmol·m−2·s−1 of far-red (FR) radiation.
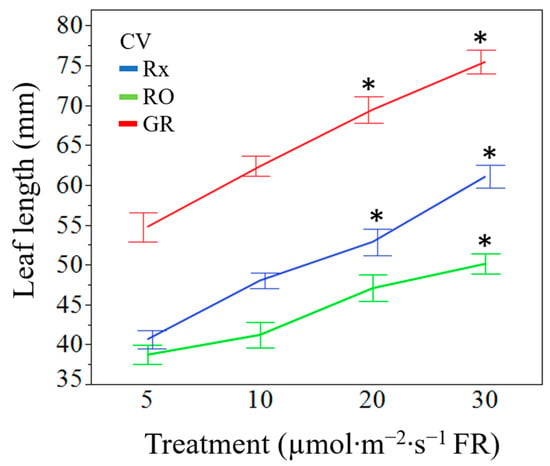
Figure 6.
Cultivar separated leaf length (mm) means of ‘Rex’ (Rx), ‘Red Oak’ (RO), and ‘Grand Rapids’ (GR) lettuce seedlings grown under white light containing 195 µmol·m−2·s−1 of PAR with 5 µmol·m−2·s−1 (control), 10 µmol·m−2·s−1, 20 µmol·m−2·s−1, or 30 µmol·m−2·s−1 of far-red (FR) radiation. Error bars are constructed using one standard error from the mean. Means with asterisk (*) are significantly different from the control (5 µmol·m−2·s−1) within the cultivar using Tukey’s honestly significance difference test at p ≤ 0.05.
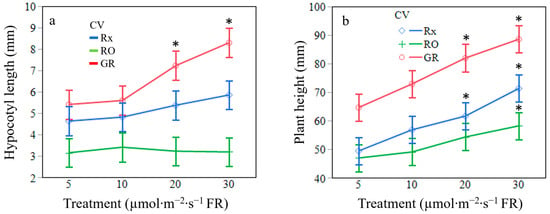
Figure 7.
(a) Hypocotyl length (mm) and (b) plant height (mm) of ‘Rex’ (Rx), ‘Red Oak’ (RO), and ‘Grand Rapids’ (GR) lettuce seedlings grown under white light containing 195 µmol·m−2·s−1 of PAR with 5 µmol·m−2·s−1 (control), 10 µmol·m−2·s−1, 20 µmol·m−2·s−1, or 30 µmol·m−2·s−1 of far-red (FR) radiation. Error bars are constructed using one standard error from the mean. Means with an asterisk (*) are significantly different from the control of the same CV using Tukey’s honestly significance difference test at p ≤ 0.05.
3.3. Seedling Coloration
Leaf coloration of ‘Red Oak’ lettuce seedlings at 12 d was altered by supplemental FR radiation (Figure 8a). L* coordinates (dark vs. light) increased with increased FR radiation (p = 0.0147) indicating that leaf coloration was lighter under increased FR applications. a* (green vs. red) coordinates were similar under all radiation treatments (p = 0.1027) indicating that ‘Red Oak’ seedlings were a similar hue of red across all radiation treatments. b* (blue vs. yellow) coordinates were significantly affected by FR radiation treatments (p = 0.0100) indicating that lower levels of FR radiation produced plants that were bluer and increasing supplemental FR radiation produced a yellowing effect.
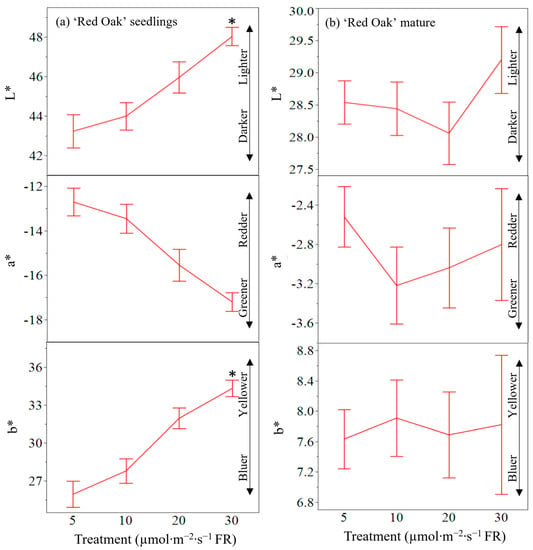
Figure 8.
