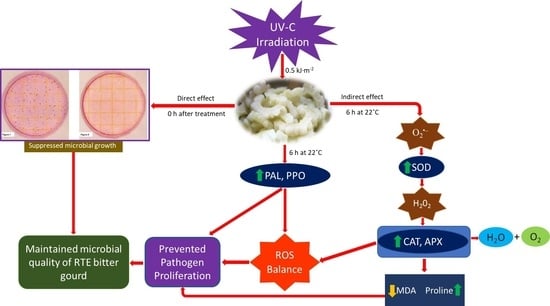
The Effects of UV-C Irradiation and Low Temperature Treatment on Microbial Growth and Oxidative Damage in Fresh-Cut Bitter Gourd (Momordica charantia L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fruit Materials and Fresh-Cut Processing
2.2. Microbiota Analysis
2.3. Treatments Application
2.3.1. UV-C Treatment
2.3.2. Low Temperature
2.3.3. UV-C Combined with Low Temperature
2.4. Microbiological Load Determinations
2.5. Determination of Reactive Oxygen Species (ROS) Production
2.5.1. H2O2 Content Analysis
2.5.2. Analysis of O2•− and H2O2 and Scavenging Activity
2.6. Analysis of Antioxidant Enzymes Activities
2.7. Determination of Defense-Related Compound Content
2.7.1. MDA Content Analysis
2.7.2. Proline Content Analysis
2.8. Analysis of PAL Activity
2.9. Analysis of PPO Activity
2.10. Statistical Analysis
3. Results
3.1. Microorganisms Grew on the Surface of Fresh-Cut Bitter Gourd
3.2. Effect of UV-C Irradiation Treatment on the Total Viable Bacterial Count of Fresh-Cut Bitter Gourd
3.3. The Effect of Low-Temperature Treatment on the Total Viable Bacterial Count of Fresh-Cut Bitter Gourd
