Abstract
This study aimed to identify the aromatic compounds present in the different aroma types of different table grape varieties and deeply understand the changes in the aromatic compounds during the growth and development process. The skin and flesh of three table grape varieties (‘Kyoho’, ‘Shine Muscat’, and ‘Ryuho’) in different growth and development stages were selected to determine their aromatic compounds using headspace solid-phase microextraction gas chromatography-mass spectrometry and principal component analysis. The results showed that the aromatic compounds of the ‘Kyoho’ and ‘Ryuho’ grapes were similar, mainly containing C6 compounds and esters, whereas ‘Shine Muscat’ was characterized by C6 compounds and terpenes. The levels of aromatic compounds in the skin were higher than those in the flesh. The content of esters in ‘Ryuho’ was significantly higher than that in ‘Kyoho’ and ‘Shine Muscat’. This showed that ‘Ryuho’ combines the advantages of the parents in its aroma composition. Selecting suitable parents for hybridization is one method for obtaining new varieties with a special aroma. This provides a theoretical basis for future molecular hybrid breeding and molecular-assisted breeding, as well as molecular biology research on aroma synthesis and metabolism in table grapes.
1. Introduction
The grape (Vitis spp.) is a perennial deciduous vine [1]. Owing to its high economic value, it is cultivated worldwide. Recently, there have been frequent reports that table grapes, wine, and other grape products are beneficial to human health [2,3]. The demand for high-quality grapes has increased significantly, and in-depth research on grape quality is increasing [4,5]. Commercially cultivated grapes can be classified as either table or wine grapes [6]. More than 10,000 grape varieties are known worldwide; among them, 13 varieties cover more than one-third of the world’s grape cultivation area [7]. Of these 13 varieties, ‘Kyoho’ has small national coverage, but its cultivation area is more than 350,000 hectares, ranking it first.
‘Kyoho’ (‘Ishiharawase’ × ‘Centennial’, 1937) is a widely planted and popularized grape variety [1,8]. Because of its large grain size and good quality, it has been introduced and popularized all over the world. ‘Ryuho’ (‘Golden Muscat’ × ‘Kuroshio’, tetraploid) is the second generation of ‘Kyoho’. It has a strawberry flavor, a sweet and good taste, and is juicy [9]. However, due to its thin skin and soft flesh, it is not resistant to storage and transportation, and the mature fruits easily rot; because of these and other shortcomings, this variety has not been widely cultivated. Nevertheless, considering the in-depth studies on the laws of grape aroma synthesis and metabolism, the research value of ‘Ryuho’ with a strong aroma will appear. ‘Shine Muscat’ (‘Akitsu-21’ × ‘Hakunan’, diploid) was manufactured by the National Institute of Fruit Tree Science (NIFTS) in Japan in 1988 [10] and is mainly for table use. It has large grains, a high sugar concentration, a muscat flavor, and a thin flesh crust and has become one of the new and alternative varieties in the grape industry.
Aroma is one of the most important characteristics of grape quality [11]. More than 800 aromatic compounds were detected in grapes, including terpenoids, alcohols, aldehydes, acids, ketones, esters, etc. [12,13]. In addition to the C6 compounds, linalool, and C13 compounds, ethyl 2-methyl butyrate and ethyl butyrate were the main aroma-contributing compounds in the three varieties of the ‘Kyoho’ series [14]. With a special and strong aroma, the ‘Ryuho’ grape has a high research value, which requires further study. Influenced by the wine industry, most studies have focused on the concentration of aromatic compounds in wine grapes in the mature stage, and there are only a few studies on the anabolism of the aromatic compounds in table grapes. Moreover, studying the laws of aroma synthesis and metabolism in the process of grape growth and development will help understand the pathway of grape aroma metabolism and the related genes [15].
In this experiment, the ‘Kyoho’, ‘Shine Muscat’, and ‘Ryuho’ varieties were chosen to analyze the aromatic compounds, qualitatively and quantitatively, in the growth and development process of table grapes. They were also used to compare the differences in the aromatic compounds between the skin and flesh; this will provide a theoretical basis to further understand the quality differences and characteristics among the table grape varieties and will have practical significance in genetic breeding, the selection of suitable harvest times, and the cultivation of new varieties.
2. Materials and Methods
2.1. Plant Materials
‘Kyoho’, ‘Shine Muscat’, and ‘Ryuho’ grapes were obtained from an orchard in the Tsukuba Plant Innovation Research Center (T-PIRC) of the University of Tsukuba, Ibaraki, Japan (36.116N, 140.094E). The grapevines were undergoing standardized cultivation in a rain-proof greenhouse in this orchard. Bloom time for this experiment was noted when 50% of the flowers had opened, which was 3 June 2017 for ‘Kyoho’ and ‘Ryuho’, and 10 June 2017 for ‘Shine Muscat’. The berries were collected from clusters 10, 30, 50, 70, 90, and 110 days after full bloom (DAFB) for ‘Kyoho’ and ‘Shine Muscat’, and 10, 30, 50, 70, 90, and 95 DAFB for ‘Ryuho’. Because of the different mature stages, ‘Kyoho’ and ‘Shine Muscat’ were harvested at 110 DAFB and ‘Ryuho’ was harvested at 95 DAFB. The berries were randomly collected from three different clusters. One part was used to measure grain quality and the other was used to analyze the aromatic compounds in the berries. After sampling, the skin and flesh of the berries were immediately separated, frozen in liquid nitrogen, and stored at −80 °C for later use.