Lab color space analysis (L*, light–dark; a*, green–red; b*; blue–yellow) for ‘Red Oak’ lettuce (a) seedling and (b) mature foliage grown under seedling treatments of white light containing 195 µmol·m−2·s−1 of PAR with 5 µmol·m−2·s−1 (control), 10 µmol·m−2·s−1, 20 µmol·m−2·s−1, or 30 µmol·m−2·s−1 of far-red (FR) radiation. Mature plants were finished under 295 µmol·m−2·s−1 (λ = 400–799 nm) of white light. Data points represent means. Error bars are constructed using one standard error from the mean. Means with asterisk (*) are significantly different from the control (5 µmol·m−2·s−1) using Tukey’s honestly significance difference test at p ≤ 0.05.
3.4. Mature Plant Mass
After the 10-d FR treatments, seedlings were transplanted and grown in a common environment with the control spectrum (2.5% FR) until they were subsequently harvested at the mature stage. Mature head fresh weights numerically increased (16%) with increasing supplemental FR radiation (Table 4). However, fresh weights were statistically similar at all FR treatment levels (p = 0.0554). Dry weights of mature lettuce heads increased when seedlings were given higher levels of supplemental FR radiation (p = 0.0224). Seedlings that received 20 µmol·m−2·s−1 and 30 µmol·m−2·s−1 of supplemental FR radiation had 10% and 14% higher dry biomass at harvest (35 d), respectively. Moisture content was not affected by FR treatment levels (p = 0.7518).

Table 4.
Mature plant mass characteristics. Fresh weight (FW), dry weight (DW), and moisture content of ‘Rex’, ‘Red Oak’, and ‘Grand Rapids’ mature lettuce heads grown under white light containing 195 µmol·m−2·s−1 of PAR supplemented with 5 µmol·m−2·s−1, 10 µmol·m−2·s−1, 20 µmol·m−2·s−1, or 30 µmol·m−2·s−1 of far-red (FR) radiation treatment (TRT). Mature plants were finished under 295 µmol·m−2·s−1 (λ = 400–799 nm) of white light.
3.5. Mature Plant Morphology
Leaf area, specific leaf area, plant height, head diameter, and stem length of mature lettuce heads were similar amongst seedling FR treatments by the time of mature head harvest (Table 5). All of these characteristics increased numerically with increased supplemental FR radiation; however, none were of significant value. The number of leaves on mature lettuce heads with tip-burn on each head were similar for all FR seedling treatments.

Table 5.
Mature plant morphology characteristics for ‘Rex’, ‘Red Oak’, and ‘Grand Rapids’ mature lettuce heads grown under white light containing 195 µmol·m−2·s−1 of PAR with 5 µmol·m−2·s−1 (control), 10 µmol·m−2·s−1, 20 µmol·m−2·s−1, or 30 µmol·m−2·s−1 of far-red (FR) radiation treatment (TRT). Mature plants were finished under 295 µmol·m−2·s−1 (λ = 400–799 nm) of white light. Specific leaf area (SLA) was calculated as leaf area divided by leaf dry weight (DW).
3.6. Mature Plant Coloration
L*a*b* coordinates of mature ‘Red Oak’ lettuce foliage (Figure 8b) were similar across all treatments (p = 0.4860, 0.6996, and 0.9891, respectively).