3.4. The Effect of UV-C Followed by Low-Temperature Treatment on the Bacterial Population of Fresh-Cut Bitter Gourd
3.5. The Effect of UV-C on the Production of ROS in Fresh-Cut Bitter Gourd
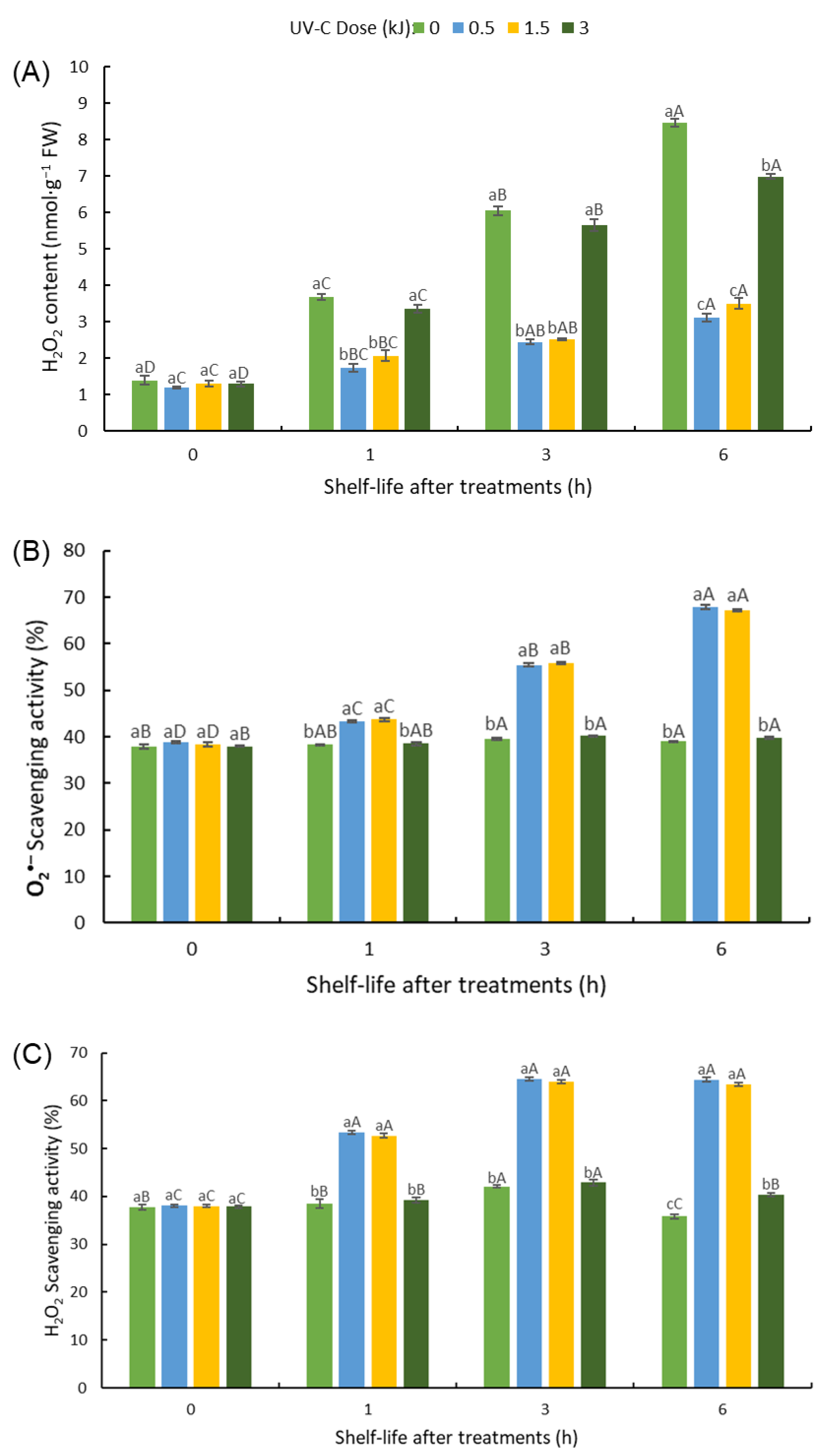
3.5.1. H2O2 Content in RTE Fresh-Cut Bitter Gourd
3.5.2. O2•− and H2O2 Scavenging Activity in UV-C-Treated RTE Fresh-Cut Bitter Gourd
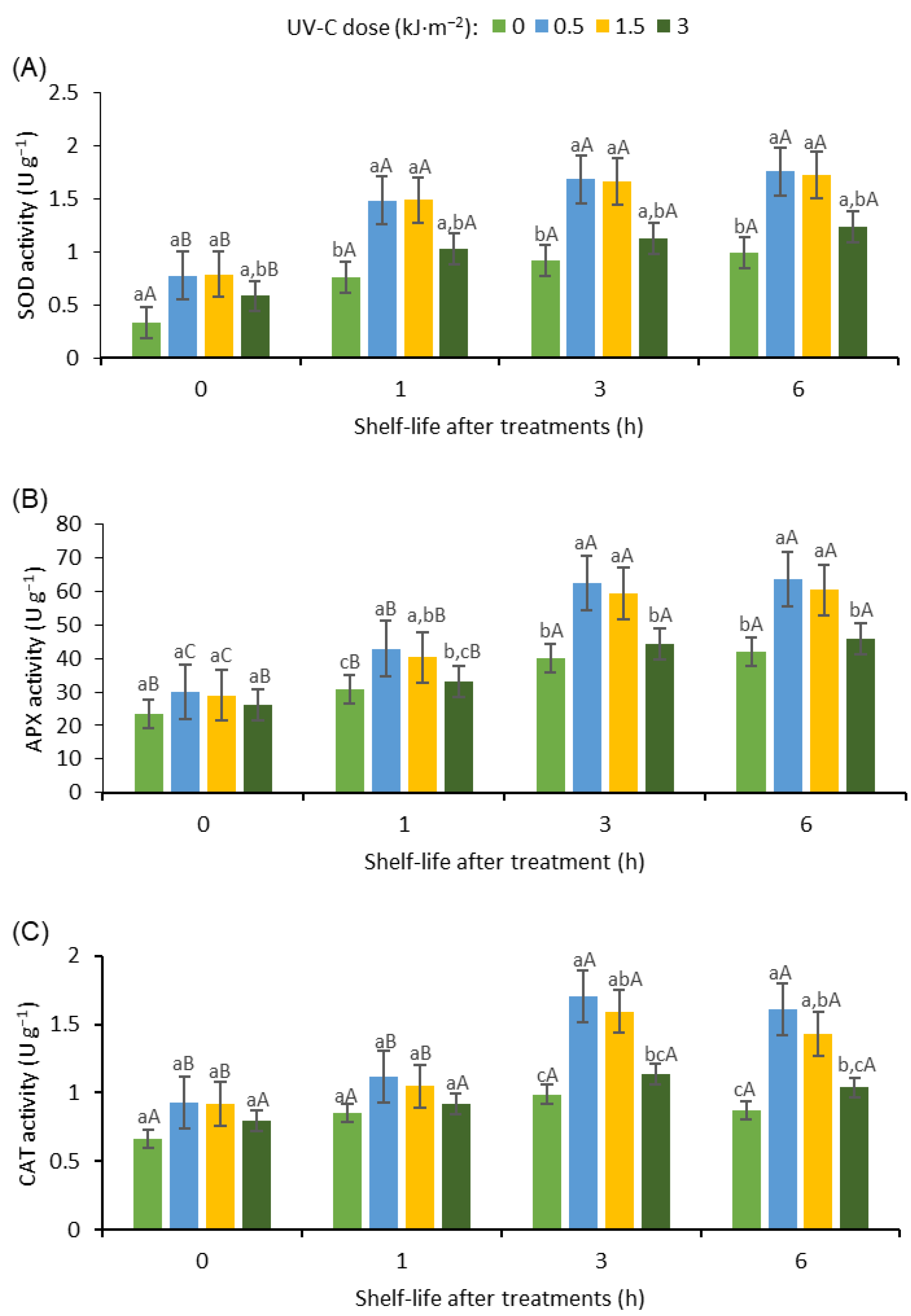
3.6. The Effect of UV-C Irradiation on the Activity of Antioxidant Enzymes of Fresh-Cut Bitter Gourd
3.7. The Effect of UV-C on the Defense-Related Compounds of Fresh-Cut Bitter Gourd
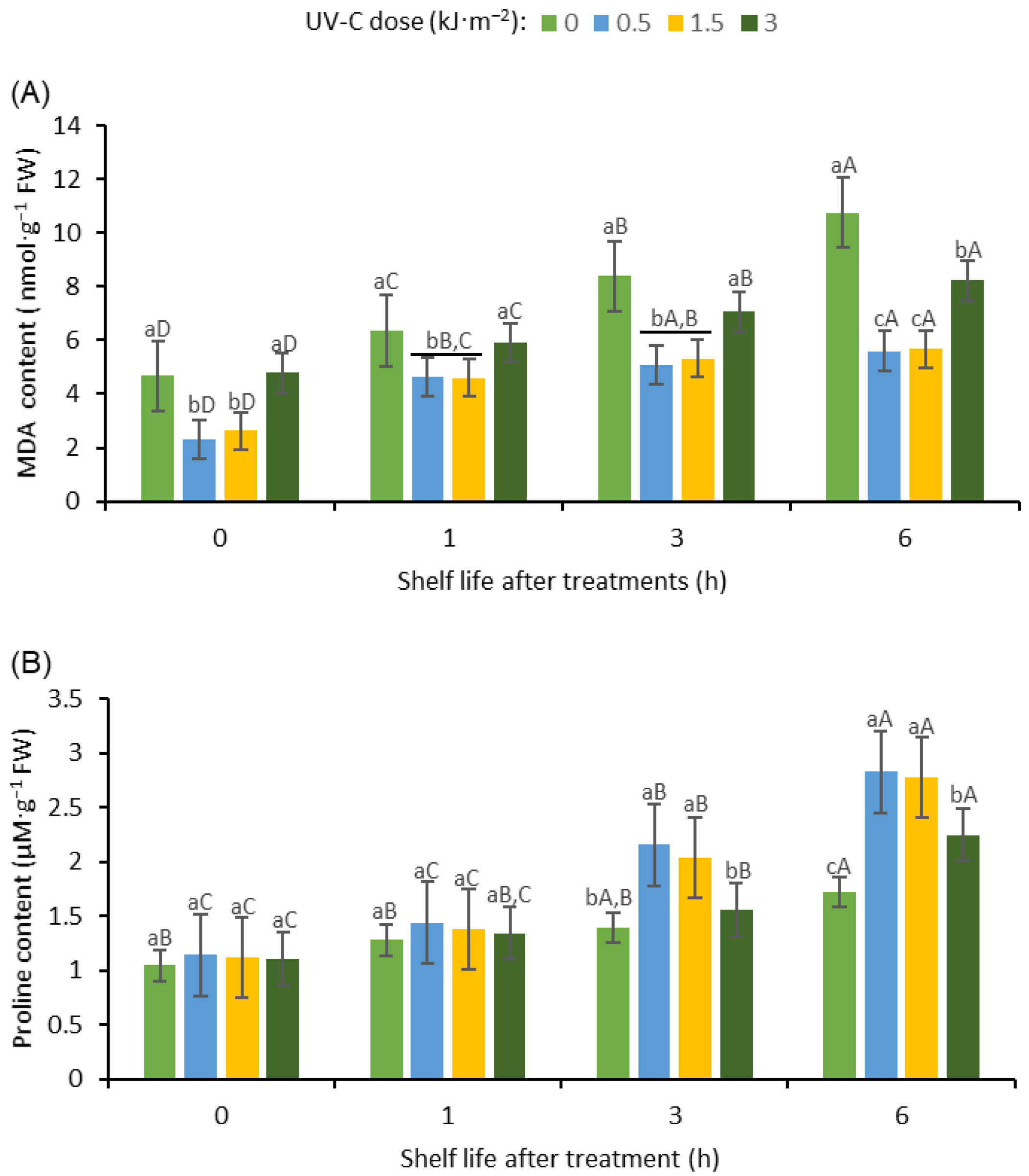
3.7.1. MDA Content
3.7.2. Proline Content
3.8. The Effect of UV-C on the Activity of Enzymes Involved in the Phenylpropanoid Pathway of Fresh-Cut Bitter Gourd
3.8.1. Phenylalanine Ammonia-Lyase (PAL)
3.8.2. Polyphenol Oxidase (PPO)
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lucas, E.A.; Dumancas, G.G.; Smith, B.J.; Clarke, S.L.; Arjmandi, B.H. Health benefits of bitter melon (Momordica charantia). In Bioactive Foods in Promoting Health, 1st ed.; Watson, R.R., Preedy, V.R., Eds.; Academic Press: London, UK, 2010; pp. 525–549. [Google Scholar] [CrossRef]