2.2. Fruit Quality
The length and width of the berries were measured using an electronic digital caliper (Shinwa, Niigata, Japan), and the weight of the individual berries was measured using an electronic balance (Shinko Denshi, Tokyo, Japan). A PR-101 α sugar meter (Atago, Tokyo, Japan) was used to measure the total soluble solids (TSS). The fruit shape index indicates the shape of the grapes: vertical diameter (mm) and horizontal diameter (mm). Three independent biological replicates were used for each experiment.
2.3. Extraction of the Aromatic Compounds
The aromatic compounds of the grape grain homogenate were extracted using headspace solid-phase microextraction (HS-SPME) [15,16]. Four grams of the grape skin or flesh, preserved at −80 °C, was ground into powder in liquid nitrogen using a mortar and pestle. An internal standard solution was prepared by diluting 1 μL of octanol with 5 mL of hexane. In a 100 mL headspace bottle, 1 μL of the internal standard solution, 5 mL of distilled water, and 4 g of NaCl were mixed in a water bath shaker at 25 °C, for 30 min. The 50/30 μm divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS) extraction fiber of the SPME (57328-u, Supelco, GA, USA) head was inserted into the bottle through the rubber pad and kept above the sample liquid surface. The distance between the fiber and liquid level was maintained at 3 cm. Extraction of the aromatic compounds was performed at 25°C, for 30 min. Three independent biological replicates were used for each experiment.
2.4. Qualitative and Quantitative Analysis of the Aromatic Compounds
Gas chromatography-mass spectrometry (GC-MS) analysis was performed using a Thermo Scientific FOCUS GC and a 110-5383 MS, with a DB-WAX (250°C, 30 m × 250 mm × 0.25 μm) chromatographic column (Agilent Technologies, Santa Clara, CA, USA). The chromatographic conditions were as follows: (1) the sample inlet temperature was 250°C, (2) helium was used as the carrier gas at 1 mL/min in the constant flow mode, and (3) the GC inlet was set in the splitless mode. The column temperature was initially set at 40°C for 5 min, followed by an increase to 220°C at a rate of 6 °C/min. The temperature was then held at 220 °C for 5 min and thereafter increased by 4 °C/min to 240°C. For the mass spectrometry, the ion source temperature was 230°C and the quadrupole temperature was 150°C. The ionization mode was set as electron ionization (EI), the electric energy was 70 eV, and the electron ionization mass spectra were obtained by scanning from 30 to 350 m/z.
Qualitative and quantitative methods were used to identify the mass spectra of the unknown compounds. Qualitative methods included matching the spectra with those found in the NIST Mass Spectral Library and combining them with an artificial map spectral analysis. Quantitative methods included calculating the aromatic compounds present using the peak area normalization relative to percentage content with the following formula:
Content of each compound (μg/kg) = peak area of each compound/peak area of internal standard × internal standard concentration (μg/L) × internal standard dosage (L)/sample volume (kg).
2.5. Data Analysis
Microsoft Office Excel 2016 (Microsoft, Washington, DC, USA), SPSS 17.0 (IBM, Chicago, IL, USA), and the JMP software (JMP, Tustin, CA, USA) were used to analyze the data. One-way analysis of variance (ANOVA) and t-tests were used to analyze the differences in fruit quality and aromatic compound formation among the three varieties.
3. Results
3.1. Fruit Gowth and Development
The growth and development of ‘Kyoho’, ‘Shine Muscat’, and ‘Ryuho’ are shown in Figure 1. The color-changing period (veraison) of the three varieties was approximately 60 DAFB. The grain length, as shown in Figure 2, was ‘Ryuho’ (35 mm) > ‘Shine Muscat’ > ‘Kyoho’ (Figure 2A). The grain width of ‘Ryuho’ and ‘Kyoho’(30 mm) was significantly larger than that of ‘Shine Muscat’ (25 mm) (Figure 2B). The single grain weight of ‘Ryuho’ and ‘Kyoho’ at maturity was about 15 g, which was significantly larger than the ‘Shine Muscat’ berries (10 g) (Figure 2D). The fruit shape indices of the three varieties were ‘Shine Muscat’(1.3–1.4) > ‘Ryuho’ (1.2), and ‘Kyoho’(0.9) (Figure 2C). The total soluble solids of the three varieties reached 20% at maturity, and there were no significant differences among the three cultivars (Figure 2E). This indicates that all three cultivars reached the maturity stage (TSS > 15%).
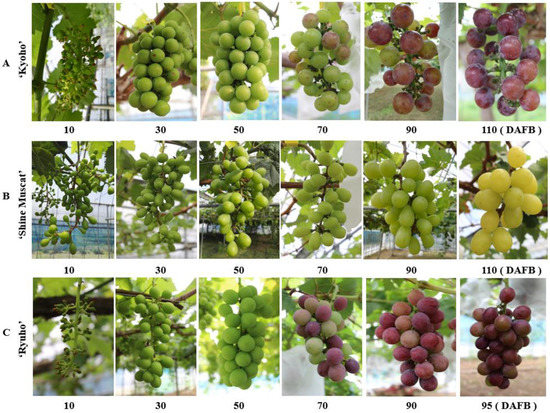
Figure 1.