4. Discussion
With regards to plant production, crop growth rate (CGR; g dry biomass per m2 ground per day) is largely dependent on radiant energy [,]. Distinctly, CGR is a metric comprised of two components: net assimilation rate (NAR; grams of dry mass per m2 of leaf per day) and leaf area index (LAI; m2 of leaf area per m2 of ground) []. Leaf area is intuitively an integral component of LAI. High blue light environments reduce leaf area through inhibition of cell division and cell expansion [,]. Through increased additions of supplemental FR radiation, the current study decreased ratios of B:FR and R:FR which produced seedlings with increased leaf area, LAI, and thus increased CGR. These results are consistent with Meng and Runkle [] who reported that substituting FR radiation for B radiation promoted leaf expansion and increased shoot mass. Additionally, Legendre and van Iersel [] reported increased leaf area and canopy cover as the FR ratio increased in relation to white LED light.
Biomass gain is closely correlated with radiation capture efficiency, or the fraction of radiation intercepted by photosynthetic plant parts []. At the time of transplant, all seedling cultivars were larger under supplemental FR radiation, yet their developmental stage was no further along as demonstrated by leaf number. In this study, the broadening of leaves was closely associated with higher fresh weights amongst all cultivars. Furthermore, the dry biomass of seedlings remained similar amongst treatments. These two factors could indicate that the increased fresh weight was merely an increase in the vacuolar sequestration of water. Therefore, the increased leaf area may be due to cellular expansion triggered by the cell vacuole exerting turgor pressure on the cell wall. This postulate is partially correct. Plant cells do grow in response to turgor pressure from the cell vacuole []. Nevertheless, cell enlargement is typically coupled by commensurate organelle growth []. If plants were only taking on increased fresh mass without additional dry mass, plant moisture content would increase with supplemental FR treatments. Remarkably, moisture content was similar across FR treatments (Table 4), indicating that this was not merely a vacuolar sequestration of water. Furthermore, SLA, a metric that indicates the amount of leaf area a plant builds with a given amount of leaf biomass, was also similar amongst all FR treatments at the seedling stage (Table 5). This suggests that plant carbohydrates were allocated similarly with respect to density to seedling structural components across the treatments.
In CEA head lettuce production, healthy and vigorous transplant cohorts are fundamental when trying to maximize harvestable yields on a schedule. The strategy proposed in this study was based on concentrating intensive energy inputs (radiant energy) during a developmental stage when lettuce plants are spaced at their highest density. On a practical level, this strategy maximizes the number of individual plants receiving a resource input while minimizing energy output. Though this work did accomplish the goal of producing vigorous transplants, it did not translate to significantly higher fresh-weight yields at the time of harvest. That being said, dry mass was significantly increased, and the margin of fresh mass was numerically close to a statistically significant value, p = 0.0554. In comparison to the control, mature heads that received 10 µmol·m−2·s−1, 20 µmol·m−2·s−1, or 30 µmol·m−2·s−1 of supplemental FR during the seedling stage had 11%, 14%, and 16% higher fresh weights, respectively. Additionally, individual cultivars responded differently to FR treatments. At the time of harvest, ‘Rex’ and ‘Red Oak’ heads that were exposed to 30 µmol·m−2·s−1 of FR at the seedling stage had 16% and 33% higher fresh weights, respectively, compared to the control. The same comparison for ‘Grand Rapids’ was only a 7% increase in fresh weight. If the same mixed model is run while excluding ‘Grand Rapids’ from the data set, the main effect of FR seedling treatment would be a significant factor on mature head fresh weight (p = 0.0183). The same adjustment to the leaf area model’s data set would yield a similar result.
The fact that removing ‘Grand Rapids’ from the data set validated the hypothesis, suggests that this cultivar’s morphology is affected by the spectrum to a lesser degree than the other cultivars. At the time of transplant, the hypocotyl length of ‘Grand Rapids’ increased drastically when the ratio of R:FR decreased to 3.3 (or B:FR decreased to 1.9). Furthermore, leaf length and total plant height increased steadily as both ratios decreased. These morphological responses to FR treatments were more pronounced in ‘Grand Rapids’ when compared to both ‘Rex’ and ‘Red Oak’ cultivars at the same stage. However, when higher levels of supplemental FR were removed from the growth spectrum (post-transplant), the ratio of B light, which increased from 17% to 20%, overcame the SAS-induced photomorphogenic responses in ‘Grand Rapids’.