- Pan, Y.; Chen, L.; Pang, L.; Chen, X.; Jia, X.; Li, X. Ultrasound treatment inhibits browning and improves antioxidant capacity of fresh-cut sweet potato during cold storage. Rsc Adv. 2020, 10, 9193–9202. [Google Scholar] [CrossRef]
- Srilatha, V.; Reddy, K.; Reddy, R.; Anitha, T.; Mamatha, N.C.; Kumari, P.L. Chemical interventions for extending shelf life of minimally processed bitter gourd (Momordica charantia L.). Pharma Innov. 2021, 10, 746–753. [Google Scholar] [CrossRef]
- Wang, L.; Li, Q.; Cao, J.; Cai, T.; Jiang, W. Keeping quality of fresh-cut bitter gourd (Momordica charantia L.) at low temperature of storage. J. Food Process. Preserv. 2007, 31, 571–582. [Google Scholar] [CrossRef]
- Olaimat, A.N.; Holley, R.A. Factors influencing the microbial safety of fresh produce: A review. Food Microbiol. 2012, 32, 1–19. [Google Scholar] [CrossRef]
- Ministry of Food and Drug Safety (MFDS). Statistics for Foodborne Illness Outbreaks. Available online: https://www.foodsafetykorea.go.kr/portal/healthyfoodlife/foodPoisoningStat.do?menu_no=3724&menu_grp=MENU_NEW02 (accessed on 7 May 2020).
- Centers for Disease Control and Prevention (CDC). National Outbreak Reporting System (NORS). Available online: https://www.cdc.gov/norsdashboard/ (accessed on 15 June 2020).
