Impact of Blue Light on Plant Growth, Flowering and Accumulation of Medicinal Flavones in Scutellaria baicalensis and S. lateriflora
Abstract
1. Introduction
2. Materials and Methods
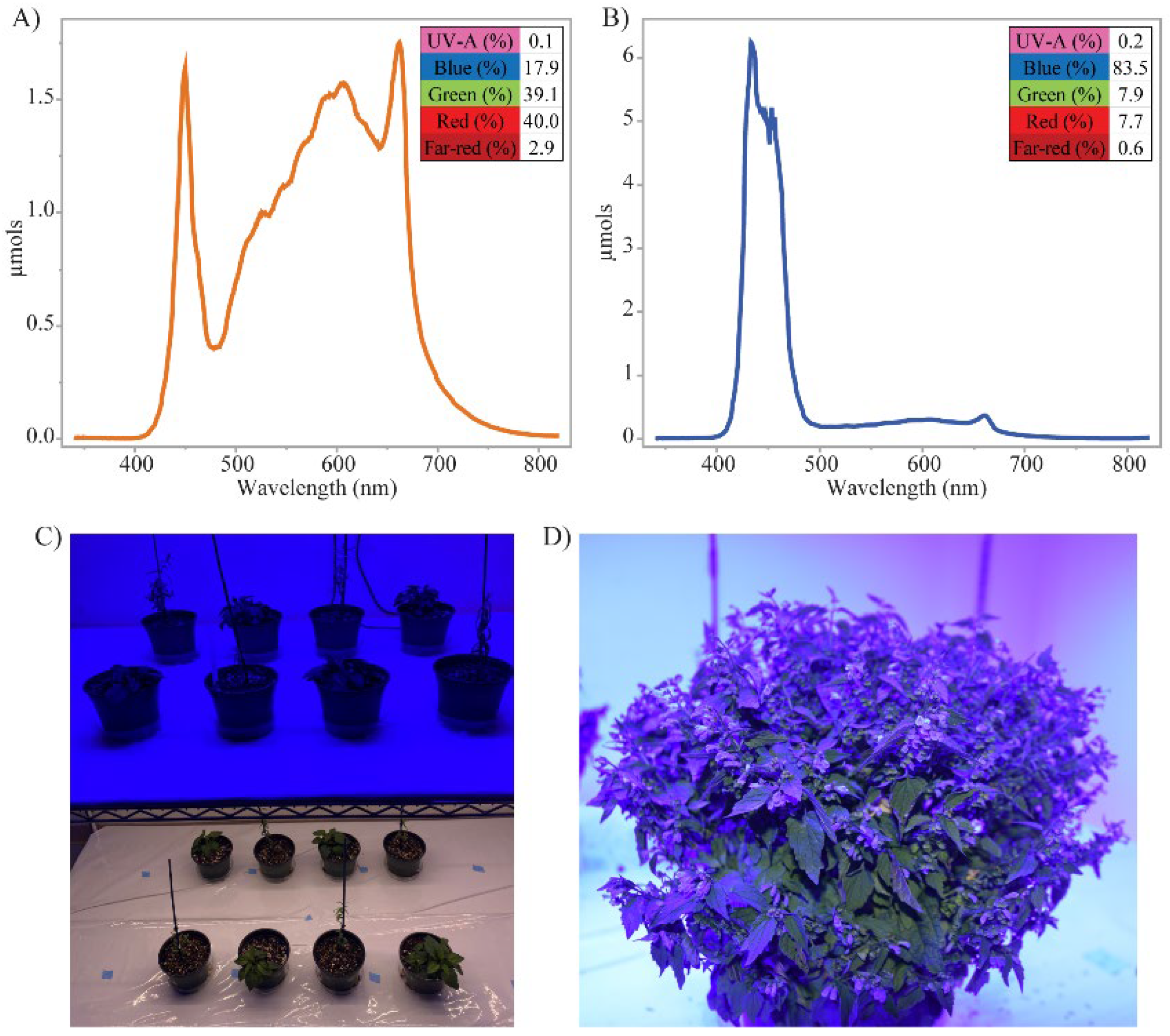
2.1. Plant Material and Growth Conditions
2.2. Morphological and Plant Growth Data Collection
2.3. Extraction and Identification of Flavonoids
2.4. Statistical Analysis
3. Results and Discussion
3.1. Blue Light Impacts Plant Growth and Flowering
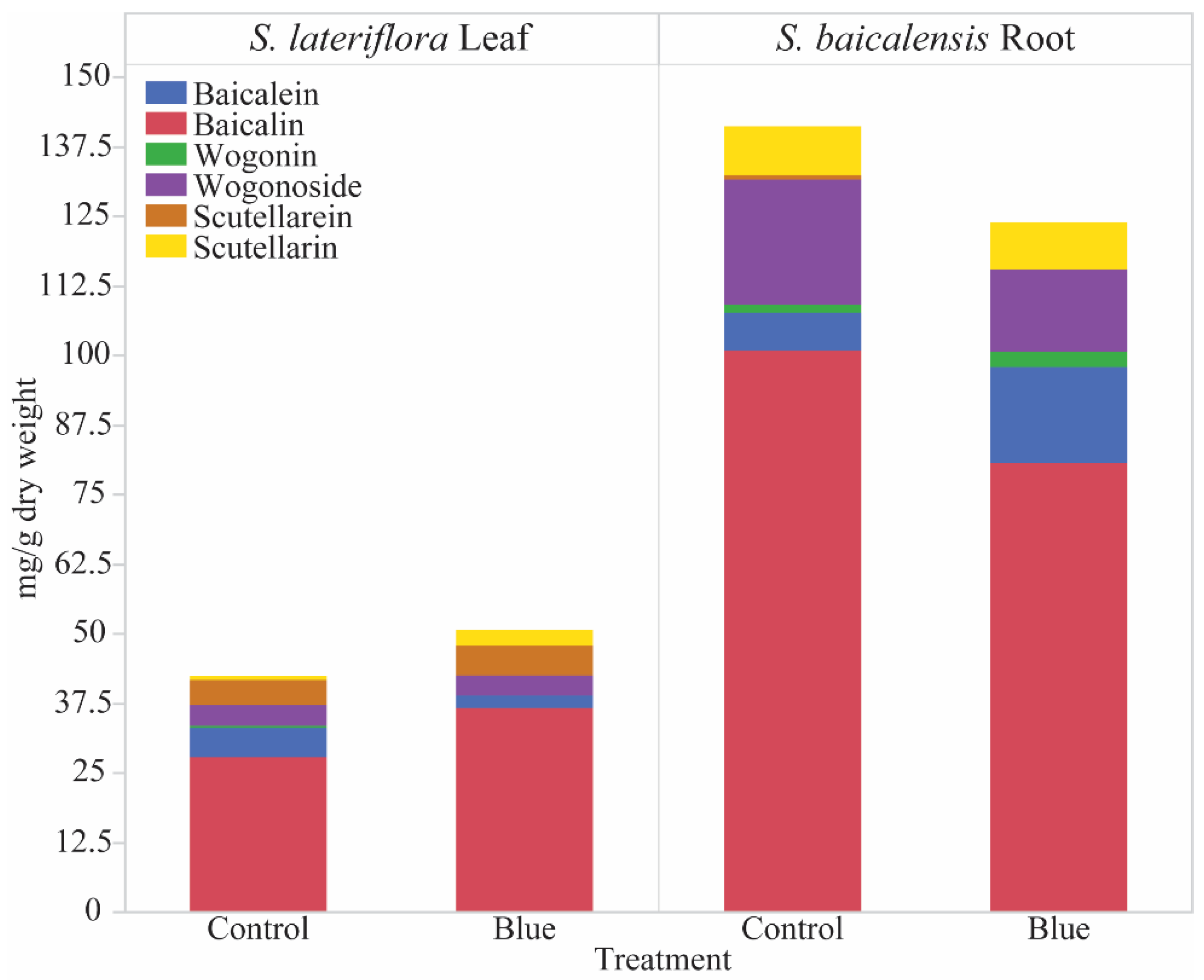
3.2. Blue Light Impact on Flavone Profiles
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alexandru Suchar, V.; Robberecht, R. Integration and scaling of UV-B radiation effects on plants: The relative sensitivity of growth forms and interspecies interactions. J. Plant Ecol. 2018, 11, 656–670. [Google Scholar] [CrossRef]
- Kalaitzoglou, P.; van Ieperen, W.; Harbinson, J.; van der Meer, M.; Martinakos, S.; Weerheim, K.; Nicole, C.C.S.; Marcelis, L.F.M. Effects of continuous or end-of-day far-red light on tomato plant growth, morphology, light absorption, and fruit production. Front. Plant Sci. 2019, 10, 322. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; van Iersel, M.W. Photosynthetic Physiology of Blue, Green, and Red Light: Light Intensity Effects and Underlying Mechanisms. Front. Plant Sci. 2021, 12, 328. [Google Scholar] [CrossRef] [PubMed]
- McCree, K.J. The action spectrum, absorptance and quantum yield of photosynthesis in crop plants. Agric. Meteorol. 1971, 9, 191–216. [Google Scholar] [CrossRef]
- Inada, K. Action spectra for photosynthesis in higher plants. Plant Cell Physiol. 1976, 17, 355–365. [Google Scholar] [CrossRef]
- Evans, J.R. The Dependence of Quantum Yield on Wavelength and Growth Irradiance. Funct. Plant Biol. 1987, 14, 69–79. [Google Scholar] [CrossRef]
- Hogewoning, S.W.; Wientjes, E.; Douwstra, P.; Trouwborst, G.; van Ieperen, W.; Croce, R.; Harbinson, J. Photosynthetic Quantum Yield Dynamics: From Photosystems to Leaves. Plant Cell 2012, 24, 1921–1935. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, L.; Zou, H.; Qiu, L.; Zheng, Y.; Yang, D.; Wang, Y. Effects of Light on Secondary Metabolite Biosynthesis in Medicinal Plants. Front. Plant Sci. 2021, 12, 2892. [Google Scholar] [CrossRef]
- Dou, H.; Niu, G.; Gu, M.; Masabni, J.G. Effects of Light Quality on Growth and Phytonutrient Accumulation of Herbs under Controlled Environments. Horticulturae 2017, 3, 36. [Google Scholar] [CrossRef]