An additional factor to consider is the vast amount of G light in the control spectrum. The base white LED produced about 44% of its photons in the G region. This is a far higher percentage than ambient sunlight which is about 28% G radiation. Similar to FR, G light induces shade-avoidance responses (in the absence of FR) and yields a high photosynthetic value [,,]. Bugbee [] states that dry mass gain decreases as the fraction of B light increases above 5% to 10%. Acting through cryptochromes, B and G light have antagonistic effects [,]. The compounded effects of FR and G light may have been too great for the inhibitory effects of B light in reducing biomass gain at the seedling stage in ‘Grand Rapids’. Meng et al. [] reported that G light was not as potent as FR at inducing shade signals. Upon transplant and removal of higher levels of FR radiation, B light inhibited G responses resulting in reduced photon capture and reduced CGR leading up to harvest.
Spectral effects on the marketability factors of lettuce are not entirely understood. Plant survivability depends on a transition to a reproductive life phase. However, in lettuce cultivation, this transition, also known as bolting, is undesirable as it creates an unmarketable bitter taste []. Environmental stimuli, phytohormones, life stage, and the expression of flowering-related genes affect the onset of bolting []. The early signs of bolting begin with rapid stem elongation []. Spalholz [] reported vastly increased stem lengths in ‘Red Oak’ lettuce plants grown under 25% and 36% FR starting after 18 d. These results were not corroborated in the current study which did not find an impact of FR at the seedling stage and bolting (i.e., there were no statistical differences in stem length at the mature stage). This difference is likely due to plants in this study being exposed during a juvenile stage vs. an older stage. Spalholz’s experiment also exposed plants to a longer photoperiod (18 h) and provided fixed quantum distribution treatments throughout the entire crop cycle at lower TPFD (total photon flux density; λ = 400–799 nm) which resulted in slow CGR which may have caused the plants to senesce older leaves earlier in the life cycle. In addition to bolting, the calcium disorder tip-burn can cause economic losses. Increasing the growth rate with high light almost always results in tip-burn []. This study, however, did not see qualitative increases in tip burn at the time of harvest between FR seedling treatments (Table 5).
The pigmentation of ‘Red Oak’ foliage resulted in different coloration between FR treatments at the seedling stage. Coordinates for L* revealed leaves that were lighter in color as treatments of FR radiation increased in supplemental intensity. In the same pattern, b* coordinates increased, indicating leaves were more yellow as FR treatments increased in seedlings. Inversely, though not a significant result, a* coordinates decreased meaning red leaves were less red as FR treatments increased. In this study, coloration is used as a proxy for chlorophyll and anthocyanin content. To that end, these seedling results are consistent with previous studies [,]. The desired phenotype for red-leafed lettuce is dark red foliage. Since FR radiation treatments reduced the desirable appearance increased yields produce a dichotomy in decision making. Unlike other mass and morphological characteristics, leaf coloration showed no trends whatsoever by the time of harvest. The results indicate that reduction in coloration pigmentation can easily be overcome through spectral manipulation.
5. Conclusions
The present study represents the first known literature on increasing FR during the lettuce seedling stage with potential impacts toward mature harvest. Treating lettuce seedlings with supplemental FR radiation-induced shade avoidance symptoms resulting in increased fresh weight, leaf length, leaf area, and plant height without increasing specific leaf area or moisture content at the transplant stage. Post-transplant under a common spectrum containing only 2.5% FR radiation, dry biomass was increased by the time of harvest (35 d), however, fresh weight, leaf area, plant height, and plant diameter were similar amongst different FR seedling treatments. The current study shows promising results using FR radiation to increase radiation capture efficiency in the early plant stages. Further research prolonging the duration of FR treatments will likely yield more positive results in fresh and dry mass gains.