- European Food Safety Authority (EFSA). Shiga toxin-producing E. coli (STEC) O104:H4 2011 Outbreaks in Europe: Taking Stock. EFSA J. 2011, 9, 2390. [Google Scholar] [CrossRef]
- King, L.A.; Nogareda, F.; Weill, F.X.; Mariani-Kurkdjian, P.; Loukiadis, E.; Gault, G.; de Valk, H. Outbreak of Shiga toxin–producing Escherichia coli O104: H4 associated with organic fenugreek sprouts, France, June 2011. Clin. Infect. Dis. 2012, 54, 1588–1594. [Google Scholar] [CrossRef] [PubMed]
- Castro-Rosas, J.; Cerna-Cortés, J.F.; Méndez-Reyes, E.; Lopez-Hernandez, D.; Gómez-Aldapa, C.A.; Estrada-Garcia, T. Presence of faecal coliforms, Escherichia coli and diarrheagenic E. coli pathotypes in ready-to-eat salads, from an area where crops are irrigated with untreated sewage water. Int. J. Food Microbiol. 2012, 156, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Faour-Klingbeil, D.; Murtada, M.; Kuri, V.; Todd, E.C. Understanding the routes of contamination of ready-to-eat vegetables in the Middle East. Food Control 2016, 62, 125–133. [Google Scholar] [CrossRef]
- Lee, H.H.; Hong, S.I.; Kim, D. Microbial reduction efficacy of various disinfection treatments on fresh-cut cabbage. Food Sci. Nutr. 2014, 2, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Preetha, P.; Deepa, J.; Venilla, P. Influence of temperature on the respiration rate of fresh-cut bitter gourd. Trends Biosci. 2014, 7, 3189–3192. [Google Scholar]
- Hu, X.; Chen, Y.; Wu, X.; Liu, W.; Jing, X.; Liu, Y.; Qin, W. Combination of calcium lactate impregnation with UV-C irradiation maintains quality and improves antioxidant capacity of fresh-cut kiwifruit slices. Food Chem. X 2022, 14, 100329. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Aguilar, G.A.; Villegas-Ochoa, M.A.; Martinez-Tellez, M.A.; Gardea, A.A.; Ayala-Zavala, J.F. Improving antioxidant capacity of fresh-cut mangoes treated with UV-C. J. Food Sci. 2007, 72, S197–S202. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Zhen, W.; Chen, Q.; Fu, M. UV-C irradiation inhibits surface discoloration and delays quality degradation of fresh-cut stem lettuce. LWT-Food Sci. Technol. 2021, 147, 111533. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, A.; Zhao, J.; Shang, J.; Zhu, Z.; Wu, X.; Zha, D. Transcriptome profiling reveals potential genes involved in browning of fresh-cut eggplant (Solanum melongena L.). Sci. Rep. 2021, 11, 16081. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, X.; Han, C.; Ji, N.; Jin, P.; Zheng, Y. UV-C treatment maintains quality and enhances antioxidant capacity of fresh-cut strawberries. Postharvest Biol. Technol. 2019, 156, 110945. [Google Scholar] [CrossRef]
- Prajapati, U.; Asrey, R.; Varghese, E.; Singh, A.K.; Singh, M.P. Effects of postharvest ultraviolet-C treatment on shelf-life and quality of bitter gourd fruit during storage. Food Packag. Shelf Life 2021, 28, 100665. [Google Scholar] [CrossRef]
- Pombo, M.A.; Rosli, H.G.; Martínez, G.A.; Civello, P.M. UV-C treatment affects the expression and activity of defense genes in strawberry fruit (Fragaria × ananassa, Duch.). Postharvest Biol. Technol. 2011, 59, 94–102. [Google Scholar] [CrossRef]
- Birmpa, A.; Sfika, V.; Vantarakis, A. Ultraviolet light and ultrasound as non-thermal treatments for the inactivation of microorganisms in fresh ready-to-eat foods. Int. J. Food Microbiol. 2013, 167, 96–102. [Google Scholar] [CrossRef]
- Erkan, M.; Wang, C.Y.; Krizek, D.T. UV-C irradiation reduces microbial populations and deterioration in Cucurbita pepo fruit tissue. Environ. Exp. Bot. 2001, 45, 1–9. [Google Scholar] [CrossRef]
- Lee, J.Y.; Yang, S.Y.; Yoon, K.S. Control measures of pathogenic microorganisms and shelf-life extension of fresh-cut vegetables. Foods 2021, 10, 655. [Google Scholar] [CrossRef]