- Ramakrishna, A.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef]
- Stevenson, P.C.; Nicolson, S.W.; Wright, G.A. Plant secondary metabolites in nectar: Impacts on pollinators and ecological functions. Funct. Ecol. 2017, 31, 65–75. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Sales, C.; Beltrán, J.; Gómez-Cadenas, A.; Arbona, V. Activation of Secondary Metabolism in Citrus Plants Is Associated to Sensitivity to Combined Drought and High Temperatures. Front. Plant Sci. 2017, 7, 1954. [Google Scholar] [CrossRef] [PubMed]
- Biała, W.; Jasiński, M. The phenylpropanoid case—It is transport that matters. Front. Plant Sci. 2018, 9, 1610. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wen, K.S.; Ruan, X.; Zhao, Y.X.; Wei, F.; Wang, Q. Response of Plant Secondary Metabolites to Environmental Factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef]
- Shalaby, S.; Horwitz, B.A. Plant phenolic compounds and oxidative stress: Integrated signals in fungal-plant interactions. Curr. Genet. 2015, 61, 347–357. [Google Scholar] [CrossRef]
- Martens, S.; Mithöfer, A. Flavones and flavone synthases. Phytochemistry 2005, 66, 2399–2407. [Google Scholar] [CrossRef]
- Lattanzio, V.; Lima, G.; Cicco, V. De Antifungal activity of phenolics against fungi commonly encountered during storage. Ital. J. Food Sci. 1994, 6, 23–30. [Google Scholar]
- McNally, D.J.; Wurms, K.V.; Labbé, C.; Quideau, S.; Bélanger, R.R. Complex C-glycosyl flavonoid phytoalexins from Cucumis sativus. J. Nat. Prod. 2003, 66, 1280–1283. [Google Scholar] [CrossRef]
- Hostetler, G.L.; Ralston, R.A.; Schwartz, S.J. Flavones: Food sources, bioavailability, metabolism, and bioactivity. Adv. Nutr. 2017, 8, 423–435. [Google Scholar] [CrossRef]
- Singh, M.; Kaur, M.; Silakari, O. Flavones: An important scaffold for medicinal chemistry. Eur. J. Med. Chem. 2014, 84, 206–239. [Google Scholar] [CrossRef]
- Sharma, A.; Tuli, H.S.; Kashyap, D.; Sharma, A.K. Flavones: Flavonoids Having Chemico-Biological Properties with a Preview into Anticancer Action Mechanism. In Bioactive Natural Products for the Management of Cancer: From Bench to Bedside; Springer Nature: Berlin, Germany, 2019; pp. 71–89. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhang, Y.; Wang, G.; Hill, L.; Weng, J.-K.; Chen, X.-Y.; Xue, H.; Martin, C. A specialized flavone biosynthetic pathway has evolved in the medicinal plant, Scutellaria baicalensis. Sci. Adv. 2016, 2, e1501780. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Li, P.; Liu, S.; Liu, Q.; Li, Y.; Sun, Y.; He, C.; Xiao, P. Traditional uses, ten-years research progress on phytochemistry and pharmacology, and clinical studies of the genus Scutellaria. J. Ethnopharmacol. 2021, 265, 113198. [Google Scholar] [CrossRef] [PubMed]
- Cathcart, M.C.; Useckaite, Z.; Drakeford, C.; Semik, V.; Lysaght, J.; Gately, K.; O’Byrne, K.J.; Pidgeon, G.P. Anti-cancer effects of baicalein in non-small cell lung cancer in-vitro and in-vivo. BMC Cancer 2016, 16, 707. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Zhan, S.; Wang, Y.; Zhou, G.; Liang, H.; Chen, X.; Shen, H. Baicalin, the major component of traditional Chinese medicine Scutellaria baicalensis induces colon cancer cell apoptosis through inhibition of oncomiRNAs. Sci. Rep. 2018, 8, 14477. [Google Scholar] [CrossRef]
- Zhang, Z.; Lian, X.Y.; Li, S.; Stringer, J.L. Characterization of chemical ingredients and anticonvulsant activity of American skullcap (Scutellaria lateriflora). Phytomedicine 2009, 16, 485–493. [Google Scholar] [CrossRef]
- Millspaugh, C.F. American Medicinal Plants Charles F Millspaugh; Dover Publications: New York, NY, USA, 1974; ISBN 0486230341. [Google Scholar]
- Upton, R.; Dayu, R.H. Skullcap Scutellaria lateriflora L.: An American nervine. J. Herb. Med. 2012, 2, 76–96. [Google Scholar] [CrossRef]
- Cole, I.B.; Alan, A.R.; Saxena, P.K.; Murch, S.; Cao, J.; Murch, S.J. Comparisons of Scutellaria baicalensis, Scutellaria lateriflora and Scutellaria racemosa: Genome Size, Antioxidant Potential and Phytochemistry. Planta Med. 2008, 74, 474–481. [Google Scholar] [CrossRef]
- Costine, B.; Zhang, M.Z.; Chhajed, S.; Pearson, B.; Chen, S.X.; Nadakuduti, S.S. Exploring native Scutellaria species provides insight into differential accumulation of flavones with medicinal properties. Sci. Rep. 2022, 12, 13201. [Google Scholar] [CrossRef]
- Liu, Y.; Fang, S.; Yang, W.; Shang, X.; Fu, X. Light quality affects flavonoid production and related gene expression in Cyclocarya paliurus. J. Photochem. Photobiol. B Biol. 2018, 179, 66–73. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, G.; Wang, G.; Cao, F. Ginkgo biloba l. Responds to red and blue light: Via phenylpropanoid and flavonoid biosynthesis pathway. Forests 2021, 12, 1079. [Google Scholar] [CrossRef]