Author Contributions
Conceptualization, N.J.E.; methodology, N.J.E. and N.S.M.; formal analysis, N.J.E.; writing—original draft preparation, N.J.E.; writing—review and editing, N.J.E. and N.S.M.; supervision, N.S.M.; project administration, N.S.M.; funding procurement, N.S.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by U.S. Department of Agriculture NIFA-SCRI, grant number 2018-51181-28365.
Data Availability Statement
The data presented in this study are openly available in FigShare at 10.6084/m9.figshare.24239182.
Acknowledgments
We wish to express gratitude to Kendra Hutchins and Nicholas Kaczmar for their technical support. We would also like to thank the members of the Mattson CEA Lab at Cornell University for their assistance in data collection. Lastly, we would like to thank and honor Marc van Iersel for his advice and wisdom during the experimental design phase: forever in our hearts, forever in our memories. Rest in peace dear friend.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gómez, C.; Currey, C.D.; Dickson, R.W.; Kim, H.J.; Hernández, R.; Sabeh, N.C.; Raudales, R.E.; Brumfield, R.G.; Laury-Shaw, A.; Wilke, A.K.; et al. Controlled environment food protection for urban agriculture. HortScience 2019, 54, 1448–1458. [Google Scholar] [CrossRef]
- McAvoy, R.J.; Janes, H.W. Alternative production strategies for greenhouse tomatoes using supplemental lighting. Sci. Hortic. 1988, 35, 161–166. [Google Scholar] [CrossRef]
- Moe, R.; Grimstad, S.O.; Gislerød, H.R. The use of artificial light in year round production of greenhouse crops in Norway. Acta Hortic. 2006, 711, 35–42. [Google Scholar] [CrossRef]
- Pinho, P.; Hytönen, T.; Rantanen, M.; Elomaa, P.; Halonen, L. Dynamic control of supplemental lighting intensity in a greenhouse environment. Light. Res. Technol. 2013, 45, 295–304. [Google Scholar] [CrossRef]
- Harbick, K.; Albright, L.D.; Mattson, N.S. Electrical savings comparison of supplemental lighting control systems in greenhouse environments. In Proceedings of the 2016 ASABE Annual International Meeting, Orlando, FL, USA, 17–20 July 2016. [Google Scholar]
- Lee, K.; Elliott, C.; Pattison, M. Energy Savings Potential of SSL in Agricultural Applications; U.S. Department Energy: Washington, DC, USA, 2020. [Google Scholar]
- Mitchell, C.A.; Dzakovich, M.P.; Gomez, C.; Lopez, R.; Burr, J.F.; Hernandez, R.; Kubota, C.; Currey, C.J.; Meng, Q.; Runkle, E.S.; et al. Light-emitting diodes in horticulture. In Horticultural Reviews; Janick, J., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2015; Volume 43, pp. 1–87. [Google Scholar] [CrossRef]
- Johnson, J.; Bugbee, B. Double-Ended High Pressure Sodium Fixtures Decline Less than 6% over 2 Years and 5000 Hours. Publications 2017, Paper 7. Available online: https://digitalcommons.usu.edu/cpl_pubs/7 (accessed on 9 June 2023).
- Katzin, D.; Marcelis, L.F.M.; van Mourik, S. Energy savings in greenhouses by transition from high-pressure sodium to LED lighting. Appl. Energy 2021, 281, 116019. [Google Scholar] [CrossRef]
- Shelford, T.J.; Both, A.J. On the technical performance characteristics of horticultural lamps. AgriEngineering 2021, 3, 716–727. [Google Scholar] [CrossRef]
- Morrow, R.C. LED lighting in horticulture. HortScience 2008, 43, 1947–1950. [Google Scholar] [CrossRef]
- Stober, K.; Lee, K.; Yamada, M.; Pattison, M. Energy Savings Potential of SSL in Horticultural Applications; U.S. Department Energy: Washington, DC, USA, 2017.