- Cantwell, M.I.; Suslow, T.V. Postharvest handling systems: Fresh-cut fruits and vegetables. In Postharvest Technology of Horticultural Crops; Adel, K., Ed.; University of California Agriculture and Nature Resources Publication 3311: Oakland, CA, USA, 2002; pp. 445–463. [Google Scholar]
- Tatsika, S.; Karamanoli, K.; Karayanni, H.; Genitsaris, S. Metagenomic characterization of bacterial communities on ready-to-eat vegetables and effects of household washing on their diversity and composition. Pathogens 2019, 8, 37. [Google Scholar] [CrossRef] [PubMed]
- Junglee, S.; Urban, L.; Sallanon, H.; Lopez-Lauri, F. Optimized assay for hydrogen peroxide determination in plant tissue using potassium iodide. Am. J. Anal. Chem. 2014, 5, 730. [Google Scholar] [CrossRef]
- Mathur, A.; Verma, S.K.; Purohit, R.; Gupta, V.; Dua, V.K.; Prasad, G.B.K.S.; Mathur, D.; Singh, S.K.; Singh, S. Evaluation of in vitro antimicrobial and antioxidant activities of peel and pulp of some citrus fruits. J. Biotechnol. Biother. 2011, 1, 1–17. [Google Scholar]
- Ruch, R.J.; Cheng, S.J.; Klaunig, J.E. Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis 1989, 10, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.B.; Al Maiman, S.A.; Elkhatim, K.A.S.; Elbadr, N.A.; Alsulaim, S.; Osman, M.A.; Ahmed, I.A.M. Effect of UV-C radiation treatment on microbial load and antioxidant capacity in hot pepper, fennel and coriander. LWT-Food Sci. Technol. 2020, 134, 109946. [Google Scholar] [CrossRef]
- Kant, C.; Turan, M. Hydrogel substrate alleviates salt stress with increased antioxidant enzymes activity of bean (Phaseolus vulgaris L.) under salinity stress. Afr. J. Agric. Res. 2011, 6, 715–724. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Kato, M.; Shimizu, S. Chlorophyll metabolism in higher plants VI. Involvement of peroxidase in chlorophyll degradation. Plant Cell Physiol. 1985, 26, 1291–1301. [Google Scholar] [CrossRef]
- Promyou, S.; Supapvanich, S. Combinative effect of salicylic acid immersion and UV-C illumination on chilling injury-related factors of longan (Dimocarpus longan Lour.) fruit. Int. J. Fruit Sci. 2020, 20, 133–148. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.A.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Moreno, C.; Andrade-Cuvi, M.J.; Zaro, M.J.; Darre, M.; Vicente, A.R.; Concellón, A. Short UV-C treatment prevents browning and extends the shelf-life of fresh-cut carambola. J. Food Qual. 2017, 2017, 2548791. [Google Scholar] [CrossRef]
- Abu-Zaid, A.A.; Sehrawy, M.H.; Mahmoud, H.; Nemari, A.H. Detection of Klebsiella pneumonia in raw food and their antibiotic resistance. Adv. Environ. Biol. 2016, 10, 80–92. [Google Scholar]
- Puspanadan, S.; Afsah-Hejri, L.; Loo, Y.Y.; Nillian, E.; Kuan, C.H.; Goh, S.G.; Chang, W.S.; Lye, Y.L.; John, Y.H.T.; Rukayadi, Y.; et al. Detection of Klebsiella pneumoniae in raw vegetables using most probable number-polymerase chain reaction (MPN-PCR). Int. Food Res. J. 2012, 19, 1757. [Google Scholar]
- Ruiz-Roldán, L.; Rojo-Bezares, B.; Lozano, C.; López, M.; Chichón, G.; Torres, C.; Sáenz, Y. Occurrence of Pseudomonas spp. in raw vegetables: Molecular and phenotypical analysis of their antimicrobial resistance and virulence-related traits. Int. J. Mol. Sci. 2021, 22, 12626. [Google Scholar] [CrossRef] [PubMed]
- Barry-Ryan, C.; Pacussi, J.M.; O’beirne, D. Quality of shredded carrots as affected by packaging film and storage temperature. J. Food Sci. 2000, 65, 726–730. [Google Scholar] [CrossRef]
- World Health Organization. Prioritization of Pathogens to Guide Discovery, Research and Development of New Antibiotics for Drug-Resistant Bacterial Infections, Including Tuberculosis (No. WHO/EMP/IAU/2017.12). Available online: https://www.who.int/publications/i/item/WHO-EMP-IAU-2017.12 (accessed on 5 May 2023).