- Taulavuori, K.; Pyysalo, A.; Taulavuori, E.; Julkunen-Tiitto, R. Responses of phenolic acid and flavonoid synthesis to blue and blue-violet light depends on plant species. Environ. Exp. Bot. 2018, 150, 183–187. [Google Scholar] [CrossRef]
- Liu, H.K.; Chen, Y.Y.; Hu, T.T.; Zhang, S.J.; Zhang, Y.H.; Zhao, T.Y.; Yu, H.E.; Kang, Y.F. The influence of light-emitting diodes on the phenolic compounds and antioxidant activities in pea sprouts. J. Funct. Foods 2016, 25, 459–465. [Google Scholar] [CrossRef]
- Ying, Q.; Jones-Baumgardt, C.; Zheng, Y.; Bozzo, G. The Proportion of Blue Light from Light-emitting Diodes Alters Microgreen Phytochemical Profiles in a Species-specific Manner. HortScience 2021, 56, 13–20. [Google Scholar] [CrossRef]
- Kawka, B.; Kwiecień, I.; Ekiert, H. Influence of Culture Medium Composition and Light Conditions on the Accumulation of Bioactive Compounds in Shoot Cultures of Scutellaria lateriflora L. (American Skullcap) Grown In Vitro. Appl. Biochem. Biotechnol. 2017, 183, 1414–1425. [Google Scholar] [CrossRef]
- Stepanova, A.; Solov’yova, A.; Salamaikina, S. Influence of spectral light composition on flavones formation in callus culture of Scutellaria baicalensis georgi. Pharmacogn. Mag. 2020, 16, 156. [Google Scholar] [CrossRef]
- Yeo, H.J.; Park, C.H.; Park, S.Y.; Chung, S.O.; Kim, J.K.; Park, S.U. Metabolic analysis of root, stem, and leaf of scutellaria baicalensis plantlets treated with different led lights. Plants 2021, 10, 940. [Google Scholar] [CrossRef]
- Wilson, S.B.; Steppe, C.; Deng, Z.; Druffel, K.; Knox, G.W.; van Santen, E. Landscape Performance, Flowering, and Female Fertility of Eight Trailing Lantana Varieties Grown in Central and Northern Florida. HortScience 2020, 55, 1737–1743. [Google Scholar] [CrossRef]
- Askey, B.C.; Liu, D.; Rubin, G.M.; Kunik, A.R.; Song, Y.H.; Ding, Y.; Kim, J. Metabolite profiling reveals organ-specific flavone accumulation in Scutellaria and identifies a scutellarin isomer isoscutellarein 8-O-β-glucuronopyranoside. Plant Direct 2021, 5, e372. [Google Scholar] [CrossRef]
- Wollaeger, H.M.; Runkle, E.S. Growth of Impatiens, Petunia, Salvia, and Tomato Seedlings under Blue, Green, and Red Light-emitting Diodes. HortScience 2014, 49, 734–740. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Rapid Suppression of Growth by Blue Light. Plant Physiol. 1981, 67, 584–590. [Google Scholar] [CrossRef]