- Pattison, P.M.; Tsao, J.Y.; Brainard, G.C.; Bugbee, B. LEDs for photons, physiology, and food. Nature 2018, 563, 493–500. [Google Scholar] [CrossRef]
- Radetsky, L. LED and HID Horticultural Luminaire Testing Report; Lighting Research Center, Rensselaer Polytechnic Institute: Troy, NY, USA, 2018. [Google Scholar]
- Design Lights Consortium. DLC Qualified Products List: Horticultural Lighting. Available online: https://qpl.designlights.org/horticulture. (accessed on 26 October 2021).
- Kusuma, P.; Pattison, M.P.; Bugbee, B. From physics to food: Current and potential LED efficacy. Hortic. Res. 2020, 7, 1–9. [Google Scholar] [CrossRef]
- Gioia, D.M.; Kim, H.H.; Wheeler, R.M.; Mitchell, C.A. Plant productivity responses to LED lighting. HortScience 2008, 43, 1951–1956. [Google Scholar] [CrossRef]
- McCree, K.J. The action spectrum, absorptance and quantum yield of photosynthesis in crop plants. Agric. Meteorol. 1972, 9, 191–216. [Google Scholar] [CrossRef]
- Sager, J.C.; Smith, W.O.; Edwards, J.L.; Cyr, K.L. Photosynthetic efficiency and phytochrome equilibria determination using spectral data. Trans. ASAE 1988, 31, 1882–1889. [Google Scholar] [CrossRef]
- Emerson, R.; Lewis, C.M. The dependence of the quantum yield of Chlorella photosynthesis on wavelength of light. Am. J. Bot. 1943, 30, 165–178. [Google Scholar] [CrossRef]
- Emerson, R.; Chalmers, R.; Cederstrand, C. Some factors influencing the long-wave limit of photosynthesis. Proc. Natl. Acad. Sci. USA 1957, 43, 133–143. [Google Scholar] [CrossRef]
- Zhen, S.; Bugbee, B. Far-red photons have equivalent efficiency to traditional photosynthetic photons: Implications for redefining photosynthetically active radiation. Plant Cell Environ. 2020, 43, 1259–1272. [Google Scholar] [CrossRef]
- Zhen, S.; van Iersel, M. Far-red light is needed for efficient photochemistry and photosynthesis. J. Plant Physiol. 2017, 209, 115–122. [Google Scholar] [CrossRef]
- McCree, K.J. Significance of enhancement for calculations based on the action spectrum for photosynthesis. Plant Physiol. 1971, 49, 704–706. [Google Scholar] [CrossRef]
- Sager, J.C.; Edwards, J.L.; Klein, W.H. Light energy utilization efficiency for photosynthesis. Trans. ASAE 1982, 25, 1737–1746. [Google Scholar] [CrossRef]
- Sager, J.C. Spectral effects on the growth of lettuce under controlled environment conditions. Acta Hortic. 1984, 148, 889–896. [Google Scholar] [CrossRef]
- Wada, M. Chloroplast movement. Plant Sci. 2013, 210, 177–182. [Google Scholar] [CrossRef]
- Pocock, T. Light-emitting diodes and the modulation of specialty crops: Light sensing and signaling networks in plants. HortScience 2015, 50, 1281–1284. [Google Scholar] [CrossRef]
- Sakamoto, K.; Nagatani, A. Nuclear localization activity of phytochrome B. Plant J. 1996, 10, 859–868. [Google Scholar] [CrossRef]
- Fankhauser, C. The phytochromes, a family of red/far-red absorbing photoreceptors. J. Biol. Chem. 2001, 276, 11453–11456. [Google Scholar] [CrossRef]
- Smith, H. Phytochrome and Photomorphogenesis; McGraw Hill: London, UK, 1975. [Google Scholar]