Developmental changes in the ‘Kyoho’, ‘Shine Muscat’, and ‘Ryuho’ clusters. (A) ‘Kyoho’, (B) ‘Shine Muscat’, (C) ‘Ryuho’.
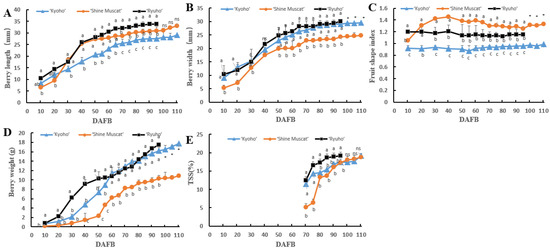
Figure 2.
The fruit quality of ‘Kyoho’, ‘Shine Muscat’, and ‘Ryuho’. (A) Grain length, (B) grain width, (C) fruit shape index, (D) single grain weight, (E) total soluble solids. ‘Kyoho’ and ‘Shine Muscat’ were harvested at 110 DAFB, and ‘Ryuho’ was harvested at 95 DAFB. n = 3. ns: not significant; p < 0.05 (*) according to a t-test between ‘Kyoho’ and ‘Shine Muscat’ at 110 DAFB. Letters represent a significant difference, p < 0.05, according to Tukey’s test. The error bars represent the standard error (SE).
3.2. The Total Aromatic Compounds in the Three Varieties
The changes in the contents of the volatile aromatic compounds during grain development in ‘Kyoho’, ‘Shine Muscat’, and ‘Ryuho’ are shown in Figure 3. The total aroma content of ‘Ryuho’ skin reached its peak (1182.23 mg/kg) at 70 DAFB, and thereafter decreased (Figure 3A). The aroma content of ‘Kyoho’ skin reached a small peak (538.22 mg/kg) at 70 DAFB but reached its peak (926.27 mg/kg) at 110 DAFB. The aromatic content of ‘Shine Muscat’s’ skin increased gradually with the growth and development of the berries, reaching its peak (1077.22 mg/kg) at 110 DAFB. The total aromatic content of ‘Ryuho’ flesh was significantly higher than that of ‘Kyoho’ and ‘Shine Muscat’ and reached its peak (1062.16 mg/kg) at 90 DAFB, and thereafter decreased (Figure 3B). The total aromatic content of ‘Kyoho’ flesh reached its peak (477.54 mg/kg) at 110 DAFB. The total aroma content of ‘Shine Muscat’s’ flesh reached a small peak (265.87 mg/kg) at 70 DAFB but reached its peak (505.49 mg/kg) at 110 DAFB.
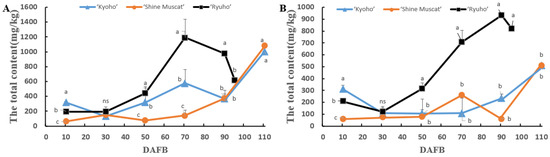
Figure 3.
The total content of various volatile aromatic compounds during the growth and development of ‘Kyoho’, ‘Shine Muscat’, and ‘Ryuho’. (A) Skin, (B) flesh. n = 3. ‘Kyoho’ and ‘Shine Muscat’ were harvested at 110 DAFB, and ‘Ryuho’ was harvested at 95 DAFB. ns: not significant; p < 0.05 (*) according to a t-test between ‘Kyoho’ and ‘Shine Muscat’ at 110 DAFB. Letters represent a significant difference, p < 0.05, according to Tukey’s test. The error bars represent the standard error (SE). Letters at harvesting time represent significant differences among three cultivars.
3.3. The Proportion of Each Aroma Type in the Three Varieties
The proportions of C6 alcohols, C6 aldehydes, and esters in the three varieties were up to 85.33%. higher (Figure 4) The proportion of esters increased with grain development, whereas the proportion of aldehydes decreased. The proportion of alcohols and terpenes fluctuated throughout the developmental period. The proportions of C6 alcohols and C6 aldehydes in the foxy type variety ‘Kyoho’ skin (45.27–63.78%) and ‘Ryuho’ skin (28.06–44.84%), and the muscat type variety ‘Shine Muscat’ skin (38.99–66.17%) are shown in Figure 4A–C. The proportions of C6 compounds in ‘Kyoho’ flesh (36.37–68.14%), ‘Ryuho’ flesh (5.88–44.84%), and ‘Shine Muscat’ flesh (38.99–66.17%) are shown in Figure 4D,E. The proportion of esters in the flesh of ‘Shine Muscat’ reached a maximum at 70 DAFB and thereafter decreased.
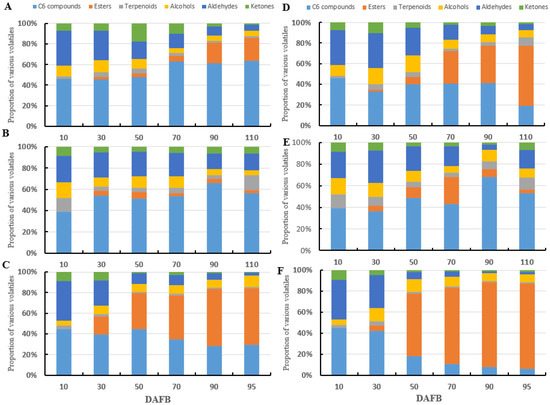
Figure 4.