- Kalaitzoglou, P.; Taylor, C.; Calders, K.; Hogervorst, M.; van Ieperen, W.; Harbinson, J.; de Visser, P.; Nicole, C.C.S.; Marcelis, L.F.M. Unraveling the effects of blue light in an artificial solar background light on growth of tomato plants. Environ. Exp. Bot. 2021, 184, 104377. [Google Scholar] [CrossRef]
- Kang, W.H.; Park, J.S.; Park, K.S.; Son, J.E. Leaf photosynthetic rate, growth, and morphology of lettuce under different fractions of red, blue, and green light from light-emitting diodes (LEDs). Hortic. Environ. Biotechnol. 2016, 57, 573–579. [Google Scholar] [CrossRef]
- Mu, R.; Lyu, X.; Ji, R.; Liu, J.; Zhao, T.; Li, H.; Liu, B. GmBICs Modulate Low Blue Light-Induced Stem Elongation in Soybean. Front. Plant Sci. 2022, 13, 803122. [Google Scholar] [CrossRef] [PubMed]
- Fraser, D.P.; Hayes, S.; Franklin, K.A. Photoreceptor crosstalk in shade avoidance. Curr. Opin. Plant Biol. 2016, 33, 1–7. [Google Scholar] [CrossRef]
- Pooam, M.; Arthaut, L.D.; Burdick, D.; Link, J.; Martino, C.F.; Ahmad, M. Magnetic sensitivity mediated by the Arabidopsis blue-light receptor cryptochrome occurs during flavin reoxidation in the dark. Planta 2019, 249, 319–332. [Google Scholar] [CrossRef]
- Kharshiing, E.V.; Ibapalei, O.; Mawphlang, L.; Lama, V.; Bhattacharjee, R.; Sahoo, L. Manipulation of light environment for optimising photoreceptor activity towards enhancing plant traits of agronomic and horticultural importance in crops. J. Hortic. Sci. Biotechnol. 2022, 97, 535–551. [Google Scholar] [CrossRef]
- Eskins, K. Light-quality effects on Arabidopsis development. Red, blue and far-red regulation of flowering and morphology. Physiol. Plant. 1992, 86, 439–444. [Google Scholar] [CrossRef]
- Shibuya, T.; Takahashi, T.; Hashimoto, S.; Nishiyama, M.; Kanayama, Y. Effects of overnight radiation with monochromatic far-red and blue light on flower budding and expression of flowering-related and light quality-responsive genes in Eustoma grandiflorum. J. Agric. Meteorol. 2019, 75, 160–165. [Google Scholar] [CrossRef]
- Park, Y.G.; Jeong, B.R. How Supplementary or Night-Interrupting Low-Intensity Blue Light Affects the Flower Induction in Chrysanthemum, a Qualitative Short-Day Plant. Plants 2020, 9, 1694. [Google Scholar] [CrossRef]
- El-Esawi, M.; Arthaut, L.D.; Jourdan, N.; D’Harlingue, A.; Link, J.; Martino, C.F.; Ahmad, M. Blue-light induced biosynthesis of ROS contributes to the signaling mechanism of Arabidopsis cryptochrome. Sci. Rep. 2017, 7, 13875. [Google Scholar] [CrossRef]