- Chavez Herrera, V.R.; Rosas De Silva, M.F.; Orendain Alcaraz, H.; Ceja Espiritu, G.; Carrazco Peña, K.; Melnikov, V. Death related to Cedecea lapagei in a soft tissue bullae infection: A case report. J. Med. Case Rep. 2018, 12, 328. [Google Scholar] [CrossRef]
- Huang, H.; Ge, Z.; Limwachiranon, J.; Li, L.; Li, W.; Luo, Z. UV-C treatment affects browning and starch metabolism of minimally processed lily bulb. Postharvest Biol. Technol. 2017, 128, 105–111. [Google Scholar] [CrossRef]
- Fonseca, J.M.; Rushing, J.W. Effect of ultraviolet-C light on quality and microbial population of fresh-cut watermelon. Postharvest Biol. Technol. 2006, 40, 256–261. [Google Scholar] [CrossRef]
- Cote, S.; Rodoni, L.; Miceli, E.; Concellón, A.; Civello, P.M.; Vicente, A.R. Effect of radiation intensity on the outcome of postharvest UV-C treatments. Postharvest Biol. Technol. 2013, 83, 83–89. [Google Scholar] [CrossRef]
- Chun, H.H.; Kim, J.Y.; Song, K.B. Inactivation of foodborne pathogens in ready-to-eat salad using UV-C irradiation. Food Sci. Biotechnol. 2010, 19, 547–551. [Google Scholar] [CrossRef]
- D’hallewin, G.; Schirra, M.; Pala, M.; Ben-Yehoshua, S. Ultraviolet C irradiation at 0.5 kJ/m−2 reduces decay without causing damage or affecting postharvest quality of star ruby grapefruit (C. paradisi Macf.). J. Agric. Food Chem. 2000, 48, 4571–4575. [Google Scholar] [CrossRef] [PubMed]
- Manzocco, L.; Plazzotta, S.; Maifreni, M.; Calligaris, S.; Anese, M.; Nicoli, M.C. Impact of UV-C light on storage quality of fresh-cut pineapple in two different packages. LWT-Food Sci. Technol. 2016, 65, 1138–1143. [Google Scholar] [CrossRef]
- Fan, L.; Song, J. Microbial quality assessment methods for fresh-cut fruits and vegetables. Stewart Postharvest Rev. 2008, 4, 10. [Google Scholar]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Zhou, Q.; Chen, C.; Zhou, F.; Hu, W.; Zhao, L.; Xu, Y. Correlation between enzymatic browning inhibition by UV-C treatment and reactive oxygen species metabolism of fresh-cut apples. Shipin Kexue/Food Sci. 2019, 40, 102–109. [Google Scholar]
- Chen, C.; Jiang, A.; Liu, C.H.; Zhao, Q.Q.; Zhang, Y.H.; Hu, W.Z. Effect of UV-C on the browning of fresh-cut Huangguan pear. Sci. Agric. Sin. 2020, 53, 5081–5090. [Google Scholar]
- Ouhibi, C.; Attia, H.; Nicot, P.; Lecompte, F.; Vidal, V.; Lachaâl, M.; Urban, L.; Aarrouf, J. Effects of nitrogen supply and of UV-C irradiation on the susceptibility of Lactuca sativa L to Botrytis cinerea and Sclerotinia minor. Plant Soil 2015, 393, 35–46. [Google Scholar] [CrossRef]
- Pongprasert, N.; Sekozawa, Y.; Sugaya, S.; Gemma, H. The role and mode of action of UV-C hormesis in reducing cellular oxidative stress and the consequential chilling injury of banana fruit peel. Int. Food Res. J. 2011, 18, 741–749. [Google Scholar]
- Yang, Z.; Cao, S.; Su, X.; Jiang, Y. Respiratory activity and mitochondrial membrane associated with fruit senescence in postharvest peaches in response to UV-C treatment. Food Chem. 2014, 161, 16–21. [Google Scholar] [CrossRef]
- Lv, Y.; Fu, A.; Song, X.; Wang, Y.; Chen, G.; Jiang, Y. 1-Methylcyclopropene and UV-C treatment effect on storage quality and antioxidant activity of ‘Xiaobai’ apricot fruit. Foods 2023, 12, 1296. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, F.; Wang, J.; Yang, Q.; Wang, P.; Zhao, H.; Wang, J.; Wang, C.; Xu, X. Salicylic acid inhibits the postharvest decay of goji berry (Lycium barbarum L.) by modulating the antioxidant system and phenylpropanoid metabolites. Postharvest Biol. Technol. 2021, 178, 111558. [Google Scholar] [CrossRef]
- Jin, P.; Wang, H.; Zhang, Y.; Huang, Y.; Wang, L.; Zheng, Y. UV-C enhances resistance against gray mold decay caused by Botrytis cinerea in strawberry fruit. Sci. Hortic. 2017, 225, 106–111. [Google Scholar] [CrossRef]