The proportions of various volatile aromatic compounds in ‘Kyoho’, ‘Shine Muscat’, and ‘Ryuho’. (A) The skin of ‘Kyoho’, (B) the skin of ‘Shine Muscat’, (C) the skin of ‘Ryuho’, (D) the flesh of ‘Kyoho’, (E) the flesh of ‘Shine Muscat’, (F) the flesh of ‘Ryuho’. n = 3.
3.4. The Content Changes of the Different Aromatic Compounds
Thirty aromatic compounds were identified in the berries of ‘Kyoho’, ‘Shine Muscat’, and ‘Ryuho’, including C6 alcohols and C6 aldehydes (6), esters (5), terpenes (4), alcohols (except C6, 4), aldehydes (except C6, 8), and ketones (3).
Most of the aromatic compounds in ‘Shine Muscat’ gradually accumulated in the skin with growth and development, especially from 70 DAFB (Figure 5). The anabolic trend of aromatic compounds in ‘Ryuho’ generally increased first and thereafter decreased. For example, the C6 alcohols and aldehydes, terpenes (limonene and p-cymene), esters (ethyl butyrate, ethyl 2-butenoate, ethyl hexanoate, ethyl octanoate), alcohols (ethanol, 1-octen-3-ol, and 2-ethyl-1-hexanol), and ketones (5-methyl-4-hexen-3-one) showed a peak at 70 DAFB. Ethyl acetate and 4-terpineol exhibited peaks at 90 DAFB. The concentrations of (E)-2-hexenal, (Z)-2-hexen-1-ol, ethanol, phenyl ethyl alcohol, and esters, in ‘Kyoho’ increased gradually with growth and development. In addition, 3-hexenal, (Z)-3-hexen-1-ol, p-cymene, and 4-terpineol exhibited peaks at 70 DAFB, which thereafter decreased.
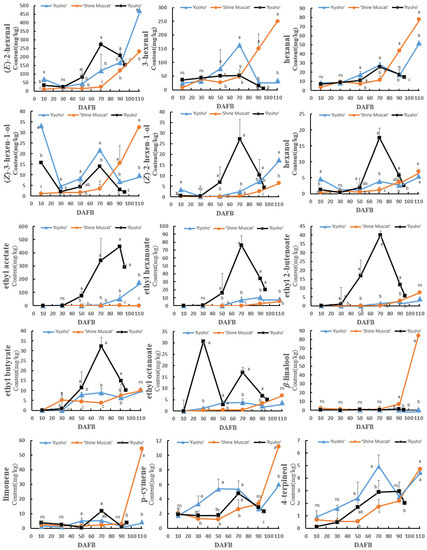
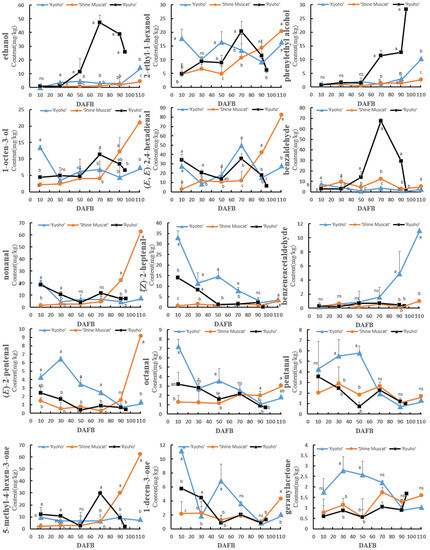
Figure 5.
The concentration changes of different aromatic compounds in the skins of ‘Kyoho’, ‘Shine Muscat’, and ‘Ryuho’ during growth and development. n = 3. ns: not significant; p < 0.05 (*) according to a t-test between ‘Kyoho’ and ‘Shine Muscat’ at 110 DAFB. ‘Kyoho’ and ‘Shine Muscat’ were harvested at 110 DAFB, and ‘Ryuho’ was harvested at 95 DAFB. Letters represent a significant difference, p < 0.05, according to Tukey’s test. The error bars represent the standard error (SE). Letters at harvesting time represent significant differences among three cultivars.
The change trends of the aromatic compounds in ‘Shine Muscat’s’ flesh were similar to those in its skin; most of them increased gradually with growth and development, especially the terpenes (Figure 6). Some aromatic compounds in ‘Ryuho’s’ flesh decreased first, then increased, and finally decreased, such as (E)-2-hexenal, hexanol, the (Z)-3-hexen-1-ol of the C6 compounds, and nonanal, which peaked at 70 DAFB. The concentrations of (E)-2-hexenal, 4-terpineol, ethanol, 2-ethyl-1-hexanol, and phenyl ethyl alcohol peaked at 90 DAFB. During growth and development, the concentrations of 3-hexenal, (E)-2-pentenal, octanal, (Z)-2-heptenal, and (E,E)-2,4-hexadienal decreased gradually. The concentration of esters in ‘Kyoho’ increased with growth and development; however, the concentration of aldehydes decreased.