- Consentino, L.; Lambert, S.; Martino, C.; Jourdan, N.; Bouchet, P.E.; Witczak, J.; Castello, P.; El-Esawi, M.; Corbineau, F.; d’Harlingue, A.; et al. Blue-light dependent reactive oxygen species formation by Arabidopsis cryptochrome may define a novel evolutionarily conserved signaling mechanism. New Phytol. 2015, 206, 1450–1462. [Google Scholar] [CrossRef] [PubMed]


| S. baicalensis Leaves | S. baicalensis Roots | S. lateriflora Leaves | ||||
|---|---|---|---|---|---|---|
| Flavone | Control | Blue | Control | Blue | Control | Blue |
| Wogonoside | ND | ND | 22.38 ± 4.02 | 14.86 ± 2.04 | 3.71 ± 0.2 | 3.62 ± 0.4 |
| Wogonin | ND | ND | 1.54 ± 0.3 | 2.71 ± 0.3 * | 0.35 ± 0.04 | ND |
| Baicalin | 1.19 ± 0.4 | 1.08 ± 0.3 | 100.77 ± 6.7 | 80.54 ± 10.2 | 27.81 ± 1.7 | 36.6 ± 2.03 * |
| Baicalein | ND | ND | 6.8 ± 1.3 | 17.3 ± 2.5 * | 5.29 ± 0.8 * | 2.29 ± 0.5 |
| Chrysin | ND | ND | ND | 0.1 ± 0.01 | 0.11 ± 0.02 | 0.07 ± 0.01 |
| Scutellarin | 15.63 ± 2.6 | 21.66 ± 4.0 | 8.71 ± 0.6 | 8.41 ± 0.7 | 0.72 ± 0.2 | 2.85 ± 0.2 * |
| Scutellarein | 1.38 ± 0.4 | 0.83 ± 0.02 | 0.85 ± 0.02 | ND | 4.5 ± 0.2 | 5.34 ± 0.2 * |
| Apigenin | ND | ND | ND | ND | 0.41 ± 0.07 | 0.43 ± 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costine, B.; Zhang, M.; Pearson, B.; Nadakuduti, S.S. Impact of Blue Light on Plant Growth, Flowering and Accumulation of Medicinal Flavones in Scutellaria baicalensis and S. lateriflora. Horticulturae 2022, 8, 1141. https://doi.org/10.3390/horticulturae8121141
Costine B, Zhang M, Pearson B, Nadakuduti SS. Impact of Blue Light on Plant Growth, Flowering and Accumulation of Medicinal Flavones in Scutellaria baicalensis and S. lateriflora. Horticulturae. 2022; 8(12):1141. https://doi.org/10.3390/horticulturae8121141
Chicago/Turabian StyleCostine, Blake, Mengzi Zhang, Brian Pearson, and Satya Swathi Nadakuduti. 2022. "Impact of Blue Light on Plant Growth, Flowering and Accumulation of Medicinal Flavones in Scutellaria baicalensis and S. lateriflora" Horticulturae 8, no. 12: 1141. https://doi.org/10.3390/horticulturae8121141
APA StyleCostine, B., Zhang, M., Pearson, B., & Nadakuduti, S. S. (2022). Impact of Blue Light on Plant Growth, Flowering and Accumulation of Medicinal Flavones in Scutellaria baicalensis and S. lateriflora. Horticulturae, 8(12), 1141. https://doi.org/10.3390/horticulturae8121141