- Sun, T.; Ouyang, H.; Sun, P.; Zhang, W.; Wang, Y.; Cheng, S.; Chen, G. Postharvest UV-C irradiation inhibits blackhead disease by inducing disease resistance and reducing mycotoxin production in ‘Korla’ fragrant pear (Pyrus sinkiangensis). Int. J. Food Microbiol. 2022, 362, 109485. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Li, Y.; Liu, G.; He, J. UV-C delays senescence in ‘Lingwu long’ jujube fruit by regulating ROS and phenylpropanoid metabolism. Plant Physiol. Biochem. 2023, 194, 383–393. [Google Scholar] [CrossRef]
- Zhang, X.; Zheng, X.; Han, Y.; Yang, R.; Wang, Q.; Gong, D.; Li, Y.; Prusky, D.; Bi, Y. UV-C irradiation maintains cell membrane integrity at wounds of potato tubers during healing by regulating ROS homeostasis and increasing antioxidant activity. Postharvest Biol. Technol. 2023, 199, 112308. [Google Scholar] [CrossRef]
- Morales, M.; Munné-Bosch, S. Malondialdehyde: Facts and artifacts. Plant Physiol. 2019, 180, 1246–1250. [Google Scholar] [CrossRef]
- Tao, D.; Wang, J.; Zhang, L.; Jiang, Y.; Lv, M. 1-Methylcyclopropene alleviates peel browning of ‘Nanguo’ pears by regulating energy, antioxidant and lipid metabolisms after long term refrigeration. Sci. Hortic. 2019, 247, 254–263. [Google Scholar] [CrossRef]
- Artés, F.; Allende, A. Processing lines and alternative preservation techniques to prolong the shelf-life of minimally fresh processed leafy vegetables. Eur. J. Hortic. Sci. 2005, 70, 231–245. [Google Scholar]
- Lin, Q.; Xie, Y.; Liu, W.; Zhang, J.; Cheng, S.; Xie, X.; Wang, Z. UV-C treatment on physiological response of potato (Solanum tuberosum L.) during low temperature storage. J. Food Sci. Technol. 2017, 54, 55–61. [Google Scholar] [CrossRef]
- Patel, N.; Gantait, S.; Panigrahi, J. Extension of postharvest shelf-life in green bell pepper (Capsicum annuum L.) using exogenous application of polyamines (spermidine and putrescine). Food Chem. 2019, 275, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, U.K.; Islam, M.N.; Siddiqui, M.N.; Cao, X.; Khan, M.A.R. Proline, a multifaceted signalling molecule in plant responses to abiotic stress: Understanding the physiological mechanisms. Plant Biol. 2022, 24, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Mahdavian, K.; Ghorbanli, M.; Kalantaraki, K. The effects of ultraviolet radiation on the contents of chlorophyll, flavonoid, anthocyanin and proline in Capsicum annuum L. Turk. J. Bot. 2008, 32, 25–33. [Google Scholar]
- Wei, Y.; Zhou, D.; Peng, J.; Pan, L.; Tu, K. Hot air treatment induces disease resistance through activating the phenylpropanoid metabolism in cherry tomato fruit. J. Agric. Food Chem. 2017, 65, 8003–8010. [Google Scholar] [CrossRef]
- Treutter, D. Significance of flavonoids in plant resistance and enhancement of their biosynthesis. Plant Biol. 2005, 7, 581–591. [Google Scholar] [CrossRef]
- Lemoine, M.L.; Civello, P.M.; Chaves, A.R.; Martínez, G.A. Influence of a combined hot air and UV-C treatment on quality parameters of fresh-cut broccoli florets at 0 °C. Int. J. Food Sience Technol. 2010, 45, 1212–1218. [Google Scholar] [CrossRef]
- Yoruk, R.; Marshall, M.R. Physicochemical properties and function of plant polyphenol oxidase: A review. J. Food Biochem. 2003, 27, 361–422. [Google Scholar] [CrossRef]
- Li, L.; Steffens, J.C. Overexpression of polyphenol oxidase in transgenic tomato plants results in enhanced bacterial disease resistance. Planta 2002, 215, 239–247. [Google Scholar] [CrossRef]
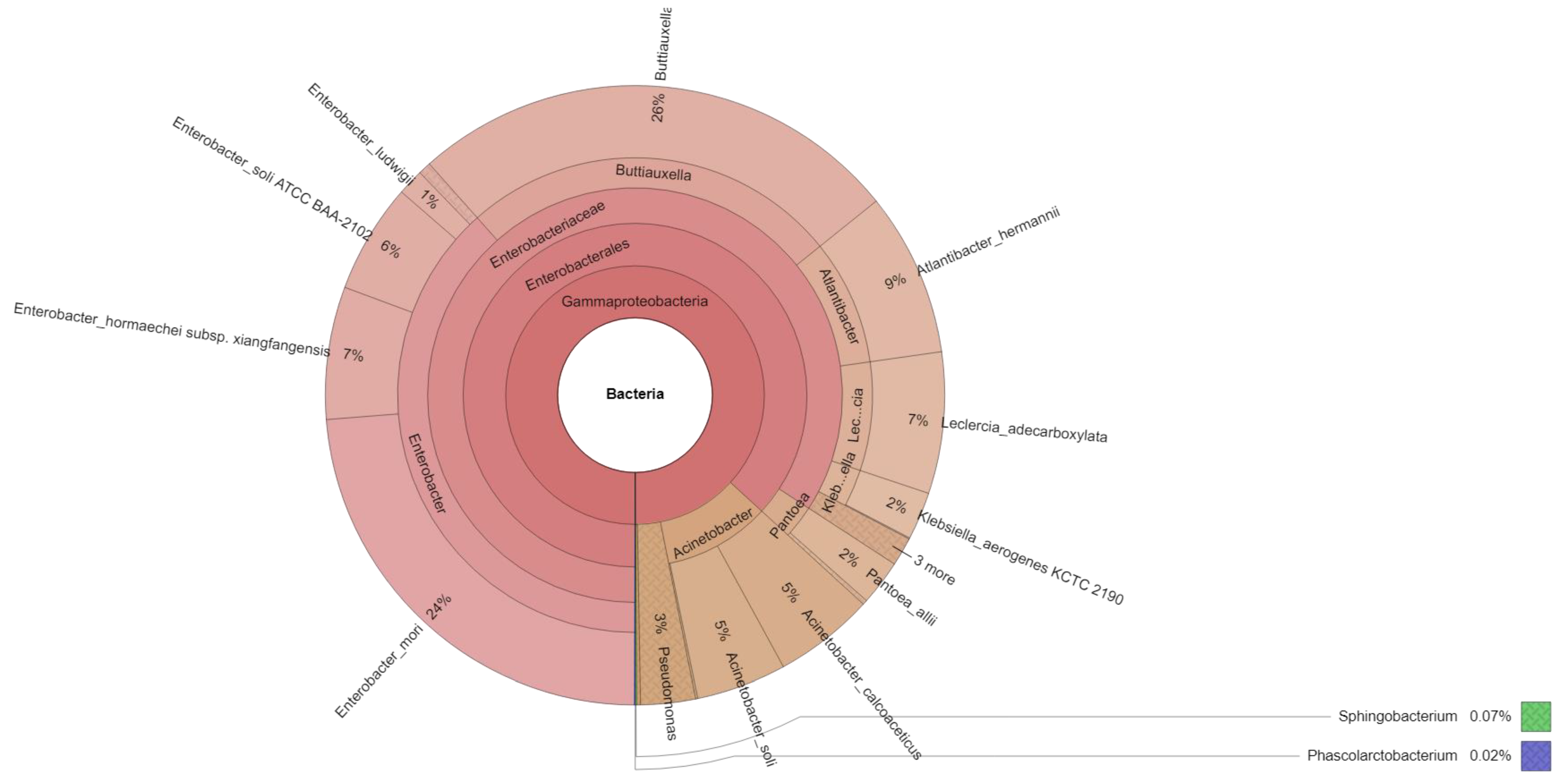

| Reads | Family | Bacterial Isolates (Genus/Species) * |
|---|---|---|
| 8 | Sphingobacteriaceae | Sphingobacterium multivorum |
| 2 | Acidaminococcaceae | Phascolarctobacterium faecium |
| 14 | Xanthomonadaceae | Xanthomonas hortorum pv. Gardneri |
| 10 | Xanthomonadaceae | Stenotrophomonas maltophilia |
| 9 | Moraxellaceae | Acinetobacter radioresistens |
| 4 | Moraxellaceae | Acinetobacter proteolyticus |
| 560 | Moraxellaceae | Acinetobacter soli |