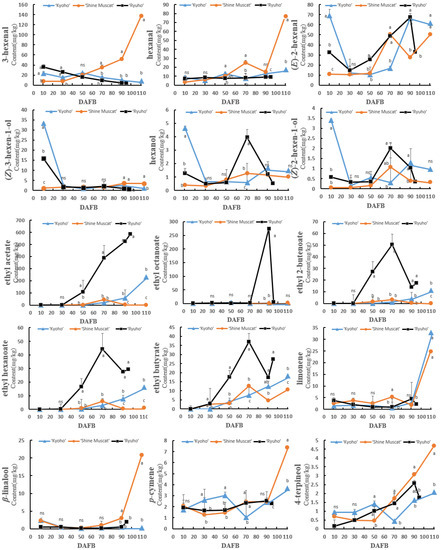
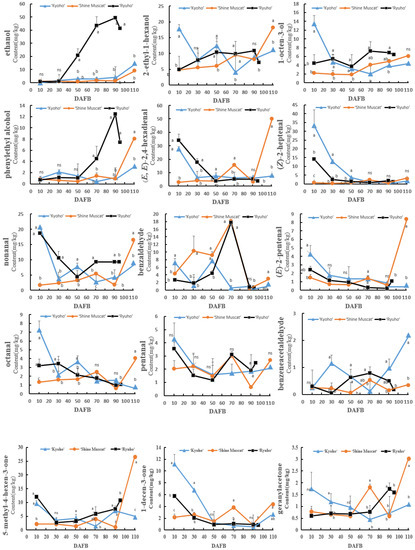
Figure 6.
The concentration changes of different aromatic compounds in the flesh of ‘Kyoho’, ‘Shine Muscat’, and ‘Ryuho’ during growth and development. n = 3. ns: not significant; p < 0.05 (*) according to a t-test between ‘Kyoho’ and ‘Shine Muscat’ at 110 DAFB. Letters represent a significant difference, p < 0.05, according to Tukey’s test. The error bars represent the standard error (SE). Letters at harvesting time represent significant differences among three cultivars.
3.5. Principal Component Analysis (PCA) of the Aromatic Compounds of the Three Varieties
The aromatic compounds in the skin and flesh of the three varieties at different growth and developmental stages of the berries were analyzed using PCA, as shown in Figure 7. The results showed that the three varieties were clearly distinguishable at different stages, based on the skin and flesh. The representative compounds of the three varieties were different during the different periods (Figure 7A). The representative aromatic compound in the ‘Shine Muscat’ skin was linalool. The aromatic compounds represented in the ‘Kyoho’ skin were C6 aldehyde and terpene. Terpenes, alcohols, and esters were the representative compounds in ‘Ryuho’, such as limonene and ethyl octanoate. The aromatic characteristics in the flesh of the three varieties were similar to those in the skin before veraison (Figure 7B). After veraison, the main representative compounds of ‘Ryuho’ were ethyl acetate, ethyl butyrate, ethyl hexanoate, ethyl octanoate, and ethanol. Terpenes and aldehydes were the representative compounds found in ‘Shine Muscat’.
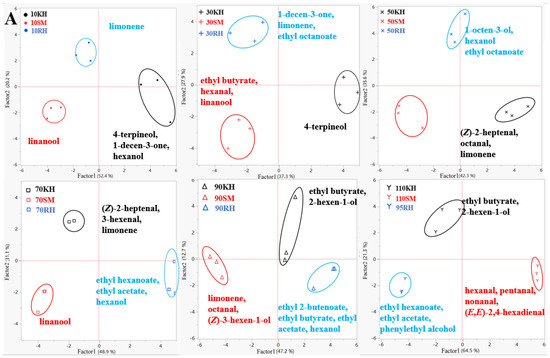
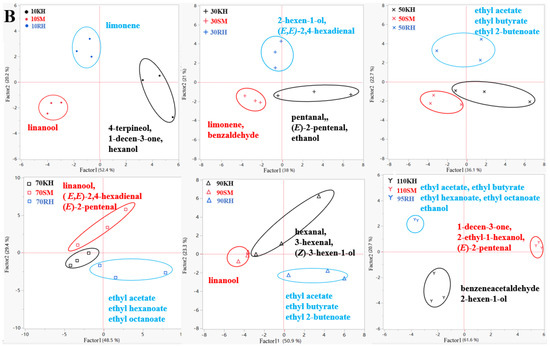
Figure 7.
The PCA of various volatile aromatic compounds in the skin and flesh of ‘Kyoho’, ‘Shine Muscat’, and ‘Ryuho’ during growth and development. (A) Skin, (B) flesh. ‘KH’ stands for ‘Kyoho’; ‘SM’ stands for ‘Shine Muscat’; ‘RH’ stands for ‘Ryuho’.
3.6. Cluster Analysis of the Aromatic Components in the Different Varieties and Growth Stages
The aromatic compounds and their relative contents of the different varieties at different growth and developmental stages were analyzed using cluster analysis. The aromatic compounds of ‘Ryuho’s’ skin were close, at 90 and 95 DAFB (Figure 8A). The compounds of ‘Kyoho’s’ skin were similar, at 90 and 110 DAFB. The compounds of ‘Kyoho’s’ and ‘Ryuho’s’ skin clustered together at 70 DAFB. In terms of the content change of the aromatic compounds, the changes in the three aldehydes in the C6 aromatic compounds were similar, and the changes in the esters and alcohols were the same, particularly ethanol and ethyl acetate. The changes in the terpenes, including β-linalool and limonene, were consistent. In the flesh, the aroma compounds of ‘Ryuho’ at 70 and 110 DAFB clustered together (Figure 8B). ‘Kyoho’ was similar to the ‘Ryuho’ grape at 70 DAFB and they clustered together at 90 DAFB. At 10 and 30 DAFB, the aroma of the flesh of the three varieties varied greatly. As in the skin, the changes in ethanol and ethyl acetate were similar in the flesh.