- Reed, J.W.; Nagpal, P.; Poole, D.S.; Furuya, M.; Chory, J. Mutations in the gene for the red/far-red light receptor phytochrome B alter cell elongation and physiological responses throughout Arabidopsis development. Plant Cell 1993, 5, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Lau, O.S.; Deng, X.W. Light-regulated transcriptional networks in higher plants. Nature Rev. Genetics 2007, 8, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Lorrain, S.; Allen, T.; Duek, P.D.; Whitelam, G.C.; Fankhauser, C. Phytochrome-mediated inhibition of shade avoidance involves degradation of growth-promoting bHLH transcription factors. Plant J. 2008, 53, 312–323. [Google Scholar] [CrossRef]
- Huq, E.; Quail, P.H. PIF4, a phytochrome-interacting bHLH factor, functions as a negative regulator of phytochrome B signaling in Arabidopsis. EMBO J. 2002, 21, 2441–2450. [Google Scholar] [CrossRef]
- Grime, J.P. Shade tolerance in flowering plants. Nature 1965, 208, 161–163. [Google Scholar] [CrossRef]
- Demotes-Mainard, S.; Peron, T.; Corot, A.; Bertheloot, J.; Le Gourrierec, J.; Pelleshi-Travier, S.; Crespel, L.; Morel, P.; Huche-Thelier, L.; Boumaza, R.; et al. Plant responses to red and far-red lights, applications in horticulture. Environ. Exp. Bot. 2016, 121, 4–21. [Google Scholar] [CrossRef]
- Djakovic-Petrovic, T.; de Wit, M.; Voesenek, L.; Pierik, R. DELLA protein function in growth responses to canopy signals. Plant J. 2007, 51, 117–126. [Google Scholar] [CrossRef]
- Finlayson, S.A.; Krishnareddy, S.R.; Kebrom, T.H.; Casal, J.J. Phytochrome regulation of branching in Arabidopsis. Plant Physiol. 2010, 152, 1914–1927. [Google Scholar] [CrossRef]
- Sasidharan, R.; Chinnappa, C.C.; Staal, M.; Elzenga, J.T.M.; Yokoyama, R.; Nishitani, K.; Voesenek, L.; Pierik, R. Light quality-mediated petiole elongation in Arabidopsis during shade avoidance involves cell wall modification by Xyloglucan endotransglucosylase/hydrolases. Plant Physiol. 2010, 154, 978–990. [Google Scholar] [CrossRef] [PubMed]
- Dale, J.E. The control of leaf expansion. Ann. Rev. Plant Physiol. Plant Mol. Biol. 1988, 39, 267–295. [Google Scholar] [CrossRef]
- Shibuya, T.; Komuro, J.; Hirai, N.; Sakamoto, Y.; Endo, R.; Kitaya, Y. Preference of sweetpotato whitefly adults to cucumber seedlings grown under two different light sources. HortTechnology 2010, 20, 873–876. [Google Scholar] [CrossRef]
- Skinner, R.H.; Simmons, S.R. Modulation of leaf elongation, tiller appearance and tiller senescence in spring barley by far-red light. Plant Cell Environ. 1993, 16, 555–562. [Google Scholar] [CrossRef]
- Kalaitzoglou, P.; van Ieperen, W.; Harbinson, J.; van der Meer, M.; Martinakos, S.; Weerheim, K.; Nicole, C.C.S.; Marcelis, L.F.M. Effects of continuous or end-of-day far-red light on tomato plant growth, morphology, light absorption, and fruit production. Front. Plant Sci. 2019, 10, 322. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Agriculture. Potatoes and Tomatoes Are the Most Commonly Consumed Vegetables; Economics Research Service, U.S. Department of Agriculture: Washington, DC, USA, 2020.