| 624 | Moraxellaceae | Acinetobacter calcoaceticus |
| 12 | Pseudomonadaceae | Pseudomonas psychrotolerans |
| 20 | Pseudomonadaceae | Pseudomonas straminea |
| 13 | Pseudomonadaceae | Pseudomonas argentinensis |
| 115 | Pseudomonadaceae | Pseudomonas parafulva NBRC 16,636 = DSM 17004 |
| 37 | Pseudomonadaceae | Pseudomonas fulva |
| 24 | Pseudomonadaceae | Pseudomonas monteilii |
| 2 | Pseudomonadaceae | Pseudomonas japonica NBRC 103,040 = DSM 22348 |
| 73 | Pseudomonadaceae | Pseudomonas plecoglossicida |
| 44 | Pseudomonadaceae | Pseudomonas taiwanensis DSM 21245 |
| 11 | Erwiniaceae | Pantoea eucrina |
| 18 | Erwiniaceae | Pantoea ananatis |
| 282 | Erwiniaceae | Pantoea allii |
| 25 | Enterobacteriaceae | Cronobacter sakazakii |
| 23 | Enterobacteriaceae | Cronobacter turicensis z3032 |
| 1017 | Enterobacteriaceae | Atlantibacter hermannii |
| 824 | Enterobacteriaceae | Enterobacter hormaechei subsp. xiangfangensis |
| 24 | Enterobacteriaceae | Citrobacter cronae |
| 79 | Enterobacteriaceae | Enterobacter cloacae subsp. Dissolvens |
| 7 | Enterobacteriaceae | Klebsiella pneumoniae |
| 163 | Enterobacteriaceae | Enterobacter ludwigii |
| 106 | Enterobacteriaceae | Cedecea lapagei |
| 683 | Enterobacteriaceae | Enterobacter soli ATCC BAA-2102 |
| 297 | Enterobacteriaceae | Klebsiella aerogenes KCTC 2190 |
| 2826 | Enterobacteriaceae | Enterobacter mori |
| 879 | Enterobacteriaceae | Leclercia adecarboxylata |
| 3069 | Enterobacteriaceae | Buttiauxella izardii |
| Dose (kJ·m−2) | Viable Bacterial Count after Exposure to Room Temperature (CFU·g−1) | |
|---|---|---|
| 0 h | 6 h | |
| 0 | 4.03 × 103 ± 0.15 a | 6.12 × 105 ± 0.15 a |
| 0.5 | 1.33 × 102 ± 0.11 b | 6.75 × 103 ± 0.07 c |
| 1.5 | 1.26 × 102 ± 0.15 b | 6.21 × 103 ± 0.11 c |
| 3.0 | 1.07 × 102 ± 0.06 b | 4.11 × 104 ± 0.03 b |
| Treatment | Viable Bacterial Count after Exposure to Room Temperature | ||
|---|---|---|---|
| Temperature | Incubation Time | (CFU·g−1) | |
| (°C) | (h) | 0 h | 6 h |
| 22 | 0 | 3.47 × 103 ± 0.30 c | 1.07 × 105 ± 0.05 a |
| 3 | 6.33 × 103 ± 1.53 c | 1.38 × 105 ± 0.74 a | |
| 6 | 1.08 × 105 ± 0.01 a | 1.39 × 105 ± 0.09 a | |
| 12 | 1.69 × 105 ± 0.03 a | 7.05 × 105 ± 0.08 a | |
| 4 | 3 | 1.47 × 102 ± 0.15 d | 1.08 × 104 ± 0.21 b |
| 6 | 9.20 × 103 ± 0.25 c | 2.42 × 104 ± 0.08 b | |
| 12 | 6.13 × 104 ± 0.55 b | 1.37 × 105 ± 0.04 a | |
| Dose (kJ·m−2) | Viable Bacterial Count after Exposure to Room Temperature (CFU·g−1) | |
|---|---|---|
| 0 h | 6 h | |
| 0 | 7.11 × 103 ± 0.31 a | 7.74 × 104 ± 0.33 a |
| 0.5 | 2.15 × 102 ± 0.5 b | 6.20 × 103 ± 0.23 b |
| 1.5 | 1.88 × 102 ± 0.23 b | 5.84 × 103 ± 0.21 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baligad, J.L.; Huang, P.-L.; Do, Y.-Y. The Effects of UV-C Irradiation and Low Temperature Treatment on Microbial Growth and Oxidative Damage in Fresh-Cut Bitter Gourd (Momordica charantia L.). Horticulturae 2023, 9, 1068. https://doi.org/10.3390/horticulturae9101068
Baligad JL, Huang P-L, Do Y-Y. The Effects of UV-C Irradiation and Low Temperature Treatment on Microbial Growth and Oxidative Damage in Fresh-Cut Bitter Gourd (Momordica charantia L.). Horticulturae. 2023; 9(10):1068. https://doi.org/10.3390/horticulturae9101068
Chicago/Turabian StyleBaligad, John Louie, Pung-Ling Huang, and Yi-Yin Do. 2023. "The Effects of UV-C Irradiation and Low Temperature Treatment on Microbial Growth and Oxidative Damage in Fresh-Cut Bitter Gourd (Momordica charantia L.)" Horticulturae 9, no. 10: 1068. https://doi.org/10.3390/horticulturae9101068
APA StyleBaligad, J. L., Huang, P.-L., & Do, Y.-Y. (2023). The Effects of UV-C Irradiation and Low Temperature Treatment on Microbial Growth and Oxidative Damage in Fresh-Cut Bitter Gourd (Momordica charantia L.). Horticulturae, 9(10), 1068. https://doi.org/10.3390/horticulturae9101068