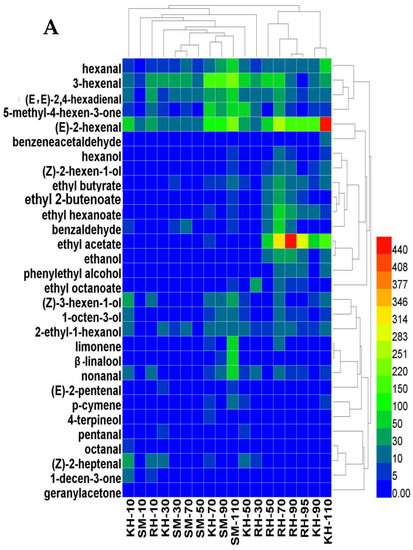
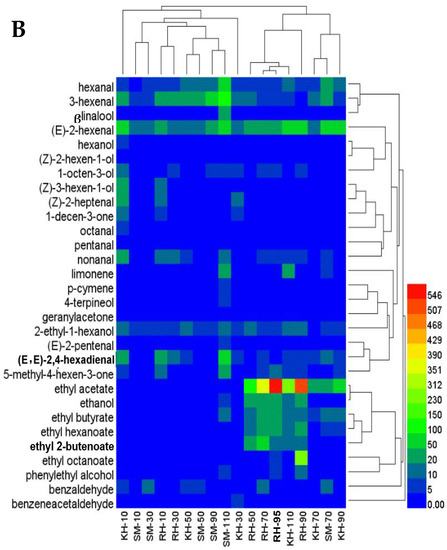
Figure 8.
Cluster analysis of the aromatic compounds in the three varieties at different development stages. (A) Skin, (B) flesh. ‘KH’ stands for ‘Kyoho’; ‘SM’ stands for ‘Shine Muscat’;‘ RH’ stands for ‘Ryuho’.
4. Discussion
Grape aroma is an important characteristic of grape quality and is key to cultivating excellent grape germplasm resources. The cultivation of new varieties with high quality and a muscat aroma will be one of the directions for table grape breeding in the future [17]. The study of fruit quality, especially the differences and inheritance of aroma substances, has important theoretical and practical significance.
4.1. Aroma Characteristics of the Different Varieties
The aroma characteristics of table grapes are floral, fruity, herbaceous, and sweet. The aromatic compounds of the different varieties of grapes are different [18]. According to the aromatic compounds, grape can be divided into muscat, non-muscat, and non-aromatic types [19]). Yang’s [20] classification was similar to that of Mateo [19], which is that they can be divided into muscat, strawberry, and no flavor. The unique muscat aroma is closely related to terpenoids and their contents [11,21]. The most abundant content observed in this variety was monoterpenoids, which mainly exist in the skin. ‘Shine Muscat’ is a muscat-flavored grape, and its aroma is closely related to terpenoids [22]. The most abundant monoterpenoids in ‘Shine Muscat’ are linalool, geraniol, nerol, citronellol, and α-terpineol [20]. Wu [22] and Tan [23] believed that linalool and geraniol were the main compounds in ‘Shine Muscat’.
Reports have shown that esters are the aromatic compounds with the highest content in the skin of the ‘Kyoho’ series grapes [8,14]. In this study, the aromatic compounds of ‘Kyoho’ were mainly C6 and esters in the skin and flesh, and the proportion of esters in the flesh was higher. The aromatic compounds in tetraploid table grapes with a muscat flavor, including ‘Zaoheibao’, ‘Qiuheibao’, ‘Wanheibao’, and ‘Tetraploid Muscat Hamburg’ as well as their diploid parents including ‘Guibao’, ‘Zaomeiguixiang’, ‘Christmas Rose’, and ‘Muscat Hamburg’, were tested [23]. Linalool and geraniol contributed the most to the eight muscat-aroma grape varieties. However, the monoterpenoids in this study were linalool and limonene, which may be related to differences in the climate conditions, soil environment, management methods, and detection and analysis.
After the diploid muscat-flavored grapes doubled, the relative content of the main aromatic substances did not double due to the chromosome doubling but was related to the richness of the parental muscat flavor. It can be inferred that in the cultivation of new varieties with good aroma, crossbreeding is better than ploidy breeding, and the key is the selection of parents.
4.2. Aroma Changes during Growth and Development and Selection of Harvest Time
The content of aromatic compounds in ‘Shine Muscat’ before veraison was low, mainly terpenes. From veraison to maturity, the aromatic compounds gradually accumulated, primarily C6 and terpenes. The aroma of the ‘Ryuho’ grape was mainly esters, which first increased and thereafter decreased. The results in Figure 3 show that, among the three grape varieties, the aromatic compounds in the skin were higher than those in the flesh.