- Legendre, R.; Iersel, M.W. Supplemental far-red light stimulates lettuce growth: Disentangling morphological and physiological effects. Plants 2021, 10, 166. [Google Scholar] [CrossRef] [PubMed]
- Spalholz, H. Development of Novel Lighting Strategies for Optimal Lettuce Growth and Quality. Ph.D. Thesis, N.C. State University, Raleigh, NC, USA, 2019. [Google Scholar]
- Chen, Z.; Han, Y.; Ning, K.; Ding, Y.; Zhao, W.; Yan, S.; Luo, C.; Jiang, X.; Ge, D.; Liu, R.; et al. Inflorescence development and the roles of LsFT in regulating bolting in lettuce (Lactuca sativa L.). Front. Plant Sci. 2018, 8, 2248. [Google Scholar] [CrossRef]
- Albright, L.D.; Both, A.J.; Chiu, A.J. Controlling greenhouse light to a consistent daily integral. Trans. ASAE 2000, 43, 421–431. [Google Scholar] [CrossRef]
- Marcelis, L.F.M.; Broekhuijsen, A.G.M. Quantification of the growth response to light quantity of greenhouse grown crops. Acta Hortic. 2006, 711, 97–103. [Google Scholar] [CrossRef]
- Bugbee, B. Toward an optimal spectral quality for plant growth and development: The importance of radiation capture. Acta Hortic. 2016, 1134, 1–12. [Google Scholar] [CrossRef]
- Beall, F.D.; Yeung, E.C.; Pharis, R.P. Far-red light stimulates internode elongation, cell division, cell elongation, and gibberellin levels in bean. Can. J. Bot. 1996, 74, 743–752. [Google Scholar] [CrossRef]
- Dougher, T.; Bugbee, B. Long-term blue light effects on the histology of lettuce and soybean leaves and stems. J. Am. Soc. Hortic. Sci. 2004, 129, 467–472. [Google Scholar] [CrossRef]
- Meng, Q.; Kelly, N.; Runkle, E.S. Substituting green or far-red radiation for blue radiation induces shade avoidance and promotes growth in lettuce and kale. Environ. Exp. Bot. 2019, 162, 383–391. [Google Scholar] [CrossRef]
- Keating, B.A.; Carberry, P.S. Resource capture and use in intercropping: Solar radiation. Field Crops Res. 1993, 34, 273–301. [Google Scholar] [CrossRef]
- Green, P. Mechanisms of cellular morphogenesis. Science 1962, 138, 1404–1405. [Google Scholar] [CrossRef]
- Chan, Y.M.; Marshall, W.F. Scaling properties of cell and organelle size. Organogenesis 2010, 6, 88–96. [Google Scholar] [CrossRef]
- Kurosaki, M. Optimizing sole-source and supplemental lighting and carbon dioxide enrichment for controlled environment production of lettuce (Lactuca sativa L.) and tomato (Solanum esculentum L.). Master’s Thesis, Cornell University, Ithaca, NY, USA, 2022. [Google Scholar]
- Lui, J.; van Iersel, M.W. Photosynthetic physiology of blue, green, and red light: Light intensity and underlying mechanisms. Front. Plant Sci. 2022, 12, 619987. [Google Scholar] [CrossRef]
- Folta, K.M. Green light stimulates early stem elongation anagonizing light-mediated growth inhibition. Plant Physiol. 2004, 135, 1407–1416. [Google Scholar] [CrossRef]
- Zhang, T.; Maruhnich, S.A.; Folta, K.M. Green light induces shade avoidance symptoms. Plant Physiol. 2011, 157, 1528–1536. [Google Scholar] [CrossRef]
- Wang, S.; Luo, C.; Sun, L.; Ning, K.; Chen, Z.; Yang, J.; Wang, Y.; Wang, Q. LsRGL1 controls the bolting and flowering times of lettuce by modulating the gibberellin pathway. Plant Sci. 2022, 316, 111175. [Google Scholar] [CrossRef] [PubMed]
- Waycott, W. Photoperiodic responses of genetically diverse lettuce accessions. J. Am. Soc. Hortic. Sci. 1995, 120, 460–467. [Google Scholar] [CrossRef]
- Frantz, J.M.; Ritchie, G.; Cometti, N.N.; Robinson, J.R.; Bugbee, B. Exploring the limits of crop productivity: Beyond the limits of tipburn in lettuce. J. Am. Soc. Hortic. Sci. 2004, 129, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Kubota, C. Effects of supplemental light quality on growth and phytochemicals of baby leaf lettuce. Environ. Exp. Bot. 2009, 67, 59–64. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).