In this experiment, the ‘Ryuho’ grapes ripened earlier than the ‘Kyoho’ and ‘Shine Muscat’ grapes. Studies have shown that C6 compounds (which are green leaf volatiles) are important in grapes and are an important indicator of grape maturity [24]. Many table varieties are used as raw materials for jams, juices, wines, and other processed products [25]. The aromatic content of ‘Ryuho’ reached its maximum value before the harvest. During production, an appropriate harvest period can be selected according to the different uses.
4.3. Research Value of ‘Ryuho’ on Aroma
The high cost and difficulty of research related to flavor phenotypes have been a huge challenge since breeding [26]. It is necessary to develop existing germplasm resources to a greater extent and collect and preserve abundant landraces, wild species, and wild relatives during breeding. ‘Ryuho’ is sweet, with a strong strawberry flavor, high and stable yield, and good quality. Compared to its sister varieties ‘Benizuiho’ and ‘Benifuji’, ‘Ryuho’ has a stronger resistance to anthrax and light fruit cracking than ‘Kyoho’ [20].
At present, little research has been conducted on the aroma synthesis of the ‘Ryuho’ grape. In this study, the concentration of aromatic compounds in ‘Kyoho’ and ‘Ryuho’ was higher than in ‘Shine Muscat’. The total aromatic content in ‘Ryuho’s’ skin and flesh were higher than in ‘Kyoho’ and ‘Shine Muscat’. The relative content of esters in the aromatic compounds of the three grape varieties was flesh > skin and ‘Ryuho’ > ‘Kyoho’ > ‘Shine Muscat’. ‘Ryuho’ has high research value and should be further studied in grape production and new variety cultivation in the future.
In short, the composition of aromatic compounds in grapes is complex. Many factors affect the formation and accumulation of aromatic compounds in grapes. Among these, the differences in the genotype are the main reason for the differences in the types and contents of the aromatic compounds. There are also differences in aromatic metabolic pathways among the grapes with different aroma types, which require further study. With the development of genomics, proteomics, and metabolomics, the specific pathways and key genes involved in grape aroma anabolism can be studied in detail.
5. Conclusions
The typical aromatic compounds of ‘Shine Muscat’ are terpenes and C6 compounds, such as linalool and 3-hexenal. The main aromatic compounds of ‘Kyoho’ and ‘Ryuho’ grapes are C6 compounds and esters such as (E)-2-hexenal and ethyl acetate. As the hybrid offspring of ‘Kyoho’, the content of esters, such as ethyl hexanoate, in ‘Ryuho’ was significantly higher than in the ‘Kyoho’ grapes, while the content of the C6 compounds was lower. ‘Ryuho’ reached maturity earlier than ‘Kyoho’ and ‘Shine Muscat’, and the highest concentration of aroma accumulation was before the ripening stage. In production, we should pay attention to selecting the appropriate harvest time according to the different demands.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae9010085/s1.
Author Contributions
S.S. designed the experiments and supervised the work; H.X. carried out the experiments and wrote the MS; Y.S. supervised the work and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available in Supplementary Material here.
Acknowledgments
We would like to thank Shigeru Matsuyama and Naoya Fukuda for their guidance in the experiments, and Kazuki Moriyama for support and assistance in the field trials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kong, Q.S. Chinese Grape Records; China Agricultural Science and Technology Press: Beijing, China, 2004. [Google Scholar]
- Averilla, J.N.; Oh, J.; Kim, H.J.; Kim, J.S.; Kim, J.S. Potential health benefits of phenolic compounds in grape processing by-products. Food Sci. Biotechnol. 2019, 28, 1607–1615. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Dey, S.; Marbaniang, D.; Pal, P.; Ray, S.; Mazumder, B. Grape seed extract: Having a potential health benefit. J. Food Sci. Technol. 2020, 57, 1205–1215. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Massonnet, M.; Sanjak, J.S.; Cantu, D.; Gaut, B.S. Evolutionary genomics of grape (Vitis vinifera ssp. vinifera) domestication. Proc. Natl. Acad. Sci. USA 2017, 114, 11715–11720. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Minio, A.; Massonnet, M.; Solares, E.; Lv, Y.; Beridze, T.; Cantu, D.; Gaut, B.S. The population genetics of structural variants in grapevine domestication. Nat. Plants 2019, 5, 965–979. [Google Scholar] [CrossRef]
- Zhong, H.; Liu, Z.; Zhang, F.; Zhou, X.; Sun, X.; Li, Y.; Liu, W.; Xiao, H.; Wang, N.; Lu, H.; et al. Metabolomic and transcriptomic analyses reveal the effects of self-and hetero-grafting on anthocyanin biosynthesis in grapevine. Hortic. Res. 2022, 9, uhac103. [Google Scholar] [CrossRef]
- Roca, P. State of the Vitiviniculture World Market. In Proceedings of the 42nd Word Congress of Vine & Wine, Geneva, Switzerland, 15–19 July 2019; Available online: https://www.bio-conferences.org/articles/bioconf/abs/2019/04/contents/contents.html (accessed on 6 January 2023).
- Ji, X.H.; Wang, B.; Wang, X.; Shi, X.B.; Liu, P.P.; Liu, F.Z.; Wang, H.B. Effects of different color paper bags on aroma development of ‘Kyoho’ grape berries. J. Integr. Agric. 2019, 18, 70–82. [Google Scholar] [CrossRef]
- Yang, Z.Y. Study on genealogy of ‘Kyoho’ grape. Sino-Overseas Grapevine Wine 2005, 2, 38–41. [Google Scholar]
- Yamada, M.; Yamane, H.; Sato, A.; Hirakawa, N.; Iwanami, H.; Yoshinaga, K.; Ozawa, T.; Mitani, N.; Shiraishi, M.; Yoshioka, M.; et al. New grape cultivar ‘Shine Muscat’. Bulletin of the National Institute of Fruit. Tree Sci. 2008, 7, 21–38. [Google Scholar]
- Xi, X.; Zha, Q.; He, Y.; Tian, Y.; Jiang, A. Influence of cluster thinning and girdling on aroma composition in ‘Jumeigui’ table grape. Sci. Rep. 2020, 10, 6877. [Google Scholar] [CrossRef]
- El Hadi, M.A.M.; Zhang, F.J.; Wu, F.F.; Zhou, C.H.; Tao, J. Advances in fruit aroma volatile research. Molecules 2013, 18, 8200–8229. [Google Scholar] [CrossRef]
- Saša, M.; Jelena, P.D.; Ristić, R.; Dušica, Ć.; Bratislav, Ć.; Tatjana, P. Volatile aroma compounds of brandy ‘Lozovača’ produced from muscat table grapevine cultivars (Vitis vinifera L.). Molecules 2019, 24, 2485. [Google Scholar] [CrossRef]
- Zhang, W.W.; Wu, Y.S.; Chen, Y.J.; Zheng, Q.Z.; Ma, C.; Xu, W.P.; Wang, S.P. Aroma characteristics of three ‘Kyoho’ grapevine series. J. Shanghai Jiaotong Univ. Agric. Sci. Ed. 2018, 36, 51–59. Available online: http://www.cqvip.com/qk/97481a/201805/676765829.html (accessed on 6 January 2023).
- Wu, Y.; Zhang, W.; Song, S.; Xu, W.; Zhang, C.; Ma, C.; Wang, L.; Wang, S. Evolution of volatile compounds during the development of Muscat grape ‘Shine Muscat’ (Vitis labrusca × V. vinifera). Food Chem. 2020, 309, 125778. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Sekozawa, Y.; Sugaya, S. Effects of gibberellin and abscisic acid on fruit quality and aromatic compound formation during fruit development in ‘Kyoho’ grapes (Vitis vinifera L. × Vitis labrusca L.). Environ. Control. Biol. 2022, 60, 67–78. [Google Scholar] [CrossRef]
- Yamada, M.; Sato, A. Advances in table grape breeding in Japan. Breed. Sci. 2016, 66, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.M. Analysis on the Constituents and Metabolic Regulations of Aroma in Table Grapes. Master’s Thesis, Shandong Agriculture University, Tai’an, China, 2013. Available online: http://cdmd.cnki.com.cn/Article/CDMD-10434-1014156803.html (accessed on 6 January 2023).
- Mateo, J.J.; Jiménez, M. Monoterpenes in grape juice and wines. J. Chromatogr. A 2000, 881, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, Y.; Liang, Z.; Fan, P.; Wu, B.; Yang, L.; Wang, Y.; Li, S. Volatiles of grape berries evaluated at the germplasm level by headspace-SPME with GC–MS. Food Chem. 2009, 114, 1106–1114. [Google Scholar] [CrossRef]
- Wu, Y.S.; Duan, S.Y.; Zhao, L.P.; Gao, Z.; Luo, M.; Song, S.R.; Xu, W.P.; Zhang, C.X.; Ma, C.; Wang, X.P. Aroma characterization based on aromatic series analysis in table grapes. Sci. Rep. 2016, 6, 31116. [Google Scholar] [CrossRef]
- Tan, W.; Tang, X.P.; Dong, Z.G.; Li, X.M. Analysis of the aromatic compounds of four tetraploid muscat flavor grapes and their diploid parents. J. Fruit Sci. 2017, 34, 435–443. [Google Scholar]
- Tan, W.; Tang, X.P.; Dong, Z.G.; Li, X.M. Analysis on fruit aromatic compounds of four seedless grape and their parents. J. Fruit Sci. 2015, 3, 440–447. [Google Scholar]
- Kang, W.H.; Xu, Y.; Cui, Y.Z. Comparative analysis of contents of C6 aldehydes and alcohols responsible for the aroma of different varieties of wine grapes. Food Sci. 2010, 31, 252–256. [Google Scholar]
- Shang, J.Y.; Tian, S.F.; Zhu, Z.Q.; Li, S.H.; Ji, X.; Gao, Y. Effects of harvesting time on fruit quality and aromatic compounds in ‘Muscat Hamburg’ grape. Acta Agric. Boreali-Sin. 2013, 28, 155–162. [Google Scholar]
- Colantonio, V.; Ferrão, L.F.V.; Tieman, D.M.; Bliznyuk, N.; Sims, C.; Klee, H.J.; Munoz, P.; Resende, M.F., Jr. Metabolomic selection for enhanced fruit flavor. Proc. Natl. Acad. Sci. USA 2022, 119, e2115865119. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).